Abstract
Thirteen of the most common lepidopteran-specific Cry proteins of Bacillus thuringiensis have been tested for their efficacy against newly hatched larvae of two populations of the spiny bollworm, Earias insulana. At a concentration of 100 μg of toxin per milliliter of artificial diet, six Cry toxins (Cry1Ca, Cry1Ea, Cry1Fa, Cry1Ja, Cry2Aa, and Cry2Ab) were not toxic at all. Cry1Aa, Cry1Ja, and Cry2Aa did not cause mortality but caused significant inhibition of growth. The other Cry toxins (Cry1Ab, Cry1Ac, Cry1Ba, Cry1Da, Cry1Ia, and Cry9Ca) were toxic to E. insulana larvae. The 50% lethal concentration values of these toxins ranged from 0.39 to 21.13 μg/ml (for Cry9Ca and Cry1Ia, respectively) for an E. insulana laboratory colony originating from Egypt and from 0.20 to 4.25 μg/ml (for Cry9Ca and Cry1Da, respectively) for a laboratory colony originating from Spain. The relative potencies of the toxins in the population from Egypt were highest for Cry9Ca and Cry1Ab, and they were both significantly more toxic than Cry1Ac and Cry1Ba, followed by Cry1Da and finally Cry1Ia. In the population from Spain, Cry9Ca was the most toxic, followed in decreasing order by Cry1Ac and Cry1Ba, and the least toxic was Cry1Da. Binding experiments were performed to test whether the toxic Cry proteins shared binding sites in this insect. 125I-labeled Cry1Ac and Cry1Ab and biotinylated Cry1Ba, Cry1Ia, and Cry9Ca showed specific binding to the brush border membrane vesicles from E. insulana. Competition binding experiments among these toxins showed that only Cry1Ab and Cry1Ac competed for the same binding sites, indicating a high possibility that this insect may develop cross-resistance to Cry1Ab upon exposure to Cry1Ac transgenic cotton but not to the other toxins tested.
Bacillus thuringiensis (Berliner) is a soil bacterium that produces a diversity of Cry proteins that are selectively toxic against a wide variety of insect pests (6). Synthetic cry genes from B. thuringiensis, modified for plant-preferred codon usage, have been introduced in a number of major crops (referred to as Bt crops) such as maize, cotton, and potato to make them insect resistant (28). Bt cotton was commercially released in the United States in 1996 and subsequently in several countries including Argentina, Australia, China, Colombia, Indonesia, Mexico, South Africa, and India (42). Cotton is currently the third most important transgenic crop in terms of surface area (after soybean and maize), involving nine million hectares in 2004 (11% of global genetically modified area). The countries that devote the largest area to Bt cotton are the United States and China, where more than half of their planted cotton is the result of biotechnological engineering (23). The European Union has recently opened its market to several products, some of them derived from Bt cotton, under the regulation on genetically modified food and feed (EC regulation no. 1829/2003; EC regulation no. 258/97-Art.5). However, no variety of Bt cotton has yet been approved for commercial planting in Europe.
The primary pests targeted by Bt cotton technology in North America are the tobacco budworm, Heliothis virescens, the cotton bollworm, Helicoverpa zea, and the pink bollworm, Pectinophora gossypiella. Throughout the rest of the world, Helicoverpa armigera is a primary pest with high resistance to organophosphate and pyrethroid insecticides that causes crop losses comparable to those caused by H. virescens in North America (29). According to the susceptibility of the above-described species to different lepidopteran-specific B. thuringiensis toxins, Cry1Ac cotton was selected as the best choice for commercial release. The second generation of Bt cotton combines Cry1Ac with a second B. thuringiensis toxin (Cry2Ab) and provides growers with a product that offers a broader spectrum of pest control and reduced chances of insects developing B. thuringiensis resistance (12, 45). Therefore, most commercially planted insect-resistant cotton contains Cry1Ac (in China, Bt cotton has been transformed to express a Cry1Ab-Cry1Ac hybrid toxin), which undoubtedly will pose an important selection pressure on the lepidopteran populations in the cotton ecosystem.
The genus Earias is widely distributed in the Old World and Australasia, and some are pests of considerable importance in many of cotton-growing countries of Africa and Asia. The spiny bollworm, Earias insulana (Boisduval), has an extremely wide range and is found throughout most of Africa and the Mediterranean region and eastwards to India, China, and Southeast Asia (38).
This species is an important component of the lepidopteran pest complex of cotton in some regions in Spain (7), Egypt (18), Israel (21), India, and Pakistan (25). Although it is a pest of cotton, it can also grow on other alternative host plants (2). Spiny bollworm causes damage by attacking terminal shoots, flower buds, and green bolls. The most serious damage to cotton is caused when larvae bore into the bolls, destroying the fiber, consuming seeds, and producing putrefaction due to the accumulation of feces and fungus. In some regions, if the attack is not controlled, E. insulana larvae can destroy all the cotton bolls in the field.
Virtually no quantitative data are available on the efficacy of single purified B. thuringiensis Cry proteins against E. insulana. In the present study, the insecticidal activity of 13 of the most common lepidopteran-specific Cry proteins was determined in terms of 50% lethal concentration (LC50) for neonate larvae of E. insulana. Assessment of the relative potency of these individual B. thuringiensis proteins is an important step in the determination of their insecticidal potential for control of this pest in cotton. Furthermore, since continuous exposure to Cry1Ac may result in the development of resistance to this toxin, we have addressed the possibility of Cry1Ac-resistant insects becoming resistant to other B. thuringiensis toxins. Considering that most cases of high levels of resistance to Cry proteins have been due to the alteration of a midgut membrane receptor (12), competition experiments between Cry1Ac and other active Cry proteins were performed to determine which toxins share a target site and therefore which toxins could lose their insecticidal properties if populations of E. insulana with an altered Cry1Ac binding site become common.
MATERIALS AND METHODS
Insects.
Two populations of E. insulana were used in this study. One colony was established in Spain from pupae obtained from a continuous laboratory culture maintained since 2001 at the Plant Protection Institute, Giza, Egypt. A second colony was started with field-collected larvae from cotton fields in Córdoba, Spain, during the summer of 2002. These colonies were continuously maintained in our laboratory in a growth chamber at 27 ± 1°C and 60% relative humidity, with a photoperiod of 14 h of light and 10 h of dark and with an artificial diet (16).
Bacillus thuringiensis Cry proteins.
The following Cry proteins were produced in recombinant B. thuringiensis strains (strain names are given in parentheses) expressing just one type of Cry protein: Cry1Aa3 (EG1273), Cry1Ab3 (EG7077), Cry1Ac4 (EG11070), Cry1Ba (EG19916), Cry1Ca2 (EG1081), Cry1Da (EG7300), Cry1Ea (EG11901), Cry1Fa1 (EG11096), and Cry1Ja1 (EG7279) (all obtained from Ecogen Inc., Langhorne, Pa.) Cry2Aa1 (EG7543) and Cry2Ab2 (EG7699) (supplied by Monsanto Co., Chesterfield, Mo.). Protoxin solubilization, trypsin activation, and toxin chromatography purification were performed as previously described (10). Cry1Ia7 was produced in recombinant Escherichia coli cultures in 2× tryptone yeast extract medium at 37°C and with constant shaking until exponential growth was achieved. The expression of the protein was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) and was purified by nickel columns. Cry1Ia7 was not trypsin activated, because this resulted in a loss of toxic activity for E. insulana larvae. Purified and activated Cry9Ca toxin (Lys mutant) was obtained from Bayer BioScience (N.V. Gent, Belgium). Protein concentration was determined by the Bradford method (5) using bovine serum albumin as a standard.
Insect bioassays.
Insect bioassays involved incorporating the toxin protein into the insect artificial diet (30). First, the susceptibility of E. insulana larvae to each Cry toxin tested was determined at a high protein concentration (100 μg/ml) by incorporating the toxin into the diet that was fed to 25 neonate larvae. A second experiment involved determining the LC50 for active Cry proteins. The concentration range used for each Cry protein was determined in preliminary bioassays. The toxin was mixed with the artificial diet of the insect when it reached 50°C and then dispensed into 24-multiwell plates. A total of 30 neonate larvae were treated with each protein concentration, and a range of five concentrations (ranging from 40 μg/ml to 0.10 μg/ml) was used for each toxin. The bioassay was performed three times. Control insects were fed artificial diet without toxin. The multiwell plates were incubated at 25°C and 60% relative humidity with a 14-h light/10-h dark photoperiod. Mortality was recorded after 6 days. Concentration-mortality data were subjected to probit regression analysis (13) in the POLO-PC program (27). To assure that Cry proteins which showed no toxicity to E. insulana were not degraded, several of these proteins were also bioassayed against larvae of known susceptible species, namely, Spodoptera exigua, Plutella xylostella, and Lobesia botrana.
Toxin purification and labeling.
For binding assays, trypsin-activated toxins (except Cry1Ia) were further purified by anion-exchange chromatography with the Mono Q HR 5/5 column using a fast protein liquid chromatograph (Pharmacia, Uppsala, Sweden). Cry1Ab and Cry1Ac were labeled with 125I by the chloramine-T method (47). Specific activities of the radio-iodinated toxins were analyzed by a sandwich enzyme-linked immunosorbent assay (47). The specific activities for 125I-labeled Cry1Ab (125I-Cry1Ab) and 125I-Cry1Ac were 2.9 mCi/mg and 1.8 mCi/mg, respectively. Cry1Ba, Cry1Ia, and Cry9Ca labeling was performed by biotinylation (Amersham Biosciences, N.J.) according to the manufacturer's instructions.
Midgut isolation and BBMV preparation.
Final-instar larvae (L5) were dissected to obtain the whole insect midguts, which were immediately frozen in liquid nitrogen and stored at −80°C until required. Brush border membrane vesicles (BBMV) were prepared by the MgCl2 precipitation method (48).
Binding experiments with 125I-Cry1Ac and 125I-Cry1Ab.
Binding experiments with E. insulana BBMV and 125I-Cry1Ac were performed as previously described (10) using the following conditions adapted for the spiny bollworm: 0.14 ng of 125I-Cry1Ac, 0.05 mg/ml BBMV, and a 1-h incubation time at room temperature in a final volume of 0.1 ml binding buffer (1 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4, 0.1% bovine serum albumin). Competition experiments were carried out with increasing concentrations of unlabeled Cry1Ab, Cry1Ac, Cry1Ba, Cry1Ia, and Cry9Ca. Radioactivity incorporated into the BBMV after washing twice with cold binding buffer was measured directly in the microtubes in which the assays were performed by using a gamma counter (Compugamma 1282; LKB).
125I-Cry1Ab binding and competition assays with unlabeled Cry1Ab and Cry1Ac were performed as described above for 125I-Cry1Ac under the appropriate conditions (1 ng of 125I-Cry1Ab and 0.15-mg/ml BBMV concentration).
Binding data analyses to obtain the dissociation constants (Kd) and the concentrations of binding sites (Rt) were performed from the homologous competition experiments using the LIGAND program (31). Graphic representations and curve fitting were performed using the Graphpad Prism v.4.0 for Windows package (Graphpad Software, San Diego, Calif.).
Binding experiments with biotinylated toxins.
Binding experiments with the biotinylated toxins were carried out by incubating 25 μg of BBMV with the appropriate amount of labeled toxin (10 ng for Cry1Ba, 14 ng for Cry1Ia, and 20 ng for Cry9Ca). An excess of at least 400-fold of unlabeled toxin was added in the homologous and heterologous competition experiments. After centrifuging the binding mixture, the pellet in the microtube containing the toxin bound to the BBMV was suspended with 10 μl electrophoresis buffer and subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were then electrotransferred onto a Hybond nitrocellulose membrane (Amersham) and blocked with 3% ECL blocking agent (Amersham) in TPBS buffer (1 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4, 0.1% Tween 20). Biotinylated toxin bound to the BBMV was detected by incubating the membrane with streptavidin conjugated to alkaline phosphatase (Roche Diagnostics, Ind.) in TPBS according to the manufacturer's recommendations. The membrane was developed with an NBT/BCIP solution (Roche) in Genius 3 color buffer (100 mM NaCl, 50 mM MgCl2, 100 mM Tris-HCl, pH 9.5).
RESULTS
Toxicity of Cry toxins.
E. insulana larvae showed different degrees of susceptibility to 13 of the most common lepidopteran-specific Cry proteins produced by B. thuringiensis. At the concentration tested (100 μg of toxin per milliliter of artificial diet), the percent mortality obtained with six of these Cry toxins (Cry1Ca, Cry1Ea, Cry1Fa, Cry1Ja, Cry2Aa, and Cry2Ab) did not differ significantly from the mortality of the control larvae, reared on a toxin-free diet, for the Egyptian and Spanish insect colonies assayed. Consequently, when a Cry toxin caused no mortality in E. insulana, it was attributed to the lack of toxic activity of that particular Cry toxin or a reduced feeding rate on toxin-contaminated diet. No mortality was observed for Cry1Aa, Cry1Ja, and Cry2Aa, but they caused an inhibition of growth. By the end of the toxicity test, all the treated larvae remained in the first instar, whereas most of the control larvae had molted to the third instar. The toxins Cry1Ca, Cry1Ja, and Cry2Aa were confirmed to be active against known susceptible species (S. exigua, P. xylostella, and L. botrana). Susceptible species were not available to confirm the integrity of the Cry1Ea, Cry1Fa, and Cry2Ab proteins, so we cannot exclude the possibility that they were degraded. All other Cry proteins (Cry1Ab, Cry1Ac, Cry1Ba, Cry1Da, Cry1Ia, and Cry9Ca) were toxic against E. insulana for both populations and resulted in larval mortality which increased with increasing toxin concentrations. In all cases, χ2 values generated in goodness-of-fit tests indicated that the probit model was appropriate for each toxin and insect colony tested (Tables 1 and 2). Probit regression lines could not be fitted in parallel, and so the relative potencies (RP) were expressed as the ratio of the LC50 values for each active Cry protein to the LC50 value for the Cry1Ac standard (39). The LC50 value of Cry1Ac toxin for E. insulana was selected as a reference because it is the usual toxin produced in transgenic cotton.
TABLE 1.
Toxicity of the active Cry proteins to neonate larvae of E. insulana from the Egyptian insect colonya
| Protein | LC50 (μg/ml) | Relative potency | 95% fiducial limits of relative potency
|
Slope ± SE | Intercept ± SE | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Cry1Ia | 21.13 | 0.052 | 0.04 | 0.06 | 3.25 ± 0.35 | 0.71 ± 0.47 |
| Cry1Da | 4.94 | 0.22 | 0.16 | 0.31 | 1.66 ± 0.16 | 3.85 ± 0.12 |
| Cry1Ac | 1.09 | 1.00 | 1.87 ± 0.17 | 4.92 ± 0.06 | ||
| Cry1Ba | 1.03 | 1.11 | 0.84 | 1.42 | 1.53 ± 0.17 | 4.98 ± 0.07 |
| Cry9Ca | 0.39 | 2.78 | 2.32 | 3.35 | 3.67 ± 0.31 | 6.49 ± 0.14 |
| Cry1Ab | 0.45 | 2.41 | 1.80 | 3.23 | 1.29 ± 0.13 | 5.44 ± 0.07 |
Parameters were obtained from the POLO-PC program (27). The χ2 value was not significant (P > 0.05) for a goodness-of-fit test for each regression. Slopes could not be fitted in parallel. The relative potency was expressed as the ratio of the LC50 value for each Cry protein to the LC50 value for Cry1Ac (39).
TABLE 2.
Toxicity of the active Cry proteins to neonate larvae of E. insulana from the Spanish insect colonya
| Protein | LC50 (μg/ml) | Relative potency | 95% fiducial limits of relative potency
|
Slope ± SE | Intercept ± SE | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Cry1Da | 4.25 | 0.06 | 0.09 | 0.04 | 1.93 ± 0.15 | −1.22 ± 0.10 |
| Cry1Ba | 0.40 | 0.65 | 0.87 | 0.50 | 2.34 ± 0.19 | 0.92 ± 0.10 |
| Cry1Ac | 0.27 | 1.00 | 1.55 ± 0.17 | 0.89 ± 0.10 | ||
| Cry9Ca | 0.20 | 1.33 | 1.01 | 1.74 | 2.71 ± 0.28 | 1.90 ± 0.20 |
Parameters were obtained from the POLO-PC program (27). The χ2 value was not significant (P > 0.05) for a goodness-of-fit test for each regression. Slopes could not be fitted in parallel. The relative potency was expressed as the ratio of the LC50 value for each Cry protein to the LC50 value for Cry1Ac (39).
In the population from Egypt, the RP of all the Cry toxins active against E. insulana indicated that Cry9Ca and Cry1Ab were the most potent toxins, with RP values of 2.78 and 2.41, respectively (Table 1). Cry1Ba toxin was also highly toxic for E. insulana, with an LC50 of 1.03 μg protein per milliliter of diet, similar to that of Cry1Ac based on the overlap of 95% confidence limits. Cry1Da and Cry1Ia were found to be 4.5 and 19.2 times, respectively, less toxic than Cry1Ac (Table 1).
In the population from Spain, the RP values indicated that Cry9Ca was significantly more toxic than Cry1Ac by a factor of 1.33, whereas Cry1Ba and Cry1Da were 1.53 and 15.69 times less toxic than Cry1Ac. The concentration-mortality relationship and the relative potencies for Cry1Ab and Cry1Ia toxins could not be determined because the Spanish colony succumbed due to a bacterial infection. The toxins assayed showed different activities for the two experimental insect populations. The estimated RP values for Cry1Ac, Cry1Ba, and Cry9Ca toxins were 3.97, 2.54, and 1.97 times significantly more toxic in the population from Spain than in the population from Egypt, respectively. However, the RP of the Cry1Da toxin was similar in both populations.
Binding of Cry proteins to BBMV of E. insulana from Egypt.
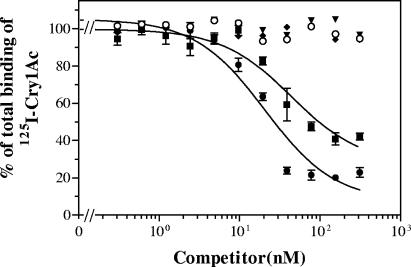
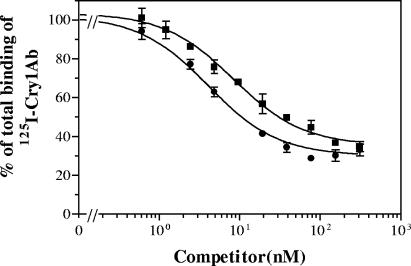
To determine if binding sites were shared by more than one toxin, competition binding assays among the active toxins were performed. Using 125I-labeled Cry1Ac, competition assays indicated that Cry1Ab was the only toxin that competed for Cry1Ac binding sites (Fig. 1). The fact that there was some 125I-Cry1Ac binding that could not be competitively displaced by unlabeled Cry1Ab (even at the highest concentration used) suggests that there is a second binding site which is specific for Cry1Ac and to which Cry1Ab does not bind. The homologous competition curve (125I-Cry1Ac versus unlabeled Cry1Ac) fitted a one-site model, which indicates that the affinities of the two proposed binding sites for Cry1Ac must be similar. Quantitative estimates gave a Kd of 1.9 ± 1.2 nM and an Rt of 19 ± 3 pmol/mg for Cry1Ac binding sites. The other toxins tested (Cry1Ba, Cry1Ia, and Cry9Ca) did not compete for the Cry1Ac binding sites.
FIG. 1.
125I-Cry1Ac binding to E. insulana BBMV at increasing concentrations of the following unlabeled competitors: Cry1Ab (▪), Cry1Ac (•), Cry1Ba (⧫), Cry1Ia (○), and Cry9C (▾). Data for the competing toxins represent the means of three experiments, and error bars are the standard errors of the means. Data for the noncompeting toxins were replicated twice, and error bars are not shown for clarity.
Competition experiments using 125I-labeled Cry1Ab confirmed the above-described results of a shared binding site for the two toxins. The total competition of Cry1Ac indicates that Cry1Ac competes for all binding sites of Cry1Ab (Fig. 2). Quantitative estimates gave a Kd of 6 ± 2 nM and an Rt of 16 ± 2 pmol/mg for Cry1Ab binding sites. The lower affinity of this toxin compared to that of Cry1Ac is in agreement with the relative positions of the competition curves of these two toxins, in which Cry1Ac is always a better competitor than Cry1Ab (Fig. 1 and 2).
FIG. 2.
125I-Cry1Ab binding to E. insulana BBMV at increasing concentrations of unlabeled Cry1Ab (▪) and Cry1Ac (•). Data are the means of three experiments, and error bars represent the standard errors of the means.
Binding of biotinylated Cry1Ba, Cry1Ia, and Cry9Ca and competition with their respective unlabeled homologs revealed that binding of these toxins to BBMV from E. insulana was specific (Fig. 3). These labeled toxins were tested with unlabeled Cry1Ac as a competitor, and in all three cases, no competition was observed, confirming the results obtained using 125I-Cry1Ac.
FIG. 3.
Binding of biotinylated toxins to E. insulana BBMV. (A) Binding of biotin-labeled Cry1Ba alone (lane 1) and in the presence of an excess of unlabeled Cry1Ba (lane 2) or unlabeled Cry1Ac (lane 3). (B) Binding of biotin-labeled Cry9Ca alone (lane 1) or in the presence of an excess of unlabeled Cry9Ca (lane 2) or unlabeled Cry1Ac (lane 3). (C) Binding of biotin-labeled Cry1Ia alone (lane 1) and in the presence of an excess of unlabeled Cry1Ia (lane 2) or Cry1Ac (lane 3).
Of the active toxins against E. insulana, Cry1Da was the only toxin for which direct binding could not be tested due to the high nonspecific binding obtained using both 125I-Cry1Da and biotin-labeled Cry1Da.
DISCUSSION
This is the first report on the insecticidal properties of individual B. thuringiensis Cry proteins against the spiny bollworm, E. insulana. Previous studies on the toxicity of B. thuringiensis isolates have examined the response of E. insulana to mixtures of toxins produced by each isolate (1, 4, 22, 32-34, 41, 43). Furthermore, the susceptibility of E. insulana to the Cry1Ac protein has been reported in assays performed using the MVPII (Dow Agrosciences, Calif.) bioinsecticide, which contains this protein expressed in and encapsulated by transgenic Pseudomonas fluorescens (36, 49).
Information on the insecticidal spectrum, potency, and mode of action of individual Cry proteins is critical to identify the most appropriate gene(s) for use in the development of B. thuringiensis-based control agents and insect-resistant transgenic plants. Among the Cry proteins known, only a limited number were included in this study. These Cry proteins were chosen because their activity towards species of the order Lepidoptera is well established. In our study, only 6 of the 13 Cry proteins assayed were toxic for E. insulana larvae. Cry9Ca was the most potent toxin for both colonies of E. insulana. For the colony from Egypt, Cry1Ab showed similar toxicity to Cry9Ca, followed jointly by Cry1Ba and Cry1Ac and finally by Cry1Da and Cry1Ia. In the population from Spain, the most active toxin was Cry9Ca, followed in decreasing order by Cry1Ac, Cry1Ba, and Cry1Da (Cry1Ab and Cry1Ia were not tested).
The occurrence of differential susceptibility to B. thuringiensis toxins has been demonstrated for numerous other insect pests, including other species of the genus Earias. A report on the related species Earias vitella indicated that Cry1Ab was more toxic than Cry1Aa and Cry1Ac (25). Our results with E. insulana showed that for the population from Egypt, Cry1Ab was also the most toxic among the assayed Cry1A proteins, with a relative potency 2.41 times higher than that of Cry1Ac. In contrast, the Cry1Aa toxin caused no mortality in our assays but resulted in larval growth inhibition, probably due to reduced feeding rates on toxin-contaminated diet, in both populations.
Variation in susceptibility to B. thuringiensis toxins has been reported among geographically distinct populations of a given species (15). In studies of 15 species of insects, only P. xylostella populations exhibited major differences in susceptibility that can be attributed to previous exposure to B. thuringiensis in the field (44). However, it is sometimes difficult to distinguish natural variation among susceptible populations from low to moderate resistance (12, 24). The present study did not address resistance per se because the relationship between the history of exposure and susceptibility was not examined. However, we report here on the baseline susceptibility to different individual Cry proteins in two different geographical populations of E. insulana, which might be a useful reference to measure changes in susceptibility given possible future exposure to Bt cotton crops.
Some of the proteins that showed activity against E. insulana (Cry1Ab and Cry1Ac) are present in strain HD1 of B. thuringiensis var. kurstaki (which contains Cry1Aa, Cry1Ab, Cry1Ac, and Cry2Aa), which is the active ingredient of many formulated bioinsecticides for the control of cotton pests including E. insulana (11). Evidently, the activity of the strain HD1 against E. insulana must be due to the presence of one or all of these proteins. A field population systematically treated with HD1-based insecticides may eventually result in the appearance of insects resistant to these Cry1A toxins.
The protection against E. insulana conferred by Cry1Ac in Bollgard cotton has been proven in field trials in southern Spain (35). Cry1Ac expressed in cotton provided better protection against E. insulana feeding damage than the conventional chemical insecticides. In transgenic plants, the efficacy of a determined Cry protein on a susceptible target insect is determined by the expression level required for effective control. Cry1Ac levels in Bollgard cotton declined steadily as the growing season progressed, ranging from 57.1 μg/g (dry weight) at 53 days after planting to 6.7 μg/g at 116 days after planting in fruit and from 163.4 μg/g at 53 days after planting to 34.5 μg/g at 116 days after planting in terminal foliage (17). If the results of our study are extrapolated for comparison with the expression level of Cry1Ac in transgenic plants, 1 μg of protein/ml of diet is equivalent to 6.23 μg of protein/g (dry weight) of diet (1 ml of diet had a dry weight of 160.4 mg). Therefore, the LC50 of Cry1Ac would be equivalent to 6.8 μg/g in the E. insulana population from Egypt and 1.7 μg/g in the population from Spain. The levels of Cry1Ac are sufficient to control E. insulana during the entire growing season; however, the relationship between Cry1Ac and its activity in the plant will likely be influenced by non-B. thuringiensis plant factors which, along with Cry1Ac, may be affected by the type and age of the plant tissue in question (17).
A possible risk in the use of transgenic plants is the potential development of pest resistance. One strategy to delay the development of resistance is the use of transgenic crops simultaneously expressing two or more insecticidal proteins with different modes of action (40). For this strategy to work, the two insecticidal proteins must not share key steps in the mode of action. Since the alteration of binding to midgut receptors seems to be the most important mechanism of resistance to B. thuringiensis toxins, determination of the binding sites of the active toxins can give us information on the possible risk of insects becoming resistant to more than one toxin by a change in a single receptor. Our results with labeled toxins show that among the toxins tested, Cry1Ab was the only one that shared common binding sites with Cry1Ac. This is a feature observed in all lepidopterans (3, 8, 9, 14, 19, 20), and it explains the basis of many cases of resistance to more than one B. thuringiensis toxin (12). It is interesting that Cry1Ac, in addition to the shared sites, also seems to have binding sites not shared with Cry1Ab in E. insulana, a feature not particularly common among lepidopteran species. To our knowledge, this model of Cry1Ac having binding sites shared with Cry1Ab and binding sites not shared with this toxin has only been proposed in two other insect species, H. virescens (47) and H. armigera (10).
In conclusion, the results obtained in this work show that E. insulana is susceptible to Cry1Ab, Cry1Ac, Cry1Ba, Cry1Da, Cry1Ia, and Cry9Ca. Cry1Ab and Cry9Ca were significantly more active than Cry1Ac, the toxin currently used in Bt cotton. From a resistance management standpoint, the most active proteins are good candidates for use in the control of this pest in addition to, or combination with, Cry1Ac, except for Cry1Ab, which shares binding sites with Cry1Ac. Cry9Ca is of particular interest since besides being the most active protein, it has a wide spectrum of toxicity that includes other important cotton pests, such as H. armigera, H. virescens, and Spodoptera littoralis (26, 37, 46).
Acknowledgments
We gratefully acknowledge Jeroen Van Rie (Bayer BioScience, Gent, Belgium) for supplying Cry9Ca and Jim Baum (Monsanto, Chesterfield, Mo.) for Cry2Aa and Cry2Ab recombinant clones. We thank Noelia Gorría for insect rearing and Baltasar Escriche and Trevor Williams for critically reading the manuscript.
This study was funded by the Spanish Ministry of Science and Technology (grants AGL2000-0840 and AGL2003-09282) and the Generalitat Valenciana (grant GRUPOS2004-21). M.A.I. and A.E. received support from the Spanish Ministry of Education and Culture (grants FP2000-4923 and FP2000-5497).
REFERENCES
- 1.Abul-Nasr, S. E., E. D. Ammar, and A. I. Merdam. 1983. Field application of two strain of Bacillus thuringiensis for the control of the cotton bollworms, Pectinophora gossypiella (Saund.) and Earias insulana (Boisd.). Bull. Entomol. Soc. Egypt 11:35-39. [Google Scholar]
- 2.Abul-Nasr, S. M., M. Megahed, and A. A. M. Mabrouk. 1973. A study on the host plants of the spiny bollworm, Earias insulana (Boisd.) other than cotton and maize (Lepidoptera: Arctiidae). Bull. Entomol. Soc. Egypt 56:151-161. [Google Scholar]
- 3.Ballester, V., B. Escriche, J. L. Ménsua, G. W. Riethmacher, and J. Ferré. 1994. Lack of cross-resistance to other Bacillus thuringiensis crystal proteins in a population of Plutella xylostella highly resistant to CryIA(b). Biocontr. Sci. Technol. 4:437-443. [Google Scholar]
- 4.Bekheit, H. K., A. Abd-El-Hafez, S. H. Taher, and G. M. Moawad. 1995. Potency of some new isolates of Bacillus thuringiensis against the pink and spiny bollworms. Ann. Agric. Sci. Egypt 40:411-416. [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Crickmore, N., D. R. Zeigler, J. Feitelson, E. Schnepf, J. Van Rie, D. Lereclus, J. Baum, and D. H. Dean. 1998. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durán, J. M., M. Alvarado, E. Ortiz, A. de la Rosa, J. A. Ruiz, A. Sánchez, and A. Serrano. 2000. Contribución al conocimiento de Earias insulana (Boisduval, 1833) (Lepidoptera, Noctuidae), la oruga espinosa del algodonero, en Andalucia occidental. Bol. San. Veg. Plagas 26:215-228. [Google Scholar]
- 8.Escriche, B., J. Ferré, and F. J. Silva. 1997. Occurrence of a common binding site in Mamestra brassicae, Phthorimaea operculella, and Spodoptera exigua for the insecticidal crystal proteins Cry1A from Bacillus thuringiensis. Insect Biochem. Mol. Biol. 27:651-656. [DOI] [PubMed] [Google Scholar]
- 9.Estada, U., and J. Ferré. 1994. Binding of insecticidal crystal proteins of Bacillus thuringiensis to the midgut brush border of the cabbage looper, Trichoplusia ni (Hüber) (Lepidoptera: Noctuidae), and selection for resistance to one of the crystal proteins. Appl. Environ. Microbiol. 60:3840-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estela, A., B. Escriche, and J. Ferré. 2004. Interaction of Bacillus thuringiensis toxins with larval midgut binding sites of Helicoverpa armigera (Lepidoptera: Noctuidae). Appl. Environ. Microbiol. 70:1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadare, T. A., and N. A. Amusa. 2003. Comparative efficacy of microbial and chemical insecticides on four major lepidopterous pest of cotton and their (insect) natural enemies. Afr. J. Biotechnol. 2:425-428. [Google Scholar]
- 12.Ferré, J., and J. Van Rie. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47:501-533. [DOI] [PubMed] [Google Scholar]
- 13.Finney, D. J. 1971. Probit analysis. Cambridge University Press, Cambridge, United Kingdom.
- 14.González-Cabrera, J., B. Escriche, T. B. Tabashnik, and J. Ferré. 2003. Binding of Bacillus thuringiensis toxins in resistant and susceptible strains of pink bollworm (Pectiniphora gossypiella). Insect Biochem. Mol. Biol. 33:929-935. [DOI] [PubMed] [Google Scholar]
- 15.González-Cabrera, J., S. Herrero, A. H. Sayyed, B. Escriche, Y. B. Liu, S. K. Meyer, D. J. Wright, B. E. Tabashnik, and J. Ferré. 2001. Variation in susceptibility to Bacillus thuringiensis toxins among unselected strains of Plutella xylostella. Appl. Environ. Microbiol. 67:4610-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene, G. L., N. C. Leppla, and W. A. Dickerson. 1976. Velvetbean caterpillar: a rearing procedure and artificial medium. J. Econ. Entomol. 69:487-488. [Google Scholar]
- 17.Greenplate, J. T. 1999. Quantification of Bacillus thuringiensis insect control protein Cry1Ac over time in Bollgard cotton fruit and terminals. J. Econ. Entomol. 92:1377-1383. [Google Scholar]
- 18.Hamed Amin, A. A., M. Gergis, and M. El-Naggar. 2001. Alternative in field refuge strategies for controlling certain cotton key pests in middle Egypt. In The Entomological Society of America Annual Meeting—2001: an entomological odyssey of ESA. Entomological Society of America, San Diego, Calif.
- 19.Herrero, S., M. Borja, and J. Ferré. 2002. Extent of variation of the Bacillus thuringiensis toxin reservoir: the case of the geranium bronze, Cacyreus marshalli Butler (Lepidoptera: Lycaenidae). Appl. Environ. Microbiol. 68:4090-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrero, S., B. Oppert, and J. Ferré. 2001. Different mechanisms of resistance to Bacillus thuringiensis toxins in the Indianmeal moth. Appl. Environ. Microbiol. 67:1085-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horowitz, A. R. 1997. Impact of Bt-transgenic cotton on the main Israeli lepidopteran cotton pests. In The Annual Convention of the Israeli Fund for Advancement of Research on and Development of Pesticides. ARO, The Volcani Center, Bet Dagan, Israel.
- 22.Hussain, M., and A. Askari. 1976. Field tests of Bacillus thuringiensis and chemical insecticides for control of Earias insulana on cotton. J. Econ. Entomol. 69:343-344. [Google Scholar]
- 23.James, C. 2004. Global status of commercialized biotech/GM crops: 2004. ISAAA briefs no. 32. International Service for the Acquisition of Agri-Biotech Applications, Ithaca, N.Y.
- 24.Koziel, M. G., N. B. Carozzi, T. C. Currier, G. W. Warren, and S. V. Evola. 1993. The insecticidal crystal proteins of Bacillus thuringiensis past, present and future uses. Biotechnol. Genet. Eng. Rev. 11:171-228. [Google Scholar]
- 25.Kranthi, S., K. R. Kranthi, and N. V. Lavhe. 1999. Baseline toxicity of Cry1A toxins to the spotted bollworm, Earias vitella F. Crop. Prot. 18:551-555. [Google Scholar]
- 26.Lambert, B., L. Buysse, C. Decock, S. Jansens, C. Piens, B. Saey, J. Seurinck, K. Van Audenhove, J. Van Rie, A. Van Vliet, and M. Peferoen. 1996. A Bacillus thuringiensis insecticidal crystal protein with a high activity against members of the family Noctuidae. Appl. Environ. Microbiol. 62:80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Ora Software. 1987. POLO-PC: a user's guide to probit or logit analysis. Le Ora Software, Berkeley, Calif.
- 28.Letourneau, D. K., J. A. Hagen, and G. S. Robinson. 2002. Bt-crops: evaluating benefits under cultivation and risks from escaped transgenes in the wild, p. 33-98. In D. K. Letourneau and B. E. Burrows (ed.), Genetically engineered organisms: assessing environmental and human health impacts. CRC Press, Boca Raton, Fla.
- 29.Luttrell, R. G., G. P. Fitt, F. S. Ramalho, and E. S. Sugonyaev. 1994. Cotton pest management: part 1. A worldwide perspective. Annu. Rev. Entomol. 39:517-526. [Google Scholar]
- 30.MacIntosh, S. C., T. B. Stone, S. R. Sims, P. L. Hunst, J. T. Greenplate, P. G. Marrone, F. J. Perlak, D. A. Fischhoff, and R. L. Fuchs. 1990. Specificity and efficacy of purified Bacillus thuringiensis proteins against agronomically important insects. J. Invertebr. Pathol. 56:258-266. [DOI] [PubMed] [Google Scholar]
- 31.Munson, P. J., and D. Roadbard. 1980. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 107:220-239. [DOI] [PubMed] [Google Scholar]
- 32.Navon, A. 1989. Development of potency bioassays for selecting Bacillus thuringiensis preparations against agricultural insect pests. Isr. J. Entomol. 23:115-118. [Google Scholar]
- 33.Navon, A., S. Keren, S. Levski, A. Grinstein, and Y. Riven. 1997. Granular feeding baits based on Bacillus thuringiensis products for the control of lepidopterous pests. Phytoparasitica 25:101-110. [Google Scholar]
- 34.Navon, A., M. Klein, and S. Braun. 1990. Bacillus thuringiensis potency bioassays against Heliothis armigera, Earias insulana, and Spodoptera littoralis larvae based on standardized diets. J. Invertebr. Pathol. 55:387-393. [DOI] [PubMed] [Google Scholar]
- 35.Novillo, C., J. Soto, and J. Costa. 1999. Resultados en España con variedades de algodón, protegidas genéticamente contra las orugas de las cápsulas. Bol. San. Veg. Plagas 25:383-393. [Google Scholar]
- 36.Ramos, J., J. F. Ortiz, and O. Vargas. 2005. Susceptibilidad de las larvas de Helicoverpa armigera (Hübner) y Earias insulana (Boisduval) (Lepidoptera: Noctuidae) a la delta-endotoxina Cry1Ac de Bacillus thuringiensis (Berliner). Bol. San. Veg. Plagas 30:239-245. [Google Scholar]
- 37.Reed, J. P., and W. R. Halliday. 2001. Establishment of Cry9C susceptibility baselines for European corn borer and southwestern corn borer (Lepidoptera: Crambidae). J. Econ. Entomol. 94:397-402. [DOI] [PubMed] [Google Scholar]
- 38.Reed, W. 1994. Earias spp. (Lepidoptera: Noctuidae), p. 151-176. In G. A. Matthews and J. P. Tunstall (ed.), Insect pests of cotton. CAB International, Ascot, United Kingdom.
- 39.Robertson, J. L., and H. K. Preisler. 1992. Pesticide bioassays with arthropods. CRC Press, Boca Raton, Fla.
- 40.Roush, R. T. 1998. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philos. Trans. R. Soc. Lond. B 353:1777-1786. [Google Scholar]
- 41.Salama, H. S., and M. S. Foda. 1984. Studies on the susceptibility of some cotton pests to various strain of Bacillus thuringiensis. J. Plant Dis. Prot. 91:65-70. [Google Scholar]
- 42.Shelton, A. M., J. Z. Zhao, and R. T. Roush. 2002. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 47:845-881. [DOI] [PubMed] [Google Scholar]
- 43.Singh, G., S. C. Bhardwaj, and G. S. Dhaliwal. 1998. Evaluation of some biopesticides for the management of fruit borers, Earias spp., on okra crop. Ind. J. Ecol. 25:187-189. [Google Scholar]
- 44.Tabashnik, B. E. 1994. Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 39:47-49. [Google Scholar]
- 45.Tabashnik, B. E., T. J. Dennehy, M. A. Sims, K. Larkin, G. P. Head, W. J. Moar, and Y. Carriere. 2002. Control of resistant pink bollworm (Pectinophora gossypiella) by transgenic cotton that produces Bacillus thuringiensis toxin Cry2Ab. Appl. Environ. Microbiol. 68:3790-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Frankenhuyzen, K., L. Gringorten, and D. Gauthier. 1997. Cry9Ca1 toxin, a Bacillus thuringiensis insecticidal crystal protein with high activity against the spruce budworm (Choristoneura fumiferana). Appl. Environ. Microbiol. 63:4132-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Rie, J., S. Jansens, H. Höfte, D. Degheele, and H. Van Mellaert. 1989. Specificity of Bacillus thuringiensis delta-endotoxins, importance of specific receptors on the brush border membrane of the mid-gut of target insects. Eur. J. Biochem. 186:239-247. [DOI] [PubMed] [Google Scholar]
- 48.Worfersberger, M., P. Luethy, A. Maurer, P. Parenti, V. Sacchi, B. Giordana, and G. M. Hanozet. 1987. Preparation and characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86A:301-308. [Google Scholar]
- 49.Zidan, Z. H., M. I. Abdel-Megeed, A. A. El-Hafez, N. M. Hussein, H. M. El-Gemeiy, and M. M. Shalaby. 1998. Toxicological and histological studies of Bacillus thuringiensis, MVII against larvae of pink and spiny bollworms. Ann. Agric. Sci. Egypt 1:319-332. [Google Scholar]