Abstract
The biotransformation of HgII in pH-controlled and aerated algal cultures was investigated. Previous researchers have observed losses in Hg detection in vitro with the addition of cysteine under acid reduction conditions in the presence of SnCl2. They proposed that this was the effect of Hg-thiol complexing. The present study found that cysteine-Hg, protein and nonprotein thiol chelates, and nucleoside chelates of Hg were all fully detectable under acid reduction conditions without previous digestion. Furthermore, organic (R-Hg) mercury compounds could not be detected under either the acid or alkaline reduction conditions, and only β-HgS was detected under alkaline and not under acid SnCl2 reduction conditions. The blue-green alga Limnothrix planctonica biotransformed the bulk of HgII applied as HgCl2 into a form with the analytical properties of β-HgS. Similar results were obtained for the eukaryotic alga Selenastrum minutum. No evidence for the synthesis of organomercurials such as CH3Hg+ was obtained from analysis of either airstream or biomass samples under the aerobic conditions of the study. An analytical procedure that involved both acid and alkaline reduction was developed. It provides the first selective method for the determination of β-HgS in biological samples. Under aerobic conditions, HgII is biotransformed mainly into β-HgS (meta-cinnabar), and this occurs in both prokaryotic and eukaryotic algae. This has important implications with respect to identification of mercury species and cycling in aquatic habitats.
Mercury in the environment accumulates both naturally and because of industrial practices that began in the first half of the 20th century. Although most of the mercury becomes deposited in soils bound to clay particles or organic compounds (30), a significant amount enters aquatic food chains (17).
Mercury (HgII) carried by rainfall is the main source for this heavy metal in areas not affected directly by industry (49). Significant amounts of environmental CH3Hg+, the most toxic mercury species, can occur in these regions (13). Although the aquatic chemistry (47) and cycling of mercury in the environment have been characterized (14, 58), the effect of microbial biotransformation on the qualitative and quantitative fates of HgII is still poorly understood.
Biotransformation of HgII has the potential to affect any subsequent interactions of the metal during the mercury cycle. While the biochemistry and genetics of several Hg biotransformation mechanisms are well known (3, 14, 23, 25, 31, 43, 44), there is a paucity of quantitative data (17). It is clear that prokaryotic methylation (21) and reduction to volatile Hg0 do play important roles in the fate of HgII (16, 30); however, the quantification of Hg loss by volatilization and the degree of biotransformation leading to the retention of Hg are needed to fully understand the biogeochemistry of this heavy metal (2).
Several researchers have used cold-vapor atomic spectrometry (CVAS) of mercury in the analysis of biological samples (4, 8-12, 15, 20, 26, 27, 51). This method requires that sample Hg be reduced to Hg0. The acid reduction method introduced by Hatch and Ott (22) has been used to measure total ionic mercury, whereas the alkaline method of Magos (27) discriminates between inorganic and organic mercury, with the latter using CdCl2 cleavage of C-Hg bonds in undigested samples. Daniels and Wigfield (8-12) addressed reagent optimization, reduction conditions, signal processing, sensitivity, and HCl reagent interferences during mercury analysis.
Daniels and Wigfield (8-12) observed that l-cysteine added to HgII samples acted as a complexing agent, lowering the detectable Hg under acid conditions. Therefore, this method could provide a means by which to selectively determine sulfhydryl-bound Hg2+. Bramanti and colleagues (6) have shown differences in the reductive behaviors of HgII and HgII-thiol complexes. However, the direct identification of mercurial forms in biological samples has not been attempted using nonacidic reducing conditions. In the present study, we assessed the susceptibility of mercurial compounds and complexes to both acid and alkaline reduction. These compounds and complexes included protein and nonprotein thiol complexes, cysteine and nucleoside complexes, Hg2+, HgS, and C-Hg-bonded organomercurials.
This method of identifying mercurial species has ramifications beyond chemical observations. The toxicity of HgII to biota relies primarily on its interaction with functional and structural sulfhydryl groups of enzymes and other biological macromolecules. Furthermore, a role for detoxification by sulfhydryl compounds such as glutathione, metallothionein (MT), and phytochelatin is suspected in most systems, but direct demonstrations of these complexes are seldom available.
Many determinations of Hg under acid reduction conditions have also used a prereduction complete digestion of the biota with acid to yield total inorganic Hg in the form of HgII for determination (15, 26, 37, 52). Although this predigestion enables the accurate measurement of total inorganic Hg, it alters chemical compartmentation within the biotransformed pools of mercury. Therefore, this study also examined Hg detection in undigested Hg2+-dosed organisms under both acid and alkaline reduction conditions.
MATERIALS AND METHODS
Cold-vapor atomic spectrometry of mercury.
A cold-vapor atomic spectrometer (LDC/Milton Roy mercuryMonitor) was used to detect Hg0 at 253.7 nm. Nitrogen carrier gas governed by a Matheson flow controller at 800 ml/min was passed through the CVAS detector reference cell, removing the Hg0. This was then dried of water vapor by passage through an ice-chilled tube followed by a 3-cm bed of MgClO4, prior to obtaining readings. Absorbance was recorded with a C4100 LDC integrator. Calibration used freshly prepared HgCl2 standards and blind testing by Analytical Products Group Laboratories (Belpre, OH) ensured quality control.
Biological sample preparation.
Limnothrix planctonica (Lemm.), a prokaryotic blue-green alga in the family Oscillatoriaceae, was isolated from an epiphytic habitat on the surface of leaves of bullhead lily (Nuphar variegatum), obtained from a wetland region of Tasso Lake, Township of Lake of Bays, Ontario, Canada. The isolate was sensitive to streptomycin and penicillin G, and chromatography confirmed the presence of only chlorophyll a; these are all characteristics of cyanobacteria. The eukaryotic alga Selenastrum minutum (UTEX 2457) was obtained from the Culture Collection of Algae, University of Texas at Austin.
These microbes were cultured by inoculation into 250 ml of pH-controlled culture medium (pH 6.5) under axenic conditions at 25°C, which were maintained with a water jacket and 400 μE/m2/s of fluorescent light. This was a saturating light level with respect to algal photosynthesis. The cyanobacterial culture medium was composed of 5 mM (NH4)2S04, 1.0 mM KH2P04, 0.5 mM MgS04 · 7H2O, 0.33 mM CaCl2, 10 μM Na2EDTA, 2 μM FeCl3 · 6H2O, 1 μM MnCl2 · 4H2O, 0.2 μM ZnCl2, 0.1 μM Na2MoO4 · 2H2O, and 0.05 μM CoCl2 · 6H2O in deionized H2O.
Selenastrum minutum was grown in a general algal medium composed of 3 mM NaNO3, 0.15 mM MgSO4 · 7H2O, 0.12 mM CaCl2, O.12 mM K2HPO4, 95 μM Na2CO3, 46 μM Na2EDTA, 42 μM FeSO4 · 7H2O, 2 μM H3BO3, 0.4 μM MnSO4 · 4H2O, 0.04 μM ZnSO4 · 7H2O, 0.04 μM AlK(SO4)2 · 12H2O, 0.04 μM KBr, 0.04 μM Ni(NH4)2(SO4)2 · 6H2O, 0.02 μM CuSO4 · 5H2O, 0.02 μM Co(NO3)2 · 6H2O, 0.02 μM KI, 0.02 μM Cd(NO3)2 · 4H2O, 0.005 μM VSO4 · 2H2O, 0.004 μM Na2WO4 · 2H2O, 0.004 μM Cr(NO3)2 · 7H2O, and 0.003 μM (NH4)6Mo7O24 · 4H2O.
During the culture period, the pH was maintained by automated titration with 0.02 M HCl, a concentration which permitted pH control without adversely affecting the algae. Growth was monitored by measuring absorption, and HgCl2 doses were added when the optical density at 665 nm (OD665) reached 0.32.
(i) Mercury treatment.
Historical environmental mercury exposure rates were used to choose the treatment levels employed in this study (28). The selected cell culture density (OD665 = 0.32) is typical of eutrophic conditions, and mercury doses of 100 to 200 ppb Hg have been commonly reported in the coastal waters of industrial Asia (33, 57). This is also the cell density-to-mercury ratio of oligotrophic conditions (2 orders of magnitude lower) at an exposure of 1 ppb Hg2+. For example, the European Union permits an average monthly maximum allowable discharge of 50 ppb (28). Thus, this study emulates mercury exposure rates commonly found in the environment.
(ii) Undigested Hg samples.
Samples of 100 μl were taken from cultures of Limnothrix planctonica treated with 100 ppb HgCl2 and placed in reduction vessels under either acid or alkaline conditions as desired. The standard errors (SE) of the acid-reducible and alkaline-reducible Hg determination methods were 2.0 and 2.2%, respectively, of the mean values (n = 8). Each experiment was done in triplicate.
(iii) Total Hg of cultures using acid digestion.
Samples of between 0.5 and 1 ml were taken from HgCl2-treated cultures, introduced into vials preloaded with an equal volume of concentrated HNO3 for acid digestion, and refrigerated until analyzed (52). Vials were borosilicate glass sealed with solid Teflon-lined caps (catalog no. 2-7162/3; Supelco Ltd., Canada). The SE was 1.1% of the mean determined value (n = 8). Again, each experiment was performed in triplicate.
(iv) Culture fractionation.
Culture samples (0.5 to 5 ml) were centrifuged at 3,000 × g. Supernatant samples representing the culture media were added to concentrated HNO3 (1:1). The remaining supernatant was then aspirated off, and the pellet was resuspended to the original density with 5 mM dithioerythreitol (DTE) and recentrifuged. The DTE wash was sampled and aspirated off as described above. The pellet was then resuspended in 1 ml concentrated HNO3, transferred to a borosilicate glass vial, and sealed with a Teflon-lined cap. The SE of the medium, DTE wash, and pellet determinations were 3.2, 7.2, and 0.7, respectively, of the mean values (n = 8). Each experiment was performed in triplicate.
Airstream Hg fractionation and analysis.
A 30-cm glass condenser tube, immersed in ice, was placed between the cultures and mercury collector tubes to prevent physical carryover of Hg in the condensate. Total airstream Hg was collected by bubbling culture exhaust through two scrubber solutions composed of 100 ml demineralized H2O, 50 mg charcoal, and 25 ml concentrated HNO3. Samples were stored in borosilicate glass vials with Teflon caps and analyzed by CVAS. The error of the method was 1.4% ± 0.24% of the mean value (n = 8 samples) determined in triplicate experiments. When blanks composed of control cultures were bubbled with 0.5% CO2 in air, the total Hg collected after 12 h was below the detection limit.
The nature of the evolved mercurials from normal aerobic cultures was determined using a qualitative thermal desorption method. Charged and organic mercury compounds were trapped on carbon, and Hg0 was trapped on silver. The compounds were collected in thermal desorption tubes and purged as Hg0 into the CVAS detector.
Briefly, the thermal desorption unit consisted of an inner quartz glass tube (melting point, 1,410°C) and an outer borosilicate glass jacket. A flow of cooling air between the jacket and inner tube assisted in temperature control. Two independently controlled heater coils wrapped two sections of the inner tube, providing a maximum operating temperature of 1,064°C that was monitored using an HHM57 multimeter/thermometer (Omega, Laval, Canada).
The desorption unit was based on that of Trujillo and Campbell (53), with the following modifications. (i) The sample desorption chamber was slip-lined with heat-resistant fiberglass, reducing the standard error in temperature over 1-cm intervals to 4.7%. In the original design, hot spots created by direct contact of the tube with the chamber walls and uneven heat distribution around each coil of nichrome wire resulted in a 21.9% standard error in temperature over the same intervals. (ii) Oxygen-free nitrogen was used as a carrier gas to prevent the formation of Hg0, which can occur at high temperature. (iii) Carbotrap 20/40 mesh (Sigma-Aldrich, Oakville, Canada) replaced the now-discontinued Carbosieve B (5) as a thermal desorption matrix for the separation of organomercury (retained) and Hg0 (not retained) in airstream samples. (iv) The silver-treated support was Chromosorb P 45-60 mesh (catalog no. C4139; Sigma-Aldrich), with silver treatment by the Brashear process (46).
Standards for Hg0 were produced by reducing HgCl2 in the reduction vessel of the CVAS apparatus. CH3HgCl gas was generated by depositing known amounts in stage 2 of the thermal desorption unit and heating to 100°C, at which point it volatilizes but does not decompose (56). This gas-phase procedure was carried out in a closed system with appropriate ventilation and exhaust scrubbers. When necessary, samples were stored under nitrogen gas.
Chemical reduction of samples.
All samples, except those from the airstream described above, were reduced prior to detection by CVAS.
(i) Acid reduction.
The acid reduction method was based on that of Hatch and Ott (22). A 30-ml reduction vessel fitted with a bubbler containing 6 ml of freshly made 10% SnCl2 in 10% HNO3 was purged to baseline, followed by addition of the Hg sample.
(ii) Alkaline reduction.
The alkaline reduction method was based on the method of Magos (27). A 30-ml reduction vessel containing 6.0 ml of 10% SnCl2 in 10% HNO3 and 3.5 ml of 45% aqueous KOH was purged to baseline prior to addition of the Hg sample.
(iii) Organic mercury reduction.
Also based on the original technique of Magos (27), a CdCl2 reagent was prepared by mixing 25 g SnCl2 and 5 g CdCl2 in 30 ml deionized H2O. This was heated to boiling in a fume hood, cooled, and made up to 50 ml with deionized H2O. Two hundred microliters of this reagent was added to the alkaline reagent given above.
Synthesis and determination of mercurial standards.
Mercuric nucleoside complexes were synthesized as described by Buncel and colleagues (7). Mercuric glutathione, mercuric metallothionein, and mercuric cysteine were produced by a standard method of synthesis and purification (42). Mercuric metallothionein was made from horse kidney metallothioneins (catalog no. M-4766; Sigma, Oakville, Canada), which were chosen as a typical mixture of this globular, cysteine-rich, low-molecular-mass (9,720-Da) protein (24). Mercury contents for these mercurials were determined by CVAS. In the cases of mercuric glutathione and mercuric metallothionein, peptide bonds were quantified by measuring absorbance at 205 nm (41).
Preparation of β-mercuric sulfide (meta-cinnabar) was not required because Puratronic grade β-HgS (black, mercury II sulfide; catalog no. 12992) was available from Alfa Aesar (Ward Hill, MA). The batch was determined to be at least 99.999% pure (metals basis). Standards for determination by acid or alkaline reduction were made up by mixing a known quantity of β-HgS with a measured amount of glass powder and adding known portions of this mixture to the reduction vessel.
The role of solubility in the reduction of β-HgS to Hg0 was tested by using Na2S solvent. A saturated aqueous solution of Na2S was added dropwise to a reaction vessel containing β-HgS and 6 ml of 10% aqueous SnCl2. Acid was omitted, and bubbling with N2 avoided the reaction of Na2S with CO2 in air, either of which could evolve large enough amounts of H2S to interfere with the Hg absorption line (54).
Thin-layer chromatography.
Plates (20 cm by 20 cm by 250 μm) were prepared using a 1:2 (wt/vol) slurry of binderless silica gel (Kieselgel; Merck) and dried at 105°C for 48 h. Mercurials and biological extracts were applied as spots on the origin, and derivatized in situ with fresh 0.1% dithizone (diphenylthiocarbazone) in CCl4. The development time in cyclohexane-acetone (9:1) was 2 h at 25°C. Standards of diethylmercury, ethylmethylmercury, and methylmercuric chloride were dissolved in acetone-aqueous HgCl2 in water in Teflon vials, Hg complexes in 0.1 M ammonium formate buffer, and β-HgS in alkali (16% aqueous KOH). Preparation of extracts of biological samples involved homogenization in 4 M HCl followed by centrifugation at 16,000 × g for 10 min. Mercury compounds were then extracted into chloroform, and this was used to spot the samples on the origin of the chromatogram (1). Development of chromatograms for β-HgS was also performed using 95% ethanol-45% KOH (1:1).
Gel chromatography.
Gel chromatography was used to purify mercuric glutathione and mercuric metallothionein from their respective reactants, prior to testing compounds for resistance to acid reduction. The method was also used to separate cell extract mercurials. Prepacked Sephadex G-25 M PD-10 columns (Pharmacia, Mississauga, Canada) were previously observed to separate mercuric glutathione from the individual reactants (42). Columns were washed until the effluent gave baseline absorbance (A205) to remove any interference from the 0.15% Kathon CG preservative. Dowex 50-X8 (200-400 mesh) cation-exchange resin (Dow Chemical Co., MI) was added (1 cm) above the G-25 medium to remove excess HgII ions without hindering neutral complexes. Following elution of complexes, HgII was eluted using 2 M HCl, and finally alkali-soluble mercury was eluted with 45% KOH. All separations were conducted at 8°C as follows. Samples (0.3 to 0.5 ml) were run into the beginning of the column bed, the outflow was stoppered, and the bed was carefully overlaid with 0.5 ml of eluting buffer (0.1 M ammonium formate). A sleeve septa (1-cm diameter) sealed the top of the column, after which the outflow stopper was removed. The column was mounted under a 125-ml separatory funnel of eluting buffer that was connected by Tygon tubing to a Gilmont flow meter set at 14 ml/h. The flow meter was connected to a sterile 16-gauge needle, and the elution buffer was supplied by piercing the septa with the needle, followed by initiating the flow from the separatory funnel. Samples were collected below the column at 1-min intervals and analyzed for both mercury content by CVAS and peptide bonds by absorbance at 205 nm.
Chemical reagents.
All nonmercury reagents were carefully selected for low Hg content. Mercury-grade SnCl2 was obtained from Mallinckrodt Chemicals (Phillipsburg, NJ). All other reagents were AnalR grade or better, including nitric acid used in digestion and reduction reagents, which gave blank Hg readings below baseline. The KOH for reduction was AnalR grade and had to be purged of a minor (<1 ppb) Hg background prior to sample addition. All standard Hg compounds were obtained from BDH (Toronto, Canada) or Sigma-Aldrich (Oakville, Canada), except for Puratronic grade β-HgS (black; Alfa Aesar catalog no. 12992; 99.999% pure), which was obtained from Johnson-Mathey (Ward Hill, MA). All mercurials were analyzed by thin-layer or column chromatography to ensure their purity.
Statistics.
The data presented in Fig. 1 and 2 include standard errors. Each of these experiments was carried out at least three times, and results of a representative experiment are presented, with n = 3 for all Hg measurements. Error bars in the figures represent SE, and these are not given if the SE is less than the size of the data point symbol. Student t tests were performed when appropriate.
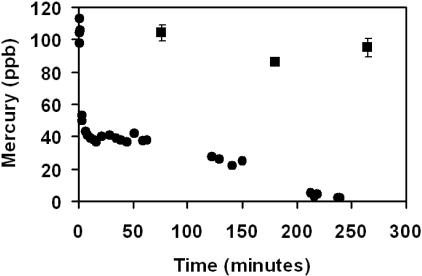
FIG. 1.
Biotransformation of a sublethal dose of HgII (120 ppb HgCl2) by the prokaryotic blue-green alga Limnothrix planctonica over 5 h, showing total (alkaline-detectable) (▪) and acid-detectable (•) inorganic Hg. The culture had an initial OD665 of 0.32. Values are means and SE; n = 3.
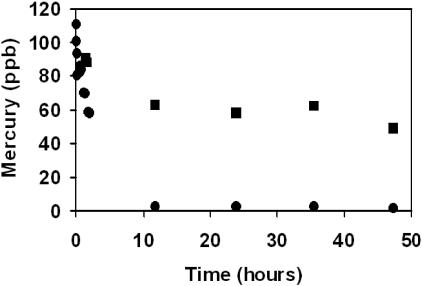
FIG. 2.
Biotransformation of 100 ppb HgII (supplied as HgCl2) by the eukaryotic alga Selenastrum minutum over 48 h, showing total (alkaline-detectable) Hg (▪) and acid-detectable (•)inorganic Hg. The culture had an initial OD665 of 0.32. Values are means and SE; n = 3.
RESULTS AND DISCUSSION
This study used cold-vapor atomic absorption spectrometry with a detection limit of 0.l ng for 1-ml samples (1, 20, 22, 26).
Biological sample preparation.
Although there are numerous methods for digestion of Hg in microbiota, HNO3 was used because of concerns over artifacts introduced by HCl digests (9) and aqua regia fumes that interfere at 253.7-nm absorbance.
Quality control was ensured by using standards of HgCl2 prepared in 10% HNO3 as well as certified standards (BDH). External quality testing determined that recoveries of blind samples ranged from 102.8 to 108.4%, which is within Environmental Protection Agency limits for quality-assured Hg analysis.
Total Hg concentrations as determined for whole culture by acid digests (1 ml sample to 1 ml concentrated HNO3) followed by either acid, alkaline, or alkaline/CdCl2 (organomercury) reduction methods were not significantly different (t test, P > 0.1; n = 4). Blue-green algae grown in pH-maintained cultures in the absence of added mercury for 24 h had a background total Hg concentration of 0.76 ± 0.1 ppb Hg, or less than 1% of the lowest experimentally applied level of Hg. Digests containing up to 50 mg (dry weight) of algal biomass at 500 ppb HgII were not significantly different from 500 ppb HgII standards alone (data not shown).
Thiol-containing compounds were used to remove cell-wall bound HgII. Comparisons of washes with DTE and cysteine showed no significant difference in the amounts of Hg removed from algal cell pellets (data not shown), and second washes removed less than 3% of the first wash. DTE was used instead of l-cysteine because the pH of the former solution was 6.5, while the latter was strongly alkaline. Single washes were used, as repeated exposure to DTE could solubilize β-HgS (55), the crystals of which may be localized in and on cell walls (32, 39).
Budget analysis showed recoveries from cultures of microorganisms ranging from 88% to 95.1% when Hg from digested whole cultures and the airstream from bubblers were accounted for. With high recoveries established, airstream Hg was thereafter determined by subtracting that in whole-culture digests from the initially applied dose. That value therefore represents a maximum amount of Hg volatilized, and any interaction with culture vessels would reduce that amount.
Thermal desorption: separation of Hg0 and methylmercury with Carbotrap and silver-treated Chromosorb P.
Carbotrap exhibited minor quantitative retention of Hg0, excellent retention of organomercurials, and passage of Hg0 in real time without drag. At an Hg0 treatment equivalent to 1 ml of 100 ppb Hg, 2.8% appeared on the Carbotrap, with the remaining 97.2% being trapped on the silver-treated Chromosorb tube. At higher doses no significant increase (t test, P > 0.25; n = 4) in the actual quantity of Hg0 retained by the Carbotrap was observed, suggesting that a saturable number of Hg0 binding sites exist and that collecting larger samples minimizes this effect. The results were not interpreted to imply organomercury generation unless 3 times this background or greater was observed on Carbotrap tubes.
A series of silver-treated Chromosorb P tubes were used to establish the number required to effectively trap Hg0. This demonstrated that 99.8% of a given Hg0 dose was trapped on the first tube.
Bloom (5) used Carbotrap to trap volatile ethylation derivatives and other organomercurials, but not methylmercuric chloride. Our tests with methylmercury found that 99.8% of a gas phase standard bound to the first silver-treated tube. Therefore, to separate organic mercury from Hg0, the silver-treated tube must follow the Carbotrap tube. With this arrangement, 96.8% ± 1.5% (mean ± SE; n = 4) of the methylmercury gas was trapped in the Carbotrap tube, while only 3.2% ± 1.5% was found on the silver tube.
The trap-and-purge analysis of Limnothrix planctonica cultures indicated that 98.8% of volatilized Hg passed through the Carbotrap tube to be trapped by silver-treated Chromosorb P, thus indicating that the volatile mercury is Hg0, with no apparent organomercurials being produced.
Thin-layer chromatography.
Diethylmercury (retention factor [Rf] = 1.0) derivatized in situ with fresh 0.1% diphenylthiocarbazone in CCl4 was red, whereas ethylmercury (Rf = 0.95) and methylmercury (Rf = 0.9) were both yellow-orange and HgII (Rf = 0.5) and β-HgS (Rf = 0.0) were purple. In situ derivitization allowed visualization of compounds throughout the separation procedure. The Hg contents of spots were confirmed using CVAS. Derivatives of other metals are known to be yellow-orange and to migrate at the solvent front (50). Because β-HgS and the major biotransformed mercurial compound in algae did not migrate in cyclohexane-acetone (9:1), their separation was also performed using 95% ethanol-45% KOH (1:1). These two mercurials also migrated together under these conditions (Rf = 0.45).
Synthesis and determination of mercurials.
A series of mercurial standards were purchased or synthesized and analytically compared to the major biotransformed compound in algae treated with HgCl2, thereby providing evidence as to the identity of this mercurial.
(i) Nucleoside phosphate.
Determination of a mercuric nucleoside complex (mercuric guanosine-5′PO4) under both reduction conditions showed a slight but significant (t test, P = 0.025; n = 4) decrease to 93.2% in detection under alkaline compared to acid reduction conditions. Furthermore, no significant difference (t test, P > 0.1; n = 4) existed between total mercury in acid digests of the complex and in direct acidic reduction of mercuric nucleoside complexes.
(ii) Thiol compounds. (a) Mercuric cysteine.
Equal amounts of Hg signal were obtained under acid and alkaline reduction. Failure to include the column chromatography purification step does produce an Hg signal depression under acid conditions, as noted by Daniels and Wigfield (9).
(b) Mercuric glutathione.
The coincident elution profiles of peptides and mercury from Sephadex G-25 M PD-10 columns were used to track and purify mercuric glutathione. A peak of mercury alone eluted later and could be removed by the addition of 1 cm of Dowex 50-X8 cation-exchange resin without affecting elution of the complex. Standards used for subsequent analytical work were collected during the elution peak of the complex.
CVAS determination of the prepared mercuric glutathione showed no difference in the quantity of mercury detected using either acid or alkaline reduction conditions (t test, P > 0.05; n = 4).
(c) Mercuric metallothionein.
Metallothioneins are proteins that are rich in thiol groups. They are functional analogues of phytochelatins, which are the cysteine-rich, low-molecular-weight polypeptides found in algae and some fungi. MTs were used because phytochelatins were not commercially available.
The coincidence of peptide bonds and mercury elution from Sephadex columns was used to purify mercuric MT. Total amounts of Hg of mercuric MT determined under either acid or alkaline reduction conditions was not significantly different (t test, P > 0.05; n = 4). However, when rates of reduction were compared, a significant difference was obtained under acid (ratio of one-half peak height to one-half peak width = 5.38 ± 0.32) versus alkaline (ratio = 133.11 ± 8.074) reduction conditions (t test, P = 0.005; n = 4). This appears to be a consequence of mercury complexing to proteins, as it was not observed with Hg-cysteine. Because the MT Hg was sensitive to acid reduction, it and phytochelatins cannot be the major biotransformed compounds in algae treated with HgCl2. The significant shift in MT Hg peak height-to-width ratios under the two reduction conditions may offer an analytical tool for future consideration.
(iii) Mercuric sulfides. (a) α-Mercuric sulfide (cinnabar).
α-HgS was neither soluble in alkali nor degraded by HNO3, whereas both the major biotransformed compound in algae and β-HgS were. In addition, this mercurial has never been observed in living organisms.
(b) β-Mercuric sulfide (meta-cinnabar).
β-HgS was detected by CVAS under alkaline but not acid reduction conditions. For example, of a 47-μg sample of β-HgS, only 0.085% of the total, or 40 ng as Hg0, was detected under acid reduction conditions, whereas under alkaline reduction conditions, this value was greater than 99.9%. This is because β-HgS is soluble in alkali but not in dilute mineral acids (56), and reduction by SnCl2, generation of Hg0, and detection by CVAS require that the mercurial be in the aqueous phase. When β-HgS was dissolved by the addition of Na2S, quantitative generation of Hg0 was also observed.
Although β-HgS is prevalent in sediments (58), evidence of its existence in aerobic biota is scarce (40). Therefore, a means of detecting and quantifying this compound in organisms would be an important analytical tool.
(c) Mercuric selenide.
HgSe did not require testing, as it has been found only in marine mammals and is soluble only in aqua regia.
Biotransformation of mercuric chloride.
This study investigated the formation of an acid reduction-resistant mercurial compound or complex similar to that previously proposed to be a product of sulfhydryl chelation by Daniels and Wigfield (9). Biosynthesis of the mercurial with these properties occurred in our algal cultures. When the cultures were dosed with mercuric chloride, initially all mercury could be recovered using both acid and alkaline reduction conditions (Fig. 1 and 2). However, with increasing exposure time, total recovery of mercury during sublethal treatments with HgCl2 could be achieved only by using alkaline reduction. During the treatment of the prokaryotic alga Limnothrix planctonica, detection under acid conditions fell quickly within a first 15-min phase, followed by a second, slower phase ending with no acid-detectable mercury at 240 min (Fig. 1). Eukaryotic cultures of the alga Selenastrum minutum (UTEX 2457, UTEX Culture Collection of Algae, University of Texas, Austin) showed the same trend on a more gradual basis, over 10 to 12 h (Fig. 2). Extraction and chromatography of the major biotransformed compound in Limnothrix planctonica exposed to 100 ppb HgII for 1 h indicated that it was β-HgS. The percentage of total Hg that migrated as β-HgS (86% ± 10% of total Hg; n = 4) was not significantly different from the percentage of total Hg that was insensitive to acid SnCl2 reduction but detected by alkaline SnCl2 reduction (79% ± 4%; n = 4).
French-pressed extracts of treated algal cultures (see above) were run on chromatographic columns to remove thiol-Hg rapidly and prior to the addition of other reagents. No Hg was associated with the ammonium formate buffer at the point when standards of protein and nonprotein thiol-Hg chelates would elute. After shifting to the basic buffer (45% KOH), virtually all sample Hg eluted from the column at the same point as β-HgS standards (data not shown).
Addition of HgII to freeze-dried cultures of prokaryotic algae suspended to the same density (OD665) as live cultures resulted in only 7% ± 2% (n = 4) of the 100 ppb dose reacting to give what appeared to be β-HgS after 40 min, and this was stable for the next 3 h. In live cultures of these cyanobacteria, 70% ± 6% of the original dose was metabolized to β-HgS within 10 min. Thus, the biotransformation was strongly dependent on active cellular metabolism.
Metal sulfide biotransformation, an idea advanced by several previous groups (18, 19, 36, 38, 45, 48, 59), was shown to produce a significant mercury pool in algae. The major biotransformed mercurial compound synthesized by algae under aerobic conditions was meta-cinnabar, or β-HgS, which has been detected by electron microscopy but not quantified in Hepatophyceae, which are close relatives of eukaryotic algae (40).
Identification of the major biotransformed mercurial compound in algae involved testing and separating mercurial compounds and complexes that have been reported or suspected to occur in biological systems on the basis of a variety of properties, including its susceptibility to reduction conditions. The compound's reactivity under acid and alkaline reducing conditions was consistent with a biological conversion of the applied HgCl2 into β-HgS (meta-cinnabar) and inconsistent with its conversion into any other tested mercurial complex or compound. Analyses by thin-layer and column chromatographies also corroborated this finding. It should be noted that failure to include purification steps for cysteine-based sulfhydryl complexes, such as glutathione and metallothionein, can generate the unknown moiety described by Daniels and Wigfield (9). This might be a product of spontaneous H2S generation leading to the precipitation of some HgII as HgS.
This is also the first report of a quantitative method to study the biosynthesis of mercuric sulfide. Determination of acid- and alkaline-reducible Hg allowed real-time measurement of cellular Hg biotransformation (Fig. 1 and 2). Hg0 was the difference between the dose and alkaline-reducible Hg, whereas HgII and its complexes comprised the acid-reducible Hg, and β-HgS was the remaining alkaline-reducible mercury. Assurance that the volatile Hg produced was Hg0 and not organomercury was obtained by separation of airstream mercurials on Carbotrap and silver-treated Chromosorb P, followed by thermal desorption. The finding that the bulk of Hg was relatively rapidly converted to a form not reducible under acid conditions indicates that thiol-Hg complexes cannot be sequestering cellular Hg over the long term.
The fate of HgII applied to algae under aerobic conditions was its conversion into Hg0 and β-HgS in algal cultures. Dissolved gaseous mercury is formed in lakes as an effect of incident sunlight, which is attributable to photosynthetic organisms present mainly in the epilimnion (2). Algal production of HgS could have important implications for the fate and metabolism of Hg; however, HgS formation has long been believed to occur primarily in sediment. Questions are also raised about the potential effects on uptake by algal grazers (29, 30, 34) and about the effect of sulfur availability on the species and solubility of mercury in general, not only in sulfidic waters (35). The present findings indicate that freshwater blue-green and green algae biotransform HgII into β-HgS under controlled laboratory conditions. The degree to which this occurs in aquatic habitats under the influence of variable environmental factors remains to be determined.
It is possible that the rapid phase of mercury sulfide formation in algae occurs because of an endogenous pool of a sulfur compound that is readily available for mercury biotransformation. Subsequent cellular production of this unidentified compound may be relatively slow, thereby accounting for the low rate of second-phase conversion. Along with investigations of this nature, it is also of interest to determine if the biotransformation process is fortuitous or if it occurs as a response to mercury exposure.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada.
We thank D. Canvin and G. vanLoon for advice throughout this research project.
Footnotes
This paper is dedicated to the memory of K. Budd.
REFERENCES
- 1.Allen, S. E., J. A. Parkinson, and A. P. Rowland. 1989. Pollutants, p. 201-239. In S. E. Allen (ed.), Chemical analysis of ecological materials, 2nd ed. Blackwell Scientific Publications, Oxford, United Kingdom.
- 2.Amyot, M., G. Mierie, D. R. S. Lean, and D. J. McQueen. 1994. Sunlight-induced formation of dissolved gaseous mercury in lake waters. Environ. Sci. Technol. 28:2366-2371. [DOI] [PubMed] [Google Scholar]
- 3.Baldi, F., M. Pepi, and M. Filippelli. 1993. Methylmercury resistance in Desulfovibrio desulfuricans strains in relation to methylmercury degradation. Appl. Environ. Microbiol. 59:2479-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baselt, R. C. 1980. Biological monitoring methods for industrial chemicals, p. 178-184. Biomedical Publications, Davis, Calif.
- 5.Bloom, N. 1989. Determination of picogram levels of methylmercury by aqueous phase ethylation, followed by cryogenic gas chromatography with cold vapour atomic fluorescence detection. Can. J. Fish. Aquat. Sci. 46:1131-1140. [Google Scholar]
- 6.Bramanti, E., A. D'Ulivo, L. Lampugnani, G. Raspi, and R. Zamboni. 1999. Cold vapour atomic fluorescence studies on the behaviour of mercury(II) and mercury(II)-thiol complexes. An alternative route for characterization of -SH binding groups. J. Anal. Atom. Spectrom. 14:179-185. [Google Scholar]
- 7.Buncel, E., C. Boone, H. Joly, R. Kumar, and A. R. Norris. 1985. Metal ion-biomolecule interactions. XII. 1H and 13C NMR evidence for the preferred reaction of thymidine over guanosine in exchange and competition reactions with mercury(II) and methylmercury(II). J. Inorg. Biochem. 25:61-73. [Google Scholar]
- 8.Daniels, R. S., and D. C. Wigfield. 1989. Cold-vapor mercury atomic absorption spectrometry. I. Reagent volume optimization. Sci. Total Environ. 89:319-323. [Google Scholar]
- 9.Daniels, R. S., and D. C. Wigfield. 1989. Cold-vapour mercury atomic absorption spectrometry. II. Acidic versus alkaline reduction. Sci. Total Environ. 89:325-329. [Google Scholar]
- 10.Daniels, R. S., and D. C. Wigfield. 1989. Cold-vapor mercury atomic absorption spectrometry. III. Signal reporting options. Sci. Total Environ. 89:331-335. [Google Scholar]
- 11.Daniels, R. S., and D. C. Wigfield. 1989. Cold-vapor mercury atomic absorption spectrometry. IV. Increased sensitivity. Sci. Total Environ. 89:37-339. [Google Scholar]
- 12.Daniels, R. S., and D. C. Wigfield. 1993. Cold-vapor mercury atomic absorption spectrometry: HCl as the cause of the double peak phenomenon. J. Anal. Toxicol. 17:196-198. [DOI] [PubMed] [Google Scholar]
- 13.Dopp, E., L. M. Hartmann, A. M. Florea, A. W. Rettenmeier, and A. V. Hirner. 2004. Environmental distribution, analysis, and toxicity of organometal(loid) compounds. Crit. Rev. Toxicol. 34:31-33. [DOI] [PubMed] [Google Scholar]
- 14.Essa, A. M. M., L. E. Macaskie, and N. L. Brown. 2002. Mechanisms of mercury bioremediation. Biochem. Soc. Trans. 30:672-674. [DOI] [PubMed] [Google Scholar]
- 15.Falandysz, J., and A. Chwir. 1997. The concentrations and bioconcentration factors of mercury in mushrooms from the Mierzeja Wislana sand-bar, Northern Poland. Sci. Total Environ. 15:221-228. [DOI] [PubMed] [Google Scholar]
- 16.Frischmuth, A., P. Weppen, and W.-D. Deckwer. 1993. Microbial transformation of mercury(II). I. Isolation of microbes and characterization of their transformation capabilities. J. Biotechnol. 29:39-55. [Google Scholar]
- 17.Gabriel, M. K., and D. G. Williamson. 2004. Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environ. Geochem. Health 26:421-434. [DOI] [PubMed] [Google Scholar]
- 18.Gadd, G. M. 1993. Interactions of fungi with toxic metals. New Phytol. 124:25-60. [Google Scholar]
- 19.Galli, U., H. Schuepp, and C. Brunold. 1994. Heavy metal binding by mycorrhizal fungi. Physiol. Plant 92:364-368. [Google Scholar]
- 20.Gill, U., L. Bigras, and H. Schwartz. 2004. Routine, automated determination of inorganic and total mercury in multimedia using cold vapor atomic absorption spectrometry. Chemosphere 56:1097-1103. [DOI] [PubMed] [Google Scholar]
- 21.Hamdy, M. K., and O. R. Noyes. 1975. Formation of methylmercury by bacteria. Appl. Microbiol. 30:424-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatch, W. R., and W. L. Ott. 1968. Determination of sub-microgram quantities of mercury by atomic absorption spectrophotometry. Anal. Chem. 40:2085-2087. [Google Scholar]
- 23.Iwahori, K., F. Takeuchi, K. Kamimura, and T. Sugio. 2000. Ferrous iron-dependent volatilization of mercury by the plasma membrane of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 66:3823-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagi, J. H. R., and B. L. Vallee. 1961. Metallothionine: a cadmium-containing, zinc-containing protein from equine renal cortex. J. Biol. Chem. 236:2435-2442. [PubMed] [Google Scholar]
- 25.King, J. K., J. E. Kostka, M. E. Frischer, and F. M. Saunders. 2000. Sulfate-reducing bacteria methylate mercury at variable rates in pure culture and in marine sediments. Appl. Environ. Microbiol. 66:2430-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locatelli, C., and G. Torsi. 2001. Heavy metal determination in aquatic species for food purposes. Ann. Chim. 91:65-72. [PubMed] [Google Scholar]
- 27.Magos, L. 1971. Selective atomic-absorption determination of inorganic mercury and methylmercury in undigested biological samples. Analyst 96:847-853. [DOI] [PubMed] [Google Scholar]
- 28.Mance, G. 1987. Pollution threat of heavy metals in aquatic environments. Elsevier Applied Science, New York, N.Y.
- 29.Mason, R. P., J. R. Reinfelder, and F. M. M. Morel. 1996. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ. Sci. Technol. 30:1835-1845. [Google Scholar]
- 30.Morel, F. M. M., A. M. L. Kraepiel, and M. Amyot. 1998. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 29:543-566. [Google Scholar]
- 31.Nascimento, A. M. A., and E. Chartone-Souza. 2003. Operon mer: bacterial resistance to mercury and potential for bioremediation of contaminated environments. Genet. Mol. Res. 2:92-101. [PubMed] [Google Scholar]
- 32.Ono, B.-I., H. Ohue, and F. Ishihara. 1988. Role of cell wall in Saccharomyces cerevisiae mutants resistant to Hg2+. J. Bacteriol. 170:5877-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panda, K. K., M. Lenka, and B. B. Panda. 1992. Monitoring and assessment of mercury pollution in the vicinity of a chloralkali plant. III. Concentration and genotoxicity of mercury in the industrial effluent and contaminated water of Rushikulya estuary, India. Mutat. Res. 280:149-160. [DOI] [PubMed] [Google Scholar]
- 34.Pickhardt, P. C., C. L. Folt, C. Y. Chen, B. Klaue, and J. D. Blum. 2002. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc. Natl. Acad. Sci. USA 99:4419-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravichandran, M., G. R. Aiken, J. N. Ryan, and M. M. Reddy. 1999. Inhibition of precipitation and aggregation of metacinnabar (mercuric sulfide) by dissolved organic matter isolated from the Florida everglades. Environ. Sci. Technol. 33:1418-1423. [Google Scholar]
- 36.Reed, R. H., and G. M. Gadd. 1990. Metal tolerance in eukaryotic and prokaryotic algae, p. 105-118. In A. J. Shaw, (ed.). Heavy metal tolerance in plants: evolutionary aspects. CRC Press, Boca Raton, Fla.
- 37.Reimann, C., H. Niskavaara, G. Kashulina, P. Filzmoser, R. Boyd, T. Volden, O. Tomilina, and I. Bogatyrev. 2001. Critical remarks on the use of terrestrial moss (Hylocomium splendens and Pleurozium schreberi) for monitoring of airborne pollution. Environ. Pollut. 113:41-57. [DOI] [PubMed] [Google Scholar]
- 38.Robinson, J. B., and O. H. Tuovinen. 1984. Mechanisms of microbial resistance and detoxification of mercury and organomercury compounds: physiological, biochemical, and genetic analyses. Microbiol. Rev. 48:95-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satake, K. 1993. Chemical form of mercury in water in a volcanic acid stream, Kashiranashigawa. Jpn. Arch. Hydrobiol. 128:169-174. [Google Scholar]
- 40.Satake, K., K. Shibata, and Y. Bando. 1990. Mercury sulphide (HgS) crystals in the cell walls of the aquatic bryophytes, Jungermannia vulcanicola Steph. and Scapania undulata (L.) Dum. Aquat. Bot. 36:325-341. [Google Scholar]
- 41.Scopes, R. K. 1982. Protein purification: principles and practice. Springer-Verlag, New York, N.Y.
- 42.Shimada, H., S. Fukudome, M. Kiyozumi, T. Funakoshi, T. Adachi, A. Yasutake, and S. Kojima. 1993. Further study of effects of chelating agents on excretion of inorganic mercury in rats. Toxicology 77:157-169. [DOI] [PubMed] [Google Scholar]
- 43.Silver, S., and T. K. Misra. 1988. Plasmid-mediated heavy metal resistances. Annu. Rev. Microbiol. 42:717-743. [DOI] [PubMed] [Google Scholar]
- 44.Silver, S., and M. Walderhaug. 1992. Gene regulation of plasmid- and chromosomal-determined inorganic ion transport in bacteria. Microbiol. Rev. 56:195-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stokes, P. M. 1983. Responses of fresh-water algae to metals, p. 87-112. In F. E. Round and D. J. Chapman (ed.). Progress in phycological research, vol. 2, Elsevier, New York, N.Y. [Google Scholar]
- 46.Strong, J. 1938. Procedures in experimental physics. Prentice-Hall, Inc., New York, N.Y.
- 47.Stumm, W., and J. J. Morgan. 1981. Aquatic chemistry: an introduction emphasizing chemical equilibria in natural waters, 2nd ed., p. 121-170, 323-417. John Wiley & Sons, Toronto, Canada.
- 48.Summers, A. O., and S. Silver. 1978. Microbial transformations of metals. Annu. Rev. Microbiol. 32:637-672. [DOI] [PubMed] [Google Scholar]
- 49.Swain, E. B., D. R. Engstrom, M. E. Brigham, T. A. Henning, and P. L. Brezonik. 1992. Increasing rates of atmospheric mercury deposition in midcontinental North America. Science 257:784-787. [DOI] [PubMed] [Google Scholar]
- 50.Takeshita, R., H. Akagi, M. Fujita, and Y. Sakagami. 1970. Detection of mercury and alkylmercury compounds by reversed-phase thin-layer chromatography. J. Chromatogr. 51:283-288. [DOI] [PubMed] [Google Scholar]
- 51.Tao, G., S. N. Willie, and R. E. Sturgeon. 1998. Determination of total mercury in biological tissues by flow injection cold vapour generation atomic absorption spectrometry following tetramethylammonium hydroxide digestion. Analyst 123:1215-1218. [DOI] [PubMed] [Google Scholar]
- 52.Tong, S.-L., and W.-K. Leow. 1980. Stationary cold-vapor atomic absorption spectrometric attachment for determination of total mercury in undigested fish samples. Anal. Chem. 52:581-583. [DOI] [PubMed] [Google Scholar]
- 53.Trujillo, P. E., and E. E. Campbell. 1975. Development of a multi-stage air sampler for mercury. Anal. Chem. 47:1629-1634. [DOI] [PubMed] [Google Scholar]
- 54.Ure, A. M. 1975. The determination of mercury by non-flame atomic absorption and fluorescence spectroscopy. Anal. Chim. Acta 76:1-26. [DOI] [PubMed] [Google Scholar]
- 55.Wang, W., and C. T. Driscoll. 1995. Patterns of total mercury concentrations in Onondaga Lake, New York. Environ. Sci. Technol. 29:2261-2266. [DOI] [PubMed] [Google Scholar]
- 56.Weast, R. C., and M. J. Astle. (ed.). 1982. Handbook of chemistry and physics, 63rd ed., p. B120. CRC Press, Boca Raton, Fla.
- 57.Wetzel, R. G., and G. E. Likens. 1991. Limnological analyses, 2nd. ed. Springer-Verlag, New York, N.Y.
- 58.Winfrey, M. R., and J. W. M. Rudd. 1990. Environmental factors affecting the formation of methylmercury in low pH lakes. Environ. Toxicol. Chem. 9:853-869. [Google Scholar]
- 59.Wood, J. M., and H. K. Wang. 1985. Strategies for microbial resistance to heavy metals, p. 81-98. In W. Stumm (ed.) Chemical processes in lakes. Wiley-Interscience, Toronto, Canada.