Abstract
Sphingomonas wittichii RW1 is able to catabolize 1,2,3,4-tetrachlorodibenzo-p-dioxin (H. B. Hong, Y. S. Chang, I. H. Nam, P. Fortnagel, and S. Schmidt, Appl. Environ. Microbiol. 68:2584-2588, 2002). Here we demonstrate the aerobic bacterial catabolism of the ubiquitous toxic diaryl ether pollutant 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin by this strain. The products of this biotransformation were identified as tetrachlorocatechol and 2-methoxy-3,4,5,6-tetrachlorophenol by comparing mass spectra recorded before and after n-butylboronate and N,O-bis(trimethylsilyl)-trifluoroacetamide derivatization with those of authentic compounds. Additional experiments showed that the less-chlorinated 1,2,3,7,8-pentachlorodibenzo-p-dioxin was not transformed by the strain RW1. The importance of substitution patterns for the degradability of individual congeners was illustrated by the fact that the 1,2,3-trichlorodibenzo-p-dioxin was catabolized to yield 3,4,5-trichlorocatechol, whereas the 2,3,7-trichlorodibenzo-p-dioxin was not attacked.
Polychlorinated aromatic compounds such as the polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/Fs) are among the most problematic environmental pollutants because of their chemical inertness and recalcitrance, lipid solubility, and toxicity (1, 3, 8, 13). Consequently, numerous studies have been carried out to elucidate the aerobic (7, 9, 19) bacterial degradation of PCDD/Fs, compounds produced by incineration processes and as unwanted by-products of the synthesis of pesticides and herbicides (4, 17). However, naturally occurring microorganisms have evolved to degrade and mineralize many, but by no means all, of these compounds. For example, several PCDD/Fs are degraded very slowly or not at all (12). In addition to genera such as Terrabacter (6, 14) and Pseudomonas (6), several strains belonging to the genus Sphingomonas were reported to use diaryl ethers such as dibenzofuran and dibenzo-p-dioxin as the sole source of carbon and energy (3). Some of these isolates are examples for effective biocatalysts that can even catabolize some di-, tri-, and tetrachlorinated dibenzo-p-dioxins and dibenzofurans (6, 7, 9, 19). However, the aerobic catabolism of important environmental pollutants such as 1,2,3,7,8-penta- or 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin, giving rise to the formation of corresponding metabolites, has not been demonstrated previously.
The catabolism of dibenzofurans and dibenzo-p-dioxins is in Sphingomonas wittichii RW1 initiated by a ring-hydroxylating dioxygenase (2, 18, 20), introducing two hydroxyl groups in the accessible angular positions of the diaryl ether, thereby producing unstable hemiacetals. The labile intermediate spontaneously decays in the case of dibenzo-p-dioxins to yield the corresponding 2,2′,3-trihydroxydiphenyl ether, which is further processed, yielding a catechol and the corresponding 2-hydroxy-cis,cis-muconic acid (3, 21). However, despite the fact that previous transformation experiments monitoring the disappearance of selected mono- to tetrachlorinated congeners of dibenzofurans and dibenzo-p-dioxins have indicated some depletion of tetrachlorodibenzo-p-dioxins is due to bacterial activity (7, 15), there is still a lack of information concerning the aerobic bacterial catabolism of higher chlorinated dibenzo-p-dioxins (i.e., Cln, where n > 4) such as the ubiquitous pollutants 1,2,3,7,8-penta- or 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin. In fact, these two congeners are known to exhibit very long half-lives compared to low chlorinated congeners (12) and were therefore selected.
MATERIALS AND METHODS
Biotransformation experiments.
The procedures used to produce resting cells of S. wittichii RW1 to be used in biotransformation experiments were essentially those described previously (5, 7, 9). However, due to the poor solubility of 1,2,3,7,8-penta- and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin in most organic solvents, these two substrates were dissolved prior to experiments in an acetone-toluene-nonane mixture (1:1:1 by volume) at a concentration of 5 mg/ml unless indicated otherwise. An appropriate aliquot of this stock solution was then added to a sterilized Erlenmeyer flask, and the solvent mixture was evaporated by flushing the flask with N2. For turnover experiments with resting cells, 100-ml portions of a cell suspension (optical density at 578 nm of 8.0) were added to 1-liter Erlenmeyer flasks containing 5 mg of 1,2,3-tri-, 2,3,7-tri-, 1,2,3,4-tetra-, 1,2,3,7,8-penta-, or 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin and incubated at 160 rpm and 28°C for 5 days. To compare the biodegradability of 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin with other congeners, substrate mixtures containing equal amounts (0.5 mg of each compound) of dibenzo-p-dioxin, 2,7-di-, 1,2,3-tri-, 1,2,3,4-tetra-, 1,2,3,7,8-pentachlorodibenzo-p-dioxin, and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin were also used. This biotransformation was monitored and quantified by using 100-ml Erlenmeyer flasks; each flask contained 10 ml of cell suspension (optical density at 578 nm of 8.0) and 0.5 mg of each substrate. Every 24 h, a set of triplicate flasks was removed, 2 ml of ortho-phosphoric acid was added to each flask to stop the reaction, and the flasks were immediately frozen and stored at −70°C. After incubation for 120 h, all flasks were thawed, and the contents were extracted as described previously (7). However, for routine determination of recovery rates, 500 μg of 2-chlorodibenzo-p-dioxin and 5 ng of 2,3,4,5-tetrachlorophenol were added to each flask prior to extraction. Extracts obtained from the flasks and the corresponding controls were dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. Aliquots were analyzed directly with a liquid chromatography-mass spectrometry system as described below. However, specifically for the gas chromatography-mass spectrometry analysis and detection of chlorinated catechols, samples were derivatized prior to measurement by the addition of n-butylboronic acid, followed by incubation at 50°C for 10 min (10, 16), or they were derivatized by reaction with BSTFA [N,O-bis(trimethylsilyl)-trifluoroacetamide] at 60°C for 1 h to form trimethylsilyl (TMS) derivatives (7, 22).
Analytical methods.
Metabolites present in the extracts obtained from the biotransformation experiments were detected and characterized initially using a liquid chromatography-mass spectrometry (Qstar Pulsar; Applied Biosystems, Foster City, Calif.) system consisting of a reversed-phase high-performance liquid chromatograph (Agilent 1100; Agilent, Waldbronn, Germany) equipped with a Lichrocart RP-18 column (125 by 30 mm; 5 μm; Merck, Darmstadt, Germany) and then by using mass and UV-visible light detection after filtration of the samples with a 2-ml syringe through a 0.45-μm-pore-size syringe filter (Millipore). The aqueous solvent system (flow rate, 1.0 ml/min) contained 0.1% (wt/vol) acetic acid and 60% methanol. In addition, the extracts of turnover experiments with 1,2,3-tri- and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin were analyzed by nanospray electrospray ionization (ESI)-tandem mass spectrometry (MS/MS) in positive mode by using an ESI mass spectrometry quadruple-time-of-flight (Qstar Pulsar) system. The derivatives obtained were analyzed by high-resolution gas chromatography ion trap mass spectrometry without further purification. Gas chromatography-mass spectrometry measurements were carried out by using a Trace GC 2000 system (Thermoquest, Austin, Tex.) linked to a Finnigan Polaris Q mass spectrometer (Thermoquest) with a 60-m DB-5 column. The initial temperature, 60°C, was maintained for 2 min; the temperature was then increased to 310°C over 10 min and held at that temperature for an additional 10 min. In all experiments, which were generally performed three times, heat-inactivated (75°C for 20 min) and poisoned (10 mM NaN3) cultures were used as controls. Metabolites were identified and quantified by comparison of their mass spectra, retention times, and peak areas with those of authentic standards.
Chemicals.
Dibenzofuran, 2,3,4,5-tetrachlorophenol, BSTFA, and n-butylboronic acid were obtained from Sigma-Aldrich (St. Louis, Mo.). 3,4,5-Trichlorocatechol, 3,4,5,6-tetrachlorocatechol, dibenzo-p-dioxin, 2-mono-, 2,7-di-, 1,2,3-tri-, 2,3,7-tri-, 1,2,3,4-tetra-, 1,2,3,7,8-penta-, and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin were purchased from Accustandard (New Haven, Conn.) or Sigma-Aldrich. The solvents used and ortho-phosphoric acid were obtained from Merck. All chemicals used were of the highest quality commercially available.
RESULTS AND DISCUSION
Biotransformation of 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin.
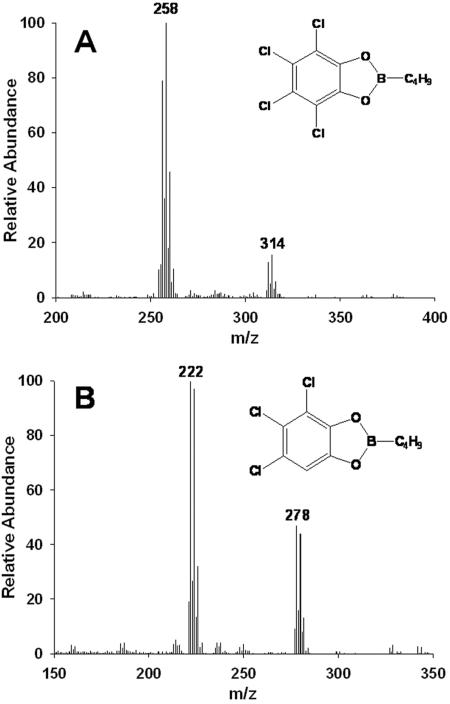
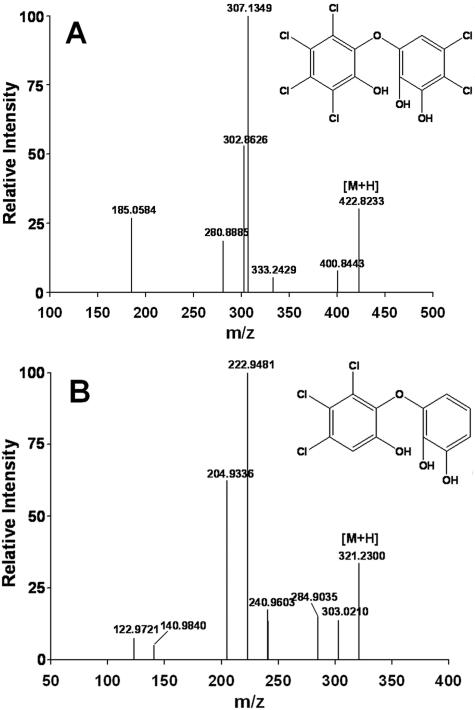
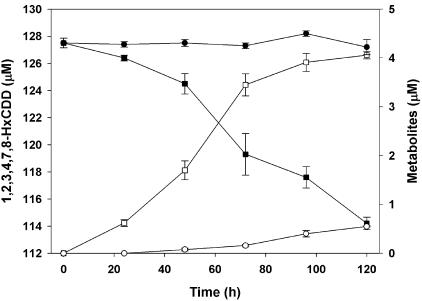
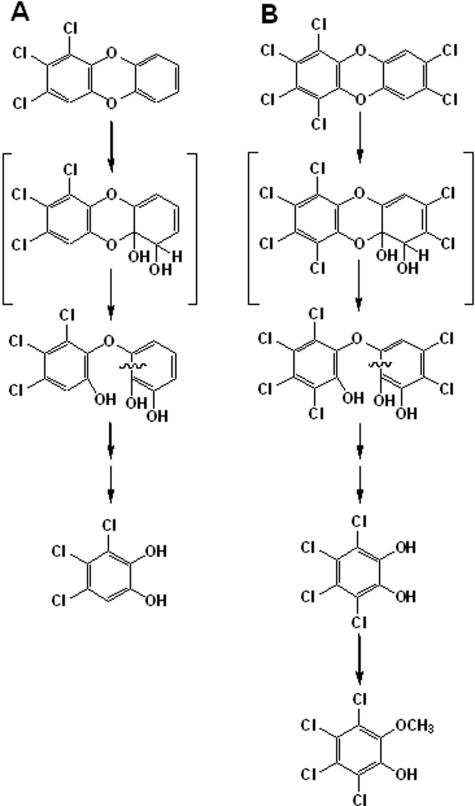
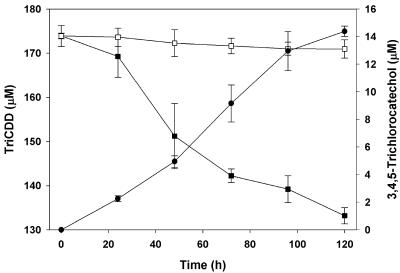
The catabolism of 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin by S. wittichii RW1 gave rise to two polar metabolites. The two metabolites obtained from 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin were identified as 3,4,5,6-tetrachlorocatechol and 2-methoxy-3,4,5,6-tetrachlorophenol by comparison to the mass spectra of authentic standards. Thus, for the first metabolite, diagnostic peaks were observed in the case of the n-butylboronic-derivative at m/z 314 [M+] and m/z 258 [M+-C4H8], and the mass spectrum was indistinguishable from that of the n-butylboronic acid derivative of authentic 3,4,5,6-tetrachlorocatechol (Fig. 1A). We also carried out derivatization of the same polar metabolite with BSTFA and found diagnostic peaks (data not shown) as reported previously for the TMS derivative of 3,4,5,6-tetrachlorocatechol (7). The other polar metabolite detected was characterized as 2-methoxy-3,4,5,6-tetrachlorophenol {TMS derivative with diagnostic peaks at m/z 334 (M+), m/z 319 (M+ - CH3), m/z 304 (M+ - CH3 - CH3), and m/z 289 [M+ - (CH3)3}, and the mass spectrum of this metabolite was indistinguishable from that of the authentic standard. Since boron derivatives can be obtained from (cis-) ortho-diolic compounds (aromatic diols and nonaromatic dihydrodiols) but not from phenols, only the TMS derivative of this metabolite was formed. By using nanospray ESI-MS/MS we detected trace amounts of a compound exhibiting an m/z of 422.82 [M+H] (35Cl), thus indicating the presence of a hexachlorotrihydroxydiphenyl ether (Fig. 2A). This is not unexpected since the catabolism of chlorinated dioxins by S. wittichii RW1 proceeds via the corresponding chlorinated trihydroxydiphenyl ethers (7). Monitoring the biotransformation over time (Fig. 3) revealed that after 120 h of incubation ca. 28% of the 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin consumed had been converted into 3,4,5,6-tetrachlorocatechol and ca. 4.5% had been converted into 2-methoxy-3,4,5,6-tetrachlorophenol. Surprisingly, the less-chlorinated 1,2,3,7,8-pentachlorodibenzo-p-dioxin was not transformed to a detectable extent in our experiments. Accordingly, metabolite formation was observed neither in poisoned and heat-inactivated controls nor in experiments with active cells under conditions identical to those used for the biotransformation of 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin. The present results, showing the formation of 3,4,5,6-tetrachlorocatechol and 2-methoxy-3,4,5,6-tetrachlorophenol in the presence of the hexachlorinated dibenzo-p-dioxin, are in good agreement with the previously described biotransformation of 1,2,3,4-tetrachlorodibenzo-p-dioxin by this strain (7). In this earlier study we showed that the nonsubstituted angular sites of the benzene rings in 1,2,3,4-tetrachlorodibenzo-p-dioxin were the targets for initial attack. Although in 1,2,3,7,8-pentachlorodibenzo-p-dioxin chlorine substituents do not occupy all available sites on the higher chlorinated ring in 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin only one benzene ring has unoccupied angular sites. The detection and identification of 3,4,5,6-tetrachlorocatechol as the degradation product is therefore consistent with an initial attack taking place at the less-substituted ring (Fig. 4).
FIG. 1.
(A) Identification of 3,4,5,6-tetrachlorocatechol as metabolic intermediate produced from 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin by S. wittichii RW1 by mass spectrometry as an n-butylboronate derivative of the detected metabolite; (B) identification of 3,4,5-trichlorocatechol as metabolic intermediate produced from 1,2,3-trichlorodibenzo-p-dioxin by S. wittichii RW1 by mass spectrometry as an n-butylboronate derivative of the detected metabolite.
FIG. 2.
(A) Identification of a hexachlorotrihydroxydiphenyl ether as an metabolic intermediate produced from 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin by S. wittichii RW1 by ESI-MS/MS; (B) identification of a trichlorotrihydroxydiphenyl ether as an metabolic intermediate produced from 1,2,3-trichlorodibenzo-p-dioxin by S. wittichii RW1 by ESI-MS/MS.
FIG. 3.
Biotransformation of 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin by S. wittichii RW1. Depletion of substrate and production of metabolic intermediates. Symbols: ▪, 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin; □, 3,4,5,6-tetrachlorocatechol; ○, 2-methoxy-3,4,5,6-tetrachlorophenol; •, poisoned control.
FIG. 4.
Proposed pathways for the biotransformation of 1,2,3-trichlorodibenzo-p-dioxin (A) and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin (B) by S. wittichii RW1.
Biotransformation of 1,2,3-trichlorodibenzo-p-dioxin.
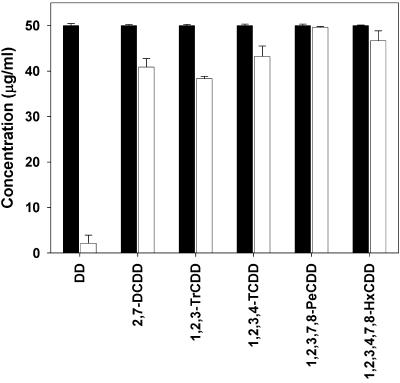
To evaluate whether substitution patterns do impact the biodegradability of these compounds we used two less-chlorinated congeners, namely, 1,2,3- and 2,3,7-trichlorodibenzo-p-dioxin (Fig. 5). Also, whereas 1,2,3-trichlorodibenzo-p-dioxin was transformed to yield a trichlorotrihydroxydiphenyl ether (exhibiting an m/z of 321 [M+H] [35Cl], as determined by ESI-MS/MS) (Fig. 2B) and 3,4,5-trichlorocatechol (identified by mass spectrometry; see Fig. 1B), 2,3,7-trichlorodibenzo-p-dioxin was not metabolized. In addition to general molecular properties that govern the transfer of substrates into the bacterial cells (molecular weight, size of the molecules, etc.) and thus limit the rate of turnover, biotransformation behavior might in addition be influenced by other molecular properties. It is interesting that from the PCDD congeners tested in the present study the two that were not transformed (2,3,7-tri- and 1,2,3,7,8-pentachlorodibenzo-p-dioxin) showed the lowest calculated ΔGf values (<2 kcal/mol, see reference 11), which again indicates the importance of substitution patterns for biodegradability. However, additional biotransformation experiments clearly showed the ability of S. wittichii RW1 to transform the degradable congeners even when a mixture of six individual dibenzo-p-dioxins with different substitution patterns is used (Fig. 6).
FIG. 5.
Biotransformation of 1,2,3-trichlorodibenzo-p-dioxin and 2,3,7-trichlorodibenzo-p-dioxin in separate experiments by S. wittichii RW1. Depletion of substrate and production of metabolic intermediates are shown. Symbols: ▪, 1,2,3-trichlorodibenzo-p-dioxin; □, 2,3,7-trichlorodibenzo-p-dioxin; •, 3,4,5-trichlorocatechol.
FIG. 6.
Biotransformation of a mixture of six dibenzo-p-dioxins by S. wittichii RW1. The dioxin mixture contained equal amounts (50 μg/ml) of each dioxin congener. Bars: ▪, amount of each congener at 0 h; □, amounts detected after incubation for 120 h. Each dioxin congener was transformed to a corresponding metabolite.
The present study is the first report to demonstrate the aerobic bacterial catabolism of a hexachlorinated dibenzo-p-dioxin together with an identification of resulting metabolites. In addition, it provides evidence that substitution patterns clearly influence the degradability of these chemicals. Future work should clarify how and to what extent inherent molecular properties of these noxious compounds impact their aerobic biodegradability.
Acknowledgments
This project was supported by the Ministry of Environment of the Republic of Korea as “The Eco-Technopia 21 Project” and “The BK21 Project.”
REFERENCES
- 1.Brzuzy, L. P., and R. A. Hites. 1996. Global mass balance for polychlorinated dibenzo-p-dioxins and dibenzofurans. Environ. Sci. Technol. 30:1797-1804. [DOI] [PubMed] [Google Scholar]
- 2.Bünz, P. V., and A. M. Cook. 1993. Dibenzofuran 4,4a-dioxygenase from Sphingomonas sp. strain RW1: angular dioxygenation by a three-component enzyme system. J. Bacteriol. 175:6467-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bünz, P. V., and S. Schmidt. 1997. The microbial degradation of halogenated diaryl ethers. Biotechnol. Adv. 15:621-632. [DOI] [PubMed] [Google Scholar]
- 4.Buser, H. R., and H. P. Bosshardt. 1976. Determination of polychlorinated dibenzo-p-dioxins and dibenzofurans in commercial pentachlorophenols by combined gas chromatography-mass spectrometry. J. Assoc. Off. Anal. Chem. 59:562-569. [PubMed] [Google Scholar]
- 5.Fortnagel, P., H. Harms, R. M. Wittich, S. Krohn, H. Meyer, V. Sinnwell, H. Wilkes, and W. Francke. 1990. Metabolism of dibenzofuran by Pseudomonas sp. strain HH69 and the mixed culture HH27. Appl. Environ. Microbiol. 56:1148-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habe, H., J. S. Chung, J. H. Lee, K. Kasuga, T. Yoshida, H. Nojiri, and T. Omori. 2001. Degradation of chlorinated dibenzofurans and dibenzo-p-dioxins by two types of bacteria having angular dioxygenases with different features. Appl. Environ. Microbiol. 67:3610-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong, H. B., Y. S. Chang, I. H. Nam, P. Fortnagel, and S. Schmidt. 2002. Biotransformation of 2,7-dichloro- and 1,2,3,4-tetrachlorodibenzo-p-dioxin by Sphingomonas wittichii RW1. Appl. Environ. Microbiol. 68:2584-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaiser, J. 2000. Just how bad is dioxin? Science 288:1941-1944. [DOI] [PubMed] [Google Scholar]
- 9.Keim, T., W. Francke, S. Schmidt, and P. Fortnagel. 1999. Catabolism of 2,7-dichloro- and 2,4,8-trichlorodibenzofuran by Sphingomonas sp. strain RW1. J. Ind. Microbiol. Biotechnol. 23:359-363. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch, N. H., and H.-J. Stan. 1994. Gas chromatographic-mass spectrometric determination of chlorinated cis-1,2-dihydrocyclohexadienes and chlorocatechols as their boronates. J. Chromatogr. A. 684:277-287. [Google Scholar]
- 11.Lee, J. E., W. Choi, and B. J. Mhin. 2003. DFT calculation on the thermodynamic properties of polychlorinated dibenzo-p-dioxins: intramolecular Cl-Cl repulsion effects and their thermochemical implications. J. Phys. Chem. A 107:2693-2699. [Google Scholar]
- 12.McLachlan, M. S., A. P. Sewart, J. R. Bacon, and K. C. Jones. 1996. Persistence of PCDD/Fs in a sludge-amended soil. Environ. Sci. Technol. 30:2567-2571. [Google Scholar]
- 13.Meharg, A. A., and D. Osborn. 1995. Dioxins released from chemical accidents. Nature 375:353-354. [DOI] [PubMed] [Google Scholar]
- 14.Monna, L., T. Omori, and T. Kodama. 1993. Microbial degradation of dibenzofuran, fluorene, and dibenzo-p-dioxin by Staphylococcus auriculans DBF63. Appl. Environ. Microbiol. 59:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiner, G., T. Wiedmann, H. Schimmel, and K. Ballschmiter. 1997. Influence of the substitution pattern on the microbial degradation of mono- to tetrachlorinated dibenzo-p-dioxins and dibenzofurans. Chemosphere 34:1315-1331. [Google Scholar]
- 16.Seeger, M., B. Camara, and B. Hofer. 2001. Dehalogenation, denitration, dehydroxylation, and angular attack on substituted biphenyls and related compounds by a biphenyl dioxygenase. J. Bacteriol. 183:3548-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin, D., S. Choi, J. E. Oh, and Y. S. Chang. 1999. Evaluation of polychlorinated dibenzo-p-dioxin/dibenzofuran emission in ten municipal solid waste incinerators. Environ. Sci. Technol. 33:2657-2666. [Google Scholar]
- 18.Wilkes, H., W. Francke, R. M. Wittich, H. Harms, S. Schmidt, and P. Fortnagel. 1992. Mechanistic investigation on microbial degradation of diaryl ethers—analysis of isotope-labeled reaction products. Naturwissenschaften 79:269-271. [Google Scholar]
- 19.Wilkes, H., R. M. Wittich, K. N. Timmis, P. Fortnagel, and W. Francke. 1996. Degradation of chlorinated dibenzofurans and dibenzo-p-dioxins by Sphingomonas sp. strain RW1. Appl. Environ. Microbiol. 62:367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittich, R. M., H. Wilkes, V. Sinnwell, W. Francke, and P. Fortnagel. 1992. Metabolism of dibenzo-p-dioxin by Sphingomonas sp. strain RW1. Appl. Environ. Microbiol. 58:1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittich, R. M. 1998. Degradation of dioxin-like compounds by microorganisms. Appl. Microbiol. Biotechnol. 49:489-499. [DOI] [PubMed] [Google Scholar]
- 22.Yu, J., R. C. Flagan, and J. H. Seinfeld. 1998. Identification of products containing -COOH, OOH, and -C=O in atmospheric oxidation of hydrocarbons. Environ. Sci. Technol. 32:2357-2370. [Google Scholar]