Abstract
Microbial nitrate-dependent Fe(II) oxidation is known to contribute to iron biogeochemical cycling; however, the microorganisms responsible are virtually unknown. In an effort to elucidate this microbial metabolic process in the context of an environmental system, a 14-cm sediment core was collected from a freshwater lake and geochemically characterized concurrently with the enumeration of the nitrate-dependent Fe(II)-oxidizing microbial community and subsequent isolation of a nitrate-dependent Fe(II)-oxidizing microorganism. Throughout the sediment core, ambient concentrations of Fe(II) and nitrate were observed to coexist. Concomitant most probable number enumeration revealed the presence of an abundant nitrate-dependent Fe(II)-oxidizing microbial community (2.4 × 103 to 1.5 × 104 cells g−1 wet sediment) from which a novel anaerobic, lithoautotrophic, Fe(II)-oxidizing bacterium, strain 2002, was isolated. Analysis of the complete 16S rRNA gene sequence revealed that strain 2002 was a member of the beta subclass of the proteobacteria with 94.8% similarity to Chromobacterium violaceum, a bacterium not previously recognized for the ability to oxidize nitrate-dependent Fe(II). Under nongrowth conditions, both strain 2002 and C. violaceum incompletely reduced nitrate to nitrite with Fe(II) as the electron donor, while under growth conditions nitrate was reduced to gaseous end products (N2 and N2O). Lithoautotrophic metabolism under nitrate-dependent Fe(II)-oxidizing conditions was verified by the requirement of CO2 for growth as well as the assimilation of 14C-labeled CO2 into biomass. The isolation of strain 2002 represents the first example of an anaerobic, mesophilic, neutrophilic Fe(II)-oxidizing lithoautotroph isolated from freshwater samples. Our studies further demonstrate the abundance of nitrate-dependent Fe(II) oxidizers in freshwater lake sediments and provide further evidence for the potential of microbially mediated Fe(II) oxidation in anoxic environments.
Naturally abundant iron (Fe) minerals exert a significant influence on soil and sediment geochemistry (13). As a result, much consideration has been vested in iron redox reactions in aquatic and sedimentary environments. Reduction of iron in natural systems mediated by both abiotic and biotic mechanisms has been well documented (13). In anoxic, nonsulfidogenic environments, microbially mediated reductive dissolution of iron oxides significantly impacts iron biogeochemistry, yielding aqueous Fe(II) and solid-phase Fe(II)-bearing minerals (29). The reoxidation of Fe(II) in these environments at circumneutral pH is not limited to abiotic or biotic reactions involving molecular oxygen (O2) (18, 41). Microbially catalyzed anaerobic Fe(II) oxidation presents a biological mechanism promoting the reoxidation of Fe(II) in anoxic environments (45), potentially contributing to a dynamic microbially mediated anoxic iron redox cycle (48, 52).
Nitrate-dependent Fe(II) oxidation can significantly influence soil and sediment mineralogy and geochemistry. In addition to soluble Fe(II), solid-phase Fe(II)-bearing minerals, including surface-bound Fe(II) and solid-phase crystalline Fe(II) minerals, have also been shown to be subject to oxidation while serving as an energy source for microbial metabolism (8, 16, 43, 45, 51, 52). One study demonstrated the anaerobic bio-oxidation of as much as 52% of the Fe(II) content of almandine [Fe3Al2(SiO4)3] and 17% of that of staurolite [(Fe,Mg,Zn)2Al9(Si,Al)4O22(OH)2], two common clay minerals previously considered to be recalcitrant to biological weathering (8).
The end products of anaerobic microbial oxidation of Fe(II) can also influence soil and sediment mineralogy and geochemistry through the formation of a broad variety of environmentally relevant Fe(III)-bearing minerals which can regulate trace element and contaminant solubility in natural environments via adsorption, coprecipitation, and redox mechanisms (19, 28, 42, 50). Previous studies have identified the biogenic formation of minerals such as ferric oxyhydroxide, goethite, hematite, iron hydrogen carbonate, and maghemite (8, 27, 47, 52). Chaudhuri and colleagues (8) demonstrated the bio-oxidative formation of magnetite (Fe3O4), a mixed Fe(II)-Fe(III) mineral with magnetic properties that is known to be responsible for natural remnant magnetization of deep-sea sediments and other sediments (1, 21, 34). Formation of magnetite under anaerobic Fe(II)-oxidizing conditions presents an alternative mechanism to the bioreduction of Fe(III) for the biogenic formation of this geologically important mineral. Abiotic redox interactions between biogenic Fe(III) oxides can result in the solubilization of U(IV) with production of aqueous U(VI) (19). Alternatively, the precipitation of biogenic Fe(III) oxides provides a mechanism for the immobilization of heavy metals and metalloids through coprecipitation or physical envelopment and also provides a reactive surface with an adsorptive affinity for anions (e.g., PO43−) and cations (e.g., Zn2+, As5+, Co2+, and U6+) (28, 42, 50). Previous studies have demonstrated that heavy metals and radionuclides, including U(VI), are rapidly removed (as much as 80% of the initial 100 μM within 5 days) from solution during anaerobic nitrate-dependent microbial Fe(II) oxidation in association with the biogenic Fe(III) oxides (28). As such, the anaerobic formation of biogenic Fe(III) oxide-containing minerals has been identified as a plausible bioremediative strategy for permanently immobilizing heavy metals and radionuclides (11, 28).
Anaerobic biological oxidation of Fe(II) mediated by microbial communities was only recently described, and very little is currently known regarding the diversity of organisms capable of this metabolism. Previous studies have demonstrated that anoxic Fe(II) oxidation is mediated by some anoxygenic phototrophs (14, 25, 53) as well as by various nitrate- or perchlorate-respiring organisms (3, 5, 8, 45, 52). Microbial communities capable of nitrate-dependent Fe(II) oxidation have been observed in a variety of freshwater and saline environments (3, 6, 15, 19, 23, 24, 26, 39, 42, 46, 52). However, the microorganisms sustaining this metabolism in situ are virtually unknown. The oxidation of Fe(II) coupled to denitrification is energetically favorable at neutral pH (ΔG°′ = −103.5 kJ/mole electron), but at present autotrophic growth under anaerobic, nitrate-dependent, Fe(II)-oxidizing conditions has been demonstrated with only one pure culture isolate, a hyperthermophilic archeon (23). Several putative mesophilic nitrate-dependent Fe(II)-oxidizing bacteria have been described previously; however, growth coupled to this metabolism either was not demonstrated or did not occur in the absence of an organic carbon and energy source (5, 8, 16, 19, 43, 45, 52).
Here we demonstrate a freshwater environment capable of chemically supporting a nitrate-dependent Fe(II)-oxidizing microbial community that includes a novel bacterium capable of coupling autotrophic growth to the oxidation of Fe(II) and the reduction of nitrate. This organism represents the first pure culture example of a mesophilic, neutrophilic lithoautotroph capable of anaerobic nitrate-dependent Fe(II) oxidation.
MATERIALS AND METHODS
Sediment geochemistry.
A sediment core from a small freshwater lake, Campus Lake, at Southern Illinois University, Carbondale, IL, was collected and immediately returned to the laboratory for processing. The sediment core was sectioned at 1.5-cm intervals in an anoxic glove bag (95:5 N2:H2 atmosphere). Samples were taken for analysis of Fe(II), total Fe, nitrate, nitrite, and sulfate content and for most probable number (MPN) enumeration of the nitrate-dependent Fe(II)-oxidizing community. Terminal electron-accepting process (TEAP) analyses were performed using [2-14C]acetate in the presence and absence of molybdenum (20 mM), a specific inhibitor of microbial sulfate reduction, as previously described (10).
MPN enumeration.
One gram of sediment from each sediment core interval was added to 9 ml anoxic (80:20 N2:CO2 headspace) bicarbonate-buffered (pH 6.8) freshwater basal medium prepared as previously described (5) and containing 5 mM nitrate and 0.1 mM acetate as the electron acceptor and the additional carbon source, respectively. Ferrous chloride was added as the electron donor from an anoxic (100% N2 atmosphere), filter sterilized (0.22-μm sterile nylon filter membrane) stock solution (1 M) to achieve a final concentration of 10 mM. Following the addition of 1 g sediment, sodium pyrophosphate (final concentration, 0.1%) was added to the sediment slurry, which was gently shaken at room temperature for 1 h. The sediment slurry was then serially diluted in basal medium prepared as described above. After 8 weeks of incubation in the dark at 30°C, tubes positive for iron oxidation were identified by the presence of a brownish-red or brownish-green precipitate. The Most Probable Number Calculator version 4.05 (Albert J. Klee, Risk Reduction Engineering Laboratory, U.S. Environmental Protection Agency, Cincinnati, Ohio, 1996; freeware available at http://www.epa.gov/nerlcwww/other.htm) was used to enumerate the nitrate-dependent Fe(II)-oxidizing microbial community and calculate confidence limits.
Isolation of nitrate-dependent Fe(II)-oxidizing bacterium.
Tubes positive for nitrate-dependent Fe(II) oxidation at the highest dilution in the MPN enumeration series were selected for the isolation of a microorganism. Samples were streaked onto R2A agar plates (Difco catalog no. 218263), an undefined low-nutrient medium, and amended with 10 mM nitrate in an anaerobic glove bag (95:5 N2:H2 atmosphere). The plates were incubated in anaerobic jars at 30°C for 120 h for heterotrophic colony development. An Fe(II) overlay (5 ml of R2A agar containing 2 mM FeCl2) was poured over each plate following colony development, and incubation took place in an anoxic atmosphere. Colonies that exhibited Fe(II) oxidation, as identified by the development of brownish-red Fe(III) oxide precipitates on or around colonies, were selected and transferred into anoxic bicarbonate-buffered freshwater basal medium containing 10 mM nitrate, 10 mM Fe(II), and 0.1 mM acetate. After 1 week of incubation in the dark at 30°C, positive cultures were transferred into fresh anoxic bicarbonate-buffered basal medium containing 10 mM Fe(II) and 5 mM nitrate with CO2 as the sole carbon source. One culture, strain 2002, was selected for further characterization.
Nitrate-dependent Fe(II) oxidation.
Cells of strain 2002 grown anaerobically on acetate (10 mM) and nitrate (10 mM) were harvested by centrifugation (6,000 × g, 10 min), washed twice with anaerobic (100% N2 atmosphere) PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer (10 mM, pH 7.0), and resuspended to serve as an inoculum for nongrowth experiments. A washed-cell suspension of C. violaceum was prepared as described above with cells grown anaerobically (100% N2 atmosphere) on nutrient broth, glucose (10 mM), and nitrate (5 mM) as previously described (2).
The prepared washed-cell suspensions (strain 2002 or C. violaceum) were added to anaerobic PIPES (10 mM, pH 7.0) buffer amended with Fe(II) (10 mM) as the sole electron donor and nitrate (4 mM or 2.5 mM) or nitrite (2.5 mM) as the electron acceptor. Heat-killed controls were prepared by pasteurizing (80°C, 10 min) the inoculum in a hot water bath. All cell suspension incubations were performed at 30°C in the dark, and samples were collected to monitor concentrations of Fe(II), nitrate, and nitrite.
Growth of strain 2002 under nitrate-dependent Fe(II)-oxidizing conditions was verified in freshwater basal medium containing 10 mM Fe(II) and 2.2 mM nitrate with or without amendment with 0.1 mM acetate. Freshwater basal medium containing 2.2 mM nitrate without an Fe(II) source served as the negative control. Strain 2002 inoculum was grown under heterotrophic nitrate-reducing conditions in medium stoichiometrically balanced for nitrate (10 mM) and acetate (6.25 mM) in order to eliminate the transfer of reducing equivalents [Fe(II)] into the negative control.
The carbon compound required for growth of strain 2002 under nitrate-dependent Fe(II)-oxidizing conditions was determined by inoculating an anaerobic, CO2-free (100% N2 atmosphere), PIPES-buffered (20 mM, pH 7.0) culture medium containing 1 mM Fe(II)-nitrilotriacetic acid (NTA) and 0.25 mM nitrate with or without a carbon source amendment (1.0 mM HCO3− or 0.5 mM acetate). Strain 2002 was grown as described above in anaerobic, PIPES-buffered culture medium. The headspace of the inoculum was aseptically sparged for 15 min with 100% N2 to eliminate CO2 immediately prior to the initiation of the experiment.
The ability of strain 2002 to assimilate CO2 into biomass was verified by amending the nitrate-dependent Fe(II)-oxidizing growth culture medium (basal freshwater PIPES-buffered medium, 5 mM FeCl2, 2 mM nitrate, 1 mM bicarbonate; 100% He atmosphere) with H14CO3− (final concentration, 1 μmol). Rhodospirillum rubrum, grown photolithoautotrophically under an anoxic atmosphere (50:50 He:H2 atmosphere) as previously described (49), served as a positive control culture. Triplicate cultures were incubated statically in the dark for 60 h. A subsample (5 ml) was concentrated to a final volume of 0.5 ml by centrifugation (6,000 × g, 10 min). A cell extract was prepared from the concentrated sample by three 30-s pulses in a bead beater (Mini-Bead-Beater-8; Biospec Products, Bartlesville, OK) with 0.1-mm silica beads (Lysing Matrix B, Qbiogene product no. 6911-100). The lysate was chilled in an ice bath for 1 min following each pulse. The sample was then centrifuged (10,000 × g, 10 min) to remove insoluble cell debris, and the soluble cell extract was withdrawn in order to determine the protein concentration and the 14C-labeled content.
Electron donors and acceptors.
Standard anaerobic culturing techniques were employed as previously described (5) in order to test the ability of strain 2002 to utilize alternative electron donors and acceptors. Potential electron donors and acceptors were added from concentrated, anoxic, sterile, aqueous stock solutions to previously prepared anoxic freshwater basal medium, yielding the desired final concentration. Unless otherwise stated, continued growth after three successive transfers in the respective culture medium was recorded as positive. When growth was not visually apparent, positive growth was defined as a doubling in cell density as observed by direct cell counts.
Analytical techniques.
Samples collected for analysis of Fe(II) and total Fe were extracted in 0.5 N HCl overnight. The Fe(II) and total Fe in 0.5 N HCl extracts were analyzed using ferrozine (44) as previously described (33). Samples collected for Fe(II) analysis in washed-cell-suspension experiments were immediately added directly to ferrozine. Virtually all of the Fe(II) added to anoxic PIPES buffer was aqueous Fe(II) and was therefore measurable by the direct addition to ferrozine. Ion chromatography with conductivity detection (IonPac AS9-HC analytical column, Dionex DX-500 system; Dionex Corp., Sunnyvale, CA) was used to analyze nitrate, nitrite, and sulfate according to the manufacturer's instructions.
Growth was monitored by determining changes in cell density by use of direct cell counts (Petroff-Hausser counting chamber, 0.02-mm depth). Samples collected for direct cell counts were immediately fixed in formaldehyde (final concentration, 3.7%). Prior to the counting, filter-sterilized (0.2-μm nylon filter) oxalate solution [28 g liter−1 C2H2O4 · 2NH3 and 15 g liter−1 (COOH)2 · 2H2O, pH 7.0] was added to the formaldehyde-fixed sample and allowed to digest for 30 min in order to remove Fe(III) precipitates in samples which were collected from growth experiments amended with an FeCl2 solution. In cultures amended with Fe(II)-NTA, biogenic Fe(III) remained in solution. Therefore an oxalate digestion prior to direct cell counts was not necessary.
Protein concentration was determined by use of the Bradford assay (Sigma product no. B6916) as directed by the manufacturer, with bovine serum albumin as the standard. The quantification of 14C label incorporated into the organic acid biomass was done after acidifying the prepared cell extract sample with an equal volume of 0.5 N HCl. The acidified sample was vortexed and sparged with 100% N2 for 5 min. The sample was then diluted and assayed for 14C-labeled organic acid content via radio-high-performance liquid chromatography detection using an Aminex Fast Acid (100- by 7.8-mm) ion exchange column (Bio-Rad, Hercules, CA) with H2SO4 (0.02 N) as the eluent at a flow rate of 1.0 ml min−1 by use of a β-RAM radio-high-performance liquid chromatography detector (IN/US Systems, Inc., Tampa, FL). No attempt was made to identify the individual organic acids eluted from the sample. Incorporation of the 14C-labeled soluble cell organic acid content of each sample is defined as the summation of radioactivity (μCi) identified in the 14C-labeled organic acid chromatogram per μg cell protein.
16S rRNA gene sequencing and similarity analysis.
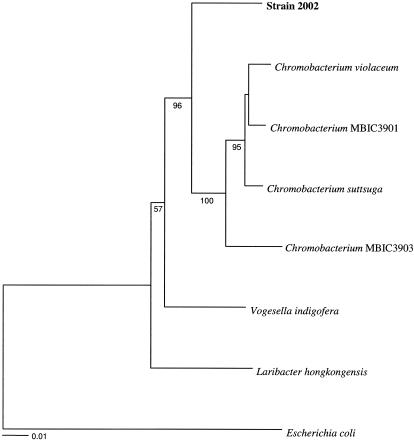
A 10-ml culture of strain 2002 maintained on 10 mM acetate and 10 mM nitrate was harvested by centrifugation (6,000 × g, 15 min). Cells were resuspended in 40 μl sterile water and lysed by the addition of 5 μl chloroform, which was followed by boiling for 10 min. Amplification, sequencing, and phylogenetic analysis of the 16S rRNA genes were performed as previously described (12). GenBank accession numbers (in parentheses) for 16S rRNA gene sequences represented in a phylogenetic tree (see Fig. 2) are as follows: Chromobacterium violaceum (M22510), Chromobacterium strain MBIC3901 (AB017487), Chromobacterium suttsuga (AY344056), Chromobacterium strain MBIC3903 (AB017489), Vogesella indigofera (AB021385), Laribacter hongkongensis (AF389085), and Escherichia coli (J01859).
FIG. 2.
Phylogenetic tree of the complete 16S rRNA gene sequence data set resulting from distance analysis of 1,548 characters using the Kimura two-parameter correction. The same topology was obtained using either parsimony or maximum likelihood. Bootstrap values based on 100 replicates are shown.
Presence of RuBisCo.
An attempt to identify the presence of ribulose-1-5-biphosphate carboxylase (RuBisCo) genes in the genome of strain 2002 was conducted via PCR amplification with primers designed to amplify form I, cbbL, and form II, cbbM, of the RuBisCo large subunit as previously described (17). The forward and reverse primers of cbbL, form I of the large subunit, i.e., 595f (forward; 5′-GACTTCACCAAAGACGACGA-3′) and 1387r (reverse; 5′-TCGAACTTGATTTCTTTCCA-3′), corresponded to positions 595 to 615 and 1387 to 1405, respectively, of Anabaena strain 7120 (17). The forward and reverse primers of cbbM, form II of the large subunit, i.e., cbbMf (5′-TCATCAARCCSAARCTSGGCCTGCGTCCC-3′) and cbbMr (5′-MGAGGTGACSGCRCCGTGRCCRGCMCGRTG-3′), corresponded to nucleotide positions 663 to 693 and 1033 to 1063, respectively, of the Riftia pachyptila endosymbiont cbbM gene (17). DNA extracts from microorganisms representing a variety of RuBisCo types, Synechococcus sp. strain PCC6301, Thiobacillus ferrooxidans, and R. rubrum, were demonstrated to produce a PCR product (17). PCR amplification of RuBisCo genes from within the Betaproteobacteria members Rhodocyclus tenuis and Dechloromonas aromatica strain RCB further confirmed the ability of these primers to yield a PCR product (data not shown).
Nucleotide sequence accession number.
The complete 16S rRNA gene sequence of strain 2002 has been deposited into GenBank under accession number AY609199.
RESULTS
Geochemical analysis and distribution of a nitrate-dependent Fe(II)-oxidizing microbial community in freshwater lake sediment.
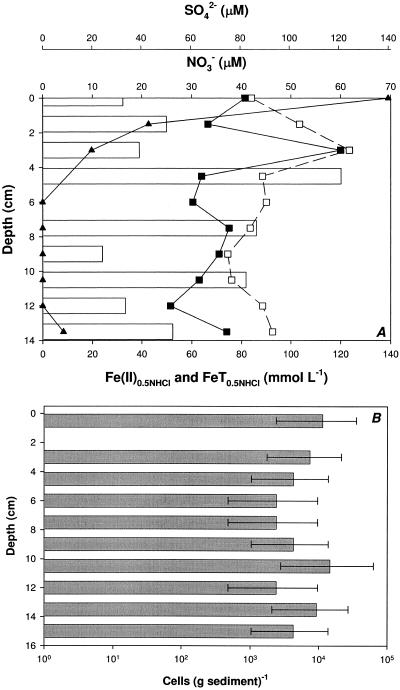
In situ geochemical analysis of a freshwater lake sediment core collected from Campus Lake in Carbondale, Illinois, revealed that the sediments were anoxic and highly reduced throughout the first 14 centimeters (Fig. 1A). Redox potential values ranged from a high of 70 mV at the sediment surface and rapidly decreased to −50 mV within the first 2 mm of depth (data not shown). Analysis of the dominant TEAP supported this observation, as [2-14C]acetate was oxidized to 14CO2 and 14CH4 in all sample incubations, indicating the presence of an active methanogenic community (data not shown). The addition of molybdate resulted in a significant decrease in the production of 14CO2, indicating that microbial sulfate reduction was also active in carbon turnover in these sediments. Consistent with this observation, the sulfate concentration decreased from the surficial sediment layer and was completely depleted at a depth of 6 cm, further indicating the activity of sulfate-reducing bacteria (Fig. 1A). Unexpectedly, detectable concentrations of both Fe(II) and nitrate were observed throughout the 14-cm-depth profile (Fig. 1A). This was not an isolated observation, as a similar geochemical profile was observed in a subsequent sediment core collected from Campus Lake (data not shown). Nitrite was not detected at any depth. Throughout the sediment core, a significant proportion (58 to 97%) of the 0.5 N HCl-extractable total Fe existed as Fe(II) (Fig. 1A) in the presence of nitrate. Coexistence of Fe(II) and nitrate is indicative of in situ environmental conditions capable of supporting a nitrate-dependent Fe(II)-oxidizing microbial community.
FIG. 1.
Chemical depth profile and nitrate, sulfate, Fe(II), and total Fe concentrations (A) and most probable number enumeration of nitrate-dependent Fe(II)-oxidizing microbial community at given depths (B) for Campus Lake sediment. (A) ▪, Fe(II); □, FeT; ▴, SO42−; bars, NO3−. The absence of bars denotes nitrate concentrations below the detection limit. For panel B, error bars associated with MPN enumeration are 95% confidence limits calculated by the Most Probable Number Calculator version 4.05 (Albert J. Klee, Risk Reduction Engineering Laboratory, United States Environmental Protection Agency, Cincinnati, Ohio, 1996; freeware available at http://www.epa.gov/nerlcwww/other.htm).
MPN enumeration of nitrate-dependent Fe(II)-oxidizing microorganisms in the sediment core revealed the presence of a microbial community ranging from 2.4 × 103 to 1.5 × 104 cells g−1 wet sediment throughout the core (Fig. 1B). No apparent correlation between the abundance of the nitrate-dependent Fe(II)-oxidizing microbial community and the ambient concentration of nitrate or of Fe(II) (Fig. 1) was recognized.
Isolation of strain 2002, a nitrate-dependent Fe(II)-oxidizing bacterium.
The most dilute tubes positive for Fe(II) oxidation in the MPN series (described above) were transferred to freshwater basal medium. Several morphologically identical cells in association with the Fe precipitates were apparent by microscopic observation of the positive enrichment. After successive transfers on basal medium amended with Fe(II) as the sole electron donor and CO2 as the sole carbon source, one isolate, strain 2002, was obtained and characterized further. Comparative analysis of the complete 16S rRNA gene sequence placed strain 2002 in the beta subclass of the Proteobacteria and in a close relationship with the common soil bacterium Chromobacterium violaceum, with 94.8% 16S rRNA gene sequence similarity (Fig. 2). Similarly to strain 2002, C. violaceum is a gram-negative, facultative, anaerobic, rod-shaped bacterium. In contrast to C. violaceum, strain 2002 was incapable of fermentative growth and did not produce the violet pigment known as violacein.
Nitrate-dependent Fe(II) oxidation by strain 2002 under nongrowth conditions.
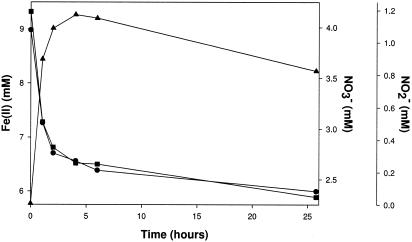
Iron(II) was rapidly oxidized, a process coupled to the stoichiometric reduction of nitrate to nitrite under nongrowth conditions by a washed-cell suspension of strain 2002 resuspended in anoxic PIPES buffer (10 mM, pH 7.0) (Fig. 3). No Fe(II) oxidation occurred in heat-killed controls or in the absence of a suitable electron acceptor (data not shown). Similarly, no reduction of nitrate occurred when Fe(II) was omitted. The molar ratio of nitrate reduced to Fe(II) oxidized (0.47) by strain 2002 was in agreement with the theoretical stoichiometry (0.5), consistent with equation 1:
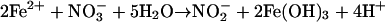 |
(1) |
No further reduction of nitrite was observed on extended incubation (Fig. 3). In addition, no oxidation of Fe(II) was observed if nitrite was provided as the sole electron acceptor in suspensions of live or heat-killed washed cells (data not shown). Similarly to strain 2002, C. violaceum oxidized Fe(II), a process coupled to the reduction of nitrate to nitrite under nongrowth conditions (data not shown). Nitrate was quantitatively reduced to nitrite; however, the decrease in Fe(II) was greater than can be accounted for by oxidation of the Fe(II) coupled to the reduction of nitrate. As with strain 2002, neither Fe(II) oxidation nor nitrate reduction was observed with heat-killed controls (data not shown).
FIG. 3.
Fe(II) oxidation coupled to nitrate reduction under nongrowth conditions [PIPES buffer (pH 7.0), 10 mM Fe(II), and 4 mM nitrate] by a suspension of live washed cells of strain 2002. ▪, Fe(II); •, NO3−; ▴, NO2−.
Nitrate-dependent Fe(II) oxidation by strain 2002 under autotrophic growth conditions.
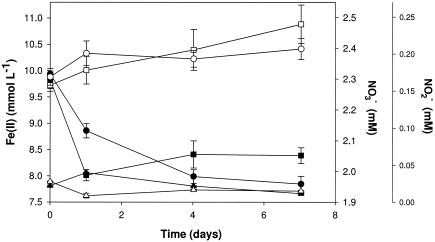
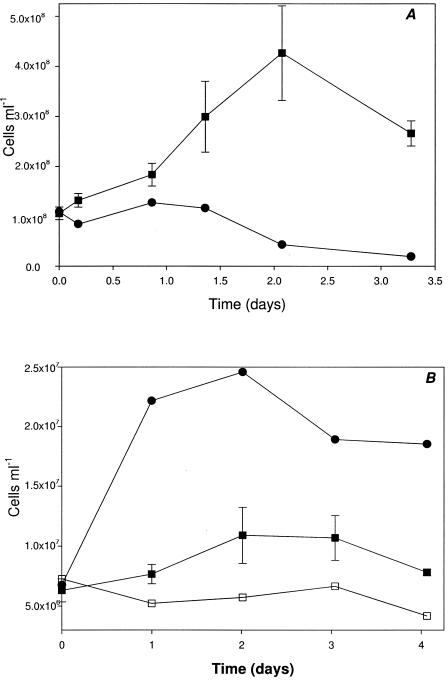
Active-growth cultures of strain 2002 under autotrophic nitrate-dependent Fe(II)-oxidizing conditions consistently oxidized 20 to 30% of the initial 10 mM Fe(II) in the culture medium. Although substantial amounts of nitrate remained, no further Fe(II) oxidation occurred after this initial period of activity (Fig. 4). In contrast to the result observed for nongrowth conditions, a transient accumulation of only a small amount of nitrite (40 μM) was observed (Fig. 4). No significant Fe(II) oxidation or nitrate reduction was observed in heat-killed controls (Fig. 4), demonstrating this reaction's dependence on the enzymatic activity of strain 2002. Similarly, no significant reduction of nitrate was observed over the course of the experiment when Fe(II) was omitted (data not shown). Upon cessation of Fe(II) oxidation, the molar ratio of Fe(II) oxidized to nitrate reduced was 0.24, which is in close agreement with the theoretical molar ratios of 0.20 and 0.25 from equations 2 and 3, respectively, suggesting the formation of gaseous nitrogen products, N2O and N2.
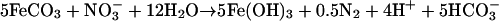 |
(2) |
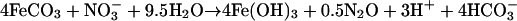 |
(3) |
FIG. 4.
Fe(II) oxidation coupled to nitrate reduction under autotrophic growth conditions by strain 2002. ▪, Fe(II) (active culture); □, Fe(II) (pasteurized culture); •, NO3− (active culture); ○, NO3− (pasteurized culture); ▴, NO2− (active culture); ▵, NO2− (pasteurized culture).
Autotrophic growth of strain 2002 under nitrate-dependent Fe(II)-oxidizing conditions.
Cell density of live cultures of strain 2002 showed a threefold increase concurrent with Fe(II) oxidation in defined bicarbonate-buffered (30 mM bicarbonate, pH 6.8) basal medium under a headspace of N2:CO2 (80:20, vol/vol) containing 10 mM Fe(II) and 2.2 mM nitrate (Fig. 5A). No organic carbon source was added, suggesting that strain 2002 obtained its carbon from dissolved CO2. Cultures grown autotrophically with CO2 as the sole carbon source could be passed through three sequential transfers without a loss of activity, demonstrating that growth was reproducible and robust (data not shown). Cell density increase was not observed when Fe(II) was omitted (Fig. 5A), indicating that growth was not a result of residual electron-donating capacity in the inoculum source and was dependent on the Fe(II) electron donor. Similar results were obtained when strain 2002 was cultured in autotrophic anaerobic (100% N2, CO2 free) PIPES-buffered (20 mM, pH 7.0) culture medium [1 mM Fe(II)-NTA and 0.25 mM nitrate] amended with 1 mM bicarbonate as the sole carbon source (Fig. 5B). No growth was observed in the absence of bicarbonate (Fig. 5B), indicating that the CO2 was assimilated as the carbon source.
FIG. 5.
(A) Autotrophic growth of strain 2002 in the presence of Fe(II) (10 mM) and NO3− (2.2 mM). ▪, Fe(II), nitrate; •, nitrate only. (B) Autotrophic and mixotrophic growth of strain 2002 under nitrate-dependent Fe(II)-oxidizing conditions in anaerobic (100% N2 headspace) PIPES-buffered basal medium. Fe(II)-NTA (1 mM), nitrate (0.25 mM), and bicarbonate (1 mM) (▪); Fe(II)-NTA (1 mM) and nitrate (0.25 mM) (□); Fe(II)-NTA (1 mM), nitrate (0.25 mM), and acetate (0.5 mM) (•). Error bars indicate standard errors for triplicate cultures.
Autotrophic metabolism was verified when strain 2002 grown in anoxic CO2-free PIPES-buffered culture medium amended with [14C]bicarbonate (specific activity, 2.5 μCi) incorporated 0.064 μCi of 14C label into the organic acid content of the cell extract per μg protein. A well-characterized photoautotroph, R. rubrum, incorporated an identical amount of 14C label per μg protein into the organic acid content of a similarly prepared cell extract. In contrast, no 14C label was detectable in the cell extract of heat-killed control cultures. This result demonstrated the ability of strain 2002 to fix CO2 under nitrate-dependent Fe(II)-oxidizing conditions. PCR amplification with primers designed to amplify form I, cbbL, and form II, cbbM, of the RuBisCo large subunit did not yield a PCR product with DNA extracted from strain 2002 (data not shown). Although not conclusive, this result suggested that an alternative CO2 fixation mechanism may be present. Growth under mixotrophic growth conditions was almost double that observed under autotrophic growth conditions (Fig. 5B). Replacement of the N2 in the headspace with He did not enhance the autotrophic cell yield beyond what was previously observed (data not shown), suggesting that the metabolism is not affected by feedback inhibition of the denitrification reaction by N2 in the headspace. Normalizing change in cell yield per electron transferred indicated that the cell yield for autotrophic growth (1.45 × 10−11 cells ml−1 per electron transferred) was approximately 63% that of mixotrophic [Fe(II)-oxidizing with 0.5 mM acetate as the carbon source] growth (2.3 × 10−11 cells ml−1 per electron transferred).
Alternative electron donors and acceptors.
In the absence of Fe(II), strain 2002 grew heterotrophically with several simple organic compounds, including acetate, propionate, butyrate, ethanol, pyruvate, and succinate as the sole carbon and energy sources. In all cases, no growth was observed when nitrate was omitted. Strain 2002 was relatively limited in the range of alternative electron acceptors used and grew on acetate aerobically or anaerobically with nitrate, nitrite, or nitrous oxide only as the alternative electron acceptor (data not shown). Reduction of Fe(III) [supplied as Fe(III)-NTA] to Fe(II) was observed in fresh anaerobic basal medium with acetate as the electron donor when inoculated (10% vol/vol) from an active anaerobic culture of strain 2002 pregrown on acetate (10 mM) and nitrate (10 mM). However, strain 2002 could not be continuously cultured under Fe(III)-reducing conditions.
DISCUSSION
Although anaerobic nitrate-dependent microbial oxidation of Fe(II) was identified nearly a decade ago, the microorganisms associated with the metabolism in situ are virtually unknown. Here we demonstrate that the geochemical conditions necessary to support an anaerobic nitrate-dependent Fe(II)-oxidizing microbial community are present in natural environments. In addition, this study provides the first example of a freshwater mesophilic lithoautotroph capable of growth through anaerobic nitrate-dependent Fe(II) oxidation.
Microbial nitrate-dependent Fe(II) oxidation in sedimentary environments.
Biogeochemical zones sequentially develop with increasing depth from the most energetically favorable to the least energetically favorable terminal electron-accepting process (20). Thus, the reduction of oxygen is followed by reductions of nitrate, Mn(IV), Fe(III), sulfate, and CO2 (7, 9, 10, 30, 31). As such, thermodynamic considerations would predict that nitrate should be completely removed in environments where Fe(II) is present. However, vertical geochemical profiles of Campus Lake sediment contained coexisting nitrate and dilute HCl-extractable Fe(II) concentrations. The coexistence of ambient nitrate and Fe(II) concentrations is not restricted to Campus Lake sediments, as similar geochemical profiles have been observed in rice paddy soil cores (40). The aforementioned thermodynamic predictions do not account for dynamic microbial metabolic processes generating nitrate. Such processes may account for the low concentrations (micromoles per liter) of nitrate observed at all depths of Campus Lake sediment, which, based on TEAP analyses, appears to be dominated by a mixed sulfate-reducing and methanogenic microbial community. Physical processes, i.e., diffusion and advection, can then contribute to the transport of both Fe(II) and nitrate across redox boundaries (4). An additional possibility is the potential for anoxic nitrification, as has been observed deep within the anaerobic zone in marine sediments (35-37). In those studies, it was suggested that ammonia oxidation coupled to the reduction of MnO2 (35-37) and possibly to that of amorphous Fe(III) oxides (36) was occurring. However, it is unknown whether or not anoxic nitrification is occurring in Campus Lake sediments. Regardless of the nitrification mechanism, nitrification processes could be expected to contribute to the formation of nitrate in Campus Lake sediments and may potentially influence nitrate-dependent Fe(II) oxidative processes in this environmental system. The contribution of nitrification to the formation of nitrate from ammonium is recognized in environments supporting a nitrate-dependent Fe(II)-oxidizing microbial community and was previously implicated as a controlling factor of nitrate-dependent Fe(II) oxidation (40). Further studies are needed to address the biogeochemical coupling between nitrification and nitrate-dependent Fe(II) oxidation processes in sedimentary environments.
The identification of the nitrate-dependent Fe(II)-oxidizing community in Campus Lake sediments (2.4 × 103 to 1.5 × 104 cells g−1 wet sediment) supports and further expands the observations of previous investigations (24, 40, 46, 52) in which it was shown that nitrate-dependent Fe(II)-oxidizing microbial communities were prevalent in several diverse environments. Previous studies have reported nitrate-dependent Fe(II)-oxidizing microbial communities at concentrations ranging from 1 × 103 to 5 × 108 cells g−1 sediment (24, 40, 46, 52). Straub and Buchholz-Cleven (46), as well as Ratering and Schnell (40), reported MPN enumeration findings of 1 × 105 to 5 × 108 cells g−1 (dry weight) sediment. This result is consistent with the size of the Fe(II)-oxidizing community in Campus Lake sediment, assuming an average (floc and consolidated layers) moisture content of the sediment of greater than 50%, which is typical for a lake sediment.
Geochemical conditions necessary to support microbially mediated anaerobic Fe(II) oxidation do occur in natural environments, given the vertical profiles of nitrate and iron in Campus Lake sediment observed in this study as well as in the rice paddy soil cores assayed by Ratering and Schnell (40). Both of these studies also established the presence of a microbial community capable of anaerobic nitrate-dependent microbial Fe(II) oxidation. Enumeration of the nitrate-dependent Fe(II)-oxidizing microbial community in Campus Lake sediments revealed that abundance did not significantly change with depth. This result is consistent with the nitrate-dependent Fe(II)-oxidizing community enumerated across redox zones (with depth) in sediment cores collected from Lake Constance (24). These results suggest that in addition to the recognized role that denitrifying/nitrate-reducing microorganisms play in nitrate-dependent Fe(II) oxidation, Fe(III)-reducing, sulfate-reducing, and methanogenic microbial communities may also potentially contribute to the reoxidation of Fe(II) in anaerobic environments. This is supported by recent reports demonstrating the metabolic ability of a model Fe(III)-reducing bacterium, Geobacter metallireducens, to contribute to the oxidation of Fe(II) coupled to nitrate reduction (19, 52). The abundance of the nitrate-dependent Fe(II)-oxidizing microbial community across various redox zones, as reported by Hauck and colleagues (24) as well as in this study, further supports the dynamic role that microbial nitrate-dependent Fe(II) oxidation may play in iron biogeochemical cycling.
Nitrate-dependent Fe(II) oxidation in pure culture.
Identification of strain 2002 presents the first opportunity to examine a lithoautotrophic, nitrate-dependent, Fe(II)-oxidizing bacterium in pure culture relative to the environmental role that microorganisms capable of this metabolism perform in most soil and sedimentary environments. Although cell density increase under Fe(II)-oxidizing conditions was limited to one doubling, repeated transfers and growth experiments demonstrated the reproducibility of this result. Attempts to create a more thermodynamically favorable culture environment by replacing the headspace gas (N2:CO2) with He:CO2 did not enhance cell yield. Interestingly, nitrate was reduced to different extents with Fe(II) as the sole electron donor under growth and nongrowth conditions. Under growth conditions, oxidation of Fe(II) coupled to nitrate reduction yielded gaseous N products consistent with those found in previous studies, which described the biological oxidation of Fe(II) coupled to denitrification (3, 8, 38, 45, 51). Although a minor concentration of nitrite was observed to transiently accumulate under growth conditions, under nongrowth conditions strain 2002 and C. violaceum quantitatively reduced nitrate to nitrite, after which no further significant biological reduction occurred. Nitrite did not serve as an electron acceptor for Fe(II) oxidation by strain 2002 under nongrowth conditions, even though nitrite did serve as a terminal electron acceptor under heterotrophic growth conditions with acetate as the sole carbon and energy source. The reason that nitrate is reduced to different extents under growth and nongrowth conditions is unknown, and this question will require more-detailed investigation in order to determine if nitrite production is simply an artifact of nongrowth conditions. Previous studies performed with the dissimilatory Fe(III)-reducing organism G. metallireducens have similarly demonstrated that the extent of nitrate reduction is directly affected by the source of the electron donor used by that organism (22). In those studies, which used a galvanic cell with graphite cathodes as the electron donor, nitrite was quantitatively produced from nitrate by G. metallireducens, an organism which normally reduces nitrate completely to ammonia when growing on acetate (32).
Environmental significance.
The results of the current study demonstrate the potential contribution in situ of nitrate-dependent Fe(II)-oxidizing microorganisms to anaerobic iron redox cycling. Furthermore, the isolation of a lithoautotrophic bacterium, strain 2002, supports the contribution of nitrate-dependent Fe(II)-oxidizing bacteria towards iron biogeochemistry in reduced environments resulting in Fe(III) mineral formation, which may include mixed-phase Fe(II)-Fe(III) minerals, such as green rust and/or magnetite (8). Given that bio-oxidation products immobilize heavy metals and radionuclide contaminants, the production of biogenic Fe(III) oxides formed as a result of this metabolism could also play a bioremediative role in contaminated sedimentary environments (28).
Acknowledgments
We thank Steven Singer, University of California, Berkeley, Calif., for the gift of Rhodospirillum rubrum.
This research was supported by the Department of Energy, Natural and Accelerated Bioremediation Program, through grants DE-FG02-98ER63592 to J.D.C. and DE-FG02-98ER62689 to L.A.A.
REFERENCES
- 1.Bazylinski, D. A., R. B. Frankel, and H. W. Jannasch. 1988. Anaerobic magnetite production by a marine, magnetotactic bacterium. Nature 334:518-519. [Google Scholar]
- 2.Bazylinski, D. A., E. Palome, N. A. Blakemore, and R. P. Blakemore. 1986. Denitrification by Chromobacterium violaceum. Appl. Environ. Microbiol. 52:696-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benz, M., A. Brune, and B. Schink. 1998. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch. Microbiol. 169:159-165. [DOI] [PubMed] [Google Scholar]
- 4.Berner, R. A. 1980. Early diagenesis. Princeton University Press, Princeton, N.J.
- 5.Bruce, R. A., L. A. Achenback, and J. D. Coates. 1999. Reduction of (per) chlorate by a novel organism isolated from paper mill waste. Environ. Microbiol. 1:319-329. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell, M. E., R. S. Tanner, and J. M. Suflita. 1999. Microbial metabolism of benzene and the oxidation of ferrous iron under anaerobic conditions: implications for bioremediation. Anaerobe 5:595-603. [Google Scholar]
- 7.Canfield, D. E., B. B. Jorgensen, H. Fossing, R. Glud, J. Gundersen, N. B. Ramsing, B. Thamdrup, J. W. Hansen, L. P. Nielsen, and P. O. J. Hall. 1993. Pathways of organic carbon oxidation in three continental margin sediments. Mar. Geol. 113:27-40. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri, S. K., J. G. Lack, and J. D. Coates. 2001. Biogenic magnetite formation through anaerobic biooxidation of Fe(II). Appl. Environ. Microbiol. 67:2844-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen, T. H., P. L. Bjerg, S. A. Banwart, R. Jakobsen, G. Heron, and H. Albrechtsen. 2000. Characterization of redox conditions in groundwater contaminant plumes. J. Contam. Hydrol. 45:165-241. [Google Scholar]
- 10.Coates, J. D., and L. A. Achenbach. 2001. The biogeochemistry of aquifer systems, p. 719-727. In C. J. Hurst, G. R. Knudsen, M. J. McInerney, L. D. Stetzenbach, and M. W. Walter (ed.), Manual of environmental microbiology, 2nd ed. ASM Press, Washington, D.C.
- 11.Coates, J. D., and R. Chakraborty. 2003. Anaerobic bioremediation: an emerging resource for environmental cleanup, p. 227-257. In I. M. Head, I. Singleton, and M. G. Milner (ed.), Bioremediation: a critical review. Horizon Scientific, Wymondham, United Kingdom.
- 12.Coates, J. D., U. Michaelidou, R. A. Bruce, S. M. O'Connor, J. N. Crespi, and L. A. Achenbach. 1999. Ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 65:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornell, R. M., and U. Schwertmann. 2003. The iron oxides: structure, properties, reactions, occurrences and uses, 2nd ed. Wiley-VCH, Weinheim, Germany.
- 14.Croal, L. R., C. M. Johnson, B. L. Beard, and D. K. Newman. 2004. Iron isotope fractionation by Fe(II)-oxidizing photoautotrophic bacteria. Geochim. Cosmochim. Acta 68:1227-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards, K. J., W. Bach, T. M. McCollom, and D. R. Rogers. 2004. Neutrophilic iron-oxidizing bacteria in the ocean: their habitats, diversity, and roles in mineral deposition, rock alteration, and biomass production in the deep-sea. Geomicrobiol. J. 21:393-404. [Google Scholar]
- 16.Edwards, K. J., D. R. Rogers, C. O. Wirsen, and T. M. McCollom. 2003. Isolation and characterization of novel psychrophilic, neutrophilic, Fe-oxidizing, chemolithoautotrophic α- and γ-Proteobacteria from the deep sea. Appl. Environ. Microbiol. 69:2906-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsaied, H., and T. Naganum. 2001. Phylogenetic diversity of ribulose-1,5-bisphosphate carboxylase/oxygenase large-subunit genes from deep-sea microorganisms. Appl. Environ. Microbiol. 67:1751-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emerson, D., and J. V. Weiss. 2004. Bacterial iron oxidation in circumneutral freshwater habitats: findings from the field and the laboratory. Geomicrobiol. J. 21:405-414. [Google Scholar]
- 19.Finneran, K. T., M. E. Housewright, and D. R. Lovley. 2002. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 4:510-516. [DOI] [PubMed] [Google Scholar]
- 20.Froelich, P. N., G. P. Klinkhammer, M. L. Bender, N. A. Luedtke, G. R. Heath, D. Cullen, P. Dauphin, D. Hammond, B. Hartman, and V. Maynard. 1979. Early oxidation of organic-matter in pelagic sediments of the eastern equatorial Atlantic—suboxic diagenesis. Geochim. Cosmochim. Acta 43:1075-1090. [Google Scholar]
- 21.Gibbs-Eggar, Z., B. Jude, J. Dominik, J. L. Loizeau, and F. Oldfield. 1999. Possible evidence for dissimilatory bacterial magnetite dominating the magnetite properties of recent lake sediments. Earth Planet. Sci. Lett. 168:1-6. [Google Scholar]
- 22.Gregory, K. B., D. R. Bond, and D. R. Lovley. 2004. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 6:596-604. [DOI] [PubMed] [Google Scholar]
- 23.Hafenbradl, D., M. Keller, R. Dirmeier, R. Rachel, P. Roβnagel, S. Burggraf, H. Huber, and K. O. Stetter. 1996. Ferroglobus placidus gen. nov., sp. nov. a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch. Microbiol. 166:308-314. [DOI] [PubMed] [Google Scholar]
- 24.Hauck, S., M. Benz, A. Brune, and B. Schink. 2001. Ferrous iron oxidation by denitrifying bacteria in profundal sediments of a deep lake (Lake Constance). FEMS Microbiol. Ecol. 37:127-134. [Google Scholar]
- 25.Heising, S., and B. Schink. 1998. Phototrophic oxidation of ferrous iron by a Rhodomicrobium vannielii strain. Microbiology 144:2263-2269. [DOI] [PubMed] [Google Scholar]
- 26.Kluber, H. D., and R. Conrad. 1998. Effects of nitrate, nitrite, NO and N2O on methanogenesis and other redox processes in anoxic rice field soil. FEMS Microbiol. Ecol. 25:301-318. [Google Scholar]
- 27.Lack, J. G., S. K. Chaudhuri, R. Chakraborty, L. A. Achenbach, and J. D. Coates. 2002. Anaerobic biooxidation of Fe(II) by Dechlorosoma suillum. Microb. Ecol. 43:424-431. [DOI] [PubMed] [Google Scholar]
- 28.Lack, J. G., S. K. Chaudhuri, S. D. Kelly, K. M. Kemner, S. M. O'Connor, and J. D. Coates. 2002. Immobilization of radionuclides and heavy metals through anaerobic bio-oxidation of Fe(II). Appl. Environ. Microbiol. 68:2704-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovley, D. R. 2000. Fe(III) and Mn(IV) reduction, p. 3-30. In D. R. Lovley (ed.), Environmental microbe-metal interactions. ASM Press, Washington, D.C.
- 30.Lovley, D. R., and F. H. Chapelle. 1995. Deep subsurface microbial processes. Rev. Geophys. 33:365-381. [Google Scholar]
- 31.Lovley, D. R., F. H. Chapelle, and J. C. Woodward. 1994. Use of dissolved H2 concentrations to determine the distribution of microbially catalyzed redox reactions in anoxic groundwater. Environ. Sci. Technol. 28:1205-1210. [DOI] [PubMed] [Google Scholar]
- 32.Lovley, D. R., S. J. Giovannoni, D. C. White, J. E. Champine, E. J. P. Phillips, Y. A. Gorby, and S. Goodwin. 1993. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 159:336-344. [DOI] [PubMed] [Google Scholar]
- 33.Lovley, D. R., and E. J. P. Phillips. 1987. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovley, D. R., J. F. Stolz, G. L. Nord, and E. J. P. Phillips. 1987. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 330:252-254. [Google Scholar]
- 35.Luther, G. W., B. Sundby, B. L. Lewis, P. J. Brendel, and N. Silverberg. 1997. Interactions of manganese with the nitrogen cycle: alternative pathways to dinitrogen. Geochim. Cosmochim. Acta 61:4043-4052. [Google Scholar]
- 36.Mortimer, R., S. Harris, M. Krom, T. Freitag, J. Prosser, J. Barnes, P. Anschutz, P. Hayes, and I. Davies. 2004. Anoxic nitrification in marine sediments. Mar. Ecol. Prog. Ser. 276:37-51. [Google Scholar]
- 37.Mortimer, R., M. Krom, S. Harris, P. Hayes, I. Davies, W. Davison, and H. Zhang. 2002. Evidence for suboxic nitrification in recent marine sediments. Mar. Ecol. Prog. Ser. 236:31-35. [Google Scholar]
- 38.Nielsen, J. L., and P. H. Nielsen. 1998. Microbial nitrate-dependent oxidation of ferrous iron in activated sludge. Environ. Sci. Technol. 32:3556-3561. [Google Scholar]
- 39.Ratering, S., and S. Schnell. 2000. Localization of iron-reducing activity in paddy soils by profile studies. Biogeochemistry 48:341-365. [Google Scholar]
- 40.Ratering, S., and S. Schnell. 2001. Nitrate-dependent iron(II) oxidation in paddy soil. Environ. Microbiol. 3:100-109. [DOI] [PubMed] [Google Scholar]
- 41.Roden, E. E., D. Sobolev, B. Glazer, and G. W. Luther. 2004. Potential for microscale bacterial Fe redox cycling at the aerobic-anaerobic interface. Geomicrobiol. J. 21:379-391. [Google Scholar]
- 42.Senn, D. B., and H. F. Hemond. 2002. Nitrate controls on iron and arsenic in an urban lake. Science 296:2373-2376. [DOI] [PubMed] [Google Scholar]
- 43.Sheloblina, E. S., C. G. VanPraagy, and D. R. Lovley. 2003. Use of ferric and ferrous iron containing minerals for respiration by Desulfitobacterium frappieri. Geomicrobiol. J. 20:143-156. [Google Scholar]
- 44.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 45.Straub, K. L., M. Benz, B. Schink, and F. Widdel. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 62:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Straub, K. L., and B. E. E. Buchholz-Cleven. 1998. Enumeration and detection of anaerobic ferrous iron-oxidizing, nitrate-reducing bacteria from diverse European sediments. Appl. Environ. Microbiol. 64:4846-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Straub, K. L., M. Hanzlik, and B. E. E. Buchholz-Cleven. 1998. The use of biologically produced ferrihydrite for the isolation of novel iron-reducing bacteria. Syst. Appl. Microbiol. 21:442-449. [DOI] [PubMed] [Google Scholar]
- 48.Straub, K. L., W. Schonhuber, B. Buchholz-Cleven, and B. Schink. 2004. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol. J. 21:371-378. [Google Scholar]
- 49.Wang, X., H. V. Modak, and F. R. Tabita. 1993. Photolithoautotrophic growth and control of CO2 fixation in Rhodobacter sphaeroides and Rhodospirillum rubrum in the absence of ribulose bisphosphate carboxylase-oxygenase. J. Bacteriol. 175:7109-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber, K. A. 2002. Microbial coupling between nitrogen and iron cycles: potential implications for nitrate and iron biogeochemistry and metal mobility in sedimentary environments. Dissertation. University of Alabama, Tuscaloosa.
- 51.Weber, K. A., F. W. Picardal, and E. E. Roden. 2001. Microbially catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environ. Sci. Technol. 35:1644-1650. [DOI] [PubMed] [Google Scholar]
- 52.Weber, K. A., M. M. Urrutia, P. F. Churchill, R. K. Kukkadapu, and E. E. Roden. 2006. Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ. Microbiol. 8:100-113. [DOI] [PubMed]
- 53.Widdel, F., S. Schnell, S. Heising, A. Ehrenreich, B. Assmus, and B. Schink. 1993. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature 362:834-836. [Google Scholar]