Abstract
Livestock manures contain numerous microorganisms which can infect humans and/or animals, such as Escherichia coli O157:H7, Listeria monocytogenes, Salmonella spp., and Mycobacterium avium subsp. paratuberculosis (Mycobacterium paratuberculosis). The effects of commonly used manure treatments on the persistence of these pathogens have rarely been compared. The objective of this study was to compare the persistence of artificially inoculated M. paratuberculosis, as well as other naturally occurring pathogens, during the treatment of dairy manure under conditions that simulate three commonly used manure management methods: thermophilic composting at 55°C, manure packing at 25°C (or low-temperature composting), and liquid lagoon storage. Straw and sawdust amendments used for composting and packing were also compared. Manure was obtained from a large Ohio free-stall dairy herd and was inoculated with M. paratuberculosis at 106 CFU/g in the final mixes. For compost and pack treatments, this manure was amended with sawdust or straw to provide an optimal moisture content (60%) for composting for 56 days. To simulate liquid storage, water was added to the manure (to simulate liquid flushing and storage) and the slurry was placed in triplicate covered 4-liter Erlenmeyer flasks, incubated under ambient conditions for 175 days. The treatments were sampled on days 0, 3, 7, 14, 28, and 56 for the detection of pathogens. The persistence of M. paratuberculosis was also assessed by a PCR hybridization assay. After 56 days of composting, from 45 to 60% of the carbon in the compost treatments was converted to CO2, while no significant change in carbon content was observed in the liquid slurry. Escherichia coli, Salmonella, and Listeria were all detected in the manure and all of the treatments on day 0. After 3 days of composting at 55°C, none of these organisms were detectable. In liquid manure and pack treatments, some of these microorganisms were detectable up to 28 days. M. paratuberculosis was detected by standard culture only on day 0 in all the treatments, but was undetectable in any treatment at 3 and 7 days. On days 14, 28, and 56, M. paratuberculosis was detected in the liquid storage treatment but remained undetectable in the compost and pack treatments. However, M. paratuberculosis DNA was detectable through day 56 in all treatments and up to day 175 in liquid storage treatments. Taken together, the results indicate that high-temperature composting is more effective than pack storage or liquid storage of manure in reducing these pathogens in dairy manure. Therefore, thermophilic composting is recommended for treatment of manures destined for pathogen-sensitive environments such as those for vegetable production, residential gardening, or application to rapidly draining fields.
Livestock manure from dairies is largely stored as a liquid in lagoons or as solid packed manure, and then periodically applied to agricultural land (6, 23, 36, 38). Liquid manure storage and application can potentially lead to a variety of adverse environmental effects primarily related to liquid runoff, leaching, and odors (6, 22, 31, 36). In addition, the transportation of liquid manures to distant fields can be expensive. Another concern is that livestock manures often contain numerous human and animal pathogens such as Salmonella spp., Escherichia coli O157:H7, Listeria monocytogenes, Mycobacterium avium subsp. paratuberculosis (Mycobacterium paratuberculosis), Cryptosporidium parvum, and Giardia spp. (31). Of these pathogens, the least is known about the persistence of M. paratuberculosis in manure management systems. This is primarily due to its slow growth rate and to difficulties in culturing. It is the causal agent of paratuberculosis or Johne's disease, which is a common and chronic disease of the intestines. The disease is of worldwide occurrence among cattle and has also been reported in other domesticated ruminants, wild ruminants, and nonruminant species (21). Estimated annual economic losses to the dairy industry in excess of $200 million have been reported due to animal culling, lowered milk production, reduced carcass value, and poor reproductive performance (29). Since there are no efficacious vaccines to protect against M. paratuberculosis infection, it is important to control the disease spread through reduction of pathogen loads within infected herds. M. paratuberculosis is also important due to its suspected link with Crohn's disease (a chronic inflammatory bowel disease) in susceptible humans (5).
Composting is a manure treatment approach that addresses many of the problems associated with liquid manure systems (3, 24, 31). High-temperature composting has been shown to reduce the levels of indicator pathogens in manure (10). Composts are solids and, unlike liquid manures, do not pose a surface and groundwater pollution risk when applied to tiled farm land or during adverse weather events. Composting can reduce the weight of manure by 50 to 80%, even after amendment addition is considered, resulting in less wear on rural infrastructure and reduced transportation costs. It is an aerobic process that destroys (rather than creates) odor-causing volatile compounds such as hydrogen sulfide, volatile fatty acids, and skatoles. Finally, composts can be sold into high-value residential, potting media, bedding, and organic farming markets, while liquid manures typically have a negative value. Challenges to this approach are the increased capital costs and levels of planning and management required, as well as the availability of low-cost compost amendments. However, successful examples of swine, poultry, and dairy farms exist where composting has been used to eliminate the liquid storage of manure and reduce the costs of manure disposal.
Composting systems can vary, from static to aerated piles/windrows in the open environment to fully enclosed vessels or tunnels, depending upon the material to be composted and on environmental issues and economics (16, 24). Manures are generally composted in static or aerated piles for a period of 30 to 90 days, after which they are stored in larger curing piles until use or sale off-site (16, 24).
There is great concern about the potential for pathogen contamination of agricultural products with human and animal pathogens present in manures and composts (14, 16), especially when these are used for land application and off-farm applications that may pose an infection risk to humans or animals.
The survival of pathogenic microorganisms in manures depends upon many factors including temperature, moisture, pH, physical composition of composting material, bedding type, and microbial competition (10, 35). LeJeune and Kauffman (19) reported that Escherichia coli O157:H7 in dairy manure persisted at higher concentrations in used-sawdust bedding (similar to that used as a compost amendment) than in used-sand bedding. Himathongkham et al. (11) observed an exponential linear destruction of E. coli O157:H7 and Salmonella enterica serovar Typhimurium, in which the time to kill 90% of these bacteria ranged from 6 days to 3 weeks in manure and from 2 days to 5 weeks in manure slurry. Using a bench scale composting system at 45°C, Lung et al. (20) showed complete decay of Salmonella after 48 h or E. coli O157:H7 after 72 h. However, levels of these organisms remained unchanged when the same mixture was incubated at room temperature. Larney et al. (18) reported 99.9% elimination of total coliforms and E. coli organisms from beef feedlot manures in the first 7 days of composting when average windrow temperatures ranged from 33.5 to 41.5°C. In summary, these reports indicate that many factors including temperature, manure amendments, and moisture content affect the survival of E. coli and Salmonella and that when properly composted, pathogen loads in manure decrease to undetectable levels within days to weeks but may persist for extended periods in liquid stored manures. However, to our knowledge, there are no reports on the survival of Listeria or M. paratuberculosis in composted animal wastes (2) and few studies compare the effects of different manure management approaches on the same manure.
To monitor the presence of M. paratuberculosis, a culture technique is commonly used (34). Since M. paratuberculosis is extremely slow growing (8 to 16 weeks) on artificial media, cultures are often lost because of overgrowth by other microorganisms. Additional confirmation of the presence of M. paratuberculosis in culture medium is required to rule out false positives generated by other contaminating microorganisms. PCR amplification and detection of M. paratuberculosis-specific nucleic acids has been recommended as a quick indicator of the presence of this bacterium (26, 37). The specificity of this approach has been improved by using DNA probes that target the PCR product in microtiter plate hybridization assays (26, 32). Although PCR cannot distinguish between live and dead organisms, it is frequently used as a detection tool (33).
The hypothesis for this research was that the elevated temperatures generated during the composting of manure can better kill/eliminate M. paratuberculosis and other human and animal pathogens than conventional liquid storage of manure. Therefore, the objectives of this study were to assess the persistence of M. paratuberculosis inoculated at very high levels, as well as other pathogens including Salmonella, E. coli, and Listeria naturally occurring in manure, during the treatment of manure by composting, liquid lagoon storage, or manure pack storage (low-temperature composting). Also determined were the effects of two of the most commonly used bedding and compost amendment materials (sawdust and straw) on the survival of these pathogens. To study these objectives we used culture and PCR techniques to monitor the presence of pathogenic and indicator organisms and M. paratuberculosis during simulated treatment of the same manure sample by composting, lagoon storage, and pack storage. Changes in the physicochemical properties of the manure during these treatments were also monitored.
MATERIALS AND METHODS
Treatments and experimental design.
A laboratory scale experiment was used to study the survival of the human and animal pathogens during simulated treatments. A total of five treatments and three replications of each treatment were used. The treatments are described in Table 1.
TABLE 1.
Description of experimental treatments
| Treatment | Manure type | Amendment type | Incubation temp | Initial moisture content (%) | Simulation |
|---|---|---|---|---|---|
| DM-SD-55 | Dairy manure | Sawdust | 55°C | 58 | Thermophilic composting |
| DM-SD-25 | Dairy manure | Sawdust | 25°C | 58 | Manure pack or low-temp composting |
| DM-ST-55 | Dairy manure | Straw | 55°C | 60 | Thermophilic composting |
| DM-ST-25 | Dairy manure | Straw | 25°C | 60 | Manure pack or low-temp composting |
| DM-L-RT | Dairy manure | Water | Room temp | 85 | Lagoon storage |
(i) Reactor systems.
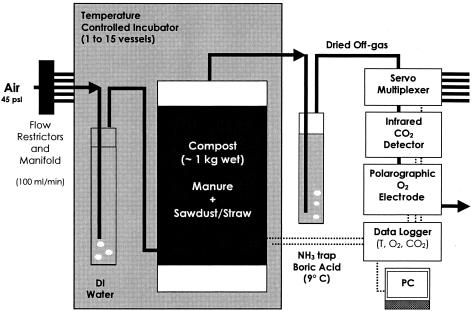
Composting for the first four treatments (Table 1) was performed using a bench scale compost reactor system (Fig. 1). The system consisted of 4-liter-capacity vessels (length 30 cm and diameter 15 cm), made of PVC pipe, placed in an incubator set at 55°C (BioCold Environmental, Inc., Fenton, MO) or 25°C (Sheldon Manufacturing Inc., Cornelius, OR). Compost mixes in the vessels were placed on two metal screens of 1-cm and 1-mm mesh sizes, respectively, supported by a perforated Plexiglas disk. To achieve an aerobic composting process for these treatments, regulated air at a −40°C dew point was restricted at a rate of 100 ml/min. This air was bubbled through bottles containing water at the incubator temperature to humidify the air entering the compost vessel and avoid drying during composting. Moist air from the bottles entered at the bottom of the vessels via tubes (TAMCO vinyl tubing; U.S. Plastic Corp.) each attached to an air inlet. Air was exhausted at the top of the vessels through tubes attached to an air outlet and bubbled through flasks containing 100 ml of 0.67 M boric acid solution with methyl red/bromocresol green indicators to trap ammonia. These flasks were placed in a water bath set at 9°C to condense moisture from the off-gas as well and to regulate the temperature of the boric acid solution. The off-gas then was analyzed for percent CO2 (Vaisala model GMT 220, range 0 to 20%) and percent O2 (Mine Safety Appliances model “ULTIMA,” range 0 to 25%). Data were automatically recorded on a Campbell Scientific model 23XL data logger for each vessel every hour. Each vessel was also equipped with a K-type thermocouple to measure temperature in the mix near the middle of the compost, and data was recorded automatically every 12 min. A separate sensor (Vaisala model HMP 235) was used to monitor room conditions in the range of 0 to 100% relative humidity and −29 to 82°C temperature.
FIG. 1.
Schematic representation of a laboratory scale compost system. A flow restrictor system was used to control aeration rates (100 ± 2 ml/min) during composting and pack storage simulations. Inlet air was bubbled through deionized water (DI) at the incubator temperature to minimize drying. Each 4-liter vessel was filled with dairy manure amended with sawdust or straw and spiked with 106 CFU/g M. paratuberculosis. Exit air passed through 0.67 M boric acid at 9°C to trap ammonia and remove water vapor and then through automated O2 and CO2 analyzers.
The simulated lagoon treatment (DM-L-RT) represented a conventional waste treatment approach shared by many dairy farms in the Midwest (1). For example, in Ohio, the manure from 46% of dairy cows is stored in liquid form in slurry tanks, earthen basins, or deep pits (F. C. Michel, Jr., J. A. Pecchia, H. M. Keener, and M. A. Berry, unpublished data). This was simulated using a diluted manure sample stored in 4-liter Erlenmeyer flasks at room temperature. The flasks were loosely covered with paper towels to allow air exchange.
(ii) Mix preparation.
Manure with sand bedding was collected from a large dairy herd (∼3,500 cows) in northeastern Ohio. Hardwood sawdust was obtained from the Ohio Agricultural Research and Development Center's dairy facility. Wheat straw was purchased from a local feed supply store. Moisture contents of the raw manure, sawdust, and straw were 73.27% (±0.12% standard deviation [SD]), 5.53% (±0.99% SD) and 6.53% (±2.37% SD), respectively. For sawdust and straw treatments, 6.5 kg of manure was mixed with 1.5 kg of sawdust or straw to achieve a moisture content of approximately 60%. About 2.6 liters of water was added to 10.4 kg of raw manure to increase the moisture content to about 85% for the lagoon storage simulation.
M. paratuberculosis inoculum was prepared from a mix suspension in phosphate-buffered saline of well-characterized isolates, three from sheep and two from humans, from the culture collection of S. Sreevatsan. The suspension cell density was determined by optical density measured at 600 nm. The M. paratuberculosis suspension was mixed into the manure used for each treatment. Compost mixes were made using M. paratuberculosis-spiked manure and sawdust or straw amendments to an initial M. paratuberculosis concentration of 106 CFU/g. On a wet-weight basis, a total of 1.115 kg sawdust compost (DM-SD-55 and DM-SD-25), 1.025 kg straw compost (DM-ST-55 and DM-ST-25), and 4 kg liquid manure (DM-L-RT) was used in each triplicate treatment. The vessels and flasks were weighed before and after filling with mixes.
Physicochemical analyses of composting material.
The treatments were incubated for 56 days at 55°C, 25°C, or room temperature (22 to 25°C). Vessels and flasks were weighed before and after removing samples at each sampling interval to calculate wet-weight loss during composting. The vessel contents were remixed before taking samples in a plastic bucket at each sampling date. Approximately 10 g of sample was dried in porcelain cups at 70°C for 24 h to determine the moisture content of each mix. Samples at day 0 and 56 were also collected and submitted to the soil testing and research laboratory at the Ohio Agriculture Research and Development Center in Wooster, Ohio, to determine initial and final pH, moisture, volatile solids, nitrogen, NH3, NO2, and carbon contents.
Boric acid solutions containing trapped ammonia from the off-gas were titrated with hydrochloric acid (0.7 N HCl) to an end point defined as that at which the color of the solution changed from blue-green to pink. Boric acid flasks with trapped ammonia and condensate were replaced with flasks containing 100 ml boric acid as needed. The percent loss of ammonia N during composting was calculated by dividing the N in the trapped solutions by the total initial N adjusted for the mass of samples removed. Volatile solids, carbon, and dry-weight losses were calculated based on an assumption that ash was not lost from the system (constant ash weight, 23) and on a mass balance at the beginning and end of the experiment.
Microbiological analyses of composting material.
Samples (approximately 100 g wet weight) were collected at 0, 3, 7, 14, 28, and 56 days after the start of the experiment for the detection of bacterial pathogens and were stored at 4°C until further processed. About 50 g of each sample was shipped to the Animal Disease Diagnostic Laboratory (ADDL), Ohio Department of Agriculture (ODA), Reynoldsburg, Ohio, on ice packs, for the isolation and identification of E. coli, Listeria, Salmonella, and M. paratuberculosis. The samples were cultured for the presence of these organisms using standard operating protocols at ADDL (27).
(i) Assessment of M. paratuberculosis survival by culture methods.
The presence of M. paratuberculosis in the treatment samples was detected using methods of Stabel et al. (34). Briefly, 2 g of sample was mixed with 35 ml of sterile distilled water and was allowed to settle for 30 min. Five milliliters of the supernatant was added to 25 ml of 0.9% HPC (hexadecylpyridinium chloride) solution. After overnight incubation, the sample was centrifuged at 900 × g for 20 min. The pellet was resuspended in 1 ml of antibiotic brew (ESP para-JEM AS) according to the manufacturer's instructions and incubated for 18 to 24 h. Samples (150 μl/tube) were inoculated into three Herrold’s egg yolk medium (HEY) tubes (Becton Dickinson, Sparks, MD) with and one tube without mycobactin J. The HEY tubes were incubated in an aerobic incubator at 35°C for 16 weeks and examined every 4 weeks for the presence of M. paratuberculosis colonies.
(ii) Assessment of survival of pathogenic microorganisms by culture methods.
Samples were screened for the presence of E. coli, Listeria, and Salmonella using standard operating protocols at ADDL, ODA, following Murray et al. (27). For E. coli, routine aerobic cultures were performed and the suspect colonies were identified using the Sensititre automated bacterial identification system (Trek Diagnostic Systems, West Lake, OH).
For Salmonella, 10 g of samples was added to 90 ml of tetrathionate broth and incubated overnight at 37°C. A loopful of this was transferred to XLT-4 (xylose-lysine-Tergitol 4) and brilliant green plates. Plates were incubated for 48 h at 37°C and examined for any suspicious colonies. The colonies were tested with Salmonella polyvalent O antisera and were identified by using the Sensititre automated bacterial identification system (Trek Diagnostic Systems, West Lake, OH). Once confirmed, the isolates were sent to National Veterinary Services Laboratories for serotyping.
For Listeria, 2 g of each sample was inoculated into University of Vermont modified Listeria enrichment broth (UVM; Difco) and incubated at 37°C for 3 days. A loopful of this broth was subcultured on blood agar, and suspicious colonies were identified using the Sensititre automated bacterial identification system (Trek Diagnostic Systems, West Lake, OH).
(iii) Assessment of M. paratuberculosis survival by PCR methods.
All samples were analyzed for the presence of M. paratuberculosis by a novel genetic approach (25, 30, 32, 37). DNA was extracted from thoroughly mixed samples following Özbek et al. (30), with slight modifications. Briefly, 5 g of sample was mixed with 35 ml of sterile water in 50-ml plastic vials. These vials were vortexed for 2 min and then shaken for 30 min on their sides at 250 rpm at 37°C. After shaking, vials were placed upright to allow contents to settle for 30 min at room temperature. Using a standard transfer pipette, 8 ml of supernatant was transferred to a 15-ml centrifuge vial, out of which 5 ml was divided into three 2-ml microcentrifuge vials. These vials were centrifuged at 14,000 rpm for 20 min at room temperature, and pellets from all three were combined and mixed in 800 μl of water and transferred to a 2-ml microcentrifuge vial containing 500 μl of hydrated, autoclaved zirconium beads (0.1 mm; BioSpec Products, Inc.). The vials were shaken on a bead beater at 4,600 rpm for 3 min and placed upright for 5 min for the beads to settle. All the supernatant was transferred to a new 1.5-ml microcentrifuge vial, and out of this, 200 μl was pipetted out to a 2-ml microcentrifuge vial for DNA extraction. The 800 μl of undiluted M. paratuberculosis suspension (as used for inoculating mixes) was used for positive control and processed with zirconium beads.
DNA was extracted using all the reagents (except ethanol) and procedures of the QIAamp DNA Stool Mini Kit (catalog no. 51504; QIAGEN, Valencia, CA). Manure used for the experiment was also included for DNA extraction, along with day 0 samples. Different manure samples, known to be free of M. paratuberculosis, were used as negative controls. DNA extracts were stored at 4°C until used.
To check for the presence of M. paratuberculosis, a 400-bp region of the insertion element IS900 was amplified from the extracted DNA using MPARA 2 (5′-GAA GGG TGT TCG GGG CCG TCG CTT AGG-3′) as the forward primer (modified from reference 25) and biotinylated MPARA 1 (5′-GAG GTC GAT CGC CCA CGT GAC-3′) as the reverse primer (modified from reference 25), respectively. Each PCR mixture contained 10 μl of DNA extract, 9.67 μl of sterile distilled water, 1.5 μl of dimethyl sulfoxide (Mallinckrodt catalog no. 5507, spectrophotometric grade), 3 μl of 10× buffer with 15 mM MgCl2 (Promega Corp., Madison, WI), 2.4 μl of 25 mM MgCl2, 0.48 μl of 10 mg/ml bovine serum albumin, 0.60 μl of a 10 μM concentration of each primer, 0.75 μl of 10 mM deoxynucleoside triphosphates, and 1 μl of HotStarTaq DNA polymerase (5 units/μl), in a final volume of 30 μl. The reaction mixture was incubated at 95°C for 15 min, cycled 36 times (at 94°C for 15 s, at 58°C for 20 s, and at 72°C for 20 s) and incubated for 7 min at 72°C in a PTC-200 thermal cycler (MJ Research Inc., Massachusetts). A PCR blank was included for each batch. DNA from an M. paratuberculosis suspension was included as a positive control, and DNA from manure free of M. paratuberculosis was used as a negative control. All PCR products were stored at 4°C until used for subsequent hybridization assay.
The biotinylated amplicons were detected by hybridizing the products to integration site-specific probe PRmpara (5′-GCG GGT GGC CAA CGA CGA GGC CGC GCT GCT GGA GTT GA-3′) coated on a microtiter plate at 100 ng/well (32). PCR products from DNA from manure free of M. paratuberculosis, PCR blanks, and hybridization negatives were used as negative controls in all batches. PCR product from M. paratuberculosis suspension DNA was used as a positive control.
Data analysis.
Means and standard deviations were calculated for composting parameters such as temperature, O2 evolution, nitrogen, carbon, ammonia, etc., as well as for M. paratuberculosis persistence results. PCR hybridization data on treatment samples were subjected to repeated-measures analysis of variance using Statistica 5.5 software (CSS:Statistica, 1999; StatSoft, Tulsa, Oklahoma). Hypotheses regarding the relative presence (optical density) of M. paratuberculosis organisms obtained with different treatments on a single date were tested through contrasts. Culture data were analyzed based on the numbers of replications showing the presence or absence of pathogens. These data were subjected to Fisher's exact test for significance using Simple Interactive Statistical Analysis (http://home.clara.net/sisa/fiveby2.htm).
RESULTS
Physicochemical changes.
Sawdust and straw amendments reduced the moisture content of the manure from 72.8% to 58.3% (±0.6%) and 60% (±0.9%) for the DM-SD and DM-ST treatments, respectively, which are near optimal results for high-rate composting (Table 2). The liquid storage simulation treatment (DM-L-RT) had a moisture content of 85% and a pH of 8.6 (±0.2) at the start of the experiment. The volatile solids and carbon content were lower in the DM-L-RT treatment than in the compost treatments, while the nitrogen content was somewhat greater due to a lack of a carbon amendment. The initial C/N ratios of the compost mixes were 30.4 for the straw-amended and 42.3 for the sawdust-amended treatments (Table 2), while the liquid treatment (DM-L-RT) had a lower mean C/N ratio of 11.7 that was similar to that of the fresh manure (C/N ratio = 10.5) due to the lack of a carbon amendment (Table 2).
TABLE 2.
Initial and final physicochemical properties of the compost mixesa
| Mix | pH | Moisture content (g/g wet) | Volatile solids (g/g dry) | Total C %b | Total N ppmb | NH3 ppmb | NO2 %b | C:N ratio |
|---|---|---|---|---|---|---|---|---|
| Initial | ||||||||
| Manure | 8.0 ± 0.1 | 72.8 ± 1.4 | 25.9 ± 1.4 | 14.6 ± 1.1 | 1.4 ± 0.2 | 8,652 | <0.5 | 10.5 ± 0.4 |
| DM-SD | 8.7 ± 0.3 | 58.3 ± 0.6 | 58.6 ± 0.8 | 28.7 ± 0.4 | 0.7 ± 0.1 | 3,728 ± 229 | <0.5 | 42.3 ± 5.1 |
| DM-ST | 8.6 ± 0.1 | 60.0 ± 0.9 | 51.2 ± 3.6 | 25.7 ± 1.3 | 0.9 ± 0.1 | 3,668 ± 287 | <0.5 | 30.4 ± 1.7 |
| DM-L-RT | 8.6 ± 0.2 | 85.1 ± 0.2 | 31.8 ± 0.2 | 16.9 ± 0.5 | 1.5 ± 0.1 | 7,881 | <0.5 | 11.7 ± 0.4 |
| Final | ||||||||
| DM-SD-55 | 7.8 ± 0.0 | 63.4 ± 0.8 | 33.7 ± 1.9 | 17.2 ± 0.7 | 1.1 ± 0.0 | 56 ± 29 | 7 ± 11 | 15.4 ± 0.8 |
| DM-ST-55 | 8.0 ± 0.1 | 62.5 ± 1.7 | 19.7 ± 0.7 | 10.2 ± 0.1 | 1.2 ± 0.0 | 256 ± 38 | <0.5 | 8.5 ± 0.3 |
| DM-SD-25 | 8.3 ± 0.1 | 67.5 ± 0.3 | 37.1 ± 1.2 | 20.1 ± 0.9 | 1.2 ± 0.0 | 291 ± 98 | <0.5 | 17.5 ± 1.2 |
| DM-ST-25 | 7.7 ± 0.1 | 68.1 ± 0.3 | 31.2 ± 0.9 | 15.5 ± 0.8 | 1.4 ± 0.1 | 122 ± 33 | 3,079 ± 183 | 11.0 ± 0.2 |
| DM-L-RT | 7.9 ± 0.4 | 83.9 ± 0.6 | 29.7 ± 1.5 | 16.1 ± 1.0 | 1.7 ± 0.1 | 17,416 ± 4,492 | <0.5 | 9.6 ± 0.2 |
Samples were submitted to the Ohio Agricultural Research and Development Center soil testing and research laboratory. Values are averages of three replications ± SDs.
Dry-weight basis.
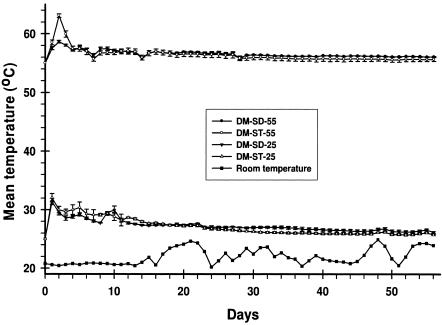
During composting, the temperatures of both compost mixes (DM-SD and DM-ST) increased from the set points of 55°C and 25°C due to microbial activity (Fig. 2). The maximum increase in temperature was observed on day 2 in the compost mixes incubated at 55°C and on day 1 for compost mixes incubated at 25°C. The mean temperature increases observed were 8°C in the DM-ST-55 treatment, 5°C in the DM-ST-25 treatment, and 4°C in the DM-SD-55 and DM-SD-25 treatments (Fig. 2). The temperatures remained 3°C to 4°C above the set points in all the compost treatments for approximately 10 days, indicating high microbial activity during this period.
FIG. 2.
Mean (n = 3) temperatures (±SD) of compost mixes (DM-ST-55, DM-SD-55, DM-ST-25, and DM-SD-25) and room temperature where liquid manure (DM-L-RT) was incubated.
The oxygen concentrations in off-gas from all of the compost treatments declined markedly during the first 10 days of composting (Fig. 3). The lowest off-gas oxygen concentrations were observed during the first 2 to 3 days in all the compost treatments, with greatest consumption of oxygen observed in the 55°C treatments (DM-ST-55 and DM-SD-55). Straw-amended compost mixes (DM-ST-55 and DM-ST-25) consumed more oxygen, at both the temperatures, than sawdust-amended compost treatments (Fig. 3). The greatest decrease in percent O2 was observed in the DM-ST-55 treatment. The oxygen concentration of the DM-L-RT treatment was not measured; however, methane gas evolution was observed during the first 20 days. Overall, the data indicate that the oxygen concentrations in the composters were above 5% during the experiment and that conditions in the composters were largely aerobic, while conditions in the liquid storage treatment were largely anaerobic as indicated by methane generation.
FIG. 3.
Average (n = 3) percentages (±SD) of O2 in the exit air during incubation of dairy manure under conditions that simulated thermophilic composting and pack storage or low-temperature composting.
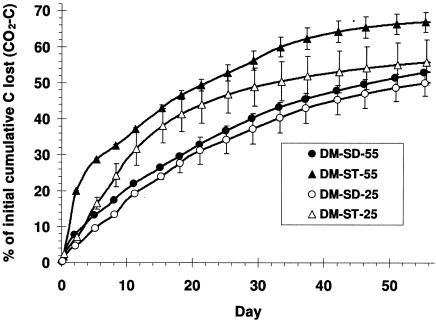
The cumulative evolution of carbon dioxide from the composters indicated that greater carbon loss occurred in the straw manure treatments incubated at both temperatures than in the sawdust-amended treatments (Fig. 4). The CO2 evolution was more rapid initially from the straw mixes and more linear from the sawdust mixes (Fig. 4). A total of 67% (±2.78%) and 55.8% (± 6.24%) of the initial carbon content was lost during incubation of DM-ST-55 and DM-ST-25, respectively. Approximately 50% of the initial carbon was evolved as CO2 from both sawdust-amended composts, indicating that, contrary to conventional thinking, in the case of sawdust-amended manure temperature has little effect on the rate of CO2 evolution during composting.
FIG. 4.
Cumulative carbon loss (CO2 − C) as the percentage of initial carbon (±SD) over time from dairy manure amended with sawdust (DM-SD-55 and DM-SD-25) or straw (DM-ST-55 and DM-ST-25) and incubated at 25°C or 55°C (n = 3). Amount of CO2 − C was determined by hourly off-gas analyses and flow rate measurements. Standard deviations are shown every fourth day for clarity. Initial total C was calculated from the initial carbon content and total weight of each mix.
The amount of NH3 emitted from the compost mixes incubated at 55°C was significantly greater than from the same composts incubated at 25°C (Fig. 5). Most of the nitrogen loss occurred during the first 10 days of composting. A total of 43.6% (±1.89%) and 33.9% (±2.0%) of the initial N content was lost as NH3 from the DM-ST-55 and DM-SD-55 treatments, respectively. However, the same mixes incubated at 25°C showed less than 5% loss of initial N content as NH3 (Fig. 5). A mass balance analysis of nitrogen loss from the liquid storage treatment (DM-L-RT) indicated no significant nitrogen loss from this treatment.
FIG. 5.
Cumulative N loss (NH3 − N) as a percentage of the initial N (±SD) from dairy manure amended with sawdust (DM-SD-55 and DM-SD-25) or straw (DM-ST-55 and DM-ST-25) and incubated at 25°C or 55°C (n = 3). The amount of initial N was calculated based on the initial mass and total N of the feed stocks used to prepare the composts.
At the end of the experiment (56 days), all the compost treatments exhibited greater moisture contents. There was a slight decrease in pH of all the mixes. The volatile-solids contents and carbon contents decreased in all the treatments, and loss was greatest in composts incubated at 55°C. There was a slight increase in percent N content in all of the compost mixes, and the C/N ratios were less than half the values observed on day 0 due to greater carbon losses. The ammonium concentration in the DM-L-RT, on the other hand, increased more than double (Table 2).
Microbiological changes. (i) Persistence of M. paratuberculosis as determined by a PCR hybridization assay.
Results of PCR amplification of IS900 followed by hybridization with IS900-specific probes showed that all the samples from all treatments were M. paratuberculosis DNA positive (Table 3). Overall, treatments differed significantly in terms of the optical density of the hybridization reactions for the relative presence of M. paratuberculosis over time (time × treatment; F = 8.03, P < 0.001). In day 0 samples, DM-L-RT treatment showed slightly low but significantly different optical densities compared to sawdust mixes (F = 6.43, P = 0.03). Day 3 samples did not differ among treatments in terms of the intensity of the hybridization reactions (P > 0.05). After 7 days of incubation, the optical density from DM-SD-55 samples was significantly lower than those of DM-SD-25 (F = 13.1, P = 0.004); however, the opposite was observed for the straw dust mixes. On day 14, DM-L-RT treatment showed significantly lower optical density values than the rest of the treatments (F = 17.24, P = 0.002), and the same was observed on day 28 (F = 38.5, P < 0.001). Furthermore, the intensity of the hybridization reaction was also low in DM-SD-55 treatment compared to DM-SD-25 (F = 11.3, P = 0.007) on day 28. On day 56, DM-ST-55 treatment showed lowest optical density and was significantly lower (F = 32.2, P < 0.001) than the same mix incubated at 25°C (DM-ST-25). A similar trend was observed for sawdust treatments.
TABLE 3.
PCR hybridization assay based on M. paratuberculosis-specific IS900 for manure treatment samples
| Treatment | Optical density (recorded at 450 mm) of hybridization reactiona,b on day:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 28 | 56 | 175 | |
| DM-SD-55 | 2.4 ± 0.2a | 3.1 ± 0.4a | 2.6 ± 0.3b | 2.6 ± 0.2a | 1.5 ± 0.4a | 1.3 ± 0.4a,c | |
| DM-ST-55 | 2.3 ± 0.1a,b | 3.2 ± 0.1a | 3.0 ± 0.2a,c | 2.5 ± 0.2a | 2.0 ± 0.2a,b | 0.8 ± 0.5a | |
| DM-SD-25 | 2.4 ± 0.2a | 3.2 ± 0.1a | 3.1 ± 0.1a | 2.6 ± 0.2a | 2.4 ± 0.4b,c | 3.0 ± 0.1b | |
| DM-ST-25 | 2.3 ± 0.1a,b | 3.3 ± 0.1a | 2.5 ± 0.1b | 2.5 ± 0.0a | 2.6 ± 0.1b,c | 2.8 ± 0.1b | |
| DM-L-RT | 2.1 ± 0.0b | 3.3 ± 0.1a | 2.7 ± 0.2b,c | 1.8 ± 0.6b | 0.9 ± 0.4d | 2.0 ± 0.8c | 0.6 ± 0.2 |
| M. paratuberculosis suspensionc | 3.00 | 3.01 | 3.37 | 3.03 | 3.00 | 3.00 | 1.44 |
| Uninoculated manure | 0.7 ± 0.2 | 1.3 | |||||
| M. paratuberculosis-negative manure (negative control) | 0.08 | 0.07 | 0.09 | 0.07 | 0.10 | 0.12 | 0.07 |
| PCR blank | 0.05 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 |
| Hybridization blank | 0.05 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 |
Values are averages of three replications ± SDs for treatments.
Statistical analysis was performed for the experimental samples and values with different letters are significantly different (P < 0.05) optical densities among treatments.
Positive control; 4.6 × 109 CFU of Mycobacterium avium subsp. paratuberculosis per extraction was used.
The source raw manure was found to be M. paratuberculosis positive although the optical density was low (0.7 ± 0.1) compared to the M. paratuberculosis-inoculated treatments. The positive controls (DNA from M. paratuberculosis suspension) had an optical density of approximately 3.00. Optical densities of manure free of M. paratuberculosis, the PCR blank, and the hybridization blank were below 0.06 (cutoff values of 0.2, 25, and 29). Since DM-L-RT samples were still culture positive on day 56, this treatment was continued through day 175. M. paratuberculosis DNA was still detected in samples from the DM-L-RT treatment on day 175, although the hybridization intensity was lower (optical density, 0.6 ± 0.2).
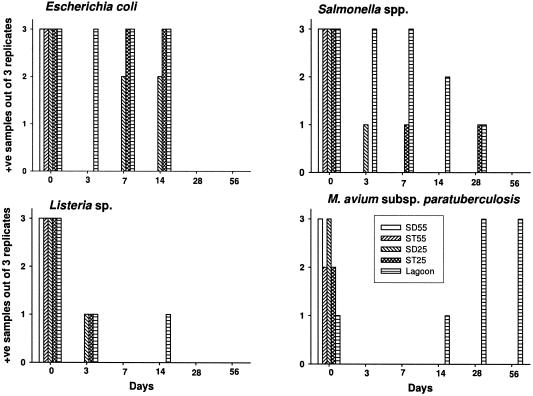
(ii) Persistence of M. paratuberculosis as determined by culturing.
Standard culture methods were used to detect M. paratuberculosis in all treatments. Contamination of some of the cultures precluded the interpretation of M. paratuberculosis cultures in some replicates at 0 day testing. As a result, treatments differed (P = 0.02) at day 0 because M. paratuberculosis could not be detected in all the replications of all the treatments, due to fungal contamination of some culture tubes (Fig. 6). Manure used for the experiment was also culture positive for M. paratuberculosis (data not shown). On days 3 and 7, all treatments were M. paratuberculosis culture negative. On day 14, M. paratuberculosis was not detected from any treatment except a single replicate from DM-L-RT treatment (average colonies, 10 per tube) (P > 0.05). On days 28 and 56, all three replications from the same treatment were positive (average colonies, 7 and 2 per tube, respectively) for M. paratuberculosis, whereas it was not detected from any other treatment (P = 0.002). The DM-L-RT treatment samples taken on day 175 were negative for the presence of viable M. paratuberculosis by culture.
FIG. 6.
Numbers of treatment replicates positive for the presence of culturable bacterial pathogens as a function of time (n = 3). Treatments were DM-SD-55 (SD55), DM-ST-55 (ST55), DM-SD-25 (SD25), DM-ST-25 (ST25), and DM-L-RT (Lagoon). +ve, positive.
(iii) Persistence of other microorganisms as determined by culture methods.
E. coli and Salmonella and Listeria species were all detected in the source manure and the initial treatment mixes (day 0) (Fig. 6). The source manure and initial treatment mixes contained both rough and smooth types of E. coli; however, only rough types were observed in later samples. The main species of Listeria detected was Listeria innocua; however, a few samples also showed the presence of L. monocytogenes. Salmonella species detected by culture typing were Salmonella cerro, which was present in a majority of the samples, and Salmonella give, which was present in some samples at later dates (DM-ST-25 and DM-L-RT).
After 3 days, E. coli was not detected in any replicates of the compost or pack storage treatments but was present in all three replications of the DM-L-RT treatment (P = 0.002). However, E. coli organisms were detected in two of the DM-SD-25 and all of the DM-ST-25 and DM-L-RT replicates on days 7 and 14 (P < 0.001 for both dates). E. coli was not detected in any treatment on days 28 and 56.
Listeria species were present in one of the three replicates of the DM-SD-25, DM-ST-25, and DM-L-RT treatments on day 3 (P = 0.05) but were not observed in any of the 55°C treatments (Fig. 6). Listeria was not detected in any treatment on day 7. On day 14, Listeria was again detected in one replicate of the DM-L-RT treatment (P > 0.05) but none of the other treatments. Thereafter, no Listeria was detected in any treatment (days 28 and 56).
Salmonella species were detectable in one replicate of the DM-SD-25 and all replications of the DM-L-RT on day 3 (P = 0.002) but were not detected in any of the other treatments on this day. On day 7, Salmonella was present in one replication of the DM-ST-25 and all replications of the DM-L-RT (P = 0.002) treatments. Salmonella was only detected in two replications of DM-L-RT on day 14 (P = 0.02). On day 28, Salmonella was absent in all the treatments except one replication of the DM-ST-25 and DM-L-RT treatments (P > 0.05). No Salmonella species were detectable in any treatment on day 56.
DISCUSSION
In this study, the persistences of four different pathogens of concern in dairy manure, M. avium subsp. paratuberculosis, Salmonella spp., Escherichia coli, and Listeria spp., were determined during composting at 55°C, packing (or low-temperature composting) at 25°C, and storage as a liquid.
Analysis of the chemical and physical properties of the initial manure, the liquid stored manure, and the composts before and after treatment showed that the composts incubated at 55°C exhibited similar patterns of CO2 and NH3 evolution, dry-weight loss, and volatile-solids loss to those observed in full-scale windrows (24). The pack (or low-temperature compost) treatments, which were identical to the 55°C compost treatments except for their incubation temperature of 25°C, showed less weight loss and O2 uptake and produced less NH3 and CO2 than the same mixes incubated at 55°C. However, the differential effects of temperature were restricted primarily to the first 10 days of incubation. Composts amended with straw had more rapid organic matter conversion and a greater increase in bulk density than sawdust-amended composts. All of the composted and pack manure treatments showed more than 50% conversion of their initial carbon to CO2. The same manure incubated as a liquid at room temperature showed no significant carbon or total-nitrogen losses during incubation.
The concentration of ammonium in the liquid stored manure (DM-L-RT) treatment rose from an initial level of 7,881 ppm to 17,416 ± 4,492 ppm (dry basis), which is within the range of concentrations found in liquid manure storage lagoons (38). The ammonium in the compost and pack treatments, on the other hand, fell from approximately 3,700 ppm (dry weight basis) initially to below 300 ppm, probably as a result of volatilization and incorporation into compost organic matter (Table 2). Extensive nitrification was observed in the DM-ST-25 treatment.
Previous studies indicate that appropriate handling of the dairy manure and elevated temperatures are important factors affecting the persistence of pathogens such E. coli and Salmonella, etc., in manure (11, 12, 13, 17). Lung et al. (20) spiked cow manure with 107 CFU/g E. coli O157:H7 and Salmonella enterica serovar Enteritidis in a laboratory experiment. They found that E. coli and Salmonella were not detectable after 72 h and 48 h, respectively, of composting at 45°C. However, cell densities of these two organisms were unchanged when the composting system was incubated at room temperature for 4 days.
Results from our study showed that composts incubated at 55°C (DM-SD-55 and DM-ST-55) had no detectable E. coli, Salmonella, or Listeria organisms after 3 days. It is worth noting that temperatures in these treatments were greater than 55°C due to self-heating. In fact, they were comparable to those (55°C to 65°C) typically observed in full-scale windrows (24). Results of DM-ST-25 and DM-SD-25 treatments, in which the temperature did not exceed 32°C, showed that 2 to 8 weeks were required to reduce the concentrations of these naturally occurring pathogens to undetectable levels in pack or low-temperature composting conditions. In the liquid storage treatment (DM-L-RT), the incubation temperature was below 25°C, yet none of the organisms were detectable after 56 days. These results indicate that the mechanism of pathogen removal during manure treatment by aerobic composting and liquid storage involves more than just elevated temperatures. Factors such as moisture content, oxygen concentration, interactions with the greater microbial community, organic matter stabilization, and compost amendment type evidently may also be involved in reducing the levels of pathogens in manure. Some studies have shown that recovery and regrowth of pathogenic microbial populations in manures is possible if incomplete inactivation occurs due to low temperatures and/or incomplete organic matter stabilization caused by drying (7, 35). In our study, however, moisture conditions and temperatures in the DM-ST-25, DM-SD-25, and DM-L-RT treatments were favorable for pathogen growth throughout the experiment but there was no evidence of such regrowth. This suggests that the pathogens were not just inactivated but unable to regrow in or recolonize these composts.
Jörgensen (15) reported that under anaerobic conditions, M. paratuberculosis can survive for 252 and 98 days in cattle slurry stored at 5°C and 15°C, respectively. Olsen et al. (28) studied survival of M. paratuberculosis during anaerobic digestion of dairy manure in biogas plants at mesophilic (35°C) and thermophilic (53°C to 55°C) conditions. Under mesophilic conditions, they were able to reisolate M. paratuberculosis at 7, 14, and 21 days but not at 28 days. At thermophilic conditions, no M. paratuberculosis was detected in as little as 3 h. To simulate anaerobic liquid storage, liquid manure (DM-L-RT) was stored at room temperature (20.2°C to 24.9°C) without mixing in a closed container in our experiment. In this treatment (DM-L-RT), M. paratuberculosis was culturable through day 56 but not on day 3, 7, or 175. Competition with heterotrophic bacteria may have reduced the M. paratuberculosis presence in the DM-L-RT treatment on days 3 and 7 or limited its detection by way of culturing. Another possible explanation for heterogeneity in samples from the same treatment could be the tendency of M. paratuberculosis organisms to form large clumps (2). Our study, along with the two above studies, indicates that M. paratuberculosis can persist for long periods in stored liquid manure under anaerobic conditions. On the other hand, no M. paratuberculosis organisms were culturable after just 3 days in the composting treatments at both incubation temperatures (DM-ST-25, DM-SD-25, DM-ST-55, and DM-SD-55). This further indicates that temperature is not the only factor reducing the persistence of these organisms.
In contrast to the culture-based assay results, M. paratuberculosis DNA was detected through day 56 in all treatments and also at day 175 in the DM-L-RT treatment. Since M. paratuberculosis was not detectable by culture after day 3 in compost and pack treatments, this suggests that cells were either dead or present below the detection limit of the conventional culture methods in these treatments. Alternately, M. paratuberculosis organisms may have been alive but unculturable due to the severe physicochemical conditions and/or microbial competition. Confirmation of M. paratuberculosis viability will require the demonstration of detectable RNA. While viability alone is insufficient to evaluate the risk of composted manure in reintroducing infection into herds or potentially exposing humans through other nonagricultural uses of compost, it will still provide key insights into the risk assessment of this potentially zoonotic organism. There are reports on M. paratuberculosis survival in milk after commercial pasteurization, indicating that M. paratuberculosis can tolerate temperatures higher than 60°C (4, 8, 9). Detection thresholds for current techniques to recover viable M. paratuberculosis may be the limiting factor in studies that evaluate the survival of this bacterium after heat treatment (33). Regardless, composting offers complex microbial interactions and competitive environmental conditions beyond just heat treatment as in pasteurization and thus may be more efficient than other manure treatments in eliminating M. paratuberculosis from heavily contaminated manure.
Overall, our study shows that the four pathogens analyzed were eliminated after 3 days of composting at 55°C. However, more than 28 days were necessary to eliminate these organisms from all treatments incubated at 25°C or less. Results show that an even longer storage period is needed to eliminate culturable M. paratuberculosis from manure stored as a liquid. M. paratuberculosis may persist for more than 2 months at unculturable levels regardless of whether the manure is composted, pack stored, or liquid stored under anaerobic conditions. Factors such as moisture content and manure stabilization appear to play a role in the elimination of the pathogens at low temperatures. The type of amendment used for dairy manure composting affected the rates and extents of organic matter conversion and initial compost bulk densities but did not have a significant effect on the survival of the pathogens. In conclusion, composting at 55°C reduces the persistence of E. coli, Listeria, M. paratuberculosis, and Salmonella organisms after 3 days. Longer periods are necessary for composts or pack manure at 25°C. Stored liquid manures showed the greatest persistence of the four pathogens tested.
Acknowledgments
This research was partly supported by USDA-IFAFS grant number 2001-52103-11302 and by the Ohio Water Development Authority. Johne's disease research in S. Sreevatsan's laboratory was supported by grants from the Johne's Disease Integrated Research Program (USDA-NRICAP) and USDA-NRI.
We acknowledge the technical support at Animal Disease Diagnostic Laboratory, ODA, Reynoldsburg, Ohio, for pathogen culture data. We thank Megan Strother for technical assistance.
REFERENCES
- 1.Barker, J. C. 1996. Management of dairy wastewater. North Carolina Cooperative Extension Service publication number EBAE 106-83. North Carolina Cooperative Extension Service, Raleigh, N.C.
- 2.Collins, M. T. 2003. Update on paratuberculosis. 1. Epidemiology of Johne's disease and the biology of Mycobacterium paratuberculosis. Ir. Vet. J. 56:565-574. [Google Scholar]
- 3.de Bertoldi, M., P. Sequi, B. Lemmes, and T. Papi (ed.). 1996. The science of composting. Blackie Academic & Professional, London, United Kingdom.
- 4.Ellingson, J. L. E., J. L. Anderson, J. J. Koziczkowski, R. P. Radcliff, S. J. Sloan, S. E. Allen, and N. M. Sullivan. 2005. Detection of viable Mycobacterium avium subsp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J. Food Prot. 68:966-971. [DOI] [PubMed] [Google Scholar]
- 5.El Zataari, F. A., M. S. Osato, and D. Y. Graham. 2001. Etiology of Crohn's disease: the role of Mycobacterium avium paratuberculosis. Trends Mol. Med. 7:247-252. [DOI] [PubMed] [Google Scholar]
- 6.Gay, S. W., D. R. Schmidt, C. J. Clanton, K. A. Janni, L. D. Jacobson, and S. Weisberg. 2003. Odor, total reduced sulfur, and ammonia emissions from animal housing facilities and manure storage units in Minnesota. Appl. Eng. Agric. 19:347-360. [Google Scholar]
- 7.Gibbs, R. A., C. J. Hu, G. E. Ho, and I. Unkovich. 1997. Regrowth of faecal coliforms and salmonellae in stored biosolids and soil amended with biosolids. Water Sci. Technol. 35:269-275. [Google Scholar]
- 8.Grant, I. R., H. J. Ball, and M. T. Rowe. 2002. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 68:2428-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant, I. R., E. I. Hitchings, A. McCartney, F. Ferguson, and M. T. Rowe. 2002. Effect of commercial-scale high-temperature, short-time pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows' milk. Appl. Environ. Microbiol. 68:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess, T. F., I. Grdzelishvili, H. Q. Sheng, and C. J. Hovde. 2004. Heat inactivation of E. coli during manure composting. Compost Sci. Util. 12:314-322. [Google Scholar]
- 11.Himathongkham, S., S. Bahari, H. Riemann, and D. Cliver. 1999. Survival of Esherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol. Lett. 178:251-257. [DOI] [PubMed] [Google Scholar]
- 12.Horan, N. J., L. Fletcher, S. M. Betmal, S. A. Wilks, and C. W. Keevil. 2004. Die-off of enteric bacterial pathogens during mesophilic anaerobic digestion. Water Res. 38:1113-1120. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, X., J. Morgan, and M. P. Doyle. 2003. Fate of Escherichia coli O157:H7 during composting of bovine manure in a laboratory-scale bioreactor. J. Food Prot. 66:25-30. [DOI] [PubMed] [Google Scholar]
- 14.Jones, D. L. 1999. Potential health risks associated with the persistence of Escherichia coli O157 in agricultural environments. Soil Use Manag. 15:76-83. [Google Scholar]
- 15.Jörgensen, J. B. 1977. Survival of Mycobacterium paratuberculosis in slurry. Nord. Vet. Med. 29:267-270. [PubMed] [Google Scholar]
- 16.Keener, H. M., W. A. Dick, and H. A. J. Hoitink. 2000. Composting and beneficial utilization of composted by-product materials, p. 316-341. In J. F. Power and W. A. Dick (ed.), Land application of agricultural, industrial, and municipal by-products. Soil Science Society of America, Madison, Wisconsin.
- 17.Kudva, I. T., K. Blanch, and C. J. Hovde. 1998. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 64:3166-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larney, F. J., L. J. Yanke, J. J. Miller, and T. A. McAllister. 2003. Fate of coliform bacteria in composted beef cattle feedlot manure. J. Environ. Qual. 32:1508-1515. [DOI] [PubMed] [Google Scholar]
- 19.LeJeune, J. T., and M. D. Kauffman. 2005. Effect of sand and sawdust bedding materials on the fecal prevalence of Escherichia coli O157:H7 in dairy cows. Appl. Environ. Microbiol. 71:326-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lung, A. J., C.-M. Lin, J. M. Kim, M. R. Marshall, R. Nordstedt, N. P. Thompson, and C. I. Wei. 2001. Destruction of Escherichia coli O157:H7 and Salmonella enteritidis in cow manure composting. J. Food Prot. 64:1309-1314. [DOI] [PubMed] [Google Scholar]
- 21.Manning, E. J., and M. T. Collins. 2001. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. Off. Int. Epizoot. 20:133-150. [DOI] [PubMed] [Google Scholar]
- 22.McCurdy, M., and K. McSweeney. 1993. The origin and identification of macropores in an earthen-lined dairy manure storage basin. J. Environ. Qual. 22:148-154. [Google Scholar]
- 23.Meyer, D. M., I. Garnett, and J. C. Guthrie. 1997. A survey of dairy manure management practices in California. J. Dairy Sci. 80:1841-1845. [DOI] [PubMed] [Google Scholar]
- 24.Michel, F. C., Jr., J. A. Pecchia, J. Rigot, and H. M. Keener. 2004. Mass and nutrient losses during the composting of dairy manure amended with sawdust and straw. Compost Sci. Util. 12:323-334. [Google Scholar]
- 25.Millar, D., J. Ford, J. Sanderson, S. Withey, M. Tizard, T. Doran, and J. Hermon-Taylor. 1996. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows' milk in England and Wales. Appl. Environ. Microbiol. 62:3446-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motiwala, A. S., M. Strother, A. Amonsin, B. Byrum, S. A. Naser, J. R. Stabel, W. P. Shulaw, J. P. Bannantine, V. Kapur, and S. Sreevatsan. 2003. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: evidence for limited strain diversity, strain sharing and identification of unique targets for diagnosis. J. Clin. Microbiol. 41:2015-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray, P. R., E. J. Barron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.). 2003. Manual of clinical microbiology, 8th ed., vol. 1 and 2. ASM Press, Washington, D.C.
- 28.Olsen, J. E., J. B. Jörgensen, and P. Nansen. 1985. On the reduction of Mycobacterium paratuberculosis in bovine slurry subjected to batch mesophilic or thermophilic anaerobic digestion. Agric. Wastes 13:273-280. [Google Scholar]
- 29.Ott, S. L., S. J. Wells, and B. A. Wagner. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40:179-192. [DOI] [PubMed] [Google Scholar]
- 30.Özbek, A., F. C. Michel, Jr., M. Strother, A. S. Motiwala, B. R. Byrum, W. P. Shulaw, C. G. Thornton, and S. Sreevatsan. 2003. Evaluation of two recovery methods for detection of Mycobacterium avium subsp. paratuberculosis by PCR: direct-dilution-centrifugation and C18-carboxypropylbetaine processing. FEMS Microbiol. Lett. 229:145-151. [DOI] [PubMed] [Google Scholar]
- 31.Pell, A. N. 1997. Manure and microbes: public and animal health problem? J. Dairy Sci. 80:2673-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sreevatsan, S., J. B. Bookout, F. Ringpis, V. S. Perumaalla, T. A. Ficht, L. G. Adams, S. D. Hagius, P. H. Elzer, B. J. Bricker, G. K. Kumar, M. Rajasekhar, S. Isloor, and R. R. Barathur. 2000. A multiplex approach to molecular detection of Brucella abortus and/or Mycobacterium bovis infection in cattle. J. Clin. Microbiol. 38:2602-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stabel, J. R. 2000. Johne's disease and milk: do consumers need to worry? J. Dairy Sci. 83:1659-1663. [DOI] [PubMed] [Google Scholar]
- 34.Stabel, J. R., S. J. Wells, and B. A. Wagner. 2002. Relationships between fecal culture, ELISA, and bulk tank milk test results for Johne's disease in US dairy herds. J. Dairy Sci. 85:525-531. [DOI] [PubMed] [Google Scholar]
- 35.Turner, C. 2002. The thermal inactivation of E. coli in straw and pig manure. Bioresour. Technol. 84:57-61. [PubMed] [Google Scholar]
- 36.Van Horn, H. H., A. C. Wilkie, W. J. Powers, and R. A. Nordstedt. 1994. Components of dairy manure management systems. J. Dairy Sci. 77:2008-2030. [DOI] [PubMed] [Google Scholar]
- 37.Vary, P. H., P. R. Andersen, E. Green, J. Hermon-Taylor, and J. J. McFadden. 1990. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne's disease. J. Clin. Microbiol. 28:933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veenhuizen, M. A., D. J. Eckert, K. Elder, J. Johnson, W. F. Lyon, K. M. Mancl, and G. Schnitkey. 1992. Ohio livestock manure and wastewater management guide. Bulletin 604. Ohio State University Extension. [Online.] http://ohioline.osu.edu/b604/.