Abstract
Epitheliocystis in leafy seadragon (Phycodurus eques), silver perch (Bidyanus bidyanus), and barramundi (Lates calcarifer), previously associated with chlamydial bacterial infection using ultrastructural analysis, was further investigated by using molecular and immunocytochemical methods. Morphologically, all three species showed epitheliocystis cysts in the gills, and barramundi also showed lymphocystis cysts in the skin. From gill cysts of all three species and from skin cysts of barramundi 16S rRNA gene fragments were amplified by PCR and sequenced, which clustered by phylogenetic analysis together with other chlamydia-like organisms in the order Chlamydiales in a lineage separate from the family Chlamydiaceae. By using in situ RNA hybridization, 16S rRNA Chlamydiales-specific sequences were detected in gill cysts of silver perch and in gill and skin cysts of barramundi. By applying immunocytochemistry, chlamydial antigens (lipopolysaccharide and/or membrane protein) were detected in gill cysts of leafy seadragon and in gill and skin cysts of barramundi, but not in gill cysts of silver perch. In conclusion, this is the first time epitheliocystis agents of leafy seadragon, silver perch and barramundi have been undoubtedly identified as belonging to bacteria of the order Chlamydiales by molecular methods. In addition, the results suggested that lymphocystis cysts, known to be caused by iridovirus infection, could be coinfected with the epitheliocystis agent.
Epitheliocystis is an infection of the gills and skin of many fish species. Sometimes it can be grossly visible as cyst-like lesions, and sometimes the cysts can be seen microscopically in gill squashes, but often the only way to detect it is through histology. Epitheliocystis has been reported worldwide, both from freshwater and marine species (12). This condition is usually benign; however, sometimes it can be associated with a high mortality, particularly in cultured fish (3, 4, 26). Due to swelling of the cells of the gills and the increase in mucus around heavily infected gills, fish can become lethargic and show respiratory distress. The causative agent of epitheliocystis replicates intracellularly in the cysts and, since 1969 epitheliocystis has been associated with chlamydia-like bacteria based on the ultrastructural characteristics of the content of the cysts (4). Attempts to identify the causative agent by using monoclonal antibodies have not been successful, and their results are often inconsistent.
In 1999 we discovered many new chlamydia-like sequences by using a universal Chlamydiales 16S rRNA gene PCR (30). Because of the unconfirmed ultrastructural association of epitheliocystis with chlamydia-like organisms we started investigation of archived epitheliocystis material of leafy seadragon (Phycodurus eques) and silver perch (Bidyanus bidyanus) and a new case of epitheliocystis in barramundi (Lates calcarifer) using this Chlamydiales-specific PCR. Ultrastructural analysis of epitheliocystis in these fish species was described previously, in leafy seadragon by Langdon et al. (19), in silver perch by Frances et al. (10), and in barramundi by Anderson and Prior (2). After we communicated our first preliminary positive findings during the Tenth International Symposium on Human Chlamydial Infections in Antalya, Turkey, in 2002 (25), Draghi et al. (6) undoubtedly identified a chlamydia-like bacterium as the cause of epitheliocystis in farmed Atlantic salmon (Salmo salar) using DNA sequence analysis and in situ hybridization (ISH). They proposed the name “Candidatus Piscichlamydia salmonis” for this bacterium.
We describe here the characterization of the epitheliocystis agents of leafy seadragon, silver perch, and barramundi by molecular and immunocytochemical methods.
(Preliminary results were presented at the Tenth International Symposium on Human Chlamydial Infections in Antalya, Turkey, in 2002.)
MATERIALS AND METHODS
Specimens.
A case of epitheliocystis in leafy seadragon (Phycodurus eques) that was previously reported (19) was further analyzed by applying molecular methods on archived paraffin-embedded gills from 1991. The material originated from a captive leafy seadragon, originally from the Esperance area, Australia, that died after a Vibrio septicemia. At necropsy, epitheliocystis of the gills was observed, and by electron microscopy chlamydia-like, as well as rickettsia-like, stages were observed in the cysts (19).
Also, a historical case of epitheliocystis in silver perch (Bidyanus bidyanus) was further analyzed by applying molecular methods on archived paraffin-embedded gills from 1998. This case was from 150 fingerlings submitted by a commercial hatchery in eastern Australia to Fisheries WA, Australia, for batch disease certification prior to export.
Analysis for the presence of Chlamydiales antigens and 16S RNA in the archived paraffin-embedded gills of both fish species were carried out in 1999 at the National Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
In 1999, a case of epitheliocystis occurred in juvenile (50 mm) barramundi (Lates calcarifer), held in a recirculating freshwater system in Perth, Western Australia. This was a mixed infection with epitheliocystis in the gills and lymphocystis in the skin (known to be caused by iridovirus, which induces hypertrophy in infected fibroblast cells [9]). The fish had an unrelated buoyancy problem, which resulted in their submission for examination at Fisheries WA, Australia. Water quality was poor with pH 6.8 and detectable levels of ammonia (0.1 mg/liter). From several affected fish, paraffin-embedded affected gills and skin, frozen ground gill and skin tissue in tissue culture medium without antibiotics, and frozen whole fish were sent to the RIVM for molecular analysis.
16S rRNA gene amplification and sequencing.
Throughout the study all possible measures were taken to avoid false-positive results and to assure the validity of the resulting 16S rRNA gene sequences, in accordance with published guidelines (28, 37). To limit introduction of nucleotide substitutions by PCR amplification, the number of amplification reactions used before cycle sequencing was carried out was kept to a minimum.
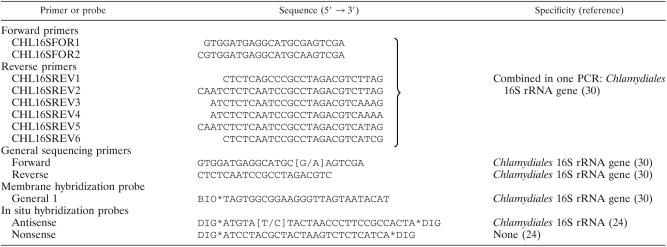
DNA was isolated from paraffin sections of gills of leafy seadragon and silver perch and from fresh homogenates of skin and gill cysts of barramundi by using the Easy-DNA kit (Invitrogen) with additional silica purification of the DNA (22). Amplification of the Chlamydiales signature sequence (7), blotting and membrane hybridization to identify specifically the Chlamydiales 16S rRNA gene, and direct cycle sequencing of both DNA strands of the amplification product were performed with primers and probes (Table 1) as described previously (30). Since PCR reagents are occasionally contaminated with Chlamydiales DNA (25), each DNA specimen was analyzed by PCR in two reactions with different pretreatment of the reagent mixture. For one PCR the reagent mixture was treated prior to addition of specimen DNA by UV illumination at 312 nm for 2.5 min to destroy possible contaminating Chlamydiales DNA in the reagents. Since UV treatment reduces the sensitivity of the PCR ∼10-fold, a second PCR was performed with a reagent mixture that was not UV treated prior to addition of specimen DNA. Only when both PCRs were positive the PCR product was considered to originate truly from the analyzed DNA specimen. When only the PCR with the untreated reagent mixture was positive the result was considered inconclusive and the analysis of the DNA specimen was repeated.
TABLE 1.
Oligonucleotide sequences used for primers and probes
Sequencing was carried out by using the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit (ABI) and the products were analyzed by using an ABI Prism 373 Sequencer (ABI) or by using an ABI Prism 3700 DNA Analyzer (ABI).
The sequences obtained were compared to sequences available in the GenBank database (National Center for Biotechnology Information) (1) to find the most similar sequences. The secondary 16S rRNA structure of each sequence was constructed by using RNAdraw version 1.1b2 (21) to check the validity of the sequences. The sequences were checked for chimera's using the “Check Chimera” option of the RDP-II (20) and mglobalCHI (17). The sequences were aligned manually using BioEdit version 5.0.9 (14), taking into account the secondary structure obtained from the SSU database available at the Antwerp rRNA Database (University of Antwerp, Antwerp, Belgium) (38). The Chlamydiales 16S rRNA signature sequences of all type strains and of all Chlamydia-like strains and molecular clones available in April 2005 at GenBank were included in the alignment. This alignment was used for similarity analysis and input in phylogenetic analysis. Phylogenetic analysis was carried out by the Jukes and Cantor method to calculate a distance matrix, followed by the neighbor-joining (NJ) method to infer a phylogenetic tree by using MEGA2 version 2.1 (18). With a selection of sequences, the tree topology was tested by maximum-likelihood and maximum-parsimony methods, and a consensus tree was drawn. Maximum-likelihood analysis was carried out by using TREE-PUZZLE version 5.2 (33). Maximum-parsimony analysis (8) was carried out by using MEGA2 (18).
Histology, ICC, and ISH.
Paraffin sections (4 μm) of HEp2 cells infected with Chlamydophila pneumoniae were used as positive controls in immunocytochemistry (ICC) and ISH as described previously (24). Of each paraffin-embedded fish tissue, 4-μm sections were stained with hematoxylin and eosin (HE) for histological examination. Consecutive 4-μm paraffin sections were used to detect chlamydial antigens by ICC using an indirect immunoperoxidase method as described previously (23). The broadly reactive Chlamydiaceae family specific anti-lipopolysaccharide (LPS) monoclonal antibodies CF-2 (Washington Research Foundation [WRF], Seattle, WA) (35) and 2.5F10 (23) and, in addition, the more specific Chlamydophila pneumoniae anti-membrane protein monoclonal antibody RR-402 (WRF) (31) were used. Irrelevant primary monoclonal antibodies of the same isotype were used as negative control antibodies.
Detection of Chlamydiales 16S rRNA by RNA ISH in 4-μm paraffin sections adjacent to those stained with ICC was carried out as described previously (24) with an antisense 3′ and 5′ DIG-labeled oligonucleotide probe specific for the order Chlamydiales 16S rRNA (Table 1), including recently discovered Chlamydia-like sequences (30). A nonsense oligonucleotide probe composed of the same nucleotides as the antisense probe but in a different sequence was used as a negative control probe (Table 1).
Nucleotide sequence accession numbers.
The partial 16S rRNA gene sequences of the epitheliocystis agents of silver perch, leafy seadragon, and barramundi are available at GenBank under accession numbers AY013394, AY013396, and AY013474, respectively.
RESULTS
Identification of novel chlamydial sequences from epitheliocystis cysts.
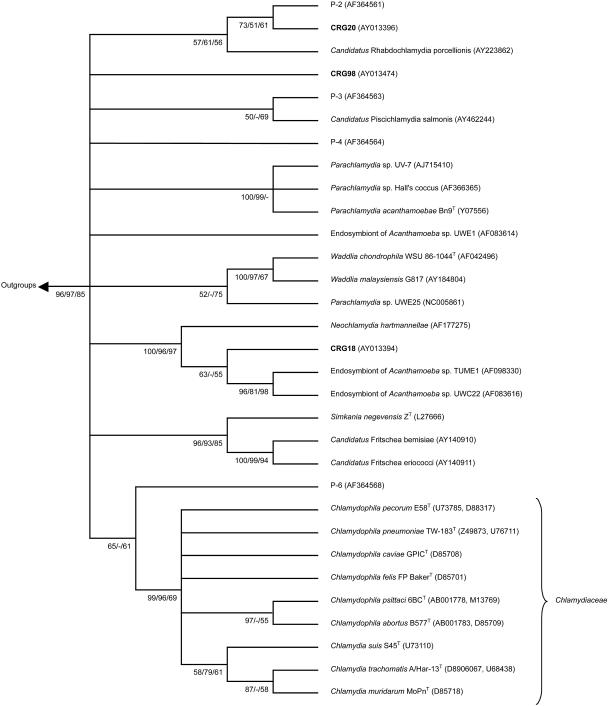
The Chlamydiales specific PCR was positive in at least two independent DNA preparations from gills from all three fish species and, surprisingly, in DNA prepared from lymphocystis skin cysts of barramundi. For barramundi, gill and skin specimens from the two analyzed fishes were positive. For each fish species a 16S rRNA sequence was identified, which appeared to be genuine after construction of their secondary structure and analysis for chimeras (not shown). The PCR products showed highest homology with the 16S rRNA signature sequence of chlamydia-like bacteria within the order Chlamydiales. The novel epitheliocystis agent 16S rRNA signature sequences were assigned the codes CRG20 for the epitheliocystis agent from leafy seadragon, CRG18 for that from silver perch, and CRG98 for that from barramundi (Table 2). Phylogenetic analysis showed that the epitheliocystis agents of all three species of fish clustered with Chlamydia-like bacteria distinct from the Chlamydiaceae (Fig. 1). Similarity analysis of carefully aligned sequences showed that CRG20 had highest similarity values (85.8 to 86.2%) with other CRG sequences isolated from human artery, water storage, and human aqueous humor (CRG numbers 12, 62, 65, and 84) (25). However, these values were low compared to those of CRG18 and CRG 98 with other chlamydia-like sequences (maximum values of 93.4 and 99.5%, respectively). Although CRG20 clustered with the P2 sequence isolated from activated sludge (15) and “Candidatus Rhabdochlamydia porcellionis” by phylogenetic analysis (Fig. 1), the similarity values of 82.3 and 77.3%, respectively, were lower than the maximum values with the CRG sequences (85.8 to 86.2%). CRG18 showed highest similarity values of 97.2 to 99.5% with CRG sequences isolated from human blood, artery and cervical smear, pig artery, and reagent controls (CRG numbers 8, 9, 19, 27, 28, 30, 70, and 71) (25) and of 97.1 to 97.6% with sequences of cultured endosymbionts Tume1 and UWC22 of Acanthamoeba sp. and Neochlamydia hartmannellae and sequences isolated from feline ocular disease (GenBank clone W258 AY225594 and clone W13 AY225593). CRG98 showed the highest similarity values of 92.0 to 93.4% with CRG sequences isolated from human throat swabs (25). By phylogenetic analysis, CRG18 clustered also with sequences isolated from koala eye and urogenital tract swabs (5). However, similarity values of CRG18 with these koala sequences (83.9 to 87.1%) were lower than the maximum values with the CRG sequences (92.0 to 93.4%). CRG98 did not cluster with any of the (provisionally) classified chlamydia-like bacteria (Fig. 1).
TABLE 2.
Results of detection of Chlamydiales bacteria in gill cysts of leafy seadragon (Phycodurus eques) and silver perch (Bidyanus bidyanus) and in gill and skin cysts of barramundi (Lates calcarifer)a
| Source | ICC with monoclonal antibodies to Chlamydiaceae LPS | ICC with monoclonal antibody to C. pneumoniae membrane protein | RNA ISH with Chlamydiales 16S rRNA-specific oligoprobe | Partial 16S rRNA sequence code (GenBank accession no.) |
|---|---|---|---|---|
| Leafy seadragon | ± or − | ND | − | CRG20 (AY013396) |
| Silver perch | − | ND | +++ | CRG18 (AY013394) |
| Barramundi | ||||
| Small gill cysts | ++ | ++ | ++++ | CRG98 (AY013474) |
| Larger gill cysts | + or − | + or − | +++ | CRG98 (AY013474) |
| Skin cysts | ± | − | ± (some dots) | CRG98 (AY013474) |
Extent of staining: ND, not done; −, none; ±, very weak; +, weak; ++, moderate; +++, intense; ++++, very intense.
FIG. 1.
Phylogenetic relationships among Chlamydiales. The tree was inferred by using maximum-likelihood (ML), neighbor joining (NJ), and maximum-parsimony (MP) analysis of a 210- to 223-bp region of the 16S rRNA Chlamydiales signature sequence. The unrooted consensus tree topology is shown. Numbers indicate the percentage of times each branch appeared in a tree during 1,000 bootstrap samples (NJ and MP) or 10,000 puzzling steps (ML) in the order (NJ/MP/ML). Multifurcations connect branches for which the relative order could not unambiguously be determined. Branches supported by a bootstrap or puzzling reliability value of ≥50% with at least two treeing methods are shown. The GenBank accession numbers are given in parentheses. Included in the inference of the phylogenetic tree were (i) sequences of all type strains of classified Chlamydiales (T), (ii) all sequences of cultured chlamydia-like organisms (endosymbionts of Acanthamoeba sp. strains UWE1, TUME1, and UWC22, Parachlamydia sp. strain UWE25, Parachlamydia sp. strain UV-7, and Parachlamydia sp. strain Hall's coccus; endosymbionts of Hartmanella vermiformis and Neochlamydia hartmannellae; “Candidatus Rhabdochlamydia porcellionis”; Waddlia malaysiensis G817; “Candidatus Fritschea bemisiae”; and “Candidatus Fritschea eriococci”), (iii) cloned sequences from activated sludge that are representatives of four new environmental chlamydial lineages suggested by Horn and Wagner (15) (P-2, P-3, P-4, and P-6), (iv) cloned sequence of “Candidatus Piscichlamydia salmonis,” and (v) outgroup species Escherichia coli (GenBank accession number AE000460) and Rickettsia prowazekii (GenBank accession number M21789).
In situ evidence for chlamydia-like organisms associated with epitheliocystis.
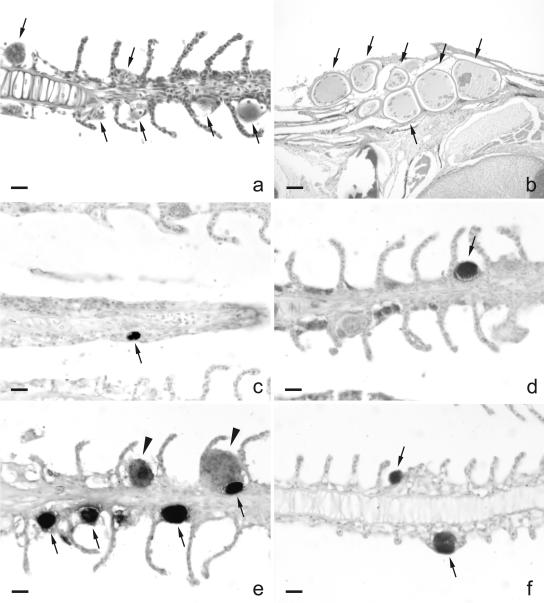
HE-stained sections of the gills of all three species showed round to oval basophilic inclusions in cells at the base of secondary lamellae (barramundi, see Fig. 2a [other species are not shown]). In silver perch these inclusions were sometimes also present in the tip of the primary lamellae. In silver perch, but not in leafy seadragon or barramundi, a proliferative response in tissue surrounding the cysts was occasionally seen that destroyed the structure of the secondary lamellae (Fig. 2f). Gill cysts were filled with fine granular material. In barramundi two types of gill cysts were seen: large cysts with loosely packed granular material and small cysts with densely packed granular material. The morphology of the skin cysts of barramundi caused by lymphocystis differed from epitheliocystis cysts in gills in that one or more cysts together were firmly encapsulated and the cysts were almost completely filled with basophilic granular and amorphous material. The sizes of these round cysts ranged from 100 to 300 μm and were much larger than the sizes of epitheliocystis cysts in the gills of barramundi, which varied from 13 to 35 μm (Fig. 2).
FIG. 2.
Detection of Chlamydiales bacteria in epitheliocystis of gills of barramundi (Lates calcarifer) and silver perch (Bidyanus bidyanus) by ICC and in situ RNA hybridization. (a) HE staining of gills of barramundi showing epitheliocystis cysts (arrows). (b) HE staining of lymphocystis cysts (arrows) in the skin of barramundi, clearly different in size and morphology from the cysts in the gills. (c and d) Some cysts in gills of barramundi stained clearly with antibodies raised against a membrane protein of Chlamydophila pneumoniae (c; arrow) and with antibodies raised against the lipopolysaccharide of Chlamydia trachomatis (d; arrow). (e and f) Abundant staining of cysts in gills of barramundi (e; arrows and arrowheads) and silver perch (f; arrows) by RNA ISH with a Chlamydiales-specific 16S rRNA oligonucleotide probe. Larger cysts in the gills of barramundi (e; arrowheads) stained less intensely than the smaller cysts (e; arrows). Bars: a and c to f, 10 μm; b, 100 μm.
The results of the in situ detection methods are summarized in Table 2. The presence of Chlamydia-like sequences in the DNA prepared from the gill specimens of silver perch and of gill and skin specimens of barramundi could be linked to the cysts in the gills of silver perch (Fig. 2f) and cysts in the gills of barramundi (Fig. 2e) by using in situ RNA hybridization with a Chlamydiales 16S rRNA specific oligoprobe. Cysts of leafy seadragon were not determined to be positive by RNA ISH. Further evidence for the association of the epitheliocystis agent with Chlamydiales bacteria came from the staining of cysts in the gills of leafy seadragon (not shown) and of the cysts in gills of barramundi by cross-reaction of antigens of the epitheliocystis agents with monoclonal antibodies raised against Chlamydia trachomatis lipopolysaccharide (Fig. 2d) and Chlamydophila pneumoniae membrane protein (Fig. 2c). Gill cysts of silver perch did not stain with these monoclonal antibodies. The larger cysts in the gills of barramundi stained less intensely than the smaller cysts using ICC and RNA ISH (Fig. 2e). The lymphocystis cysts in the skin of barramundi stained, although faintly, for chlamydial antigen using ICC, and some dots stained for chlamydial RNA using ISH (not shown), in line with the detection of the epitheliocystis agent by PCR in DNA preparations of these cysts.
DISCUSSION
Using phylogenetic analysis of products from a Chlamydiales-specific 16S rRNA gene PCR, ISH using a Chlamydiales 16S rRNA-specific probe, and ICC using monoclonal antibodies raised against Chlamydia trachomatis and Chlamydophila pneumoniae, we identified the epitheliocystis agents of leafy seadragon (Phycodurus eques), silver perch (Bidyanus bidyanus), and barramundi (Lates calcarifer) as bacteria belonging to the order Chlamydiales in a lineage separate from bacteria belonging to the family Chlamydiaceae.
The epitheliocystis cysts we found in the gills of all three species were similar in dimension as previously reported in these (10, 19) and other (13, 26, 29) species. A proliferative response was only found in silver perch as described previously in another case from this species (10), and it was similar to that in Atlantic salmon (6).
The detection of Chlamydiales specific 16S rRNA by rRNA ISH in cysts of silver perch and barramundi together with the determination of novel Chlamydiales 16S rRNA sequences from DNA extracts of affected tissue is evidence that the epitheliocystis in these fish was caused by Chlamydiales bacteria, in line with the molecular revision of Koch's postulates by Fredericks and Relman (11). In contrast, the cysts of leafy seadragon did not stain for Chlamydiales specific 16S rRNA by RNA ISH. A plausible explanation is that the RNA was degraded in the 8-year-old paraffin-embedded specimen. Studies by Risio et al. (32) and Tan et al. (36) showed a dramatic decrease in FISH signal with increasing storage time of paraffin-embedded tissue up to 10 years. Although DNA is much more stable than RNA, the ISH method with oligoprobes we used was unfortunately not sensitive enough to detect chlamydia bacteria by ISH with genomic DNA (24). However, by positive ICC for Chlamydiaceae LPS we were able to associate the amplified chlamydial 16S RNA gene fragment to cysts in the gills of leafy seadragon.
The positive ICC results, although variable, in two fish species can be explained by cross-reactivity of the used antibodies with the antigens of epitheliocystis bacteria. Previously, this has also been shown for different species of fish (6, 13). However, the results are not consistent, often reported negative (4) and depend on the antibodies used and probably on the stage of maturation of the bacteria. Our results indicate that the family-specific LPS might be present in other members of the order Chlamydiales. Even epitopes considered to be species specific for Chlamydophila pneumoniae might be present in other members of the order Chlamydiales. However, these results need confirmation in other laboratories. The negative results from monoclonal antibody staining of gill cysts in silver perch were consistent with previous reports for epitheliocystis in this species (10). However, previous staining of leafy sea dragon cysts with other monoclonal antibodies gave negative results (19), in contrast to our results.
Unfortunately, we could not confirm our data by isolation of the agent. Although various cell lines and procedures were used, isolation attempts remained negative (data not shown).
Remarkably, cysts in gills and skin of barramundi were, despite their different origin and large difference in morphology, associated with the same bacterium by PCR analysis and confirmed by ISH and ICC. This suggests that the lymphocystis cysts, known to be caused by iridovirus infection, were coinfected with epitheliocystis bacteria. The potential for mixed infections with different Chlamydiaceae strains and a porcine epidemic diarrhea virus was shown in vitro with Vero cells (African green monkey kidney cells); however, the dual infections appeared to be occasional and incidental events (34).
With increasing size of the epitheliocystis cysts of barramundi (small and large cysts in gills) a decreasing intensity of staining by ISH and ICC was observed. This corresponded with the densely packed appearance of the small cysts and loosely packed appearance of the large cysts. This could be consistent with different stages of maturation of the bacteria in the cysts. The larger the cysts the more end stages (elementary bodies) of bacteria in the cysts have been observed. Together with a reduced amount of 16S rRNA in elementary bodies (27), this may explain the reduction in ISH signal with increasing size of the cysts. Antigens may lose reactivity with antibodies that recognize early stages in the maturation of LPS or proteins (16), which may partly explain the reduction in ICC signal with increasing size of the cysts.
Despite large differences in the ultrastructure of epitheliocystis agents compared to bacteria in the Chlamydiaceae family (4, 6), in four species of fish these agents have now been identified by molecular analysis as belonging to the order Chlamydiales. Thus far, each agent has been different, suggesting species specificity of the pathogens. Draghi et al. (6) proposed the name “Candidatus Piscichlamydia salmonis” for the epitheliocystis agent of salmon. The name piscichlamydia and the association with epitheliocystis suggests that there is a universal genus specific for chlamydia bacteria that infect fish. However, the phylogenetic analysis of the 16S rRNA signature sequences of the three novel epitheliocystis agents showed them to be clearly separate from “Candidatus Piscichlamydia salmonis” and from each other, suggesting that no such universal genus exist.
In conclusion, in line with the molecular revision of Koch's postulates by Fredericks and Relman (11), our results provided evidence that epitheliocystis of leafy seadragon, silver perch, and barramundi is caused by bacteria belonging to the order Chlamydiales but separate from the family Chlamydiaceae.
Acknowledgments
We thank Siska Gielis for expert technical assistance.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, I. G., and H. C. Prior. 1992. Subclinical epitheliocystis in barramundi, Lates calcarifer, reared in sea cages. Aust. Vet. J. 69:226-227. [DOI] [PubMed] [Google Scholar]
- 3.Bradley, T. M., C. E. Newcomer, and K. O. Maxwell. 1988. Epitheliocystis associated with massive mortalities of cultured lake trout Salvelinus namaycush. Dis. Aquat. Org. 4:9-17. [Google Scholar]
- 4.Crespo, S., C. Zarza, F. Padros, and M. Marin de Mateo. 1999. Epitheliocystis agents in sea bream Sparus aurata: morphological evidence for two distinct chlamydia-like developmental cycles. Dis. Aquat. Org. 37:61-72. [DOI] [PubMed] [Google Scholar]
- 5.Devereaux, L. N., A. Polkinghorne, A. Meijer, and P. Timms. 2003. Molecular evidence for novel chlamydial infections in the koala (Phascolarctos cinereus). Syst. Appl. Microbiol. 26:245-253. [DOI] [PubMed] [Google Scholar]
- 6.Draghi, A., II, V. L. Popov, M. M. Kahl, J. B. Stanton, C. C. Brown, G. J. Tsongalis, A. B. West, and S. Frasca, Jr. 2004. Characterization of “Candidatus piscichlamydia salmonis” (order Chlamydiales), a chlamydia-like bacterium associated with epitheliocystis in farmed Atlantic salmon (Salmo salar). J. Clin. Microbiol. 42:5286-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett, K. D., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 8.Fitch, W. M. 1971. Towards defining the course of evolution: minimum change for a specific tree topology. Syst. Zool. 20:406-416. [Google Scholar]
- 9.Flugel, R. M. 1985. Lymphocystis disease virus. Curr. Top. Microbiol. Immunol. 116:133-150. [DOI] [PubMed] [Google Scholar]
- 10.Frances, J., R. Tennent, and B. F. Nowak. 1997. Epitheliocystis in silver perch, Bidyanus bidyanus (Mitchell). J. Fish. Dis. 20:453-457. [Google Scholar]
- 11.Fredericks, D. N., and D. A. Relman. 1996. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin. Microbiol. Rev. 9:18-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fryer, J. L., and C. N. Lannan. 1994. Rickettsial and chlamydial infections of freshwater and marine fishes, bivalves and crustaceans. Zool. Stud. 33:95-107. [Google Scholar]
- 13.Groff, J. M., S. E. LaPatra, R. J. Munn, M. L. Anderson, and B. I. Osburn. 1996. Epitheliocystis infection in cultured white sturgeon (Acipenser transmontanus): antigenic and ultrastructural similarities of the causative agent to the chlamydiae. J. Vet. Diagn. Investig. 8:172-180. [DOI] [PubMed] [Google Scholar]
- 14.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 15.Horn, M., and M. Wagner. 2001. Evidence for additional genus-level diversity of Chlamydiales in the environment. FEMS Microbiol. Lett. 204:71-74. [DOI] [PubMed] [Google Scholar]
- 16.Kannenberg, E. L., S. Perotto, V. Bianciotto, E. A. Rathbun, and N. J. Brewin. 1994. Lipopolysaccharide epitope expression of Rhizobium bacteroids as revealed by in situ immunolabeling of pea root nodule sections. J. Bacteriol. 176:2021-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komatsoulis, G. A., and M. S. Waterman. 1997. A new computational method for detection of chimeric 16S rRNA artifacts generated by PCR amplification from mixed bacterial populations. Appl. Environ. Microbiol. 63:2338-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 19.Langdon, J. S., K. Elliott, and B. MacKay. 1991. Epitheliocystis in the leafy sea-dragon. Aust. Vet. J. 68:244. [DOI] [PubMed] [Google Scholar]
- 20.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, J. M. Stredwick, G. M. Garrity, B. Li, G. J. Olsen, S. Pramanik, T. M. Schmidt, and J. M. Tiedje. 2000. The RDP (Ribosomal Database Project) continues. Nucleic Acids Res. 28:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matzura, O., and A. Wennborg. 1996. RNAdraw: an integrated program for RNA secondary structure calculation and analysis under 32-bit Microsoft Windows. Comput. Appl. Biosci. 12:247-249. [DOI] [PubMed] [Google Scholar]
- 22.Meijer, A., J. A. van der Vliet, L. M. Schouls, A. de Vries, P. J. M. Roholl, and J. M. Ossewaarde. 1998. Detection of microorganisms in vessel wall specimens of the abdominal aorta: development of a PCR assay in the absence of a gold standard. Res. Microbiol. 149:577-583. [DOI] [PubMed] [Google Scholar]
- 23.Meijer, A., J. A. van der Vliet, P. J. M. Roholl, S. K. Gielis-Proper, A. de Vries, and J. M. Ossewaarde. 1999. Chlamydia pneumoniae in abdominal aortic aneurysms: abundance of membrane components in the absence of heat shock protein 60 and DNA. Arterioscler. Thromb. Vasc. Biol. 19:2680-2686. [DOI] [PubMed] [Google Scholar]
- 24.Meijer, A., P. J. M. Roholl, S. K. Gielis-Proper, Y. F. Meulenberg, and J. M. Ossewaarde. 2000. Chlamydia pneumoniae in vitro and in vivo: a critical evaluation of in situ detection methods. J. Clin. Pathol. 53:904-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijer, A., and J. M. Ossewaarde. 2002. Description of a wider diversity within the order Chlamydiales than currently classified, p. 13-16. In J. Schachter, J., G. Christiansen, I. N. Clarke, M. R. Hammerschlag, B. Kaltenboeck, C.-C. Kuo, R. G. Rank, G. L. Ridgway, P. Saikku, W. E. Stamm, R. S. Stephens, J. T. Summersgill, P. Timms, and P. B. Wyrick (ed.), Chlamydial infections: proceedings of the Tenth International Symposium on Human Chlamydial Infections. International Chlamydia Symposium, San Francisco, Calif.
- 26.Miyazaki, T., Y. Fujimaki, and K. Katai. 1986. A light and electron microscopic study on epitheliocystis disease in cultured fish. Bull. Jpn. Soc. Sci. Fish. 52:199-202. [Google Scholar]
- 27.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumaier, M., A. Braun, and C. Wagener. 1998. Fundamentals of quality assessment of molecular amplification methods in clinical diagnostics. Clin. Chem. 44:12-26. [PubMed] [Google Scholar]
- 29.Nowak, B. F., and A. Clark. 1999. Prevalence of epitheliocystis in Atlantic salmon, Salmo salar L., farmed in Tasmania, Australia. J. Fish Dis. 22:73-78. [Google Scholar]
- 30.Ossewaarde, J. M., and A. Meijer. 1999. Molecular evidence for the existence of additional members of the order Chlamydiales. Microbiology 145:411-417. [DOI] [PubMed] [Google Scholar]
- 31.Puolakkainen, M., J. Parker, C.-C. Kuo, J. T. Grayston, and L. A. Campbell. 1995. Further characterization of Chlamydia pneumoniae specific monoclonal antibodies. Microbiol. Immunol. 39:551-554. [DOI] [PubMed] [Google Scholar]
- 32.Risio, M., G. De Rosa, I. Sarotto, L. Casorzo, L. Capussotti, B. Torchio, M. Aglietta, and L. Chiecchio. 2003. HER2 testing in gastric cancer: molecular morphology and storage time-related changes in archival samples. Int. J. Oncol. 23:1381-1387. [PubMed] [Google Scholar]
- 33.Schmidt, H. A., K. Strimmer, M. Vingron, and A. von Haeseler. 2002. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18:502-504. [DOI] [PubMed] [Google Scholar]
- 34.Stuedli, A., P. Grest, I. Schiller, and A. Pospischil. 2005. Mixed infection in vitro with different Chlamydiaceae strains and a cell culture adapted porcine epidemic diarrhea virus. Vet. Microbiol. 106:209-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson, A. F., and C.-C. Kuo. 1991. Evidence that the major outer membrane protein of Chlamydia trachomatis is glycosylated. Infect. Immun. 59:2120-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan, L. H., E. Do, S. M. Chong, and E. S. Koay. 2003. Detection of ALK gene rearrangements in formalin-fixed, paraffin-embedded tissue using a fluorescence in situ hybridization (FISH) probe: a search for optimum conditions of tissue archiving and preparation for FISH. Mol. Diagn. 7:27-33. [DOI] [PubMed] [Google Scholar]
- 37.von Wintzingerode, F., U. B. Göbel, and E. Stackebrandt. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21:213-229. [DOI] [PubMed] [Google Scholar]
- 38.Wuyts, J., Y. Van de Peer, T. Winkelmans, and R. De Wachter. 2002. The European database on small subunit rRNA. Nucleic Acids Res. 30:183-185. [DOI] [PMC free article] [PubMed] [Google Scholar]