Abstract
Wolbachia bacteria are common intracellular symbionts of arthropods and have been extensively studied in Drosophila. Most research focuses on two Old Word hosts, Drosophila melanogaster and Drosophila simulans, and does not take into account that some of the Wolbachia associations in these species may have evolved only after their fast global expansion and after the exposure to Wolbachia of previously isolated habitats. Here we looked at Wolbachia of Neotropical Drosophila species. Seventy-one lines of 16 Neotropical Drosophila species sampled in different regions and at different time points were analyzed. Wolbachia is absent in lines of Drosophila willistoni collected before the 1970s, but more recent samples are infected with a strain designated wWil. Wolbachia is absent in all other species of the willistoni group. Polymorphic wWil-related strains were detected in some saltans group species, with D. septentriosaltans being coinfected with at least four variants. Based on wsp and ftsZ sequence data, wWil of D. willistoni is identical to wAu, a strain isolated from D. simulans, but can be discriminated when using a polymorphic minisatellite marker. In contrast to wAu, which infects both germ line and somatic tissues of D. simulans, wWil is found exclusively in the primordial germ line cells of D. willistoni embryos. We report on a pool of closely related Wolbachia strains in Neotropical Drosophila species as a potential source for the wAu strain in D. simulans. Possible evolutionary scenarios reconstructing the infection history of wAu-like Wolbachia in Neotropical Drosophila species and the Old World species D. simulans are discussed.
Wolbachia strains are intracellular gram-negative, vertically transmitted Alphaproteobacteria that infect at least 20% of all insects (24, 47). In Drosophila, Wolbachia infections are capable of inducing cytoplasmic incompatibility (CI) or male killing (34). The CI phenotype increases the fitness of Wolbachia-infected females relative to uninfected females and drives Wolbachia through host populations. In recent years scientific interest has broadly focused on the evolutionary and functional interactions between Wolbachia and genetic model systems such as D. melanogaster and D. simulans, two well-studied Old World species belonging to the melanogaster group (42). In D. melanogaster, a single infection variant, wMel (50), had been described until not long ago (36). This infection is associated with variable levels of CI in its natural host. In D. simulans, five Wolbachia variants have been described: wRi, wHa, and wNo, which can induce CI, and wMa and wAu, which generally do not (29). Strains wMel of D. melanogaster and wAu of D. simulans are closely related in respect to the most sensitive molecular gene marker sets of wsp (50) and ftsZ (9, 35). There is a complete lack of wsp sequence polymorphism within wMel (36) and wAu (2, 23), which suggests either a strict clonality of the parasite or a recent acquisition by their host species. The phylogenetic relationship of these two Wolbachia strains has previously been analyzed (see, e.g., references 9, 21, and 50); however, the evolutionary origins of both the wAu and wMel associations remain unclear, including a possible recent acquisition from other host species after the global expansion of both Old World Drosophila species.
In contrast to the well-studied Wolbachia associations in D. melanogaster and D. simulans, little is known about the occurrence of Wolbachia among American Neotropical Drosophila strains comprising two groups of species, the saltans group and the willistoni group (Fig. 1). There are presently two conflicting reports about the occurrence of Wolbachia in Neotropical Drosophila: Bourtzis et al. (4) screened a broad range of Drosophila species derived from various labs and from the Drosophila Species Stock Center (DSSC) in Bowling Green, Ohio (now held at the University of Arizona, Tucson). In their survey only two species out of the 41 stocks comprising 30 species were infected with Wolbachia. Interestingly, none of the analyzed DSSC fly lines was infected. The six Neotropical Drosophila species surveyed, including D. willistoni, D. prosaltans, and D. sturtevanti, were uninfected (4). The Neotropical samples surveyed originated from iso- or oligofemale lines kept at the DSSC since the 1950s. In contrast, Werren et al. (46) reported that a natural population of D. willistoni collected in the early 1990s in Panama was infected with Wolbachia. Its presence in D. willistoni was recently confirmed by discovering partial fragments of a Wolbachia genome in the Trace Archive of the D. willistoni genome sequencing project (37; J. Brownlie, personal communication). The genome sequence was derived from an isofemale line collected in the early 1990s in Guadeloupe (L. Ehrman, personal communication).
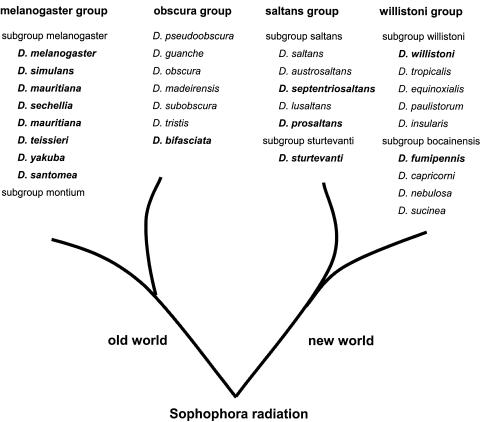
FIG. 1.
Phylogenetic relationship of the Sophophora radiation (41). The Wolbachia infection statuses of the Drosophila species shown were deduced from data published previously (melanogaster and obscura groups) and from this study (saltans and willistoni groups). In boldface are species that have been found to be infected with various Wolbachia strains based on wsp sequences (see Materials and Methods).
Here we reevaluated the Wolbachia infection status of Neotropical Drosophila species by conducting a large-scale survey. Seventy-one lines of 16 Neotropical Drosophila species belonging to the willistoni and saltans groups were searched for Wolbachia. We compared the occurrence of infection in old versus recent population samples of different geographic origins and increased replicate numbers of analyzed lines per species, as analysis of only one or a few iso- or oligofemale lines would not detect low infection frequencies in species. Different diagnostic tools such as Wolbachia-specific wsp and ftsZ PCR, Southern blot hybridizations, and immunological diagnostic methods were applied for this purpose.
MATERIALS AND METHODS
Drosophila strains.
Fly samples were kindly provided by colleagues Margaret Kidwell, Egon Bartel, Kim van der Linde, Francesco Ayala, Jeff Powell, and Peter Chabora and by the DSSC, Tucson, Ariz. (For details about geographic origin, collector's name, and date of collection, see Tables 1 to 3.) All strains were kept on standard fly food in vials at a constant temperature of 21°C.
TABLE 1.
Distribution of Wolbachia in natural populations and stocks of D. willistoni
| Region and fly line | Location; sourcea | Collection yr | PCRb | Southern blottingc |
|---|---|---|---|---|
| American continental | ||||
| Pan 02 | Panama City, Panama; KL | 2002 | + | + |
| Lag | Laguna Negra, Rocha, Uruguay; LB | 2000 | + | + |
| Apa 5.1 | Veracruz, Mexico; JS | 1998 | + | + |
| Apa 8.2 | Veracruz, Mexico; JS | 1998 | + | + |
| Pan 98 | Panama; EB | 1998 | + | + |
| JS 6.3 | Jaton Sacha near Tena, Ecuador; PO | 1997 | + | + |
| JS 1 | Jaton Sacha near Tena, Ecuador; PO | 1997 | + | + |
| Para 3 | Belem, Pará, Brazil; MM | 1997 | + | + |
| Para 4 | Belem, Pará, Brazil; MM | 1997 | + | + |
| RIP | Ribeirao Preto, Sao Paulo, Brazil; CR | 1995 | + | NDd |
| Pan 92 | BCI, Panama; EB | 1992 | + | ND |
| Manaus | Manaus, Brazil; MM | 1986 | − | − |
| wilB6 | Belize; FA | 1974 | + | + |
| wilC | Costa Rica; FA | 1971 | − | − |
| SP | Sao Pedro, Rio Grande do Sul, Brazil | 1965 | − | ND |
| WIP4 | Ipitanga, Bahia, Brazil; HW and AC | 1961 | − | − |
| 14030-0811.6 | Fairchild Gardens, FL; WH | 1959 | − | − |
| 14030-0811.1 | San Salvador, El Salvador; WH | 1955 | − | − |
| 14030-0811.0 | San Maria d'Ostuna, Nicaragua; WH | 1954 | − | − |
| 14030-0811.3 | Atlixco, Veracruz, Mexico; WH | 1947 | − | − |
| 14030-0811.2 | Royal Palm Park, FL; WH | 1941 | − | − |
| Caribbean | ||||
| wilG1-FWI | Basse Terre, Guadeloupe; PC | 2000 | + | + |
| LAntilles 6 | St. Vincent and Grenadines; HH | 1997 | + | + |
| LAntilles 3 | Grand Etang, Grenada; HH | 1997 | − | − |
| LAntilles 4 | St. Vincent and Grenadines; HH | 1997 | − | − |
| LAntilles 1 | Toro Negro, Puerto Rico; HH | 1994 | + | + |
| wilG2 | Guana Island, Virgin Islands; PC | 1991 | + | + |
| wilG1 | Basse Terre, Guadeloupe; PC | 1991 | + | + |
| wilH | Grande-Terre, Guadeloupe; PC | 1991 | − | − |
Collectors: CR, C. Rohde; EB, E. Bartel; FA, F. Ayala; HH, H. Hollocher; HW, H. Winge; KL, K. van der Linde; LB, L. Basso da Silva; MM, M. Martins; PC, P. Chabora; PO, P. O'Grady; WH, W. Heed.
Results obtained per line on individual flies from independent genomic PCRs with ftsZ and wsp primer sets (n = 6 adult females per line).
Results derived from genomic single-fly Southern blot hybridizations probed with the wsp fragment (n = 5 adult females per line).
ND, not determined.
TABLE 3.
Distribution of Wolbachia in the saltans group
| Species | Fly line, location/sourcea | Collection yr | PCRb |
|---|---|---|---|
| saltans subgroup | |||
| D. saltans | PNM; Panama City, Panama; KL | 2002 | − |
| PLR; Gamboa, Panama; KL | 2002 | − | |
| FS; Colon, Panama; KL | 2002 | − | |
| BCI; Panama; KL | 1998 | − | |
| D. austrosaltans | 14030-0771.0; Pirassununga, Brazil | 1959 | − |
| D. lusaltans | 14045-0891.0; Petionville, Haiti | NDc | − |
| D. septentriosaltans | PLR; Gamboa, Panama; KL | 2002 | + |
| PNM; Panama City, Panama; KL | 2002 | + | |
| FS; Colon, Panama; KL | 2002 | + | |
| BCI; Panama; EB | 1998 | + | |
| D. subsaltans | 14044-0872.0; Balem, Brazil | 1959 | − |
| D. prosaltans | SG; Summit Gardens, Panama; EB | 1998 | + |
| 14045-0901.3; Balboa, Panama | 1958 | − | |
| sturtevanti subgroup | |||
| D. sturtevanti | PNM; Panama City, Panama; KL | 2002 | − |
| PLR; Gamboa, Panama; KL | 2002 | − | |
| Barb 1; Turner's Hall, Barbados; HH | 1999 | + | |
| Barb 2; Turner's Hall, Barbados; HH | 1999 | − | |
| Pan 6; Panama; TM | 1999 | + | |
| Pan 12; Panama; TM | 1999 | − | |
| MI; Maria Eugenia, Panama; EB | 1998 | + | |
| SG; Summit Gardens, Panama; EB | 1998 | + |
Collectors: EB, E. Bartel; HH, H. Hollocher; KL, K. van der Linde; TM, T. Markow.
Results obtained per line on individual flies from independent genomic PCRs with ftsZ and wsp primer sets (n = 6 adult females per line).
ND, not determined.
PCR diagnostics, cloning, sequencing, and strain typing.
Genomic DNAs derived from single adult female flies were extracted according to the single-fly PCR protocol (14), and the quality of fly genomic DNAs was tested by control PCR experiments carried out with primers binding to conserved segments in exon 2 and exon 3 of the Adh gene (15). Wolbachia-specific PCRs were performed as previously described (24). In brief, 2 μl of the 50-μl single-fly sample was added to 20 μl of PCR mix (0.75 U Taq DNA polymerase [Promega] in 1× reaction buffer, 0.10 μM of each primer, and 75 μM of each deoxynucleoside triphosphate). PCR primer sets were used as described previously (24). The Wolbachia infection of D. willistoni was discriminated from wAu infection of D. simulans by the hypervariable VNTR-141 locus in wMel (primer set VNTR-141F-R), isolated by Riegler et al. (36). At least two independent PCRs were analyzed per sample. PCR fragments of the expected size were gel eluted, cloned into the pGEM-T Easy vector, and transformed into JM109 (Promega). Both strands of each clone were sequenced by GENterprise GmbH, Mainz, Germany. Wolbachia strain names were assigned to wsp sequence variants deriving from different hosts according to current standards (34, 50). This is important in order to keep the ecological origin of the Wolbachia symbiosis transparent. The highly polymorphic wsp gene undergoes homologous recombination among strains, which is problematic for an evolutionary analysis of the symbiosis (1). Therefore, we used a multilocus approach, including wsp and ftsZ genes as well as the VNTR-141 locus.
Phylogenetic analysis.
Multiple wsp sequence alignments, including the hypervariable regions (bases 217 to 252 and 520 to 582), were generated using the Clustal X program (40). Alignments were based on amino acid translations followed by manual modifications. A base substitution was included in the analysis if it occurred in two or more plasmid clones obtained from independent PCRs. Other substitutions were eliminated. The final alignment is available at ftp://ftp.ebi.ac.uk/pub/databases/embl/align/under accession number ALIGN_000917. Phylogenetic trees were constructed by applying PAUP* (39) in the absence of an available outgroup. Neighbor-joining analyses after midpoint rooting and unweighted-pair group method with arithmetic mean analyses yielded similar phylogenies, supporting the close relationship of wAu-like Wolbachia variants.
Single-fly Southern hybridization.
DNA extraction from individual 10-day-old female flies, restriction digestion with HindIII, vertical agarose gel separation, and membrane blotting were performed according to the protocol described by Junakovic (26). Nylon membranes were probed with the eluted wsp PCR fragment of wWil derived from the D. willistoni strain Pan 02 (Table 1) cloned into the pGEM-T Easy vector.
Semiquantitative genomic wsp PCRs.
The density of Wolbachia in D. willistoni was determined by semiquantitative wsp PCRs on 10 individual adult females of staged ages. After gel separation and SYBR Green I staining (Roche), the emission intensities of the obtained wsp fragments were determined and compared to wsp signal intensities derived from individual D. simulans (from a Coffs Harbor line) infected with wAu and D. simulans (from a Riverside line) infected with wRi.
Immunological studies.
Wolbachia density and tissue tropism of wWil in D. willistoni were determined using the polyclonal Wolbachia surface protein (WSP) antibody (11). WSP protein expression was analyzed via Western blotting of protein extracts derived from individual adult flies in independent replicates as well as whole-mount immunostainings on adult tissues and staged embryos (44). Rabbit anti-wsp antibody was used at a 1:500 dilution overnight at 4°C and detected after incubation with a 1:500 dilution of Alexa Fluor 488 goat anti-rabbit immunoglobulin G-labeled secondary antibody (Molecular Probes) at room temperature for 1 h. The total number of primordial germ line cells (PGCs) in stage 10 and later embryos of D. willistoni was determined using the pole cell-specific polyclonal rabbit anti-VASA antibody at a dilution of 1:1,000. Slides were stained for 3 min with 1 μg/ml DAPI (4′,6′-diamidino-2-phenylindole) (Molecular Probes), rinsed, stained with 5 μg/ml propidium iodide (Molecular Probes) for 20 min, rinsed again, and mounted with ProLong antifade medium (Molecular Probes).
Fluorescence microscopy.
Immunostainings of embryos and ovaries were examined by using a Zeiss Axiomot 2 Epifluorescence microscope. Images were processed using Photoshop 6.0 (Adobe).
Nucleotide sequence accession numbers.
The wsp sequence data derived from Neotropical Wolbachia strains were deposited in GenBank under accession numbers AY620207 to AY620229 and DQ118779, as well as AY858801 for the respective sequence from D. ananassae collected in 2002 in Sao Tome. Sequences of the diagnostic VNTR-141 loci of D. simulans (Coffs Harbor) and D. willistoni were deposited in GenBank under accession numbers DQ118777 and DQ118778, respectively.
RESULTS
Isolation of Wolbachia from D. willistoni.
Twenty-one continental American and eight Caribbean lines of D. willistoni were screened for Wolbachia by using wsp PCR and single-fly Southern hybridization. Based on both molecular methods, 12 continental and 5 Caribbean lines tested positive. All five lines originating from the DSSC as well as most lines derived from collections before the 1980s were devoid of Wolbachia (Table 1). The five DSSC-derived fly lines collected in Central America and Florida in the 1940s and 1950s and the Brazilian and Costa Rican lines collected in the 1960s and 1970s lack Wolbachia (Table 1 and Fig. 2A). The oldest sample of D. willistoni infected with Wolbachia originates from a line of flies collected in Belize in 1974 (sample wilB6). The second-oldest infected line was collected in Panama in 1992. While the Brazilian line “Manaus” originating from a collection in 1986 is uninfected, all continental lines, ranging from Mexico to Uruguay, collected in the 1990s and later harbor Wolbachia infections (Table 1). Whereas older continental lines are devoid of Wolbachia, more recent samples are universally infected. Caribbean samples of D. willistoni show a more heterogeneous infection pattern. For example, recent collections from Grenada and St. Vincent (line L'Antilles 4) in 1997 are not infected. A line collected from Grand Terre, Guadeloupe (wilH), in 1991 is uninfected, whereas another one collected on the neighboring island Basse Terre (wilG1) in the same year is infected with Wolbachia (Table 1).
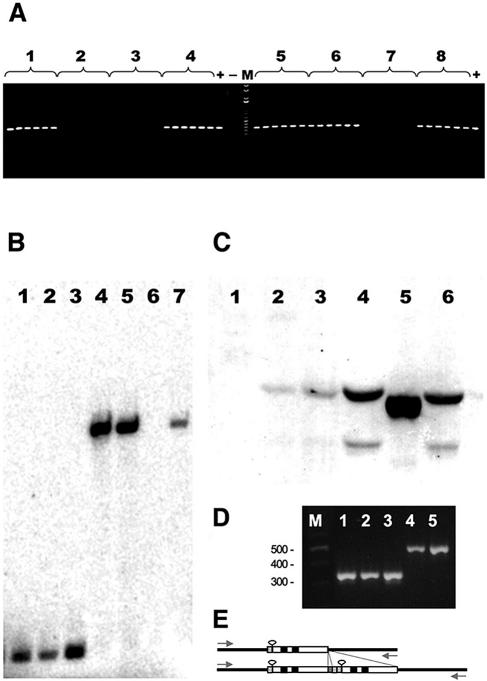
FIG. 2.
Intra- and interspecific distributions of Wolbachia in Neotropical Drosophila species. (A) Single-fly wsp PCR on eight strains of D. willistoni collected at different American locations in different years (see Table 1). For each D. willistoni strain tested, PCRs were performed separately on six individual 2-day-old female flies. Lines are as follows: 1, Pan 02; 2, wilC; 3, wilH; 4, wilB6; 5, Apa 5.1; 6, Para 4; 7, WIP4; 8, wilG1. (B) Genomic single-fly Southern blot hybridization probed with the wsp plasmid of wWil on individual 10-day-old females of D. melanogaster/wMel CS (lane 1), D. melanogaster/wMel ywc67 (lane 2), D. simulans/wRi (lane 3), D. simulans/wAu (lanes 4 and 5), D. willistoni/wWil treated with tetracycline (lane 6), and D. willistoni/wWil strain Pan 02 (lane 7). (C) Western immunoblotting using the anti-WSP antibody (1:1,000) on single-fly protein extracts derived from D. willistoni-T, the tetracycline-treated control line of JS 6.3 (lane 1), D. willistoni/wWil (lane 2), D. septentriosaltans/wSpt (lane 3), D. simulans/wAu (lane 4), D. simulans/wRi (lane 5), and D. melanogaster/wMel (lane 6). (D) VNTR-141 specific PCR on D. willistoni/wWil Pan 02 (lane 1), D. willistoni/wWil JS6.3 (lane 2), D. willistoni/wWil Para 4 (lane 3), D. simulans/wAu Coffs Harbor (lane 4), and D. simulans/wAu Yaounde 6 (lane 5). (E) Schematic comparison between the VNTR-141 loci (34) of wWil (top) and wAu (bottom). The basic unit is composed of a 15-bp repeat (stippled), a 23-bp hairpin (loop), an 18-bp insertion (hatched), and a 15-bp repeat (black). The size difference is caused by a 141-bp duplication in VNTR-141 of wAu.
Multiple wsp PCRs on individual flies from lines of D. willistoni confirmed the complete absence of Wolbachia in uninfected lines. Within infected fly lines, each individual tested was positive for Wolbachia (Fig. 2A and data not shown). These 100% infection frequencies suggest a close-to-complete vertical transmission efficiency of Wolbachia in D. willistoni hosts. This is corroborated by our observations that flies from naturally Wolbachia-infected populations of D. willistoni kept in our lab maintained a stable 100% infection frequency in the 3 years since collection.
Molecular characterization of the D. willistoni-specific Wolbachia strain wWil.
We sequenced fragments of two genes, wsp and ftsZ, from 12 Wolbachia-infected lines covering continental and Caribbean populations of D. willistoni in order to characterize the molecular structure and phylogenetic relationship of this Wolbachia association with other Wolbachia variants. Until recently these two diagnostic marker genes were regarded as the most informative for molecular Wolbachia variant classification (34). All isolated Wolbachia clones of D. willistoni were identical in their sequence. Below we refer to the strain as wWil. With respect to the wsp sequence of wWil obtained from the 12 infected lines (accession numbers AY620218 to AY620229, no sequence polymorphism could be detected. Moreover, all wsp and ftsZ sequences of D. willistoni were 100% identical to the respective wsp and ftsZ genes (accession numbers AF020067 and AY227739) of the Wolbachia variant wAu. As deduced from comparative Southern blots (Fig. 2B), the close relationship between wAu and wWil is corroborated by the conservation of the two HindIII restriction sites flanking the wsp locus.
In contrast to the identity of wWil and wAu at the wsp and ftsZ sequence level, comparative genomic single-fly Southern blots (Fig. 2B) and semiquantitative PCRs (data not shown) of infected individuals of D. willistoni and D. simulans showed clear quantitative differences. Strong signals comparable to those of wRi were obtained from wAu-infected D. simulans adults, and the intensity of wWil in similar-sized D. willistoni clearly showed a 70% reduction compared to that of wAu (Fig. 2B, lanes 4, 5, and 7). This quantitative effect was also detected at the WSP protein expression level by Western blots derived from single-fly protein extracts with the polyclonal anti-wsp antibody (Fig. 2C, lanes 2 and 4). The WSP proteins of wAu and wWil have the same molecular weight, whereas, for example, the homologues of two other Wolbachia variants that infect D. melanogaster and D. simulans (wMel and wRi, respectively) differ significantly (Fig. 2C, lanes 5 and 6).
In contrast to the wsp and ftsZ sequence identity between wWil and wAu, we were able to discriminate both strains at the genomic level by applying the recently isolated polymorphic marker VNTR-141 (36). This diagnostic marker covers the noncoding polymorphic VNTR-141 locus in wMel (positions 89003 to 90332 in the wMel chromosome). By performing VNTR-141-specific PCRs (Fig. 2D), we have obtained a 528-bp fragment from wAu (accession number DQ11877) and a 387-bp fragment from wWil (accession number DQ118778). The length difference is caused by a 141-bp duplication in wAu that is not present in wWil (Fig. 2E). Hence, wWil is closely related but not identical to wAu of D. simulans.
Extreme pole cell tropism of wWil in D. willistoni embryos.
Whole-mount immunostainings were performed on early embryos and ovaries of both fly species, using the anti-WSP antibody. In early embryos of D. simulans, wAu bacteria were detected in somatic and germ line tissues during all stages of embryonic development (Fig. 3A). Nuclei of earlier blastodermal stages were infected with Wolbachia, with some significant enrichment in the posterior pole cell region in both D. simulans and D. willistoni. Such posterior accumulations of wAu in D. simulans blastodermal embryos were reported recently (44). In contrast to this, during stages 9 and 10 of embryonic development of D. willistoni, wWil specifically targets the germ line (Fig. 3B). In early gastrulating embryos, shortly after pole cell invagination, somatic tissues of D. simulans were heavily infected by wAu (Fig. 3C). At this developmental stage, wWil bacteria in D. willistoni are selectively targeting a small number of primordial germ line cells, whereas somatic tissues are devoid of bacteria (Fig. 3D). Later, during stages 12 to 14, in the course of germ band retraction, only one lateral pair of five or six PGC nuclei was infected by wWil (Fig. 3E to H). Control immunostainings with the Drosophila germ line-specific VASA antibody (28) showed that, in contrast to D. melanogaster, the Neotropical species D. willistoni harbors a reduced number of PGCs which perfectly colocalize with wWil (data not shown). Based on the tight temporal and spatial association between the host-encoded VASA protein and WSP-expressing wWil, we assume that this intracellular parasite possesses a molecular association with the host-expressed, pole cell-specific vasa RNA or with its encoded protein.
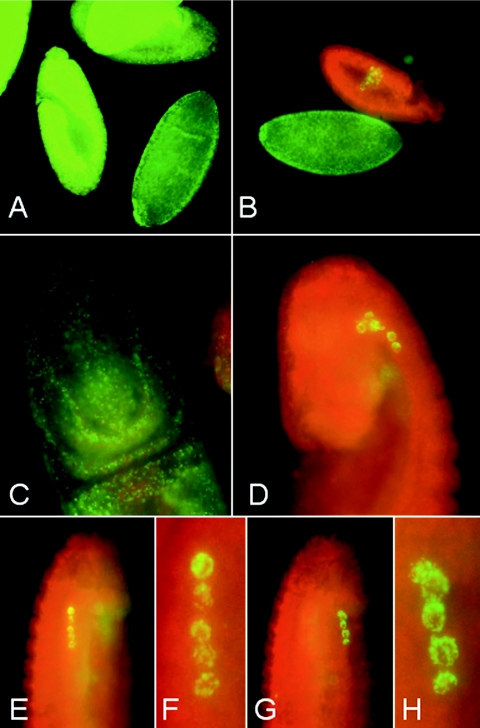
FIG. 3.
Distribution of Wolbachia in Drosophila embryos. (A and B) Whole-mount immunostainings with rabbit anti-WSP antibody (green) on early-stage embryos of D. simulans infected with wAu (A) and D. willistoni JS 6.3 infected with wWil (B). (C and D) Stage 9 to 10 embryos of D. simulans (C) and D. willistoni (D) are shown in detail. Whereas wAu in D. simulans uniformly infects both somatic and germ line cells, wWil selectively targets a very limited number of primordial germ cells. In D. willistoni, wWil is not detectable at the immunological level in somatic cells at embryonic stage 9 or later. (E to H) Lateral views of a stage 12 embryo of D. willistoni infected with wWil (E and G) and their enhanced magnifications show a row of five heavily infected primordial germ cell nuclei on both lateral sides of the embryo (F and H).
Natural polymorphism of wAu-like Wolbachia in other Neotropical Drosophila species.
We have expanded our survey into species of the willistoni group in order to search for a potential origin of the wWil detected in recent collections of D. willistoni. Besides D. willistoni, 21 fly lines derived from eight species of this group, covering both the willistoni and bocainensis subgroups (Fig. 1), were screened for the presence of Wolbachia by using the wsp primer set. With the exception of D. fumipennis, a strain kept at the DSSC since 1958, all willistoni group species sampled were negative for wsp and ftsZ PCR (Table 2). On the basis of its wsp sequence, the infection in D. fumipennis (wFum; accession number AY620207) shows only a distant relationship to wWil (Fig. 4), similar to the A subgroup Wolbachia infection of Pegoscapus longiceps (accession number AF521161).
TABLE 2.
Distribution of Wolbachia in the willistoni group
| Species | Fly line, location, sourcea | Collection yr | PCRb |
|---|---|---|---|
| willistoni subgroup | |||
| D. tropicalis | PNM; Panama City, Panama; KL | 2002 | − |
| Panama; JS | 1998 | − | |
| BCI; Panama City, Panama; EB | 1997 | − | |
| D. insularis | St. Kitts, St. Lucia; HH | NDc | − |
| D. equinoxialis | Apazapan, Veracruz, Mexico; JS | 1998 | − |
| Gigante, Panama; EB | 1997 | − | |
| PLR; Gamboa, Panama; KL | 2002 | − | |
| FS; Colon, Panama; KL | 2002 | − | |
| D. paulistorum | JS 5.2; Jaton Sacha, Tena, Ecuador; PO | 1997 | − |
| Interior; LE | 1970 | − | |
| Central americas; LE | 1959 | − | |
| 14030-0771.6; San Salvador, El Salvador | 1955 | − | |
| 14030-0771.2; Mesitas, Mexico; LE | ND | − | |
| A28; LE | ND | − | |
| bocainensis subgroup | |||
| D. capricorni | 14030-0721.1; Canal Zone, Panama | 1961 | − |
| D. sucinea | Xalapa Botanical Gardens; Mexico, JS | 1998 | − |
| 14030-791.0; Medellin, Colombia | 1958 | − | |
| D. nebulosa | Apazapan, Veracruz, Mexico; JS | 1998 | − |
| 14030-0761.0; Palmira, Columbia | ND | − | |
| 14030-0761.1; San Jose, Costa Rica | ND | − | |
| D. fumipennis | 14030-0751.1; Arima Valley, Trinidad | 1958 | + |
Collectors: EB, E. Bartel; HH, H. Hollocher; JS, J. Silva; KL, K. van der Linde; LE, L. Ehrman; PO, P. O'Grady.
Results obtained per line on individual flies from independent genomic PCRs with ftsZ and wsp primer sets (n = 6 adult females per line).
ND, not determined.
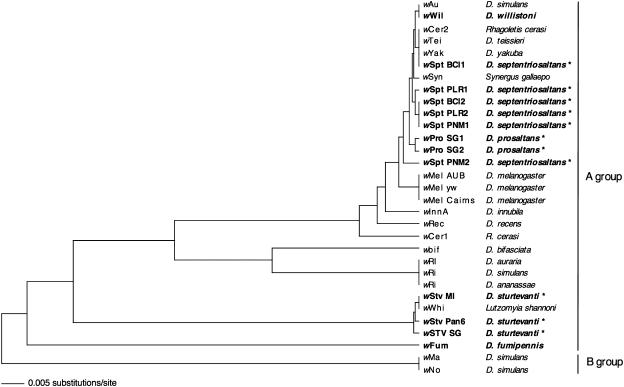
FIG. 4.
Unweighted-pair group method with arithmetic mean cladogram based on wsp sequence alignment, including the hypervariable region of the Wolbachia strains (50) derived from Neotropical Drosophila species (boldface) and from earlier reported host species (lightface). Host species found to harbor polymorphic Wolbachia variants are indicated by asterisks.
In contrast to the absence of wWil infections in the willistoni group, three out of the seven tested species belonging to the saltans group harbor Wolbachia (Table 3). The two saltans subgroup members D. septentriosaltans and D. prosaltans are infected with wWil-related Wolbachia strains, designated wSpt and wPro, respectively. The wsp sequence of the Wolbachia strain wPro SG1 (accession number AY620208) isolated from D. prosaltans shows 97.9% homology to wWil of D. willistoni and is almost identical (99.0%) to the wSpt PNM2 strain (AY622214) of D. septentriosaltans. Below we refer to these Neotropical strains wWil, wSpt, and wPro (Fig. 4) as wAu-like Wolbachia because of their close phylogenetic relationship with wAu of D. simulans.
Six wSpt wsp sequences were isolated from three different D. septentriosaltans lines collected in Panama between 1998 and 2002 (Table 3). At least four different wSPT subtypes can be distinguished according to their wsp sequences (Fig. 4): wSPT BCI1 (accession number AY620209) is identical to wCer2 (accession number AF418557) of the cherry fruit fly Rhagoletis cerasi (33) and to wTei (accession number AY291347) and wYak (accession number AY291348) of D. teissieri and D. yakuba, respectively (8). The variant wSpt PLR1 (accession number AY620211) clusters with wSpt PLR2 (accession number AY620212), BCI2 (accession number AY620210), and PNM1 (accession number AY620213). The latter three wsp clones are identical at the sequence level but stem from three different Panamanian D. septentriosaltans populations (Table 3). The fourth subtype, wSpt PNM2 (accession number AY620214), is the most divergent variant positioned between wMel (accession number AF020072) of D. melanogaster and the wAu-like Wolbachia clade (Table 4). All lines of D. septentriosaltans tested are multiply infected with wsp variants of wSpt. For example, individual flies from the PNM strain from Panama City harbor at least two different types of wsp sequences. Each wsp variant sequenced seems to be part of an intact open reading frame encoding a 196-amino-acid (aa) section of the WSP protein. The observed wsp sequence polymorphism of wSpt variants within D. septentriosaltans is manifested even at the protein level (Table 4). With respect to the WSP consensus sequence wBCL1 (accession number AY620209), two amino acid substitutions are found, i.e., in the sequence of wSpt PLR1, PLR2, BCI2, PNM1, and PNM2 at consensus position aa 24 (Tyr to His) and in the variant PLR1 (accession number AY620211) at position 126 (Asp to Gly).
TABLE 4.
Variable nucleotide and amino acid sites in the wsp sequence of the closely related wAu-like Wolbachia strains of Drosophila
| Strain | Nucleotide at variable position in wsp DNA consensusa
|
Strain | Amino acid at variable position in wsp amino acid sequence
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 68 | 70 | 258 | 263 | 333 | 340 | 363 | 377 | 426 | 520 | 529 | 536 | 538 | 23 | 24 | 88 | 114 | 126 | 174 | 177 | 179 | 180 | ||
| Consensus | C | T | T | G | T | G | A | A | T | G | A | T | A | Consensus | T | Y | G | A | D | D | R | V | T |
| wAu | A | wAu | T | ||||||||||||||||||||
| wWil | A | wWil | T | ||||||||||||||||||||
| wPro SG1 | G | C | A | wPro SG1 | S | H | E | ||||||||||||||||
| wPro SG2 | G | C | wPro SG2 | S | H | ||||||||||||||||||
| wSpt PLR1 | C | G | wSpt PLR1 | H | G | ||||||||||||||||||
| wSpt PLR2 | C | wSpt PLR2 | H | ||||||||||||||||||||
| wSpt BCI2 | C | wSpt BCI2 | H | ||||||||||||||||||||
| wSpt PNM1 | C | wSpt PNM1 | H | ||||||||||||||||||||
| wSpt PNM2 | C | C | C | G | wSpt PNM2 | H | |||||||||||||||||
| wSpt BCI1 | wSpt BCI1 | ||||||||||||||||||||||
| wTei | wTei | ||||||||||||||||||||||
| wYak | wYak | ||||||||||||||||||||||
| wCer2 | wCer2 | ||||||||||||||||||||||
| wMel | A | G | C | G | wMel | N | G | A | A | ||||||||||||||
Position 1 of the consensus sequence corresponds to position 164 in the wsp sequence of wAu of D. simulans (accession number AF020067).
At least two wsp variants of wPro were isolated from the D. prosaltans SG line from Panama. Both wPro variants share a host species diagnostic substitution at aa 23 (Thr to Ser), and wProSG1 has a substitution at aa 88 (Table 4).
wStv Wolbachia in D. sturtevanti.
Our survey yielded another new Wolbachia variant, wStv, which was isolated from D. sturtevanti, a member of the sturtevanti subgroup (Fig. 1). The distribution pattern of the wStv infection within its host species is patchy; e.g., wStv is present in the isofemale line Pan 6 (accession number AY620216) but is absent from Pan 12 (Table 3). As deduced from wsp sequence data wStv belongs to A-group Wolbachia but is distantly related to the wAu-like variants (Fig. 4). Three closely related but distinctive variants of wStv were isolated as singly occurring infections from three Panamanian populations (accession numbers AY620215, AY620216, and AY620217) (Fig. 4). Interestingly, the wsp sequence of wStv MI (accession number AY620215) collected in Maria Eugenia, Panama, is identical to that of wWhi (accession number AF237886) isolated from the phlebotomine sand fly Lutzomyia shannoni in Colombia (31). Those authors proposed, based on an extensive data set showing that other non-American populations of L. shannoni are free of Wolbachia, that L. shannoni probably acquired wWhi recently from another host in America.
DISCUSSION
wWil infection of D. willistoni.
Our survey shows that Neotropical Drosophila species belonging to the willistoni and saltans groups are infected with various A-group Wolbachia strains. In wsp and ftsZ sequence analysis, wWil of D. willistoni is identical to wAu of D. simulans. However, wWil can be discriminated from wAu by the VNTR-141 polymorphism and the strict pole cell tropism in its natural host. Hence, wWil is closely related but not identical to wAu of D. simulans. Our biogeographic analysis suggests that the infection is absent in D. willistoni stocks collected before the 1970s. Two alternative hypotheses may explain this result, i.e., a stochastic loss in the stocks or a recent invasion in the field. All five DSSC-derived D. willistoni samples tested negative for wWil (Table 1) and were kept under artificial lab conditions since the 1940s and 1960s. The DSSC collection was moved first from Texas to Ohio and then to Arizona. We cannot exclude the possibility that the Wolbachia infection was present in all lines but was then stochastically lost in independent lines in the course of their long-term stock maintenance due to stress factors, starvation, dramatic reduction of population size, or application of antibiotics. This hypothesis cannot completely be dismissed; however, we have three arguments against it: (i) wWil infections in D. willistoni lines were completely stable under our lab conditions for more than 3 years, (ii) the DSSC contains infected Drosophila lines originating from equally old collections (e.g., D. fumipennis), and (iii) overall ratios of infected versus uninfected D. melanogaster fly lines in several other stock centers stayed constant over the last 80 years (36). Hence, we are in favor of the hypothesis of recent spreading, for which we can add three supporting observations: (i) the lack of sequence variation of all available wWil markers obtained from our samples suggests clonality of the infection and recent acquisition by horizontal transfer from an external source; (ii) individual adult flies of two alcohol samples of D. willistoni (DSSC stock numbers 14030-0811.4 and 14030-0811.5; kindly provided by S. J. Castrezana, Drosophila Species Stock Center, Tucson, Ariz.) collected in Mexico in the 1950s were uninfected, whereas control PCRs with Adh-specific primer sets were successful; and (iii) the two old strains wilC and wilB6, collected by F. Ayala in Central America in the 1970s, are uninfected and infected, respectively (Table 1). Additional analysis of D. willistoni populations collected between the 1970s and 1990s would doubtlessly improve our data set, although these strains would be difficult or impossible to obtain.
The complete absence of wAu-like Wolbachia in the related willistoni group species tested (Table 2) supports the idea that D. willistoni was infected after its speciation. Since all recently collected continental samples of D. willistoni are infected with wWil, we assume that this Wolbachia strain reached fixation in continental populations of D. willistoni. wWil's pole cell tropism and its 100% transmission rate, seen in lab lines, were probably crucial factors. A recent Wolbachia-driven process should also be detected in the biogeographic distribution of mitochondrial variation, but this has not yet been looked at in the context of Wolbachia infections. A departure from an expected ratio of mitochondrial versus nuclear DNA polymorphism has been reported when comparing different populations of D. willistoni, and a selective mitochondrial sweep has been suggested as one plausible reason (38; J. Silva and M. Kidwell, personal communication).
wAu-like Wolbachia originated in saltans group species.
We found polymorphic but closely related wsp sequences of wPro and wSpt in the host species D. prosaltans and D. septentriosaltans, respectively. This implies that these Wolbachia variants are an outcome of old associations with Neotropical Drosophila species. Independent multiple horizontal transfers with closely related Wolbachia strains are less likely. The progenitor of wPro and wSpt presumably infected the common ancestor of both host species before speciation and subsequently diverged at the wsp sequence level in the course of long-term vertical transmission. Host-specific diagnostic sites within wsp correspond with our hypothesis (Table 4). Therefore, we suggest that wAu-like variants evolved in the American Neotropical saltans group species and are potential donors for the horizontal transmission to D. willistoni. A similar event has been suggested for Wolbachia associations among the Old World sibling species D. simulans and D. sechellia, where original Wolbachia infections in an original species have not yet yielded a sequence divergence in wsp in the sibling species (6).
Recent horizontal transfer into D. similans: origin of the wAu infection.
Non-CI-inducing wAu of D. simulans (17) is found worldwide, including in Australia, Madagascar, Cameroon, parts of Europe and Japan, Ecuador, Jamaica, and the southern United States (2, 3, 7, 23). The overlapping geographic distribution of populations of D. simulans, D. willistoni, and other Neotropical Drosophila species in Central America, together with wsp and fstZ sequence identity of the two Wolbachia variants wAu and wWil, strongly suggests a recent horizontal transfer of Wolbachia from an original native Neotropical Drosophila-Wolbachia guild to the immigrating Old World species D. simulans. To date D. willistoni can be regarded as the most likely donor species of this transfer. Recent transfers of transposable elements between D. willistoni and another immigrating Old World Drosophila species, D. melanogaster, have been shown for the canonical P transposon (10, 18), and for the retrotransposon copia (13, 25). Furthermore, the male-killing bacterium Spiroplasma poulsonii of the D. willistoni group species D. nebulosa has recently infected immigrating D. melanogaster populations in Brazil (30). Extensive phylogenetic studies of hosts and their parasites suggest horizontal transmission of Wolbachia variants between distantly related insect species (5, 16, 43, 46). Furthermore, it has been experimentally demonstrated that Wolbachia can be shuffled horizontally within and between Trichogramma parasitoid species (19, 20).
In agreement with the hypothesis of an American origin of wAu and opposed to an African origin (7) is the extensive analysis of mitochondrial variation in D. simulans. wAu is globally associated with the mitochondrial-siII haplotype of D. simulans (23). However, some African populations of D. simulans harboring the siII haplotype are uninfected. Ballard proposed recently that uninfected flies migrated to Ecuador and acquired wAu in a horizontal transmission event from an unknown host source (2). Subsequently, wAu spread throughout natural populations of D. simulans worldwide. The infection model outlined by Ballard, based on mitochondrial haplotypes and geographic distribution of wAu-infected D. simulans, is in line with our hypothesis that a Neotropical species such as D. willistoni could be the donor species of wAu.
In summary, we suggest a potential evolutionary scenario: wAu-like variants evolved in the guild of the Neotropical saltans group, being vertically transmitted and/or horizontally shuffled between related host species over a long period of time. More recently, a proto-wAu-like strain, the ancestor of wWil, infected horizontally a locally isolated population of D. willistoni, most likely in Central America. In this population, wWil evolved perfect maternal transmission through an extreme tissue tropism towards the germ line of D. willistoni. Within the last 300 years, immigrating D. simulans flies from Africa may have become infected by wWil or by another wAu-like strain from infected Neotropical Drosophila species through vectors such as parasitoid wasps (20, 43). wAu-infected D. simulans has then spread worldwide (2). An alternative source for wAu is an acquisition from outside the closely related Wolbachia pool of Neotropical Drosophila species, but if so, the fact that Neotropical Drosophila species are infected with closely related Wolbachia strains will need to be explained. It is unclear how wWil and wAu drove themselves through host populations. Presently, neither wAu in D. simulans nor wWil in D. willistoni is able to induce measurable levels of CI (17; W. J. Miller, unpublished data). The possibility that they did so in the past cannot be excluded. As reported by Ballard and coworkers, wAu seems to induce weak levels of CI in some infected populations of D. simulans from Florida (3, 23, 29). Alternatively, the driving force for the spreading of wAu-like strains could be a positive fitness contribution to their hosts that remains to be elucidated. The phenotypes of the Neotropical Wolbachia strains still need to be elucidated. The wAu-like strains are nested within the Mel cluster (50) of closely related Wolbachia strains that have a variety of phenotypic effects in other host species. Based on the wsp sequence, the variant wSpt BCI1 of D. septentriosaltans is identical to the infection of the African species D. yakuba and D. teissieri (27) and to wCer2 of R. cerasi (33). Whereas wCer2 causes strong CI in R. cerasi and in transinfected Ceratitis capitata (49) and intermediate CI in transinfected D. simulans (35), wTei and wYak do not induce CI but are able to fully rescue the wRi mod function in their original host (48). Wolbachia infections of D. melanogaster (32, 50) and the quinaria group member D. recens induce CI (45). The strain wInnA causes male killing in the related D. innubila (22), where it is regarded as an ancestral infection (12).
The present paper shows the complexity of evolutionary dynamics of Wolbachia in Neotropical Drosophila species and its success in colonizing the Old World species D. simulans. Both wWil and wAu successfully colonized natural populations of D. willistoni in America and of D. simulans globally. The detailed understanding of the evolutionary “jump-and-go” dynamics of Wolbachia will have important implications for practical applications of this symbiont as a vector system and in biological pest control management.
Acknowledgments
This work was supported by FWF grant P13384-GEN from the Austrian Science Foundation.
We are very much obliged to Petra Zwirn, Edeltraud Kehrer, Ingrid Gerstl, and Sabine Lagger for excellent technical support and assistance. We highly appreciate the valuable material contributions by Margaret Kidwell, Andrew Holyake, Egon Bartel, Kim van der Linde, Peter Chabora, and Francesco Ayala, who donated numerous Neotropical Drosophila lines, and by Paul Lasko and Kostas Bourtzis, who provided the anti-VASA and anti-WSP antibodies, respectively. Moreover, we thank Margaret Kidwell, Joana Silva, Andrew Holyoake, and Filipa Vala for stimulating discussions and Filipa Vala, Jeremy Brownlie, and two anonymous reviewers for valuable comments on earlier versions of the manuscript.
REFERENCES
- 1.Baldo, L., and J. H. Werren. 2005. Mosaic nature of Wolbachia surface protein. J. Bacteriol. 187:5406-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard, J. W. O. 2004. Sequential evolution of a symbiont inferred from the host: Wolbachia and Drosophila simulans. Mol. Biol. Evol. 21:428-442. [DOI] [PubMed] [Google Scholar]
- 3.Ballard, J. W. O., J. Hatzidakis, T. L. Karr, and M. Kreitman. 1996. Reduced variation in Drosophila simulans mitochondrial DNA. Genetics 144:1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourtzis K., A. Nirgianaki, G. Markakis, and C. Savakis, C. 1996. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics 144:1063-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle, L., S. L. O'Neill, H. M. Robertson, and T. L. Karr. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796-1799. [DOI] [PubMed] [Google Scholar]
- 6.Charlat, S., A. Nirgiaanki, K. Bourtzis, and H. Merçot. 2002. Evolution of Wolbachia-induced cytoplasmic incompatibility in Drosophila simulans and D. sechellia. Evol. Int. J. Org. Evol. 56:1735-1742. [DOI] [PubMed] [Google Scholar]
- 7.Charlat, S., L. Le Chat, and H. Merçot. 2003. Characterization of non-cytoplasmic incompatibility inducing Wolbachia in two continental African populations of Drosophila simulans. Heredity 90:49-55. [DOI] [PubMed] [Google Scholar]
- 8.Charlat, S., J. W. O. Ballard, and H. Merçot. 2004. What maintains noncytoplasmic incompatibility inducing Wolbachia in their hosts: a case study from a natural Drosophila yakuba population. J. Evol. Biol. 17:322-330. [DOI] [PubMed] [Google Scholar]
- 9.Charlat, S., M. Riegler, I. Baures, D. Poinsot, C. Stauffer, and H. Merçot. 2004. Incipient evolution of Wolbachia compatibility types. Evol. Int. J. Org. Evol. 58:1901-1908. [DOI] [PubMed] [Google Scholar]
- 10.Daniels, S. B., K. R. Peterson, L. D. Strausbaugh, M. G. Kidwell, and A. Chovnick. 1990. Evidence for horizontal transmission of the P transposable element between Drosophila species. Genetics 124:339-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobson, S. L., K. Bourtzis, H. R. Braig, B. F. Jones, W. Zhou, F. Rousset, and S. L. O'Neill. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29:153-160. [DOI] [PubMed] [Google Scholar]
- 12.Dyer, K. A., and, J. Jaenike. 2004. Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila: molecular evidence from the host and parasite genomes. Genetics 168:1443-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flavell, A. 1999. Long terminal repeat transposons jump between species. Proc. Natl. Acad. Sci. USA 96:12211-12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gloor, G. B., C. R. Preston, D. M. Johnson-Schlitz, N. A. Nassif, R. W. Phillis, W. K. Benz, H. M. Robertson, and W. R. Engels. 1993. Type I repressors of P element mobility. Genetics 135:81-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagemann S., E Haring, and W. Pinsker. 1996. Repeated horizontal transfer of P transposons between Scaptomyza pallida and Drosophila bifasciata. Genetica 98:43-51. [DOI] [PubMed] [Google Scholar]
- 16.Heath, B. D., R. D. Butcher, W. G. Whitfield, and S. F. Hubbard. 1999. Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr. Biol. 9:313-316. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann, A. A., D. Clancy, and J. Duncan. 1996. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 76:1-8. [DOI] [PubMed] [Google Scholar]
- 18.Houck, M. A., J. B. Clark, K. R. Peterson, and M. G. Kidwell. 1991. Possible horizontal transfer of Drosophila genes by the mite Proctolaelaps regalis. Science 253:1125-1129. [DOI] [PubMed] [Google Scholar]
- 19.Huigens, M. E., R. F. Luck, R. H. G. Klaassen, M. Maas, M. Timmermans, and R. Stouthamer. 2000. Infectious parthenogenesis. Nature 405:178-179. [DOI] [PubMed] [Google Scholar]
- 20.Huigens, M. E., R. P. de Almeida, P. A. Boons, R. F. Luck, and R. Stouthamer. 2004. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. R. Soc. London B 271:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iturbe-Ormaetxe, I., G. R. Burke, M. Riegler, and S. L. O′Neill. 2005. Distribution, expression, and motif variability of ankyrin domain genes in Wolbachia pipiens. J. Bacteriol. 187:5136-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaenike, J., K. A. Dyer, and L. K. Reed. 2003. Within-population structure of competition and the dynamics of male-killing Wolbachia. Evol. Ecol. Res. 5:1023-1036. [Google Scholar]
- 23.James, A. C., and J. W. O. Ballard. 2000. Expression of cytoplasmic incompatibility in Drosophila simulans and its impact on infection frequencies and distribution of Wolbachia pipientis. Evol. Int. J. Org. Evol. 54:1661-1672. [DOI] [PubMed] [Google Scholar]
- 24.Jeyaprakash, A., and M. A. Hoy. 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 4:393-405. [DOI] [PubMed] [Google Scholar]
- 25.Jordan, I. K., L. V. Matyunina, and J. F. McDonald. 1999. Evidence for the recent horizontal transfer of long terminal repeat transposons. Proc. Natl. Acad. Sci. USA 96:12621-12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Junakovic, N. 2004. Southern blot analysis of individual Drosophila flies. Methods Mol. Biol. 260:41-57. [DOI] [PubMed] [Google Scholar]
- 27.Lachaise, D., M. Harry, M. Solignac, F. Lemeunier, V. Benassi, and M. L. Cariou. 2000. Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from Sao Tome. Proc. R. Soc. London B 267:1487-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang, L., W. Diehl-Jones, and P. Lasko. 1994. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development 120:1201-1211. [DOI] [PubMed] [Google Scholar]
- 29.Merçot, H., and S. Charlat. 2004. Wolbachia infections in Drosophila melanogaster and D. simulans: polymorphism and levels of cytoplasmic incompatibility. Genetica 120:51-59. [DOI] [PubMed] [Google Scholar]
- 30.Montenegro, H., V. N. Solferini, L. B. Klaczko, and G. D. Hurst. 2005. Male-killing Spiroplasma naturally infecting Drosophila melanogaster. Insect Mol. Biol. 14:281-287. [DOI] [PubMed] [Google Scholar]
- 31.Ono, M., H. R. Braig, L. E. Munstermann, C. Ferro, and S. L. O'Neill. 2001. Wolbachia infections of phlebotomine sand flies (Diptera: Psychodidae). J. Med. Entomol. 38:237-241. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds, K. T., and A. A. Hoffmann. 2002. Male age, host effect and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet. Res. 80:79-87. [DOI] [PubMed] [Google Scholar]
- 33.Riegler, M., and C. Stauffer. 2002. Wolbachia infections and superinfections in cytoplasmically incompatible populations of the European cherry fruit fly Rhagoletis cerasi (Diptera, Tephritidae). Mol. Ecol. 11:2425-2434. [DOI] [PubMed] [Google Scholar]
- 34.Riegler, M., and S. L. O′Neill. 2004. The genus Wolbachia. In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, and E. Stackebrandt (ed.), The Prokaryotes: an evolving electronic resource for the microbiological community. Springer-Verlag, New York, N.Y. http://141.150.157.117:8080/prokPUB/chaphtm/454/COMPLETE.htm.
- 35.Riegler, M., S. Charlat, C. Stauffer, and H. Merçot. 2004. Wolbachia transfer from Rhagoletis cerasi to D. simulans: investigating the outcome of host-symbiont coevolution. Appl. Environ. Microbiol. 70:273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riegler, M., M. Sidhu, W. J. Miller, and S. L. O′Neill. 2005. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr. Biol. 15:1428-1433. [DOI] [PubMed] [Google Scholar]
- 37.Salzberg, S. L., J. C. Dunning Hotopp, A. L. Delcher, M. Pop, D. R. Smith, M. B. Eisen, and W. C. Nelson. 2005. Serendipitous discovery of Wolbachia genomes in multiple Drosophila species. Genome Biol. 6:R23. (Correction, 6:402.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva, J. C. 2000. Population genetics of P transposable elements and their host species, with emphasis on Drosophila willistoni and Drosophila sturtevanti. Ph.D. thesis. University of Arizona, Tucson.
- 39.Swofford, D. L. 1998. PAUP*: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, Mass.
- 40.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Throckmorton, L. H. 1975. The phylogeny, ecology, and geography of Drosophila, p. 421-469. In R. C. King (ed.), Handbook of genetics, vol. 3. Plenum, New York, N.Y. [Google Scholar]
- 42.Val, F. C., C. R. Vilela, and M. D. Marques. 1981. Drosophilidae of the Neotropical region, p. 123-168. In M. Ashburner et al. (ed.), The genetics and biology of Drosophila, vol. 3a. Academic Press, London, United Kingdom. [Google Scholar]
- 43.Vavre, F., F. Fleury, D. Lepetit, P. Fouillet, and M. Bouletreau. 1999. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol. Biol. Evol. 16:1711-1723. [DOI] [PubMed] [Google Scholar]
- 44.Veneti, Z., M. E. Clark, T. L. Karr, C. Savakis, and K. Bourtzis. 2004. Heads or tails: host-parasite interactions in the Drosophila-Wolbachia system. Appl. Environ. Microbiol. 70:5366-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werren, J. H., and J. Jaenike. 1995. Wolbachia and cytoplasmic incompatibility in mycophagous Drosophila and their relatives. Heredity 75:320-326. [DOI] [PubMed] [Google Scholar]
- 46.Werren, J. H., D. Windsor, and L. Guo. 1995. Distribution of Wolbachia among Neotropical arthropods. Proc. R. Soc. London B 262:197-204. [Google Scholar]
- 47.Werren, J. H., and D. Windsor. 2000. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. R. Soc. London B 267:1277-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zabalou, S., S. Charlat, A. Nirgianaki, D. Lachaise, H. Mercot, and K. Bourtzis. 2004. Natural Wolbachia infections in the Drosophila yakuba species complex do not induce cytoplasmic incompatibility but fully rescue the wRi modification. Genetics 167:827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zabalou, S., M. Riegler, M. Theodorakopoulou, C. Stauffer, C. Savakis, and K. Bourtzis. 2004. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA 101:15042-15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou, W., F. Rousset, and S. L. O′Neill. 1998. Phylogeny and PCR based classification of Wolbachia strains using wsp sequences. Proc. R. Soc. London B 265:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]