Abstract
The lumbricid earthworms (annelid family Lumbricidae) harbor gram-negative bacteria in their excretory organs, the nephridia. Comparative 16S rRNA gene sequencing of bacteria associated with the nephridia of several earthworm species has shown that each species of worm harbors a distinct bacterial species and that the bacteria from different species form a monophyletic cluster within the genus Acidovorax, suggesting that there is a specific association resulting from radiation from a common bacterial ancestor. Previous microscopy and culture studies revealed the presence of bacteria within the egg capsules and on the surface of embryos but did not demonstrate that the bacteria within the egg capsule were the same bacteria that colonized the nephridia. We present evidence, based on curing experiments, in situ hybridizations with Acidovorax-specific probes, and 16S rRNA gene sequence analysis, that the egg capsules contain high numbers of the bacterial symbiont and that juveniles are colonized during development within the egg capsule. Studies exposing aposymbiotic hatchlings to colonized adults and their bedding material suggested that juvenile earthworms do not readily acquire bacteria from the soil after hatching but must be colonized during development by bacteria deposited in the egg capsule. Whether this is due to the developmental stage of the host or the physiological state of the symbiont remains to be investigated.
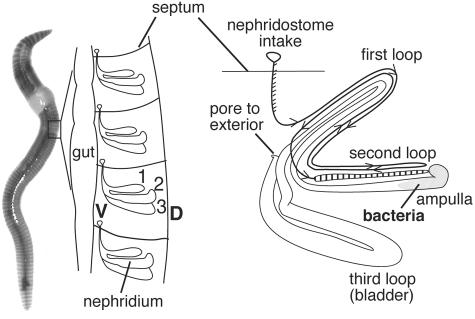
The mixing and processing of soils by earthworms worldwide alter soil structure, nutrient availability, and microbial activities through diverse feeding strategies and burrowing habits (10, 11, 12). There is an increasing appreciation that the alteration of soils involves synergistic interactions between earthworms and microorganisms. One of the better-studied interactions is the interaction with the bacteria that are ingested from the soil and transit the gut. Numerous studies have examined the activity and fate of microorganisms in the earthworm gut and casts (fecal pellets) by employing both culture-based and molecular methods (13, 14, 16, 17, 27, 31). A less well-characterized, and apparently specific, association was reported in 1926 by Knop (18), who presented microscopic observations of dense populations of rod-shaped bacteria in the ampullas of the nephridia of 30 species of lumbricids (representing Europe, Asia, Africa, and North and South America). The nephridia are paired osmoregulatory-excretory organs on the internal body wall of each segment of a worm (ca. 200 nephridia per worm) that pass fluid from the coelom to the outside through a continuous winding tube that forms three major loops (20) (Fig. 1). An ampulla is formed by a widening of the second loop. Since members of the Lumbricidae tend to dominate earthworm populations in temperate regions (11), this apparently ubiquitous association likely has functional significance for the host and possibly influences soil chemistry.
FIG. 1.
Anatomy of earthworm nephridia and location of the associated bacteria. (Left) Schematic diagram of an earthworm (dorsal) with enlargement of one half of the body, showing the location of nephridia within the segments. The numbers indicate the loops. (Right) Schematic drawing of a nephridium, showing the arrangement of the three loops. The arrowheads indicate the direction of flow.
Since discovery of the association, new findings regarding bacterial colonization of earthworm nephridia have been sparse. Our initial characterization of this association using comparative 16S rRNA gene sequence analysis revealed that the bacteria colonizing the nephridia form a monophyletic group within the genus Acidovorax (28). Individuals of the same species of earthworm yielded symbiont sequences with >99% identity, even if the individuals were from different continents (Germany and United States), whereas different species of worms harbor distinct sequence types of the associated Acidovorax sp. bacteria, suggesting that there was cospeciation from a common ancestor. Specific and stable bacterial associations with host animals usually involve a reliable means for transferring the bacterial symbiont to the next generation. Although a function for the Acidovorax sp. nephridial bacteria has not been described yet, the apparent ubiquitous and specific association implies that the earthworms have mechanisms to ensure transmission of the bacteria from the parents to the progeny.
There are two general modes of microbial symbiont transmission, horizontal and vertical, and the details of the mechanisms vary among taxa. In horizontal transmission, a juvenile stage acquires the symbiont from the environment rather than directly from the parent, and there is mixing of symbiont populations from multiple individual hosts or environmental sources. For example, in vestimentiferan tubeworm-sulfide-oxidizing bacterium and bobtail squid (Euprymna scolopes)-Vibrio fischeri associations, juveniles are released into the environment without their symbionts, and survival depends upon acquiring an appropriate strain of bacteria from the seawater (9, 22, 23). In contrast, transmission of the intracellular Buchnera and Wolbachia symbionts of insects is ensured by direct (vertical) transfer via the eggs (3, 6, 25). Among annelids, there is a diverse array of symbiotic associations with bacteria, ranging from surface colonization to obligate symbioses in which the entire gut tract has been replaced by an organ containing chemoautotrophic endosymbionts (reviewed in reference 4), but there has been speculation concerning the mechanisms of transmission for only a few of these associations. Transmission directly from the parent to the eggs has been shown for at least one member of the gutless marine oligochete Tubificidae Inanidrilus leukodermatus, in which subcuticular bacteria occur in conspicuous pads associated with the ovipores and appear in the mucus surrounding the egg during deposition (15).
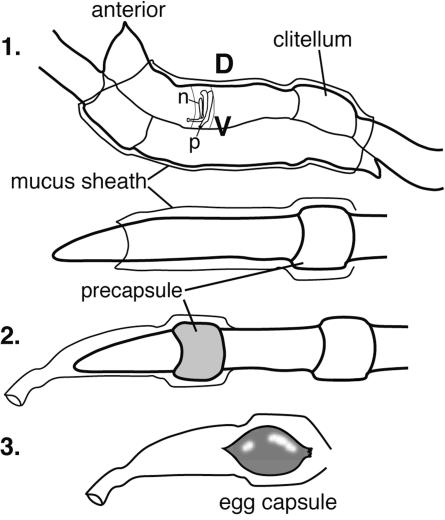
Knop's initial observations of bacterial cells in the mucus of mating earthworms suggested that transmission might occur by direct incorporation into the egg capsules (18). During earthworm mating, a gelatinous mucus sheath is formed externally around both worms, sperm is exchanged, and a precapsule forms around the clitella of the individual worms (Fig. 2). The eggs, sperm, and albumin are expelled through pores into this precapsule, which then slides off the anterior end to form the mature egg capsule with a sealed chitinous shell. Formation of the capsule occurs externally, and entrainment of soil bacteria could explain the presence of bacteria in the mucus and capsule, along with bacteria possibly originating from the nephridia. Nonspecific entrainment of soil bacteria has been suggested to occur by culture-based characterization of egg capsule contents that reported that there was 108 CFU/g capsule that included diverse colony types (34). This study did not identify Acidovorax spp. among the isolates obtained. Scanning electron microscope analyses have since revealed bacteria on the surfaces of developing embryos within egg capsules (7). However, no previous study confirmed that the bacteria in the mucus or capsules are symbiotic bacteria originating from the nephridia.
FIG. 2.
Earthworm mating and egg capsule formation. (Diagram 1) The worms adhere to each other on the ventral side. A nephridium is shown for a single segment to illustrate the orientation of the nephridia. (Diagram 2) The precapsule forms around the clitellum and slides off the anterior end of the worm. (Diagram 3) The capsule chitinous shell matures. D, dorsal; V, ventral; n, nephridium; p, nephridial pore.
The main objective of this study was to establish the mode of transmission from adult to progeny of nephridial symbionts in Eisenia fetida, a common earthworm used in composting systems. We employed in situ hybridization, bacterial 16S rRNA gene amplification, cloning, and sequencing and examined both capsules and newly emerging juveniles to demonstrate that (i) symbiotic bacteria are shed by the adult worms during mating, (ii) newly formed egg capsules contain Acidovorax sp. bacterial cells at high densities, (iii) juveniles emerge from the capsules fully colonized, and (iv) juveniles do not acquire bacteria from the environment after hatching under the conditions tested.
MATERIALS AND METHODS
Laboratory rearing of E. fetida.
E. fetida adults (Yelm Earthworm and Castings Farm, Yelm, WA) were maintained at 20 to 23°C at a density of approximately 60 worms per liter in a mixture containing autoclaved hydrated (with distilled H2O [diH2O]) coir (mulched coconut husks; Coconut Palm Resources, Inc., Hillsboro, OR) and 5 g oatmeal per liter. The bedding was changed approximately every 7 to 10 days.
Collection of egg capsules and hatchlings.
Newly deposited capsules (∼1 day old) were obtained by removing capsules daily from bedding material and using a dissection microscope to confirm that no developed embryos were present. The capsules were then maintained in petri dishes on Whatman no. 1 filters moistened with distilled water. Hatchlings emerge approximately 20 days after egg capsule formation (this study). Upon hatching, juveniles were placed in petri dishes containing fresh bedding material.
Specimen collection and fixation.
Specimens for in situ hybridization (eggs, juveniles, and mucus) were fixed in buffered 4% paraformaldehyde (50 mM phosphate buffer, 0.15 M NaCl; pH 7.4) for 2 h at room temperature or overnight at 4°C, washed in phosphate buffer, and stored at −20°C in 70% ethanol until analysis. Worms were anesthetized in 5% ethanol, opened along the dorsal midline, and pinned to expose the nephridia, and the gut was carefully removed prior to fixation in paraformaldehyde. Fresh mucus was collected from six nonmating adults by placing the animals in a clean plastic petri dish following an initial rinse in sterile diH2O to remove surface debris and old mucus. Mucus (500 μl) was collected with a sterile pipette and immediately fixed (1:1). The solution was then centrifuged at 10,000 × g with an Eppendorf tabletop centrifuge for 10 min, the supernatant was removed, and the pellet was resuspended in 500 μl phosphate buffer (filtered with a 0.2-μm filter). Following a second centrifugation, the pellet was resuspended in 20 μl diH2O (sterile filtered), and 3-μl aliquots were dried on coated slides (Erie Scientific Cel-line eight-well 6-mm slides with ADCELL bioadhesive coating) prior to in situ hybridization.
Newly laid egg capsules (<1 to 2 days old) remaining in the sheath were left intact for fixation. After fixation and washes, the egg capsules were removed from the sheaths, and their contents (∼5 μl) were dried onto slides prior to hybridization. The sheaths were laid out flat on slides and dried prior to hybridization. For the most part, this method preserved the distribution and form of bacterial aggregates within the capsules and the mucus sheaths.
FISH.
The presence of bacterial cells in the mucus gel sheaths of mating worms and egg capsules was analyzed by fluorescent in situ hybridization (FISH) with a series of fluor-labeled oligonucleotide probes. The Cy3-LSB 145 probe (S-G-Acidov-0145-a-A-18 [probe and primer designations as described in reference 33]) identifies a subset of the Acidovorax group that includes the earthworm bacterial symbiont strains, the Acidovorax facilis cluster, and Acidovorax wohlfahrtii (28, 29). The fluorescein (FL) or Cy5-EUB 338 probe (S-D-Bact-0338-a-A-18) identifies most members of the bacterial domain (2). Probes specific for the Betaproteobacteria (Cy3-BET42a; L-D-Beta-1027-a-A-16) and Gammaproteobacteria (FL-GAM42a; L-D-Gamma-1027-a-A-16) were also used together to control for specificity (21). These two probes differ by one internal nucleotide and target the same region of the 23S rRNA with GAM42a as the competitor for BET42a.
FISH was performed by using a previously described protocol under stringent conditions for the probes used (26). Specifically, specimens were incubated in hybridization buffer (35% formamide, 0.9 M NaCl, 20 mM Tris-HCl [pH 8], 0.01% sodium dodecyl sulfate) with 5 ng/μl probe at 46°C for 2 h and then placed in wash buffer (80 mM NaCl, 20 mM Tris-HCl [pH 8], 5 mM EDTA, 0.01% sodium dodecyl sulfate) at 48°C for 30 min with one change after 15 min. The specimens were then mounted in Vecta Shield (Vector Labs, Inc.) and probe-conferred fluorescence was imaged using excitation at the appropriate wavelength with a Zeiss LSM Pascal laser scanning confocal microscope (Carl Zeiss, Jena, Germany).
Antibiotic treatment of egg capsules.
A number of antibiotic treatment regimens were tested for the ability to eliminate the symbiont without significant embryo mortality. Curing efficacy was determined by FISH of hatchlings. Toxicity was evaluated by monitoring hatching success and embryo development. In these studies we examined both the concentration and treatment duration, initially testing gentamicin, kanamycin (50, 100, 120, and 150 μg/ml), and equal amounts of gentamicin and ampicillin together up to a concentration of 100 μg/ml for 4, 7, and 10 days. These antibiotics were selected based on past success with gentamicin treatment of other invertebrates and the use of kanamycin and ampicillin as genetic markers for possible future genetic manipulations of the symbiont. Egg capsules were collected, rinsed in diH2O, and placed in clean petri dishes with Whatman no. 1 filter paper. Five milliliters of antibiotic solution in diH2O was added and changed daily. Control egg capsules were treated in a similar manner with diH2O alone. After the treatment period ended, egg capsules or hatchlings were removed from exposure to the antibiotic.
Colonization of treated and control hatchlings (newly emerged), juveniles, and adults was assessed by FISH with both the Cy3-LSB 145 probe and the FL-EUB 338 probe. Approximately one-half of each worm was examined (30 to 45 segments; two nephridia per segment), and the nephridia were counted as colonized or clear. Colonization was assessed in hatchlings collected from petri dishes immediately after emergence and at 1, 4, and 8 weeks and 3 months of age.
Exposure of juveniles to Acidovorax symbiotic bacteria shed by adults.
Inoculation of newly emerged juvenile worms via exposure to environmental populations of the symbiont was examined by incubating aposymbiotic egg capsules and hatchlings with bedding material containing colonized adults. The term aposymbiotic indicates capsules and worms that did not carry bacterial symbionts and were not exposed to antibiotics. The term cured refers to worms that were exposed to antibiotics during development. Fifteen to twenty staged egg capsules (17 to 18 days old) recovered from cured adults were placed in 150 ml of bedding that had been worked by colonized worms for 6 to 10 days and to which six adult colonized worms were added (five replicates; 10 juveniles assayed from each replicate). These egg capsules were not exposed to antibiotics. Control containers contained the same bedding, six adults, and 10 to 15 colonized control egg capsules (17 to 18 days old). The juveniles were allowed to hatch and remain in the bedding for 4 weeks, and then they were collected and assessed for colonization by FISH. After the first week and every week thereafter, approximately one-half of the bedding was exchanged with fresh bedding to maintain the health of the worms and to remove capsules deposited by the adults.
Amplification, cloning, and sequencing of 16S rRNA genes.
The identity of bacteria detected by FISH in the egg capsules was confirmed by comparing bacterial 16S rRNA gene sequences recovered from the egg capsules and nephridia. DNA was extracted from the pooled contents of five freshly deposited capsules (QIAGEN QIAquick tissue extraction kit). The nearly complete 16S rRNA gene sequence was amplified using primers GM3 (S-D-Bacteria-8-b-S-16) and GM4 (S-*-Univ-1492-b-A-16) (24) with the following reaction mixture (20 μl): 2 μl 10× Fermentas buffer, 2 μl of a 25 mM MgCl2 solution, 2 μl of a 4% bovine serum albumin solution, 0.4 μl of a 10 μM primer solution, 0.4 μl of an equal mixture of each deoxynucleoside triphosphate (10 mM), and 0.1 μl (5 U/μl) of Taq polymerase (MBI Fermentas, Hanover, MD). The thermocycler program (model PTC-100; MJ Research, Inc.) consisted of 94°C for 5 min, followed by 28 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min and final extension at 72°C for 5 min. The PCR products were ligated into pCR4 TOPO (1 μl of salt solution and 1 μl of vector) and transformed into Escherichia coli DH5α-T1 One Shot TOP10 competent cells (TOPO-TA cloning kit; Invitrogen) according to the manufacturer's protocol. Clone libraries were screened by amplified rRNA gene restriction analysis (1). Inserts were amplified from isolated plasmids by PCR using primers GM3 and GM4 and the conditions described above, except that the reaction volume was 11 μl and 23 cycles were used. Following amplification, 1 U of enzyme (HaeIII and MspI separately) was added along with 1.5 μl 10× NEB buffer 2 (New England Biolabs, Inc., Beverly, MA) and enough water to bring the volume to 15 μl; the mixture was incubated overnight at 37°C and then electrophoresed on a 3% NuSieve agarose gel with 1× Tris-borate-EDTA buffer with ethidium bromide and visualized with a UV transilluminator. Clones with unique patterns were sequenced using the MegaBACE 1000 DNA analysis system and reagents according to manufacturer's recommendations (Molecular Dynamics, Sunnyvale, CA). Primers GM3 and GM4 and selected internal primers (19) were used to obtain the complete sequences of both strands of each cloned 1,460-bp amplicon, and Sequencher (Gene Codes Corp.) was used to assemble the overlapping reads.
Phylogenetic analysis of cloned sequences.
Complete sequences were aligned with a 50% conservation filter for Betaproteobacteria using the ARB software package (www.arb-home.de) and were compared to sequences that we previously determined for nephridial bacteria from E. fetida, Lumbricus terrestris L., Aporrectodea tuberculata Eisen, Octolasion lacteum Savigny (28), and other representatives of the genus Acidovorax. The aligned sequences were then analyzed using PAUP V. 4.0beta 10 (30) to infer phylogenetic relationships from approximately 1,400 bp using maximum parsimony and maximum likelihood with 1,000 bootstrap resamplings to generate a bootstrap value for each node. The 16S rRNA sequence of Comamonas testosteroni was used as the outgroup.
Nucleotide sequence accession numbers.
The nucleotide sequences of egg capsule DNA 16S rRNA gene clones A2 and A5 have been deposited in the GenBank database under accession numbers DQ093612 and DQ093613, respectively.
RESULTS
Acidovorax sp. cells in mucus and egg capsules.
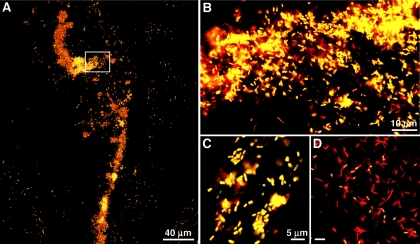
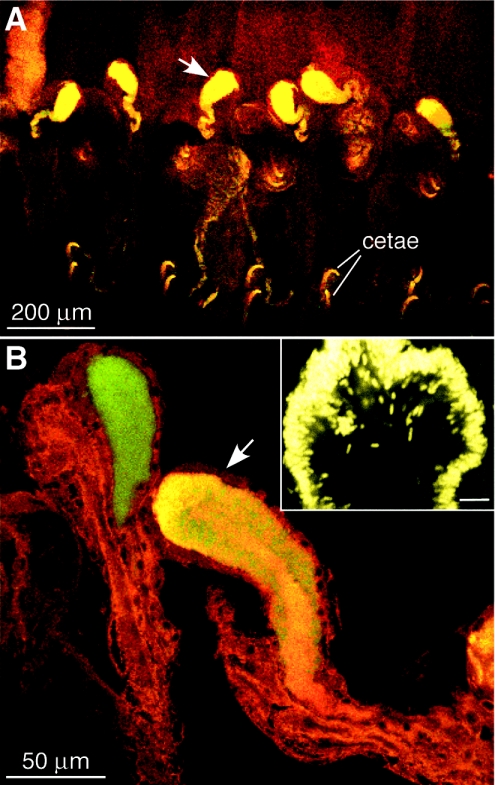
The Acidovorax probe hybridized to bacterial cells in the mucus, mucus sheaths (n = 8), and capsules (n = 12) produced by adult E. fetida. The size and morphology of these cells were similar to the size and morphology of cells observed in the nephridia. Acidovorax-positive cells occurred in occasional patches as scattered cells or aggregated streams of cells in the mucus sheaths collected from mating worms (not shown). Bacterial cells having diverse morphologies that were labeled only with the Eub 338 probe were also observed throughout the mucus sheaths. In contrast to the sparse distribution in the mucus, dense aggregates and clouds of Acidovorax-positive cells (LSB 145 probe) were observed throughout the egg capsule albumin (Fig. 3). Analysis of homogenized capsule contents revealed that cells that were labeled only with the Eub 338 probe were also abundant (about 30 to 40% of the cells) (Fig. 3D). However, due to the dense aggregation of both the Acidovorax sp. cells and other unidentified cells within the albumin, more accurate quantification has not been completed yet. In contrast, Acidovorax cells were not detected by FISH in the mucus sheaths (n = 5) or egg capsules (n = 13) from cured adults, indicating that Acidovorax-positive cells in specimens and eggs recovered from colonized adults did not originate from the bedding. Bacteria detected in the mucus sheaths (mixed ages from newly formed to 1 day old and older) by the Eub 338 probe alone (LSB 145 negative) varied in morphology and density.
FIG. 3.
False color images of bacterial cells detected by FISH in egg capsules of E. fetida using both Cy3-LSB 145 and FL-Eub 338. (A) laser scanning confocal microscope image of a large aggregate representative of aggregates detected in the egg capsule albumin. (B to D) Yellow cells, Acidovorax; red cells, Eub 338 only. (B) Enlargement of the area in the box in panel A. (C) Acidovorax cells, showing morphology similar to the morphology of cells observed in ampullas of E. fetida nephridia. (D) Abundant cells labeled with only Eub 338.
Antibiotic treatments and timing of colonization.
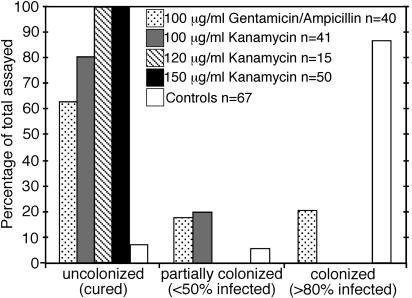
Preliminary treatments with 50 and 100 μg/ml gentamicin and with gentamicin and ampicillin together did not completely clear bacteria from juveniles and resulted in hatchlings that remained partially colonized. However, gentamicin at concentrations greater than 100 μg/ml was toxic and resulted in arrested development. Kanamycin was found to be fully effective and nontoxic at concentrations of 120 and 150 μg/ml given in a 10-day treatment in the latter half of development. The results for 10-day treatments are summarized in Fig. 4. Preliminary data from ineffective shorter treatments are not shown.
FIG. 4.
Proportion of nephridia containing bacteria after 10-day antibiotic treatments of E. fetida egg capsules as detected by FISH. The percentages of infected worms indicate the percentages of nephridia that were colonized by bacteria within a worm.
After hatching, the nephridia of control juveniles were fully colonized by the symbiotic bacteria (n = 30), indicating that they were colonized during development in the egg capsule (Fig. 4 and 5). Several trial antibiotic treatments yielded a mixture of cured, partially cured (<50% of nephridia colonized), and fully colonized (>80% of nephridia colonized) hatchlings (Fig. 4). Over the 3-month growth period, various levels of colonization were observed, and the extent of colonization did not increase to full colonization. For example, treatment with 100 μg/ml gentamicin and ampicillin resulted in the following ratios of cured worms to partially cured worms to colonized worms: at 2 days, 56:22:22 (n = 9); at 18 days, 60:20:20 (n = 10); at 1 month, 60:0:40 (n = 5); and at 2 months, 98:2:0 (n = 9). Three petri dishes from the 50-μg/ml gentamicin treatment contained both individuals that were fully colonized and individuals that were fully cured growing together for up to 3 months without cross infection. For example, two dishes each contained two fully colonized and three fully cured individuals, and an additional dish contained one of each type of worm. Within partially colonized individuals (found in all age groups up to 3 months old), infected nephridia appeared within segments adjacent to segments containing uncolonized nephridia (Fig. 5B), suggesting that nephridia within a worm do not cross-inoculate each other at this stage of development. Bacterial cells were not detected by FISH using the Eub 338 probe in nephridia lacking the Acidovorax symbiont. A low level (∼8%) of uncolonized controls was found throughout these studies. Of the matching controls assayed at 2 to 3 months, 90% were fully colonized (n = 19).
FIG. 5.
Detection of bacteria in juvenile earthworm nephridia by FISH using the Acidovorax-specific Cy3-LSB 145 probe and the FL-Eub 338 probe. (A) Six segments of a hatchling with Acidovorax cells (yellow) detected in ampullas (arrow). (B) Nephridia of adjacent segments in a partially cured juvenile, one colonized (arrow) and the other not colonized. (Inset) Cross section of a colonized ampulla.
Exposure of juveniles to Acidovorax shed by adults.
After worms emerged and grew for 4 weeks in the presence of colonized adults, the nephridia of all but four of the aposymbiotic juveniles remained free of bacteria as detected by FISH (n = 50). Fully colonized juveniles (n = 4) were observed in only two of five replicates. All but three of the control colonized juveniles remained colonized (n = 30), and the negative control aposymbiotic capsules placed in fresh bedding without colonized adults remained uncolonized (n = 35). Under the conditions tested, the adults mated and deposited new egg capsules, indicating that they were shedding nephridial bacteria, and the juveniles were routinely observed in direct contact with the adults.
Amplification, cloning, and sequencing of 16S rRNA genes.
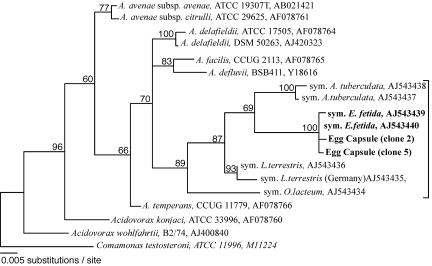
A total of 60 16S rRNA gene clones amplified from pooled egg capsule (n = 5) DNA were screened for similarity by amplified rRNA gene restriction analysis. Two distinct patterns (pattern 1, 30%; pattern 2, 18%) were obtained for clones matching Acidovorax sequences from E. fetida, representing ∼50% of the clones screened, which was consistent with in situ hybridization observations. The two sequences were 99.6% identical but differed in the location of an MspI restriction site. The sequence of the pattern 1 clones exhibited 99.46 and 99.73% identity with E. fetida sequences recovered from nephridia in our previous study (accession no. AJ543439 and AJ543440) and 95.5 to 97% identity with Acidovorax symbiont sequences obtained previously from other earthworm species (accession no. AJ543434 to AJ543438). Phylogenetic relationships (Fig. 6) among different earthworm symbiont sequences from our previous analysis and the sequences newly recovered from the E. fetida egg capsules were consistent with previous results (28). The egg capsule clone sequences grouped with sequences previously determined for the E. fetida nephridial symbiont.
FIG. 6.
Phylogenetic relationships among earthworm symbionts, E. fetida capsule bacteria, and other members of the genus Acidovorax. The maximum-likelihood topology was determined from sequences aligned with the ARB software package (www.arb-home.de) and analyzed with PAUP V. 4.0beta 10 (30). Bootstrap values (1,000× sampling) are indicated at the nodes. Unsupported branch patterns have been collapsed. C. testeroni was used as the outgroup. The bracket indicates the earthworm nephridial bacterium cluster.
DISCUSSION
The data presented here provide evidence that there is direct transfer of earthworm nephridial bacteria from the adults to the egg capsules and that there is a critical period of colonization within the egg capsule. Acidovorax cells (LSB 145 probe positive) comprised the majority of the cells in egg capsules; these cells occurred in dense clouds and aggregates throughout the albumin but were sparse in the mating gel sheath and shed mucus. Although the LSB 145 probe could not distinguish between the symbiotic species and closely related Acidovorax spp. possibly present in the bedding, the high density of Acidovorax cells in the egg capsules examined shortly after formation could not be accounted for by contamination from bedding. Additionally, Acidovorax cells were not detected in samples from cured worms, verifying that the Acidovorax detected in capsules from colonized worms originated from the nephridia. The high density of symbiotic Acidovorax cells observed in the egg capsule by FISH was supported by the abundance of egg capsule-derived 16S rRNA gene clone sequences that were more than 99% identical to E. fetida nephridial sequences, confirming that there was direct deposition of nephridial bacteria into egg capsules. The recovery of two distinct sequence variants from both the nephridia and the egg capsules suggested that there is either variation among rRNA operon sequences or some strain variation. The level of 16S rRNA gene sequence variability within a given species of earthworm symbiont has not been characterized yet. Taken together, these findings indicate that there is an active process that is specific to egg capsule formation by which bacteria are deposited into the egg albumin.
Bacteria distinct from Acidovorax were also present in the egg capsules of both cured and colonized worms, as determined by FISH and examination of clone libraries (Fig. 3 and data not shown), suggesting that bacteria from the environment are incorporated during capsule formation. This is not surprising, since the capsule girdles the worm during formation and then slides off the anterior end (Fig. 2). Although the source of these other cell types remains to be confirmed, clearly the embryo develops in the presence of multiple bacterial populations, some of which may have specific roles in the life history of the earthworm. For example, other investigators have reported the presence of antibacterial factors in the egg capsule of E. fetida (5, 8, 32) that might be of host or bacterial origin and protect the embryos from bacterial overgrowth. Because we examined freshly laid capsules, we do not know whether the numbers of Acidovorax and/or other bacterial species in the capsule change over the course of development of the embryos. Previous direct cell counting of egg capsule contents suggested that the numbers do not change significantly over the course of development (34), but this remains to be investigated further.
Our studies also indicate that there is a developmental period for specific colonization of the nephridia by Acidovorax present in the egg capsule and that colonization by Acidovorax, or other bacteria, does not readily occur after hatching. The nephridia of hatchlings from colonized capsules were fully colonized upon emergence, indicating that they were inoculated while they were in the capsule sometime during embryo development. Although acquisition of bacteria from the soil offers an alternative route of inoculation, evidence does not support this. The nephridia of aposymbiotic hatchlings, juveniles, and adults remained free of bacteria in all experiments, even when the animals were exposed to colonized worms for up to 3 months. These treatments included a set of aposymbiotic capsules that had not been exposed to antibiotics; thus, interference by antibiotics could not explain the lack of inoculation. Mixed colonization states after antibiotic treatments persisted over a 3-month period, establishing that juveniles hatching together and growing in close contact did not cross-inoculate each other, nor did the nephridia cross-infect within the same worm. Since the matched control worms growing under the same conditions in all treatments retained their bacteria, the patterns of nephridial colonization within a single worm likely did not result from secondary loss. Although the few fully colonized worms in the hatchling exposure treatment experiments (4 of 50 worms found in two of five bins) suggested that low-level infection after hatching is possible, it is also possible that a few hatchling worms were incidentally transferred with the bedding from the colonized adult bins at the beginning of the experiment.
In conclusion, our data imply that there is a developmental stage of the earthworm embryo (and/or a specific physiological state of the bacterial symbiont) within the egg capsule that ensures specific colonization. The specific developmental timing and the details of the colonization events remain to be characterized. Following hatching, acquisition of the symbiont or other bacteria from a free-living stage is difficult or does not occur, suggesting that general protective mechanisms that prevent infection after hatching are activated. Together, our observations revealed what appear to be highly evolved mechanisms, combining active deposition of the symbiont in the egg capsule and selective colonization during development, to accomplish direct (vertical) transfer of bacterial symbionts from parents to offspring.
Acknowledgments
We thank Andreas Schramm for helpful discussions and Frederick An for technical assistance.
This work was funded by grant GM20177 from NIH (to S.K.D.) and by NSF grant IBN-0345049 (to D.A.S. and S.K.D.).
REFERENCES
- 1.Alves, A., O. Santos, I. Henriques, and A. Correia. 2002. Evaluation of methods for molecular typing and identification of members of the genus Brevibacterium and other related species. FEMS Microbiol. Lett. 213:205-211. [DOI] [PubMed] [Google Scholar]
- 2.Amann, R. I., L. Krumholz, and D. A. Stahl. 1990. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 172:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, P., N. A. Moran, and L. Baumann. 2000. Bacteriocyte-associated endosymbionts of insects. In M. Dworkin (ed.), The prokaryotes, a handbook on the biology of bacteria: ecophysiology, isolation, identification, applications, 3rd ed. [Online.] Springer-Verlag, New York, N.Y. http://141.150.157.117:8080/prokPUB/chaprender/jsp/showchap.jsp?chapnum=243.
- 4.Bright, M., and O. Giere. 2005. Microbial symbiosis in Annelida. Symbiosis 38:1-45. [Google Scholar]
- 5.Brown, B. A., and M. J. Mitchell. 1981. Role of the earthworm, Eisenia fetida, in affecting survival of Salmonella enteritidis ser. Typhimurium. Pedobiologia 22:434-438. [Google Scholar]
- 6.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience Publishers, John Wiley & Sons, New York, N.Y.
- 7.Daane, L. L., J. A. E. Molina, and M. J. Sadowsky. 1998. Scanning electron microscopy of the microflora in egg capsules of the earthworm Eisenia fetida. Pedobiologia 42:79-87. [Google Scholar]
- 8.Day, G. M. 1950. The influence of earthworms on soil microorganisms. Soil Sci. 69:175-184. [Google Scholar]
- 9.Di Meo, C. A., A. E. Wilbur, W. E. Holben, R. A. Feldman, R. C. Vrijenhoek, and S. C. Cary. 2000. Genetic variation among endosymbionts of widely distributed vestimentiferan tubeworms. Appl. Environ. Microbiol. 66:651-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doube, B. M., and G. G. Brown. 2004. Life in a complex community: functional interactions between earthworms, organic matter, microorganisms, and plants, p. 213-240. In C. A. Edwards (ed.), Earthworm ecology, 2nd ed. St. Lucie Press/CRC Press, Boca Raton, Fla.
- 11.Edwards, C. A. (ed.). 2004. Earthworm ecology, 2nd ed. St. Lucie Press/CRC Press, Boca Raton, Fla.
- 12.Edwards, C. A., and P. J. Bohlen (ed.). 1996. Biology and ecology of earthworms, 3rd ed. Chapman and Hall, London, United Kingdom.
- 13.Egert, M., S. Marhan, B. Wagner, S. Scheu, and M. W. Friedrich. 2004. Molecular profiling of 16S rRNA genes reveals diet-related differences of microbial communities in soil, gut, and casts of Lumbricus terrestris L. (Oligochaeta: Lumbricidae). FEMS Microbiol. Ecol. 48:187-197. [DOI] [PubMed] [Google Scholar]
- 14.Furlong, M. A., D. R. Singleton, D. C. Coleman, and W. B. Whitman. 2002. Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl. Environ. Microbiol. 68:1265-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giere, O., and C. Langheld. 1987. Structural organization, transfer and biological fate of endosymbiotic bacteria in gutless oligochaetes. Mar. Biol. 93:641-650. [Google Scholar]
- 16.Horn, M. A., A. Schramm, and H. L. Drake. 2003. The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms. Appl. Environ Microbiol. 69:1662-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihssen, J., M. A. Horn, C. Matthies, A. Gößner, A. Schramm, and H. L. Drake. 2003. N2O-producing microorganisms in the gut of the earthworm Aporrectodea caliginosa are indicative of ingested soil bacteria. Appl. Environ Microbiol. 69:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knop, J. 1926. Bakterien und Bakteroiden bei Oligochäten. Z. Morphol. Oekol Tiere 6:588-624. [Google Scholar]
- 19.Lane, D. S. 1990. 16S and 23S rRNA sequencing, p. 115-148. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley, New York, N.Y.
- 20.Laverack, M. S. 1963. The physiology of earthworms. Pergamon Press Ltd., New York, N.Y.
- 21.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K. H. Schleifer. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 22.McFall-Ngai, M. J., and E. G. Ruby. 1998. Sepiolids and vibrios: When first they meet. Bioscience 48:257-265. [Google Scholar]
- 23.McMullin, E., S. Hourdez, S. W. Schaeffer, and C. R. Fisher. 2003. Phylogeny and biogeography of deep-sea vestimentiferans and their bacteril symbionts. Symbiosis 34:1-41. [Google Scholar]
- 24.Muyzer, G., A. Teske, C. O. Wirsen, and H. W. Jannasch. 1995. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch. Microbiol. 164:165-172. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill, S., A. A. Hoffman, and J. H. Werren (ed.). 1997. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom.
- 26.Pernthaler, J., F. O. Glöckner, W. Schönhuber, and R. Amann. 2001. Fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. Methods Microbiol. 30:207-226. [Google Scholar]
- 27.Schönholzer, F., D. Hahn, B. Zarda, and J. Zeyer. 2002. Automated image analysis and in situ hybridization as tools to study bacterial populations in food resources, gut and cast of Lumbricus terrestris L. J. Microbiol. Methods 48:53-68. [DOI] [PubMed] [Google Scholar]
- 28.Schramm, A., S. K. Davidson, J. A. Dodsworth, H. L. Drake, D. A. Stahl, and N. Dubilier. 2003. Acidovorax-like symbionts in the nephridia of earthworms. Environ. Microbiol. 5:804-809. [DOI] [PubMed] [Google Scholar]
- 29.Schweitzer, B., I. Huber, R. Amann, W. Ludwig, and M. Simon. 2001. α- and β-Proteobacteria control the consumption and release of amino acids on lake snow aggregates. Appl. Environ. Microbiol. 67:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swofford, D. L. 2003. PAUP*, phylogenetic analysis using parsimony (*and other methods) version 4.0b 10. Sinauer Associates. Sunderland, Mass.
- 31.Toyota, K., and M. Kimura. 2000. Microbial community indigenous to the earthworm Eisenia foetida. Biol. Fertil. Soils 31:187-190. [Google Scholar]
- 32.Valembois, P., P. Roch, and M. Lassegues. 1986. Antibacterial molecules in annelids, p. 74-93. In M. Brehelin (ed.), Immunity in invertebrates. Springer-Verlag, Berlin, Germany.
- 33.Wheeler Alm, E., D. B. Oerther, N. Larsen, D. A. Stahl, and L. Raskin. 1996. The oligonucleotide probe database. Appl. Environ. Microbiol. 62:3557-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zachmann, J. E., and J. A. E. Molina. 1993. Presence of culturable bacteria in cocoons of the earthworm Eisenia fetida. Appl. Environ. Microbiol. 59:1904-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]