Abstract
Heterotrophic bacteria and fungi are widely recognized as crucial mediators of carbon, nutrient, and energy flow in ecosystems, yet information on their total annual production in benthic habitats is lacking. To assess the significance of annual microbial production in a structurally complex system, we measured production rates of bacteria and fungi over an annual cycle in four aerobic habitats of a littoral freshwater marsh. Production rates of fungi in plant litter were substantial (0.2 to 2.4 mg C g−1 C) but were clearly outweighed by those of bacteria (2.6 to 18.8 mg C g−1 C) throughout the year. This indicates that bacteria represent the most actively growing microorganisms on marsh plant litter in submerged conditions, a finding that contrasts strikingly with results from both standing dead shoots of marsh plants and submerged plant litter decaying in streams. Concomitant measurements of microbial respiration (1.5 to 15.3 mg C-CO2 g−1 of plant litter C day−1) point to high microbial growth efficiencies on the plant litter, averaging 45.5%. The submerged plant litter layer together with the thin aerobic sediment layer underneath (average depth of 5 mm) contributed the bulk of microbial production per square meter of marsh surface (99%), whereas bacterial production in the marsh water column and epiphytic biofilms was negligible. The magnitude of the combined production in these compartments (∼1,490 g C m−2 year−1) highlights the importance of carbon flows through microbial biomass, to the extent that even massive primary productivity of the marsh plants (603 g C m−2 year−1) and subsidiary carbon sources (∼330 g C m−2 year−1) were insufficient to meet the microbial carbon demand. These findings suggest that littoral freshwater marshes are genuine hot spots of aerobic microbial carbon transformations, which may act as net organic carbon importers from adjacent systems and, in turn, emit large amounts of CO2 (here, ∼870 g C m−2 year−1) into the atmosphere.
Analyses of microbial functions in ecosystems have placed emphasis on the capacities of microbes as decomposers and their roles in nutrient cycling (12, 16, 24). While these functions are clearly important, a sizeable fraction of organic matter degraded and assimilated by microbes is normally not respired or mineralized but utilized for cellular growth. This suggests that microbial secondary production constitutes a significant route of carbon and nutrient fluxes in ecosystems. Further, microbes tend to be enriched in nitrogen and phosphorus compared to the organic matter they degrade (63), suggesting that microbial biomass provides nutritious food for particle-ingesting consumers. Accordingly, the significance of microorganisms as secondary producers and food web components has long been central to concepts of carbon cycling in the pelagic zone of lakes and oceans (11, 12, 61). For benthic habitats and soils, in contrast, where most microbes occur globally (74) and most organic matter is turned over, information on annual microbial productivity is exceedingly scarce. Importantly, no annual estimates are available that differentiate between bacterial and fungal production, preventing assessment of the specific roles of these two groups of microorganisms to food web dynamics and carbon flows in ecosystems.
Wetlands dominated by emergent vegetation are often characterized by a complex physical structure and exceptionally high levels of plant production (29, 40) with up to 10 kg of shoot dry mass produced per square meter and per year (32, 72). Since only a small fraction of the plant tissue is consumed by herbivores during the growing season (40), most enters the detrital pool after plant senescence and death (25, 39, 47), the resulting accumulation of organic matter providing ample scope for secondary microbial production.
Some estimates of bacterial production in sediments have highlighted the importance of carbon flows through benthic bacterial biomass (up to 160 g C m2 year−1) (36, 41). In macrophyte-dominated wetlands, additional biomass may be produced in epiphytic biofilms (46, 67), the water column (43), and particularly plant litter (39). Fungal production associated with litter likewise can be sizeable, as shown for small streams (ca. 12 to 15 g C m−2 year−1) (42, 65) and standing dead plant shoots in salt marshes (535 g C m2 year−1) (50). These data reveal that both bacterial and fungal production can indeed be large and that in view of the paucity of comprehensive data, the current understanding of carbon flow in nonpelagic ecosystems is correspondingly incomplete (52).
The main objectives of the present study were (i) to provide an estimate of total annual carbon production of heterotrophic microbes in aerobic aquatic compartments of a physically complex system, a freshwater marsh dominated by Phragmites australis (Cav.) Trin. ex Steud., a cosmopolitan, highly productive wetland plant; (ii) to assess the relative contribution of different marsh compartments (surficial sediment, submerged plant litter, epiphytic biofilms, and water); (iii) to determine the relative contribution of bacteria and fungi in the plant litter compartment; (iv) to depict the seasonal dynamics of microbial production; and (v) to relate total microbial carbon demand to primary production of the marsh plants to assess the importance of heterotrophic bacteria and fungi to carbon flow in the ecosystem.
MATERIALS AND METHODS
Study site.
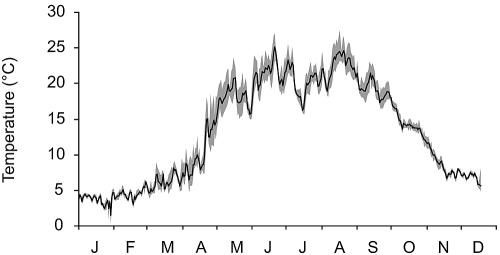
The study was conducted in a littoral marsh on the eastern shore of Lake Hallwil, a eutrophic, meromictic lake on the Swiss Plateau (47°17′N, 8°14′E) at 449 m above sea level (Table 1). The common reed, Phragmites australis, extended 850 m along the shore, with the width of the stand varying between 6 and 20 m and the water depth averaging 1 m at the lakeward margin and 35 to 70 cm in the center. Temperature in the marsh was recorded at 30-min intervals with 8-bit Minilog-TR data loggers (Vemco, Nova Scotia, Canada) deployed in the litter layer at a ca. 60-cm water depth (Fig. 1).
TABLE 1.
Morphometry of Lake Hallwil and water chemistry within a Phragmites marsh on the eastern shorea
| Parameter | Mean | Range |
|---|---|---|
| Lake morphometry | ||
| Catchment area (km2)b | 128 | |
| Lake surface area (km2) | 10 | |
| Mean depth (m) | 28 | |
| Maximum depth (m) | 46 | |
| Volume (km3) | 0.3 | |
| Theoretical water residence time (a) | 3.8 | |
| Water chemistry | ||
| Conductivity (μS cm−1) | 286 | 223-421 |
| Alkalinity (mmol liter−1 at 25°C) | 2.4 | 1.5-3.9 |
| pH | 8.3 | 7-9 |
| Chlorophyll a and b (μg liter−1) | 8.5 | 0.5-18.6 |
| SRP (μg liter−1) | 2.0 | 0-12 |
| TP (μg liter−1) | 40 | 18-188 |
| NH4+-N (μg liter−1) | 23 | 0.6-163.4 |
| NO2−-N (μg liter−1) | 2.5 | 0-9 |
| NO3−-N (μg liter−1) | 330 | 0-1,240 |
| DON (μg liter−1) | 384 | 275-941 |
| DOC (mg liter−1) | 7.2 | 1.6 |
Samples were taken monthly in each of the six sampling plots (n = 72). SRP, soluble reactive phosphorus; TP, total phosphorus; DON, dissolved organic nitrogen.
Including the catchment of upstream Lake Baldegg.
FIG. 1.
Range (gray area) and average (black line) of daily temperature in the litter layer within the investigated littoral marsh.
Experimental design and sampling.
A stratified random sampling design was used to delineate six plots (each, 2.8 by 1.3 m) within the marsh. Two plots were situated at the lakeward side (A), two were situated in the center (B), and two were situated landward (C) in the randomly determined order ABCBAC. Within each plot, sampling areas 30 cm in diameter were randomly chosen at each sampling occasion, which were visited not more than once during the study. Monthly samples were taken in four aerobic compartments: the water column, epiphytic biofilms on submerged reed stems, the plant litter layer, and the aerobic top sediment layer underneath the plant litter. Depth-integrated water samples were taken with an acid-washed polyvinyl chloride tube and placed in 1-liter glass bottles. Submerged stems of P. australis were clipped off just above the sediment surface, and the submerged portions were cut in three 10-cm sections and inserted in sterile tubes containing lake water. Sediment samples were collected with a hand-held corer modified after Davis and Steinman (13) with a diameter of 6.5 cm. The vertical extension of the aerobic sediment layer was determined directly in the field with a calibrated oxygen microelectrode (model O2NAD-1; Toepffer Lab Systems, Göppingen, Germany), and the aerobic sediment was subsequently sampled to that depth (0 to 29 mm) and transferred to autoclaved glass vials. Plant litter was collected with a custom-made drum sampler (30 cm in diameter) that allowed sampling without the destruction of plant shoots. The material enclosed by the cylinder was pumped over a 1-mm mesh screen, rinsed with lake water, and placed in plastic boxes. Samples from all compartments were stored in cool boxes and processed immediately upon arrival at the laboratory.
Sample processing.
Volumes between 400 to 700 ml (depending on particle concentration) were filtered through precombusted (450°C; 4 h) fiberglass filters (GF/F; Whatman, Maidstone, United Kingdom) for chemical water analyses and chlorophyll determination. Filters for chlorophyll analysis were stored overnight at 4°C in 90% ethanol. The filtrate was used for chemical analyses. For dissolved organic carbon (DOC) analysis, 20 ml of water was filtered through precombusted GF/F filters. The filtrate was collected in a precombusted (550°C; 4 h) glass tube with a Teflon-lined screw cap, acidified with 200 μl of 1 M HCl, and stored at 4°C. Ten milliliters of water was preserved with buffered (0.1% sodium pyrophosphate) 2% formalin and stored in glass vials at 4°C for bacterial biomass determination. Three milliliters of water was transferred to scintillation vials to estimate bacterial production. Submerged stem sections of P. australis with attached epiphytic biofilms were cut into three 1-cm pieces and placed in tubes containing 3 ml of filtered (0.2-μm-pore-size) lake water. These subsamples served to determine bacterial production. To determine bacterial biomass, the epiphytic biofilm on the remaining stem sections was carefully scraped off with a scalpel and collected in a graduated tube, the volume was adjusted to 50 ml with filtered lake water, the tube was vortexed, and a representative subsample (5 ml) was taken with a reversed glass pipette and preserved with buffered formalin. Two 0.5-ml aliquots of the aerobic sediment were transferred to glass vials with sterile 1-ml syringes with the luer end cut off. One aliquot was preserved with 10 ml of buffered formalin to determine bacterial biomass, and the other was adjusted to 1 ml with filtered lake water to measure bacterial production. Representative plant litter subsamples were cut into small pieces with a razor blade, and subsamples (each, 500 mg [wet weight]) were preserved in 10 ml of buffered formalin for bacterial biomass determination. Additional subsamples (each, 80 mg [wet weight]) were placed into glass vials containing 1 ml of filtered lake water to determine bacterial production. The remaining plant litter was frozen, freeze-dried, weighed, and ground to pass a 0.5-mm mesh screen. The resulting powder was used to determine carbon content.
Chemical analyses.
Total organic carbon of plant litter was determined with a CHN analyzer (Vario EL elemental analyzer; Elementar, Hanau, Germany). The accuracy of the instrument was 99.2% as determined by a certified sediment standard (no. 8704; National Institute of Standards and Technology, Gaithersburg, Md.). Acetanilide was used as a routine standard.
Conductivity and pH of the marsh water were measured in the field with a multiparameter field meter (meter 340i; WTW, Weilheim, Germany). Alkalinity was determined by titration with 0.1 M HCl to pH 4.3. DOC was analyzed by high temperature catalytic oxidation with a total organic carbon analyzer (TOC-5000A; Shimadzu, Kyoto, Japan). Samples were acidified with 1 M HCl and sparged for 7 min to remove the inorganic carbon, and a 100-μl aliquot was injected on top of the catalyst (0.5% Pt; 680°C). The system was calibrated with freshly prepared potassium hydrogen phthalate (C8H5KO4) standards in the range of 100 to 1,000 μM. Reported DOC values are averages of three replicate injections from each sample. Analyses of soluble reactive phosphorus, total phosphorus, ammonia, nitrite, nitrate, and dissolved nitrogen were determined according to German Standard Methods for the Examination of Water, Wastewater and Sludge (15). Chlorophyll was extracted in 90% ethanol by heating for 10 min in a water bath at 80°C and subsequent treatment in an ultrasonic bath for 10 min. Chlorophylls a and b were separated and quantified by reversed-phase high-performance liquid chromatography using 49.5% methanol, 45% ethylacetate, and 5.5% water (vol/vol/vol) as the mobile phase (45), with detection at 430 and 460 nm, respectively.
Bacterial production and growth rates.
Bacterial production was estimated as leucine incorporation into bacterial protein (35). A specifically adapted protocol involving ultrasonication and NaOH extraction ensured high protein extraction efficiencies and a good signal-to-noise ratio (generally >10:1) even in the presence of normally strongly interfering substances derived from plant litter and sediments rich in organic matter (9). Basic assumptions underlying the method, such as linearity of leucine incorporation, substrate saturation, and isotope dilution, were checked for each substrate (9). Specific activities, saturation levels, and incubation volumes used in the present study are summarized in Table 2. Leucine incorporation rates were converted to bacterial production rates using a conversion factor for freshwater bacteria (1.44 kg C mol−1 (10), which is similar to the factor reported for marine bacterioplankton (60).
TABLE 2.
Conditions used for [3H]leucine incubations to determine bacterial production in four aerobic compartments of a littoral freshwater marsh
| Compartment | Incubation vol (ml) | Total concn (μM) | Sp act (Bq mmol−1) |
|---|---|---|---|
| Water | 3 | 0.15 | 5.8-7.8 · 1011 |
| Epiphyton | 3 | 0.3 | 3.1-4.4 · 1011 |
| Sediment | 1 | 50 | 6.1-7.8 · 109 |
| Plant litter | 1 | 50 | 6.9-7.8 · 109 |
To determine bacterial biomass, bacterial cells were detached from epiphyton, sediment, and plant litter with an ultrasonic probe as detailed in Buesing and Gessner (8). Appropriate aliquots (each, 10 to 350 μl) were filtered onto aluminum oxide filters (0.02-μm pore size, Anodisc; Whatman) supported by a backing filter (0.45-μm pore size; Millipore HAWP). The Anodisc filter was placed on a drop of the fluorescent stain SYBR Green II (stock solution diluted 400 fold; Molecular Probes, Eugene, Oreg.) for 15 min, blotted dry, and transferred to a slide (53). A drop of antifade mounting solution (50% glycerol, 0.1% p-phenylenediamine, 50% phosphate-bufferd saline [120 mM NaCl-10 mM NaH2PO4, pH 7.5) was added, and slides were viewed under a Zeiss Axioskop 2 epifluorescence microscope equipped with a 100-W high-pressure bulb and a Chroma light filter set (no. 41001) for SYBR Green II (excitation filter, 480 nm; beam splitter, 505 nm; emission filter, 530 nm). Cell numbers and biovolumes were determined for 10 to 20 microscopic fields (typically >400 cells) with an image analysis system (Visitron Systems, Puchheim, Germany). Digital images were captured with a 12-bit cooled slow-scan charge-coupled device camera (Photometrics SenSys 0400; Roper Scientific, Trenton, N. J.) controlled by MetaMorph Imaging Software (Universal Imaging Corp., Downingtown, Pa.), which was also used for analyzing the acquired images (7). Fluorescence images were corrected by background subtraction for shading resulting from uneven illumination (contrast stretched). Cell edges were sharpened by the application of a low-pass filter, which was subtracted from the original picture. The optimal threshold level for detecting cells from background was set manually. The resulting binary picture was then edited in an overlay mode with the original gray image. Green-fluorescing bacterium-shaped particles with a diameter of >0.3 μm were considered to be bacteria. Cells were counted, and the area and perimeter of each cell was determined to derive cell length (l) and width (w). This indirect procedure to determine l and w is generally necessary, since imaging software overestimate real cell dimensions severely, especially for curved bacteria. Cell volumes (V) of individual cells were calculated under the assumption that cells were cylinders with hemispherical ends (20). Cellular carbon (C, in picograms) was derived from cell volumes (V, in cubic micrometers) according to the allometric relation C = 0.12 · V0.7 (54). Growth rates were calculated as P/B ratios, where P is the bacterial production rate and B is the bacterial biomass.
Fungal production and growth rates.
Fungal biomass, growth rate, and production were estimated by determining ergosterol concentrations and rates of [14C]acetate incorporation into ergosterol (28). Samples were incubated for 5 h at ambient lake temperatures in 4 ml of filtered lake water containing sodium [14C]acetate (Amersham, Little Chalfont, Buckinghamshire, United Kingdom); the specific activity of added acetate was 3.7 107 Bq mmol−1 (27). The total added acetate concentration was 5 mM. Controls containing buffered formalin were treated in the same way. Incubations were stopped by placing samples on ice and collecting them immediately on GF/F filters. Filters were rinsed with 15 ml of GF/F-filtered lake water, frozen, freeze-dried, and weighed.
Ergosterol was extracted in alkaline methanol (8 g KOH per liter; 80°C for 30 min), cleaned, concentrated, and quantified by solid-phase extraction (30) and high-performance liquid chromatography. The ergosterol fractions of three separate injections (50 μl each) were collected, combined, and mixed with 10 ml Packard Emulsifier Scintillator Plusä, and radioactivity was measured with a Packard Tri-Carb 1600CA liquid scintillation counter with corrections for quenching.
Ergosterol concentrations were converted to fungal biomass by assuming an ergosterol concentration of 7.3 mg ergosterol g−1 fungal dry mass as determined for two fungal strains (Stagonospora sp. and Leptosphaeria sp.) growing on standing-dead Phragmites shoots at the study site (K. A. Kuehn et al., unpublished data). This conversion factor is similar to the average of a range of other saprophytic fungi (17, 26, 48). To convert fungal dry mass to fungal carbon, a factor of 0.43 g C g−1 dry mass was applied (17). Fungal production was calculated from rates of acetate incorporation into ergosterol using a conversion factor of 12.6 μg fungal biomass per nmol acetate incorporated (49). Growth rates of fungi were calculated as P/B ratios, as for bacteria.
Microbial respiration.
Microbial respiration was determined as oxygen consumption at ambient lake temperature with six oxygen microelectrodes (model 5357; YSI, Yellow Springs, Ohio). Samples were placed in glass chambers, which were subsequently filled with known volumes of filtered (GF/F) littoral lake water (each, about 30 ml), taking care not to enclose air bubbles. A stainless steel grid was positioned on top of the samples to prevent swirling of litter pieces. After acclimation to the target temperature, the linear decrease in oxygen concentration was recorded every 30 s for 30 min. Electrode drift was monitored for each chamber before and after respiration measurements, but corrections of measured gross rates for drift were not necessary.
Rates of O2 consumption were converted to rates of CO2 evolution, assuming a respiratory quotient of 0.85 mol CO2 per mol O2. After measurements, samples were dried (105°C overnight) and weighed to the nearest 0.1 mg. Sample dry mass was converted to carbon, based on the carbon content determined for parallel samples. Microbial growth efficiency was calculated for each sample as the sum of bacterial and fungal production divided by the sum of total microbial respiration and bacterial plus fungal production, expressed as a percentage.
Plant shoot production.
Living shoots were counted at the end of the growing season (late August to early September) in 1999, 2000, and 2001 within 4 quadrats (50 by 50 cm) immediately adjacent to each of the six delineated sampling plots. One representative shoot in each quadrat (i.e., a total of 24 shoots per year) was cut just above the sediment surface and separated into leaf blades and sheaths, adventitious roots, stems, and panicles. Fully brown dead, senescent, and green leaf blades and sheaths were also distinguished, and the number of missing leaves was recorded. Total shoot production was estimated as the sum of all fractions plus an estimate of lost and partly decayed leaves, with brown leaf blades and sheaths assumed to have lost 15% of their initial weight (25). Missing leaves were assumed to have the same average weight as the leaves remaining on shoots.
Data analysis.
Bacterial production in sediment and water were expressed in volumetric units to avoid spurious correlations (6) and to facilitate comparison with published data. Data from epiphytic biofilms were expressed on an areal substrate basis (in micrograms of bacterial C per square centimeter of stem surface area). Microbial production and respiration data for plant litter were normalized by the carbon content of the organic matter (e.g., micrograms of bacterial C per gram of plant litter C). In addition, production data for all compartments and respiration data were expressed on an areal basis (i.e., grams of bacterial C per square meter of marsh surface area). Total annual production was calculated by first linearly interpolating daily production rates per square meter of marsh surface measured in each plot under the assumption that measurements were representative for half of the time between the preceding and following sampling date. Data for each plot were then summed, and statistics were calculated on the resulting annual values.
Repeated-measure analysis of variance was used to examine temporal and spatial differences. Sampling time was used as the within-subject factor and plot position (landward, marsh center, lakeward) was used as the between-subject factor. All data were logarithmically transformed before analyses. This resulted in near-normal distributions of the residuals as checked by means of quantile-quantile plots and Shapiro-Wilks tests. Huyn-Feldt-adjusted P values are reported to account for the fact that data failed to meet the circularity conditions. These adjusted probabilities are more conservative than standard P values. Statistical analyses were made with SYSTAT 10 (SPS, Inc., Chicago, Ill.) or JMP IN 3.2.1 (SAS Institute Inc., Cary, N.C.).
RESULTS
Plant shoot production.
Annual above-ground net production of P. australis in the years 1999, 2000, and 2001 was 1,510, 850, and 1,660 g m−2 year−1, respectively. Given a carbon content of 45% (25), the average of 1,340 ± 250 g m−2 year−1 (mean ± standard error [SE]) corresponds to 603 ± 112 g C m−2 year−1.
Bacterial production and growth rates.
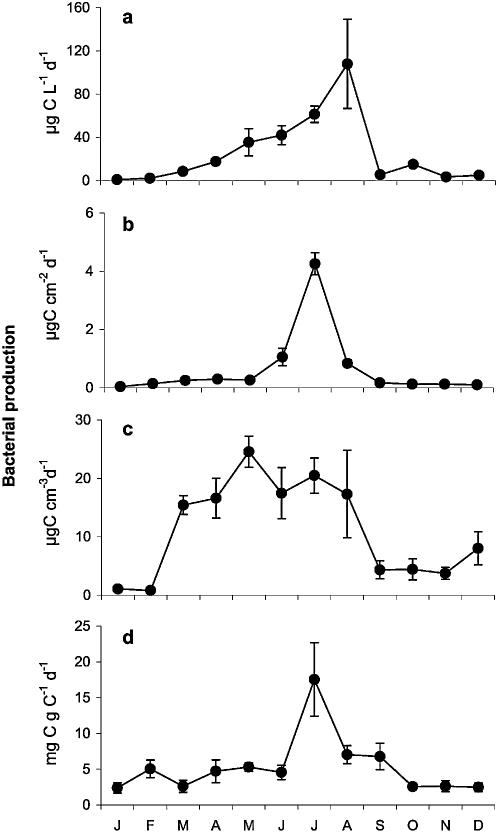
Bacterial production in the marsh water column varied seasonally over 2 orders of magnitude (Fig. 2a). The lowest rate was observed in January (1.0 μg liter−1 day−1), the highest in August (108 μg liter−1 day−1). The annual average was 25 μg liter−1 day−1. In epiphytic biofilms, bacterial production followed a similar temporal pattern (0.04 μg C cm−2 stem surface area day−1 in January and 4.3 μg C cm−2 day−1 in July), but the maximum was more distinct and was reached 1 month earlier (Fig. 2b). The annual average was 0.6 μg C cm−2 day−1. Two distinct periods of either high bacterial production (∼20 μg C cm−3 day−1; March until August) or four-times-lower production (September till February) occurred in the aerobic top sediment layer (Fig. 2c). The annual average was 12 μg C cm−3 day−1. Production rates associated with plant litter (Fig. 2d) varied between 2.6 mg C g−1 C day−1 during winter and a pronounced peak of 18.8 mg C g−1 day−1 in July, with an annual average of 5.7 mg C g−1 day−1.
FIG. 2.
Bacterial production in the water column (a), epiphyton on reed culms (b), aerobic top sediment layer (c), and plant litter layer (d) of the investigated littoral marsh. Values are means ± SE.
Rates of bacterial production expressed per square meter of marsh surface area ranged from 0.2 to 6.3 g C m−2 day−1 (average of 2.1 g C m−2 day−1) and 0.6 to 4.1 g C m−2 day−1 (average, 1.9 g C m−2 day−1) in sediment and plant litter, respectively, and were thus 2 to 3 orders of magnitude higher than in epiphytic biofilms (range, 0.27 to 33 mg m−2 day−1; average, 6.2 mg m−2 day−1) and the marsh water column (range, 0.4 to 41 mg m−2 day−1; average, 11.4 mg m−2 day−1) (Fig. 3). Bacterial production in different compartments peaked in different months, as indicated by the highly significant interaction term of compartment and time (Table 3).
FIG. 3.
Bacterial production per square meter of littoral marsh in the water column (a), epiphyton (b), sediment (c), and litter layer (d). Values are means ± SE.
TABLE 3.
Effects on bacterial production (g C m−2 day−1) of marsh habitats (littoral water, epiphytic biofilm, sediment, and plant litter), position in the marsh (landward, center, or lakeward) and time (sampling month) as analyzed by repeated-measure analysis of variancea
| Source of variation | SS | df | F |
|---|---|---|---|
| Between subjects | |||
| Compartment | 2,975.0 | 3 | 193.0*** |
| Position | 4.4 | 2 | 0.4 |
| Compartment × position | 14.3 | 6 | 0.5 |
| Error | 61.7 | 12 | |
| Within subjects | |||
| Time | 300.0 | 11 | 26.2*** |
| Time × compartment | 124.0 | 33 | 3.6*** |
| Time × position | 23.5 | 22 | 1.0 |
| Time × compartment × position | 57.5 | 66 | 0.8 |
| Error | 137.3 | 132 |
SS, sum of squares; df, degree of freedom; ∗∗∗, P < 0.001.
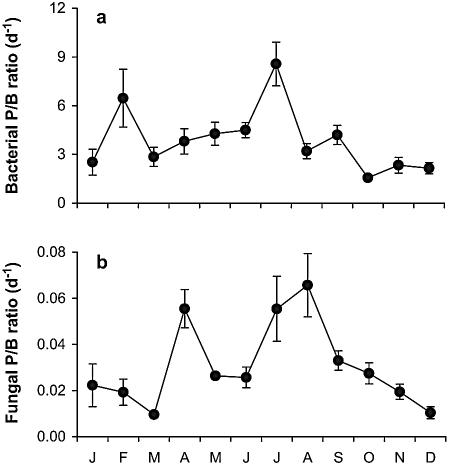
High bacterial growth rates (P/B ratios) in sediment and plant litter (average, 2.6 and 3.4 day−1, respectively) were due to both high production rates and relatively low biomass. In epiphytic biofilms on submerged reed stems and in the marsh water column, rates were 20 to 30 times lower (average, 0.12 and 0.14 day−1, respectively). Seasonal variations were caused primarily by temporal variation in production rates (Fig. 4a), although biomass also changed over time (data not shown).
FIG. 4.
Bacterial (a) and fungal (b) growth rates in the litter layer of the investigated littoral marsh. Bars indicate ± SE (for six samples).
Fungal production and growth rates.
The average production rate of fungi associated with plant litter was 0.7 mg C g−1 C day−1 with maxima in April (2.4 mg C g−1 day−1) and in July and August (1.1 and 1.0 mg C g−1 day−1) (Fig. 4b), resulting in a highly significant effect of sampling time (P < 0.001). Plot position did not affect fungal production (P = 0.98). The lower fungal production rates coupled with higher biomass resulted in growth rates (P/B ratios) ranging from 0.010 to 0.066 day−1 (annual average, 0.031 day−1), which were >100 times lower than the average bacterial growth rates.
Annual microbial production.
Annual bacterial production per square meter of marsh surface area was lowest for epiphytic biofilm on reed stems (mean ± SE, 1.67 ± 0.14 g C m−2 year−1), slightly higher for the littoral water column (4.39 ± 0.44 g C m−2 year−1), and much higher in both the aerobic top sediment layer (732 ± 72 g C m−2 year−1) and plant litter (661 ± 182 g C m−2 year−1). Fungal production on plant litter was 93 ± 36 g C m−2 year−1.
Microbial respiration and growth efficiency.
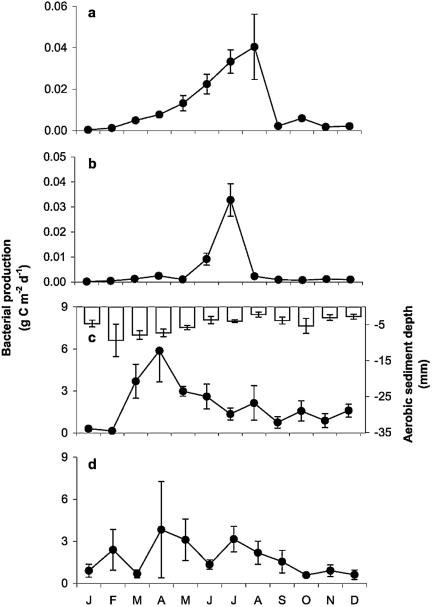
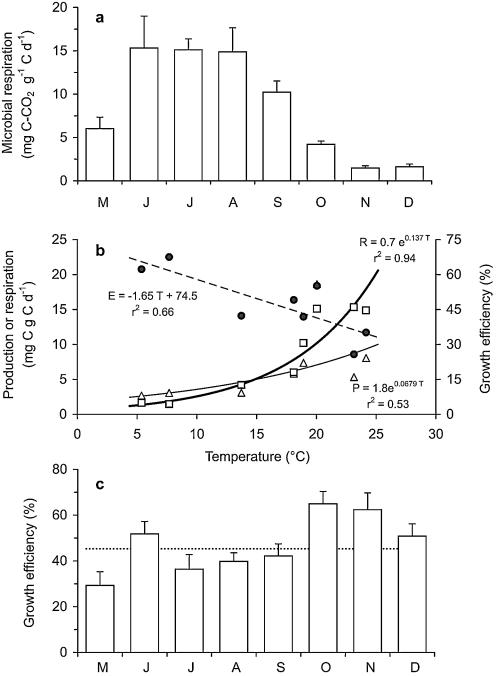
Microbial respiration associated with plant litter was determined between May and December (Fig. 5a). Rates increased sharply between May and June to a maximum of 15.3 mg C g−1 C day−1. The lowest rate (1.5 mg C g−1 plant litter C day−1) was measured in November. Respiration rates increased exponentially with water temperature between 5 and 25°C (r2 = 0.94; P < 0.001) (Fig. 5b). Annual respiration was estimated at 874 ± 58 g C m−2 year−1.
FIG. 5.
Daily microbial respiration rates on plant litter (a); microbial production (open triangles), microbial respiration (open squares), and growth efficiency (black circles) on plant litter as a function of temperature (b); and microbial growth efficiency from May to December 2000 (c). The dotted line indicates average microbial growth efficiency. Bars indicate ± SE (for six samples).
Microbial growth efficiency (ɛ) ranged from 29 to 65% (Fig. 5c). The annual average estimated as the slope of a linear regression of microbial production (i.e., bacterial plus fungal production) versus the sum of microbial production and respiration was 45.5% (r2 = 0.71; P < 0.001). Growth efficiency showed a significant negative relationship with temperature (T), where ɛ=−1.7 · T + 75 (r2 = 0.66; P < 0.01) (Fig. 5b), indicating a stronger response to temperature of microbial respiration than of production.
DISCUSSION
Our study of microbial productivity in a freshwater marsh revealed three remarkable results that have important implications for microbial dynamics and mechanisms of carbon cycling in ecosystems. First, bacterial productivity can greatly outweigh fungal productivity in decaying plant litter, a finding that contrasts with the dominance of fungi in both decaying leaves submerged in streams and standing dead shoots of marsh plants above the water level. Second, total aerobic microbial production in the marsh was driven about equally by bacteria within a thin aerobic surficial sediment layer and the much thicker overlying plant litter layer, whereas contributions by marsh bacterioplankton and epiphytic biofilms were trivial. Third, and most important to ecosystem carbon and energy flow, even elevated primary productivity within marshes can be insufficient to meet microbial carbon demands. As a consequence, a sizeable import of organic matter into the marsh would be required to balance microbial metabolic outputs.
Predominance of bacterial productivity.
The dominance of bacterial over fungal production we observed in submerged marsh plant litter is striking. The two other litter decomposition systems examined so far are characterized by the opposite pattern: the amount of bacterial productivity was typically much smaller than that of fungi on both standing dead plant shoots in marshes (39, 51) and decomposing leaves submerged in litterbags in streams (3, 4, 55, 73). The contrasting bacterial dominance observed in the present study did not result from poor fungal performance, since fungal biomass production (near 100 g C m−2 year−1) clearly was significant on submerged marsh plant litter in absolute terms (for comparisons, see references 42, 47, and 65). Rather, bacterial production outweighed even the high level of fungus production, due to high growth rates (1.36 to 7.92 day−1) and corresponding turnover times (2.8 to 15 h) that compensated for the smaller bacterial biomass. Thus, bacteria rather than fungi appear to be the primary mediators of carbon flow on submerged decomposing litter in freshwater marshes.
Magnitude and seasonal dynamics of microbial productivity and respiration.
Our high bacterial production rates in the aerobic sediment are within the range of rates measured in other freshwater sediments (36, 41, 75). Although comparable data are not available for naturally decaying plant litter in submerged conditions, data from litterbag studies show bacterial production rates 2 orders of magnitude lower (39, 51, 66). Several explanations can be offered to account for the discrepancy. First, the greater bacterial productivity we observed in the marsh may be related to a decline in fungi (which potentially are strong competitors of heterotrophic bacteria) when standing dead plant litter topples over and becomes exposed to radically different environmental conditions on the marsh surface (39; G. Van Ryckegem et al., unpublished data). Second, litter retrieved from litterbags on average is less aged than the heterogeneous mixture of decaying organic matter sampled in the present study, and it is well known that bacteria gradually gain in importance as decomposition of organic matter proceeds (62). Third, our large productivity of litter bacteria likely reflects methodological differences among studies, specifically the enhanced extraction efficiency of protein when protocols include an NaOH treatment (2, 9, 18, 37).
In contrast to the plant litter system, our bacterial production rates in the marsh water column are well within the range of bacterial production rates reported for pelagic freshwater systems, which range from 0.02 to 6.25 μg C liter−1 h−1 (11). They were generally closer to values reported from eutrophic (34) than oligotrophic waters (56, 71) and also compare well with the few data available from shallow water bodies with abundant macrophyte growth, i.e., 0.2 to 2.9 μg C liter−1 h−1 (21) or 3.78 to 9.98 μg C liter−1 h−1 (23), with an average of 4.98 μg C liter−1 h−1 (57). Similarly, in biofilms on reed stems, our average bacterial production rate was akin to rates determined in other environments, including epiphytic biofilms in a lowland river (0.75 μg cm−2 day−1) (19) and in two subtropical freshwater marshes (2.76 μg cm−2 day−1) (68) and 0.93 to 1.85 μg cm−2 day−1 (69). Thus, apart from the fast rate of growth of bacteria on plant litter, our estimates of microbial productivity are consistent with published reports.
The microbial respiration associated with plant litter in our marsh was also in good agreement with literature data (22, 38). The resulting growth efficiencies (average, 45.5%) are similar to the average of empirically determined values for bacteria growing on submerged decaying plant litter in freshwaters (41%) (reference 14 and references therein). This good correspondence of independent measures lends confidence to the accuracy of our results.
Seasonal variations of bacterial productivity in water and epiphytic biofilms were characterized by a large single peak in midsummer, similarly to the large changes reported from bacteria growing on sea grass leaves (70). However, the dynamics of bacterial productivity in sediment and plant litter was much smaller, although productivity correlated broadly with temperature in all marsh compartments. As a result of this dampened seasonal response in plant litter and sediment, total microbial productivity per square meter of marsh showed less pronounced seasonal changes than may have been expected.
Role of microbial production in marsh carbon flows.
Our invariably high bacterial production rates on a square meter basis in the aerobic sediment and plant litter are comparable to estimates for sediments in small streams (18 to 140 mg C m−2 h−1) (33) and mangroves (8.3 to 213 mg C m−2 h−1) (1), which range among the highest records in the literature. This comparison shows that the marsh we studied is an ecosystem characterized by intense microbial carbon turnover. As a result, total annual microbial secondary production (bacteria plus fungi; all compartments combined) per square meter of marsh surface amounted to 1,492 ± 199 g C. As much as 99% of this production was due to the aerobic sediment and plant litter layer, the epiphytic biofilm and the water column making trivial contributions on a marsh area basis. Given a bacterial growth efficiency of 45.5% as determined for plant litter microbes (Fig. 5c) and typical of aerobic compartments in eutrophic environments (5, 11), a total microbial carbon demand of 3,280 g C m−2 year−1 results.
On the supply side, average net shoot production of P. australis accounts for 603 ± 112 g C m−2 year−1, likely by far the greatest portion of organic carbon produced within the marsh (29). Exudates from P. australis root mats extending at the sediment surface are likely to provide some additional 60 g C m−2 year−1 (i.e., 10% of shoot production) (58), especially to bacteria in the aerobic top sediment layer. In addition, riparian trees at the landward edge of the marsh likely provided allochthonous litter inputs on the order of 50 g C m2 (29). A rough estimate of algal production on reed stems can be derived from our monthly chlorophyll measurements (Table 1) based on a specific productivity of 2 mg C mg−1 chlorophyll a h−1 determined for littoral epilithic algae (31, 44) which resulted in 210 g C m−2 year−1. Phytoplankton production within the marsh is unlikely to be important, since in two other Phragmites marshes it has been estimated at only 9 (29) and 0.53 ± 0.04 (57) g C m−2 year−1. Thus, our estimate of all primary carbon sources amounts to about 930 g C m−2 year−1.
Part of these primary carbon sources can be used multiple times because carbon converted to microbial biomass can be reutilized by heterotrophic organisms including microbes (64). In our case, the average carbon atom can be used 1.84 times until it is completely respired, according to the geometric progression
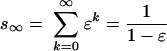 |
with a growth efficiency (ɛ) of 45.5%. Consequently, the sum of all organic carbon deriving from plant production and riparian inputs (930 g C m−2 year−1) allows for a microbial carbon assimilation of 1,710 g C m−2 year−1. This figure still leaves a deficit in the balance of 1,570 g C m−2 year−1 and thus implies additional inputs of external carbon sources of at least 714 g C m−2 year−1.
Additional organic carbon may be received from surrounding land, the subsurface, and/or the open lake. Slightly positive hydraulic heads of piezometers installed in the marsh indicate that subsurface inputs were too small to notably contribute to the budget (unpublished data). Pelagic plankton, in contrast, is likely to be an important source. The marsh is located downwind of prevailing southwestern and northwestern winds, which induce horizontal currents strong enough (30 cm s−1 on average every 3 to 4 days, as recorded with an acoustic Doppler current profiler; A. Lorke, personal communication) to transport large volumes of surface water across the lake in a conveyer belt-like fashion. Given a fetch of 1.5 km and an annual primary production in the upper meter of the pelagic zone of about 30 g C m−2 (reference 59 and A. Stöckli, personal communication), some 2,700 g C m−2 year−1 would be carried into the marsh, where seston can settle as currents slow within the vegetation stand. This mass transport across the lake and the littoral trapping effect is macroscopically most evident during blooms of the blue-green alga (cyanobacterium) Planktothrix rubescens (Fig. 6). As this model calculation illustrates, pelagic carbon inputs (2,700 g C m−2 year−1) could easily meet the microbial demand for production and respiration in the littoral marsh. Consequently, plankton of pelagic origin may be a critical carbon fraction sustaining bacterial production in the marsh. As a consequence of this inverse littoral zone-pelagic zone coupling, littoral marshes may thus act as sinks of lakeborne organic carbon and in turn emit large amounts of CO2 from microbial respiration to the atmosphere.
FIG. 6.
Bloom of Plankthotrix rubescens accumulating in the investigated littoral marsh. Photograph, courtesy of D. Steiner.
Acknowledgments
We thank R. Gächter, J. V. Ward, M. Märki, and the late R. G. Wetzel for discussion, critical reading, and valuable comments on an earlier draft of the manuscript. Many thanks also to Dany Steiner particularly for ergosterol and respiration measurements; to Othmar Fries, Katri Saukkonen, Catherine Hoyle, and numerous other colleagues for field and laboratory assistance; and to Robert Berger and his workshop team for designing and constructing sampling devices. The Baudepartement of the Canton Aargau kindly granted permission to access the protected site, and the Windsurfing School Octopus and Hotel Seerose provided logistic support which was much appreciated.
This study was partly supported by the Research Commission of the Swiss Federal Institute of Science and Technology (ETH Zurich; grant no. 0-23010-00) and the Swiss National Science Foundation (3100-050439.97).
REFERENCES
- 1.Alongi, D. M. 1988. Bacterial productivity and microbial biomass in tropical mangrove sediments. Microb. Ecol. 15:59-79. [DOI] [PubMed] [Google Scholar]
- 2.Bååth, E., M. Pettersson, and K. H. Söderberg. 2001. Adaptation of a rapid and economical microcentrifugation method to measure thymidine and leucine incorporation by soil bacteria. Soil Biol. Biochem. 33:1571-1574. [Google Scholar]
- 3.Baldy, V., E. Chauvet, J.-Y. Charcosset, and M. O. Gessner. 2002. Microbial dynamics associated with leaves decomposing in the mainstem and a floodplain pond of a large river. Aquat. Microb. Ecol. 28:25-36. [Google Scholar]
- 4.Baldy, V., and M. O. Gessner. 1997. Towards a budget of leaf litter decomposition in a first-order woodland stream. C. R. Acad. Sci. Ser. III 320:747-758. [Google Scholar]
- 5.Bastviken, D., M. Olsson, and L. Tranvik. 2003. Simultaneous measurement of organic carbon mineralization and bacterial production in oxic and anoxic lake sediments. Microb. Ecol. 46:73-82. [DOI] [PubMed] [Google Scholar]
- 6.Bird, D. F., and C. M. Duarte. 1989. Bacteria-organic matter relationship in sediments: a case of spurious correlation. Can. J. Fish. Aquat. Sci. 46:904-908. [Google Scholar]
- 7.Buesing, N. 2005. Bacterial counts and biomass determination by epifluorescence microscopy, p. 203-208. In M. A. S. Graça, F. Bärlocher, and M. O. Gessner (ed.), Methods to study litter decomposition: a practical guide. Springer, Dordrecht, The Netherlands.
- 8.Buesing, N., and M. O. Gessner. 2002. Comparison of detachment procedures for direct counts of bacteria associated with sediment particles, plant litter and epiphytic biofilms. Aquat. Microb. Ecol. 27:29-36. [Google Scholar]
- 9.Buesing, N., and M. O. Gessner. 2003. Incorporation of radiolabeled leucine into protein to estimate bacterial production in plant litter sediment epiphytic biofilms and water samples. Microb. Ecol. 45:291-301. [DOI] [PubMed] [Google Scholar]
- 10.Buesing, N., and J. Marxsen. 2005. Theoretical and empirical conversion factors for determining bacterial production in freshwater sediments via leucine incorporation. Limnol. Oceanogr. Methods 3:101-107. [Google Scholar]
- 11.Cole, J. J., S. Findlay, and M. L. Pace. 1988. Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar. Ecol. Prog. Ser. 43:1-10. [Google Scholar]
- 12.Cotner, J. B., and B. A. Biddanda. 2002. Small players, large role: microbial influence on biogeochemical processes in pelagic aquatic ecosystems. Ecosystems 5:105-121. [Google Scholar]
- 13.Davis, W. P., and A. D. Steinman. 1998. A lightweight, inexpensive benthic core sampler for use in shallow water. J. Freshw. Ecol. 13:475-479. [Google Scholar]
- 14.del Giorgio, P. A., and J. J. Cole. 1998. Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 29:503-541. [Google Scholar]
- 15.Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung. 1996. Physikalische, chemische, biologische und bakteriologische Verfahren. Aktuelles Grundwerk (Lieferung 1-63). Wasserchemische Gesellschaft, Fachgruppe in der GDCh (ed.), in Gemeinschaft mit dem Normenausschuss Wasserwesen (NAW) im DIN e.V. (ed.). Wiley-VCH, Weinheim, Germany.
- 16.Eyre, B. D., and A. J. P. Ferguson. 2002. Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic microalgae- and macroalgae-dominated warm-temperate Australian lagoons. Mar. Ecol. Progr. Ser. 229:43-59. [Google Scholar]
- 17.Findlay, S. E. G., S. Dye, and K. A. Kuehn. 2002. Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22:616-625. [Google Scholar]
- 18.Fischer, H., and M. Pusch. 1999. Use of the [14C]leucine incorporation technique to measure bacterial production in river sediments and the epiphyton. Appl. Environ. Microbiol. 65:4411-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer, H., and M. Pusch. 2001. Comparison of bacterial production in sediments, epiphyton and the pelagic zone of a lowland river. Freshw. Biol. 46:1335-1348. [Google Scholar]
- 20.Fry, J. C. 1988. Determination of biomass, p. 27-72. In B. Austin (ed.), Methods in aquatic bacteriology. Wiley, New York, N.Y.
- 21.Furtado, A. L. S., P. Casper, and F. A. Esteves. 2001. Bacterioplankton abundance, biomass and production in a Brazilian coastal lagoon and in two German lakes. An. Acad. Bras. Cienc. 37:39-49. [DOI] [PubMed] [Google Scholar]
- 22.Fuss, C. L., and L. A. Smock. 1996. Spatial and temporal variation of microbial respiration rates in a blackwater system. Freshw. Biol. 36:339-349. [Google Scholar]
- 23.Gajewski, A. J., and R. J. Chróst. 1995. Production and enzymatic decomposition of organic matter by microplankton in a eutrophic lake. J. Plankton Res. 17:709-728. [Google Scholar]
- 24.Gauci, V., E. Matthews, N. Dise, B. Walter, D. Koch, and G. Granberg. 2004. Sulfur pollution suppression of the wetland methane source in the 20th and 21st centuries. Proc. Natl. Acad. Sci. USA 34:12583-12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gessner, M. O. 2001. Mass loss, fungal colonisation and nutrient dynamics of Phragmites australis leaves during senescence and early aerial decay. Aquat. Bot. 69:325-339. [Google Scholar]
- 26.Gessner, M. O., and E. Chauvet. 1993. Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl. Environ. Microbiol. 59:205-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gessner, M. O., and E. Chauvet. 1997. Growth and production of aquatic hyphomycetes in decomposition leaf litter. Limnol. Oceanogr. 42:496-505. [Google Scholar]
- 28.Gessner, M. O., and S. Y. Newell. 2002. Biomass, growth rate, and production of filamentous fungi in plant litter, p. 390-408. In C. J. Hurst, R. L. Crawford, G. R. Knudsen, M. J. McInerney, and L. D. Stetzenbach (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 29.Gessner, M. O., B. Schieferstein, U. Müller, S. Barkmann, and U. A. Lenfers. 1996. A partial budget of primary organic carbon flows in the littoral zone of a hardwater lake. Aquat. Bot. 55:93-105. [Google Scholar]
- 30.Gessner, M. O., and A. Schmitt. 1996. Use of solid-phase extraction to determine ergosterol concentrations in plant tissue colonized by fungi. Appl. Environ. Microbiol. 62:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickman, M. 1971. Standing crops and primary productivity of the epipelon of two small ponds in North Somerset, U.K. Oecologia 6:238-253. [DOI] [PubMed] [Google Scholar]
- 32.Hocking, P. J. 1989. Seasonal dynamics of production, and nutrient accumulation and cycling by Phragmites australis (Cav.) Trin. ex Stuedel in a nutrient-enriched swamp in inland Australia. I. Whole plants. Aust. J. Mar. Freshw. Res. 40:421-444. [Google Scholar]
- 33.Hudson, J. J., J. C. Roff, and B. K. Burnison. 1992. Bacterial productivity in forested and open streams in southern Ontario. Can. J. Fish. Aquat. Sci. 49:2412-2422. [Google Scholar]
- 34.Jørgensen, N. O. G. 1992. Incorporation of [3H]leucine and [3H]valine into protein of freshwater bacteria: uptake kinetics and intracellular isotope dilution. Appl. Environ. Microbiol. 58:3638-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirchman, D. L., E. K’Nees, and R. E. Hodson. 1985. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl. Environ. Microbiol. 49:599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirschner, A. K. T., and B. Velimirov. 1999. Benthic bacterial secondary production measured via simultaneous 3H-thymidine and 14C-leucine incorporation, and its implication for the carbon cycle of a shallow macrophyte-dominated backwater system. Limnol. Oceanogr. 44:1871-1881. [Google Scholar]
- 37.Kirschner, A. K. T., and B. Velimirov. 1999. Modification of the 3H-leucine centrifugation method for determining bacterial protein synthesis in freshwater samples. Aquat. Microb. Ecol. 17:201-206. [Google Scholar]
- 38.Komínková, D., K. A. Kuehn, N. Büsing, D. Steiner, and M. O. Gessner. 2000. Microbial biomass, growth, and respiration associated with submerged litter of Phragmites australis decomposing in a littoral reed stand of a large lake. Aquat. Microb. Ecol. 22:271-282. [Google Scholar]
- 39.Kuehn, K. A., M. J. Lemke, K. Suberkropp, and R. G. Wetzel. 2000. Microbial biomass and production associated with decaying leaf litter of the emergent macrophyte Juncus effusus. Limnol. Oceanogr. 45:862-870. [Google Scholar]
- 40.Kvêt, J., and D. F. Westlake. 1998. Primary production in wetlands, p. 78-139. In D. F. Westlake, J. Kvêt, and A. Szczepanski (ed.), The production ecology of wetlands. Cambridge University Press, Cambridge, United Kingdom.
- 41.Marxsen, J. 2001. Bacterial production in different streambed habitats of an upland stream: sandy versus coarse gravelly sediments. Arch. Hydrobiol. 152:543-565. [Google Scholar]
- 42.Methvin, B. R., and K. Suberkropp. 2003. Annual production of leaf-decaying fungi in 2 streams. J. N. Am. Benthol. Soc. 22:554-564. [Google Scholar]
- 43.Moran, M. A., and R. E. Hodson. 1992. Contributions of three subsystems of a freshwater marsh to total bacterial secondary productivity. Microb. Ecol. 24:161-170. [DOI] [PubMed] [Google Scholar]
- 44.Moss, B. 1969. Algae of two Somersetshire pools: standing crops of phytoplankton and epipelic algae as measured by cell numbers and chlorophyll a. J. Ecol. 57:397-414. [DOI] [PubMed] [Google Scholar]
- 45.Murray, A. P., C. F. Gibbs, A. F. Longmore, and D. J. Flett. 1986. Determination of chlorophyll in marine waters: intercomparison of a rapid HPLC method with full HPLC, spectrophotometric and fluorometric methods. Mar. Chem. 19:211-227. [Google Scholar]
- 46.Neely, R. K., and R. G. Wetzel. 1995. Simultaneous use of 14C and 3H to determine autotrophic production and bacterial protein production in periphyton. Microb. Ecol. 30:227-237. [DOI] [PubMed] [Google Scholar]
- 47.Newell, S. Y. 1993. Decomposition of shoots of a salt-marsh grass, p. 301-325. In J. Gwynfryn Jones (ed.), Advances in microbial ecology, vol 13. Plenum Press, New York, N.Y. [Google Scholar]
- 48.Newell, S. Y. 1996. The [14C]acetate-to-ergosterol method: factors for conversion from acetate incorporated to organic fungal mass synthesized. Soil Biol. Biochem. 28:681-683. [Google Scholar]
- 49.Newell, S. Y. 2000. Methods for determining biomass and productivity of mycelial marine fungi, p. 69-91. In K. D. Hyde and S. B. Pointing (ed.), Marine mycology—a practical approach. Fungal Diversity Press, Hong Kong, China.
- 50.Newell, S. Y. 2001. Multiyear patterns of fungal biomass dynamics and productivity within naturally decaying smooth cordgrass shoots. Limnol. Oceanogr. 46:573-583. [Google Scholar]
- 51.Newell, S. Y., M. A. Moran, R. Wicks, and R. E. Hodson. 1995. Productivities of microbial decomposers during early stages of decomposition of leaves of a freshwater sedge. Freshw. Biol. 34:135-148. [Google Scholar]
- 52.Newell, S. Y., and D. Porter. 1999. Microbial secondary production from saltmarsh-grass shoots, and its known and potential fates, p. 159-185. In M. P. Weinstein and D. A. Kreeger (ed.), Concepts and controversies in tidal marsh ecology. Kluwer, Amsterdam, The Netherlands.
- 53.Noble, R. T., and J. A. Fuhrman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-118. [Google Scholar]
- 54.Norland, S. 1993. The relationship between biomass and volume of bacteria, p. 303-307. In P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (ed.), Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, Fla.
- 55.Pascoal, C., and F. Cassio. 2004. Contribution of fungi and bacteria to leaf litter decomposition in a polluted river. Appl. Environ. Microbiol. 70:5266-5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petit, M., P. Servais, and P. Lavandier. 1999. Bacterial production measured by leucine and thymidine incorporation rates in French lakes. Freshw. Biol. 42:513-524. [Google Scholar]
- 57.Reitner, B., A. Herzig, and G. J. Herndl. 1999. Dynamics in bacterioplankton production in a shallow, temperate lake (Lake Neusiedl, Austria): evidence for dependence on macrophyte production rather than on phytoplankton. Aquat. Microb. Ecol. 19:245-254. [Google Scholar]
- 58.Richert, M., S. Saarnio, Juutinen, S., J. Silvola, J. Augustin, and W. Merbach. 2000. Distribution of assimilated carbon in the system Phragmites australis-waterlogged peat soil after carbon-14 pulse labelling. Biol. Fertil. Soils 32:1-7. [Google Scholar]
- 59.Schumpelick-Deuschel, B. 1995. Einfluss der Populationsstruktur, Verteilung und Biomasse des Planktons auf das Community Grazing im Hallwilersee. Ph.D. thesis 11293. Eidgenössische Technische Hochschule Zürich, Zürich, Switzerland.
- 60.Simon, M., and F. Azam. 1989. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Progr. Ser. 51:201-213. [Google Scholar]
- 61.Simon, M., H.-P. Grossart, B. Schweitzer, and H. Ploug. 2002. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 28:175-211. [Google Scholar]
- 62.Sinsabaugh, R. L., and S. Findlay. 1995. Microbial production, enzyme activity, and carbon turnover in surface sediments of the Hudson River estuary. Microb. Ecol. 30:127-141. [DOI] [PubMed] [Google Scholar]
- 63.Sterner, R. W., and J. J. Elser. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton, N.J.
- 64.Strayer, D. 1988. On the limits to secondary production. Limnol. Oceanogr. 33:1217-1224. [Google Scholar]
- 65.Suberkropp, K. 1997. Annual production of leaf-decaying fungi in a woodland stream. Freshw. Biol. 38:169-178. [Google Scholar]
- 66.Suberkropp, K., and H. Weyers. 1996. Application of fungal and bacterial production methodologies to decomposing leaves in streams. Appl. Environ. Microbiol. 62:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theil-Nielsen, J., and M. Søndergaard. 1999. Production of epiphytic bacteria and bacterioplankton in three shallow lakes. Oikos 86:283-292. [Google Scholar]
- 68.Thomaz, S. M., and F. A. Esteves. 1997. Secondary productivity (3H-leucine and 3H-thymidine incorporation), abundance and biomass of the epiphytic bacteria attached to detritus of Typha domingensis Pers. in a tropical coastal lagoon. Hydrobiologia 357:17-26. [Google Scholar]
- 69.Thomaz, S. M., and R. G. Wetzel. 1995. [3H]leucine incorporation methodology to estimate epiphytic bacterial biomass production. Microb. Ecol. 29:63-70. [DOI] [PubMed] [Google Scholar]
- 70.Törnblom, E., and M. Søndergaard. 1999. Seasonal dynamics of bacterial biomass and production on eelgrass Zostera marina leaves. Mar. Ecol. Progr. Ser. 179:231-240. [Google Scholar]
- 71.Tulonen, T. 1993. Bacterial production in a mesohumic lake estimated from [14C]leucine incorporation rate. Microb. Ecol. 26:201-217. [DOI] [PubMed] [Google Scholar]
- 72.Wetzel, R. G., and M. J. Howe. 1999. High production in a herbaceous perennial plant achieved by continuous growth and synchronized population dynamics. Aquat. Bot. 64:111-129. [Google Scholar]
- 73.Weyers, H., and K. Suberkropp. 1996. Fungal and bacterial production during the breakdown of yellow poplar leaves in 2 streams. J. N. Am. Benthol. Soc. 15:408-420. [Google Scholar]
- 74.Whitman, W. B., D. C. Coleman, and W. J. Wiebe. 1998. Prokaryotes: the unseen majority. Proc. Natl. Acad. Sci. USA 95:6578-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wieltschnig, C., U. R. Fischer, A. K. T. Kirschner, and B. Velimirov. 2003. Benthic bacterial production and protozoan predation in a silty freshwater environment. Microb. Ecol. 46:62-72. [DOI] [PubMed] [Google Scholar]