Abstract
The ubiE gene of Geobacillus stearothermophilus V, with its own promoter, was cloned and introduced into Escherichia coli. The cloned gene complemented the ubiE gene deficiency of E. coli AN70. In addition, the expression of this gene in E. coli JM109 resulted in the evolution of volatile selenium compounds when these cells were grown in selenite- or selenate-amended media. These compounds were dimethyl selenide and dimethyl diselenide.
Selenium plays an essential role in cellular metabolism when present at controlled, defined concentrations. Higher levels of selenium, however, are toxic. The relative toxicity of oxyanions of selenium is SeO32− > SeO42− (4). Although the mechanism(s) of selenite and selenate toxicity is not understood at the molecular level, they probably act as or induce oxidizing agents that may cause general oxidative damage to the cell. In support of this possibility, proteomic analyses have shown that the response of Escherichia coli to selenite and selenate exposure involves the induction of over 20 proteins, of which 8 exhibit antioxidant properties (2).
The biomethylation of sulfur, selenium, and tellurium is a well-established phenomenon. Different biogenic, volatile, and methylated forms of these elements have been detected in the headspace of both bacterial and fungal cultures (3), and mechanisms for the reduction and methylation of these metalloids have been discussed previously (4).
For several years, we have been studying resistance to heavy metals and metalloids exhibited by soil bacteria. Our model has been the thermotolerant, plasmidless, gram-positive rod Geobacillus stearothermophilus V (formerly Bacillus stearothermophilus V), which is naturally resistant to selenite (MIC > 175 μg/ml) and tellurite (MIC ≈ 125 μg/ml) (13). Because we are interested in identifying potential genes involved in this resistance, our strategy has been to construct gene libraries of G. stearothermophilus V and to utilize them to transform metalloid-sensitive E. coli hosts, selecting for the acquisition of a metalloid resistance phenotype. Using this strategy, we have shown that cysK and iscS gene products of G. stearothermophilus V mediate low-level tellurite resistance in E. coli (11, 14) and that the ubiE gene product of this bacterium mediates resistance to tellurium toxic salts through the conversion of tellurate into volatile Te compounds (1). In this work, we communicate that the ubiE gene of G. stearothermophilus V is also responsible for the evolution of CH3SeCH3 (dimethyl selenide [DMSe]) and CH3SeSeCH3 (dimethyl diselenide [DMDSe]) into the headspace of SeO32−- or SeO42−-amended E. coli cultures.
To clone the G. stearothermophilus V ubiE gene along with its own promoter (600pr), appropriate DNA fragments were obtained by PCR using plasmid p1VH as the template (1) and primers ORF600pr, GTCGGAGCGTTTTGATTTTTGCTTCCTTTG (nucleotides 1675 to 1704, GenBank accession number AAR04820), and ORF600R (1). The amplified PCR product was cloned into a pGEM-T cloning vector, sequenced, and used for all subsequent work.
To obtain mixed selenium-sulfide compounds, the chemical reduction of CH3SSCH3 (dimethyl disulfide) in the presence of DMDSe was carried out as described previously (3, 10). Gas chromatography with fluorine-induced chemiluminescence and gas chromatography/mass spectrometry were used to detect organoselenium compounds above bacterial headspace as described elsewhere (1, 10).
ORF600 of Geobacillus stearothermophilus V encodes a UbiE methyltransferase.
Open reading frame 600 (ORF600), carrying the ubiE gene of G. stearothermophilus V is one of three major ORFs encoded in plasmid p1VH, which was originally isolated as a tellurite-resistant recombinant clone upon transformation of tellurite-sensitive E. coli with a HindIII gene library of G. stearothermophilus V (1). We reported previously that the expression of this gene in E. coli cells that were exposed to potassium tellurate resulted in the evolution of dimethyl telluride (1). To show that this gene encodes a true UbiE methyltransferase, we cloned the ubiE gene, including its promoter, into the pGEM-T cloning vector. The cloned gene was then transformed into E. coli AN70, an ubiE-deficient strain which was isolated after N-methyl-N′-nitro-N-nitrosoguanidine treatment of E. coli AB3311 (15).
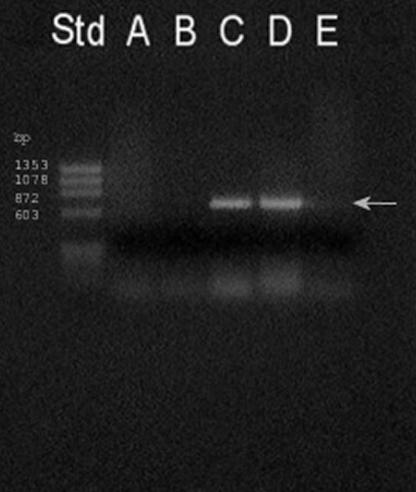
As shown in Table 1, the presence of the ubiE gene was required to overcome the difficulty for this strain to grow with succinate as the sole carbon source. The expression of the G. stearothermophilus V ubiE gene in both E. coli AN70 and E. coli JM109 was demonstrated by reverse transcription (RT)-PCR analysis (Fig. 1, arrow) using primers ORF600F and ORF600R (1).
TABLE 1.
Genetic complementation of E. colia with the ubiE gene of G. stearothermophilus V
| Strain/plasmid | M9 minimal medium supplemented with:
|
|||||
|---|---|---|---|---|---|---|
| glu | succ | glu/cas | succ/cas | glu/ye | succ/ye | |
| AN70/none | +/− | − | + | + | + | + |
| AN70/600prb | + | +/− | + | + | + | + |
Cells were streaked in solid M9 minimal medium (9) plates (25 ml) supplemented with the indicated nutrients at 0.2% final concentration and grown at 37°C for 24 h. glu, glucose; succ, succinate; cas, Casamino Acids; ye, yeast extract. −, no growth (even after 48 h); +/−, slow growth; +, normal growth. Trials were done in triplicate.
Plates contained ampicillin (100 μg/ml).
FIG. 1.
Expression of the G. stearothermophilus V ubiE gene in E. coli. Agarose gel (1.5%) electrophoresis of the RT-PCR products obtained using primers ORF600F and ORF600R, specific for the ubiE gene of G. stearothermophilus V, was performed (1). In each case, the template was 20 ng of total RNA extracted from E. coli AN70 (lane A), E. coli JM109 (lane B), E. coli AN70/600pr (lane C), or E. coli JM109/600pr (lane D). Lane E is a negative control to which no template was added. Std, molecular weight standard (HaeIII-digested Φ X174 replicative-form DNA). After incubating the RNA preparations at 25°C for 30 min with 1 unit of RQ1 DNase (Promega), the deoxyribonuclease was inactivated for 10 min at 70°C. The RT-PCR was then incubated for 1 h at 42°C and then for 30 cycles that consisted of 1 min at 95°C, 1 min at 55°C, and 1 min at 72°C, followed by a final extension of 10 min at 72°C.
A low level of growth of E. coli AN70 was also seen in M9 medium supplemented with glucose, confirming the metabolic illness of this strain; these growth difficulties were almost overcome when E. coli AN70 was grown in M9 minimal medium supplemented with yeast extract or with Casamino Acids (Table 1). Since the AN70 strain grew very poorly in the presence of Se oxyanions, the following results were obtained using E. coli JM109 as host.
The UbiE methyltransferase of G. stearothermophilus V methylates selenite and selenate salts.
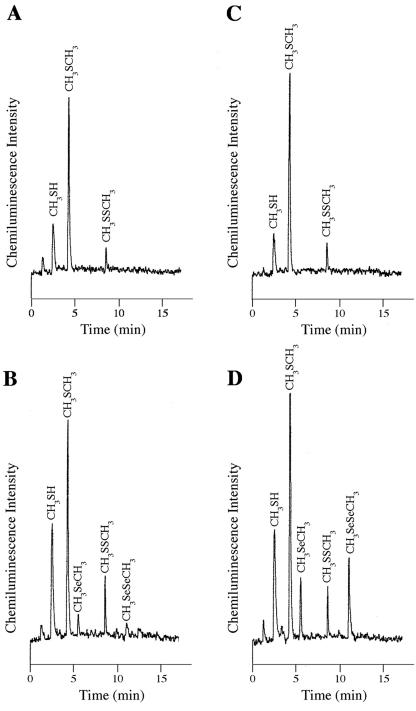
E. coli JM109 cells carrying the G. stearothermophilus ubiE gene or the cloning vector alone was grown in the presence of 0.2 mM sodium selenite or 0.2 mM sodium selenate, and culture headspaces were analyzed for the presence of volatile organoselenium compounds as described above. As shown in Fig. 2B and D, the expression of the G. stearothermophilus V ubiE gene resulted in the evolution of organoselenium compounds like DMSe and DMDSe. Totals of 2.5-fold more DMSe and 9-fold more DMDSe in the headspace of selenate-amended cultures than in those that were grown in the presence of sodium selenite were found (Fig. 2B and D). None of these volatile organoselenium derivatives was seen in the headspace of control cell cultures carrying the cloning vector alone (Fig. 2A and C).
FIG. 2.
Detection of volatile selenium compounds above the headspace of E. coli expressing the ubiE gene of G. stearothermophilus V. A to D, Fluorine-induced chemiluminescence/gas chromatography chromatogram from 1 ml of headspace of E. coli JM109 carrying pGEM-T cloning vehicle (A and C) or plasmid 600pr (B and D). Cells were grown for 48 h in the presence of 0.2 mM Na2SeO3 (A and B) or 0.2 mM Na2SeO4 (C and D). Chromatograms are representatives of triplicate runs.
These results showed that ORF600, encoding UbiE methyltransferase, is responsible for the selenium biomethylation seen in E. coli JM109/600pr. Thus, as expected and as observed with tellurite amendments (1), this recombinant E. coli strain appears to be able to reduce and methylate not only sulfur salts (as sulfate in the growth medium) to organosulfur compounds, but also selenium salts to DMSe and DMDSe.
One of the most important results of this work is the clear requirement of the expression of the G. stearothermophilus V ubiE gene for selenium biomethylation in E. coli. The control data in Table 1 and Fig. 2A and C show that the absence of this methyltransferase gene does not allow the methylation of selenium oxyanions; however, the expression of ubiE yields volatile organoselenium compounds (Fig. 2B and D).
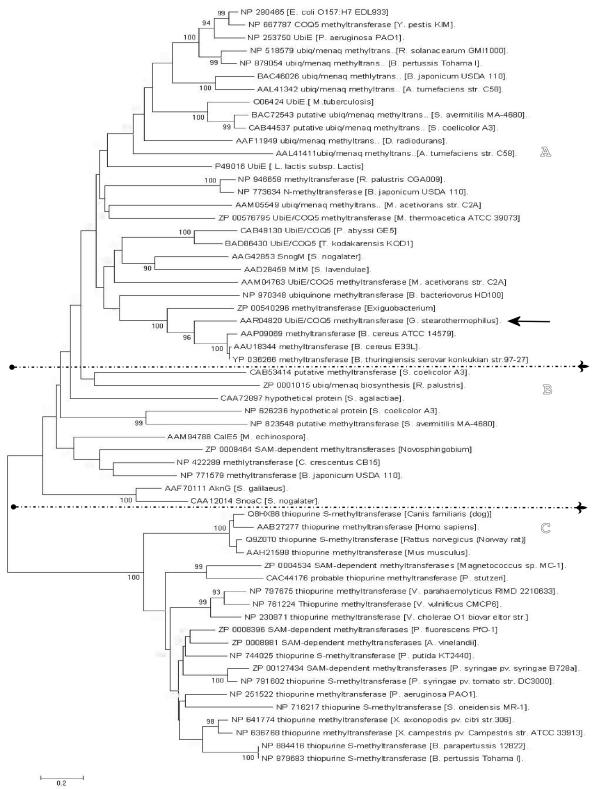
In addition to the results reported here, other methyltransferases belonging to the thiopurine methyltransferase and to the UbiE-MmtA groups that participate in metalloid biomethylation in bacteria have been described previously in the literature (6, 7, 8). A phylogenetic analysis using sequences of proteins belonging to the above groups, which included the G. stearothermophilus V methyltransferase studied here, positioned it undoubtedly within the UbiE subgroup (Fig. 3). These results suggest that G. stearothermophilus V UbiE may play a selenium-methylating function in addition to its classical C-methylation activity during ubiquinone-menaquinone biosynthesis.
FIG. 3.
Neighbor-joining phylogenetic tree of UbiE/COQ5 (A), UbiE/MmtA (B), and thiopurine methyltransferase (C) methyltransferase enzymes. Fifty-nine different sequences, including proteins belonging to the above groups, were used. Alignments of the sequences were performed using the Clustal X (12) program, and the phylogenetic tree was computed using MEGA software (version 3.1) (5). Organism names, a distance scale, bootstrap values, and GenBank accession numbers of the sequences used are indicated in the figure. The black arrow indicates the G. stearothermophilus V UbiE enzyme.
Acknowledgments
This work was supported by grant no. 1030234 from FONDECYT and DICYT (Universidad de Santiago de Chile) to C.C.V. and a Robert A. Welch Departmental Grant and Enhancement Grant for Research to J.W.S., M.F.P., and T.G.C. D.E.F. and M.A.A. were supported by doctoral fellowships from MECESUP UCH106 (Chile).
We thank Catherine F. Clarke at UCLA for providing the ubiE-deficient strain E. coli AN70.
REFERENCES
- 1.Araya, M. A., J. W. Swearingen, Jr., M. Plishker, C. P. Saavedra, T. G. Chasteen, and C. C. Vásquez. 2004. Geobacillus stearothermophilus V ubiE gene product is involved in the evolution of dimethyl telluride in Escherichia coli K-12 cultures amended with potassium tellurate but not with potassium tellurite. J. Biol. Inorg. Chem. 9:609-615. [DOI] [PubMed] [Google Scholar]
- 2.Bébien, M., G. Lagniel, J. Garin, D. Touati, A. Verméglio, and J. Labarre. 2002. Involvement of superoxide dismutases in the response of Escherichia coli to selenium oxides. J. Bacteriol. 184:1556-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chasteen, T. G., and R. Bentley. 2003. Biomethylation of selenium and tellurium: microorganisms and plants. Chem. Rev. 103:1-26. [DOI] [PubMed] [Google Scholar]
- 4.Frankenberger, W. T., Jr., and R. A. Engberg, (ed.). 1998. Environmental chemistry of selenium. Marcel Dekker, Inc., New York, N.Y.
- 5.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 6.Liu, M., R. J. Turner, T. L. Winstone, A. Saetre, M. Dyllick-Brenzinger, G. Jickling, L. W. Tari, J. H. Weiner, and D. E. Taylor. 2000. Escherichia coli TehB requires S-adenosylmethionine as a cofactor to mediate tellurite resistance. J. Bacteriol. 182:6509-6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranjard, L., C. Prigent-Combaret, S. Nazaret, and B. Cournoyer. 2002. Methylation of inorganic and organic selenium by the bacterial thiopurine methyltransferase. J. Bacteriol. 184:3146-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranjard, L., C. Prigent-Combaret, S. Favre-Bonté, C. Monnez, S. Nazaret, and B. Cournoyer. 2004. Characterization of a novel selenium methyltransferase from freshwater bacteria showing strong similarities with the calicheamicin methyltransferase. Biochim. Biophys. Acta 1679:80-85. [DOI] [PubMed] [Google Scholar]
- 9.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 10.Swearingen, J. W., Jr., M. A. Araya, M. F. Plishker, C. P. Saavedra, C. C. Vásquez, and T. G. Chasteen. 2004. Identification of biogenic organotellurides in Escherichia coli K-12 headspace gases using solid phase microextraction and gas chromatography. Anal. Biochem. 331:106-114. [DOI] [PubMed] [Google Scholar]
- 11.Tantaleán, J. C., M. A. Araya, C. P. Saavedra, D. E. Fuentes, J. M. Pérez, I. L. Calderón, P. Youderian, and C. C. Vásquez. 2003. The Geobacillus stearothermophilus V iscS gene, encoding cysteine desulfurase, confers resistance to potassium tellurite in Escherichia coli K-12. J. Bacteriol. 185:5831-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vásquez, C. 1985. Isolation and partial characterization of BstVI, a thermostable isoschizomer of XhoI. Biochem. Int. 10:655-662. [PubMed] [Google Scholar]
- 14.Vásquez, C., C. Saavedra, C. Loyola, M. Araya, and S. Pichuantes. 2001. The product of the cysK gene of Bacillus stearothermophilus V mediates potassium tellurite resistance in Escherichia coli. Curr. Microbiol. 43:418-421. [DOI] [PubMed] [Google Scholar]
- 15.Young, I. G., L. M. McCann, P. Stroobant, and F. Gibson. 1969. Characterization and genetic analysis of mutant strains of Escherichia coli K-12 accumulating the ubiquinone precursors 2-octaprenyl-6-methoxy-1,4-benzoquinone and 2-octaprenyl-3-methyl-6-methoxy-1,4-benzoquinone. J. Bacteriol. 105:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]