Abstract
To investigate whether membrane proteases are involved in the activity of Bacillus thuringiensis insecticidal toxins, the rate of pore formation by trypsin-activated Cry1Aa was monitored in the presence of a variety of protease inhibitors with Manduca sexta midgut brush border membrane vesicles and by a light-scattering assay. Most of the inhibitors tested had no effect on the pore-forming ability of the toxin. However, phenylmethylsulfonyl fluoride, a serine protease inhibitor, promoted pore formation, although this stimulation only occurred at higher inhibitor concentrations than those commonly used to inhibit proteases. Among the metalloprotease inhibitors, o-phenanthroline had no significant effect; EDTA and EGTA reduced the rate of pore formation at pH 10.5, but only EDTA was inhibitory at pH 7.5. Neither chelator affected the properties of the pores already formed after incubation of the vesicles with the toxin. Taken together, these results indicate that, once activated, Cry1Aa is completely functional and does not require further proteolysis. The effect of EDTA and EGTA is probably better explained by their ability to chelate divalent cations that could be necessary for the stability of the toxin's receptors or involved elsewhere in the mechanism of pore formation.
Bacillus thuringiensis is by far the most widely used alternative to chemical insecticides for the control of insect pests in forestry, agriculture, and public health. During sporulation, this bacterium produces insecticidal proteins that accumulate in the form of parasporal crystals. Following their ingestion by susceptible insect larvae, these protoxins are solubilized and converted to active toxins by midgut proteases. Activated toxins act by forming pores after binding to specific receptors at the surface of the insect midgut luminal membrane, leading to cell lysis, destruction of the epithelium, and death of the insect (52). In their activated form, all B. thuringiensis Cry toxins for which the crystal structure has been solved share a similar three-domain structure (7, 21, 24, 33, 34, 44). Domain I is composed of a bundle of seven anti-parallel α-helices and is generally thought to be responsible for membrane insertion and pore formation, while domains II and III are mainly composed of β-sheets and involved in the binding, specificity, and stability of the toxin (24, 33, 52).
Midgut proteases play an essential role in the activation of B. thuringiensis toxins (1, 6, 13). In the case of Cry1A toxins, the first 28 amino acid residues are removed from the N terminus and approximately half of the protoxin residues are removed from the C terminus (6). However, susceptibility of the activated toxins to further proteolysis in the midgut environment could possibly affect toxicity and explain apparent discrepancies that are occasionally observed between their in vitro and in vivo activities (15, 41, 49, 60). For example, Cry1Ca can be completely degraded when incubated with midgut juice from advanced larval instars of Spodoptera littoralis (29). Synergistic effects between B. thuringiensis toxins and protease inhibitors have been reported (39, 55), suggesting that some degradation of activated toxins occurs in the midgut (47, 55). Such degradation could be triggered by receptor binding (55).
Several in vitro studies have identified proteolytic cleavage sites within the activated toxin. These are found at the C-terminal end of helix α1 of Cry1Ac (46); between α1 and α2a of Cry1Ab (23) and Cry1Ac (35); within α2a of Cry1Ab (43) and Cry1Ac (35); between α2a and α2b of Cry1Ab (43) and Cry1Ac (35, 46); between α2 and α3 of Cry4Ba (7); between α3 and α4 of Cry2Aa (3, 45), Cry3Aa (10, 12), and Cry9Ca (32); between α5 and α6 of Cry4Aa (66), Cry4Ba (2, 68), and Cry9Aa (68); between β4- and β5-sheets of Cry11Aa (17, 67); between β6 and β7r of Cry1Aa (48); within β7r of Cry1Ab (14); and between β9 and β10 of Cry1Ac (35). The functional significance of these cleavages remains unclear. Removal of helices α1 and α2 during the crystallization of Cry4Ba did not affect its mosquito larvicidal activity compared with that of freshly activated Cry4Ba, which aligns with the N terminus of α1 in homologous toxins (7). This result suggests that cleavage within the activated toxin does not necessarily lead to a significant alteration of its insecticidal activity. In contrast, midgut enzymatic activity appears to play an important role in the mode of action of Cry3Aa, since proteolytic removal of the C-terminal end of domain III allowed the toxin to become soluble under the midgut pH conditions found in susceptible larvae (10).
Recent studies suggest that activated toxins could be further processed by midgut proteases after binding to their membrane receptors. Lightwood et al. (35) suggested that a cleavage within domain I of Cry1Ac could facilitate membrane insertion without necessarily being required for pore formation. According to Gómez et al. (23), binding of Cry1Ab to the cadherin-like receptor BT-R1 allows the proteolytic cleavage of helix α1 in domain I of the toxin, resulting in the formation of a tetrameric prepore structure. They proposed that this cleavage could be essential for pore formation. The fact that activated toxins form pores in isolated brush border membrane vesicles in the absence of soluble midgut proteases suggests that membrane proteases could be involved in the mechanism of action of these toxins. In agreement with this suggestion, Culex quinquefasciatus brush border membrane proteases have been reported to cleave the Cry11Aa toxin (17). To investigate the possibility that membrane-associated proteases are involved in toxin function, the effect of a variety of membrane protease inhibitors on the pore-forming activity of the trypsin-activated toxin Cry1Aa was studied. Pore formation was monitored in vitro with Manduca sexta midgut brush border membrane vesicles and by an osmotic swelling assay (11). The results indicate that trypsin-activated Cry1Aa is completely functional.
MATERIALS AND METHODS
Chemicals.
4-(2-Aminoethyl)benzenesulfonyl fluoride (AEBSF), antipain, aprotinin, bestatin, EGTA, leupeptin, o-phenanthroline, 4-chloromercuribenzoic acid (pCMB), pepstatin A, p-hydroxymercuribenzoate, phenylmethylsulfonyl fluoride (PMSF), trans-epoxysuccinyl-l-leucylamido-(4-guanidino)butane (E-64), N-p-tosyl-l-lysine chloromethyl ketone (TLCK), and N-p-tosyl-l-phenylalanine chloromethyl ketone (TPCK) were purchased from Sigma (St. Louis, Mo). EDTA was from Fisher Scientific (Nepean, Ontario). Protease inhibitors were solubilized in water except pepstatin A (stock solution of 29.2 mM in dimethyl sulfoxyde), pCMB (67 mM in dimethyl sulfoxide), PMSF (200 mM in ethanol), and o-phenanthroline (200 mM in methanol). A cocktail (Protease Inhibitor Cocktail Set III) containing 100 mM AEBSF, 80 μM aprotinin, 5 mM bestatin, 1.5 mM E-64, 2 mM leupeptin, and 1 mM pepstatin A solubilized in dimethyl sulfoxide was purchased from Calbiochem (San Diego, CA) and used at a final 100-fold dilution.
Preparation of membrane vesicles.
Whole midguts were isolated from fifth-instar M. sexta larvae (Carolina Biological Supply Company, Burlington, NC); freed of attached Malpighian tubules and luminal contents; rinsed thoroughly with ice-cold 300 mM sucrose, 17 mM Tris-HCl (pH 7.5), and 5 mM EGTA; and stored at −80°C until use. Brush border membrane vesicles were prepared by magnesium precipitation and a differential centrifugation technique (64). The final membrane preparation was resuspended in 10 mM HEPES-KOH (pH 7.5) and stored at −80°C until use.
Toxins.
B. thuringiensis strains HD73Cry− and HD1Cry−B and Escherichia coli strains HB101 and DH5α were transformed, respectively, with pBA1 (4), p60.5G31 (62), pMP30 (42), and pEM14 (61) to produce Cry1Aa, Cry1Ca, Cry1Ab, and Cry1Ea protoxins. Cry1Ac protoxin was produced from B. thuringiensis strain HD73. Protoxins were solubilized, trypsin activated, and purified by fast protein liquid chromatography as described previously (41, 42). N-terminal sequencing of Cry1Aa was performed by automated N-terminal Edman degradation at the Eastern Quebec Proteomic Center, Centre Hospitalier de l'Université Laval Research Center, Quebec, Canada.
Light-scattering assay.
The permeabilizing effect of B. thuringiensis toxins was analyzed using an osmotic swelling technique based on light-scattering measurements (11). Unless specified otherwise, in preparation for the experiments, vesicles were resuspended to about 90% of the desired final volume in 10 mM HEPES-KOH (pH 7.5) or 3-cyclohexylamino-1-propanesulfonic acid (CAPS)-KOH (pH 10.5) and allowed to equilibrate overnight at 4°C. Before the start of the experiments, they were diluted to a final concentration of 0.4 mg of membrane protein/ml with the appropriate buffer and enough bovine serum albumin to achieve a final concentration of 1 mg/ml. In kinetics experiments designed to monitor toxin-induced increases in membrane permeability to KCl, vesicles were first warmed up to 23°C and then rapidly mixed with a stopped-flow apparatus (Hi-Tech Scientific Co., Salisbury, United Kingdom) with an equal volume of a solution containing 150 mM KCl, 10 mM HEPES-KOH (pH 7.5), or CAPS-KOH (pH 10.5); 1-mg/ml bovine serum albumin; and a specified toxin concentration. Due to the hypertonic shock, water exits from the vesicles and their volume decreases rapidly. Then, depending on their permeability to KCl, vesicles reswell and subsequently recover some of their original volume. When protease inhibitors were used, they were added at least 10 min before the beginning of the experiments to the vesicle suspension and to the KCl solution. Since a high pH is present in the lepidopteran midgut (18, 19), most experiments were carried out at pH 10.5.
Alternatively, for incubation experiments, vesicles were incubated with the indicated concentration of toxin for 60 min at 23°C and then mixed with a solution containing 150 mM KCl, 10 mM HEPES-KOH (pH 7.5), or CAPS-KOH (pH 10.5) and 1-mg/ml bovine serum albumin. In these experiments, protease inhibitors were added to the vesicle suspension and the KCl solution 10 min before the addition of the toxin or after an incubation period of 50 min with the toxin.
Scattered light intensity was monitored at a wavelength of 450 nm at a frequency of 10 Hz with a PTI spectrofluorometer (Photon Technology International, South Brunswick, NJ) with a photomultiplier tube located at 90° from the incident light beam.
Data analysis.
As described previously (59), scattered light measurements were first converted into relative scattered light intensity where the value of 1 was attributed to the highest intensity measured in the absence of toxin and the value 0 was attributed to the lowest intensity measured with 150 pmol Cry1Aa/mg of membrane protein. Percent volume recovery was defined as 100(1 − It), where It is the relative scattered light intensity measured at a given time (t). For pore formation kinetics experiments, percent volume recovery was calculated for every experimental point. Values obtained with control vesicles, assayed without toxin, were subtracted from the experimental values measured in the presence of toxin. To take into account any influence of the inhibitors and their solvents on scattered light intensity measurements, these compounds were also added in the control experiments performed without toxin. Volume recovery curves were then fitted with a Boltzmann sigmoid with the software Origin (OriginLab Corporation, Northampton, MA). The osmotic swelling rate was taken as the maximum slope of these curves.
Data are means ± standard error of the mean (SEM) of at least three experiments, each performed with a different vesicle preparation. Experimental values for each individual experiment consisted of the average of five replicates obtained using the same vesicle preparation. Statistical comparisons were made with the two-tailed unpaired t test.
RESULTS
Effect of various protease inhibitors on the pore-forming activity of Cry1Aa.
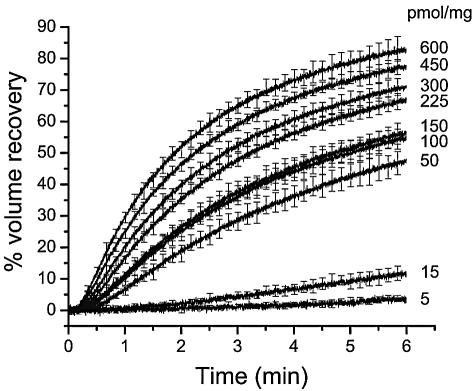
Midgut brush border membrane vesicles were rapidly mixed with an equal volume of 150 mM KCl. Following the osmotic shock, the vesicles reswelled at a rate that increased with toxin concentration (Fig. 1). To test the possible role of membrane proteases in the mode of action of B. thuringiensis toxins, the effect of a variety of protease inhibitors on the pore-forming activity of Cry1Aa was tested (Fig. 2 and Table 1). Protease inhibitors were used at a concentration known to be largely sufficient to inhibit the type of protease against which they are active (5, 51, 58, 65). Because in theory protease inhibitors could either stimulate or inhibit pore formation, a toxin concentration of 50 pmol toxin/mg of membrane protein was chosen to test all inhibitors.
FIG. 1.
Osmotic swelling of M. sexta midgut brush border membrane vesicles induced by various concentrations of Cry1Aa. Vesicles equilibrated in 10 mM CAPS-KOH (pH 10.5) and 1-mg/ml bovine serum albumin were rapidly mixed with an equal volume of 150 mM KCl, 10 mM CAPS-KOH (pH 10.5), 1-mg/ml bovine serum albumin, and the indicated concentrations of toxin (in picomoles of toxin per milligram of membrane protein). Percent volume recovery was calculated for each experimental point, and the values measured for control vesicles, assayed in the absence of toxin, were subtracted from those obtained in the presence of toxin. Data are means ± SEM of four experiments. For clarity, error bars are shown for every 100th experimental point.
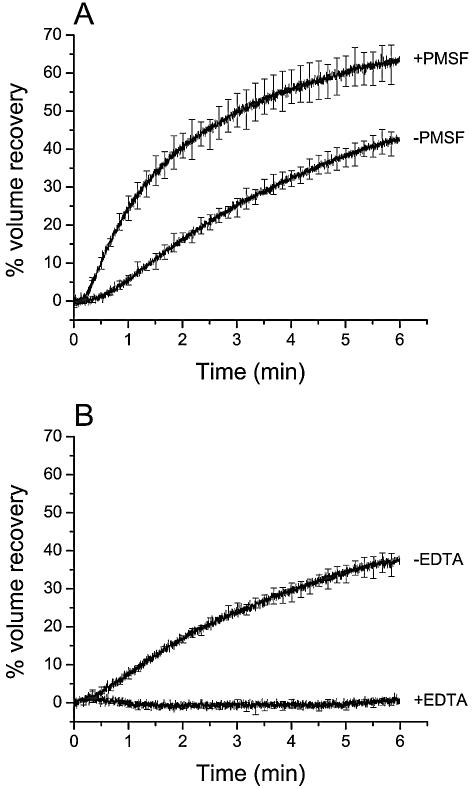
FIG. 2.
Osmotic swelling of midgut brush border membrane vesicles induced by Cry1Aa in the presence of PMSF (A) and EDTA (B). Vesicles were mixed with a solution containing 150 mM KCl, 10 mM CAPS-KOH (pH 10.5), 1-mg/ml bovine serum albumin, and 50 pmol Cry1Aa/mg of membrane protein. The vesicle suspension and the KCl solution contained 5 mM PMSF (+PMSF), 2.5% ethanol (−PMSF), or 2 mM EDTA (+EDTA). Data are means ± SEM of three experiments. For clarity, error bars are shown for every 100th experimental point.
TABLE 1.
Effect of protease inhibitors on the pore-forming activity of Cry1Aa
| Inhibitor | Concn | Osmotic swelling ratea (% control activity) |
|---|---|---|
| PMSF | 5 mM | 314 ± 60 (3)* |
| AEBSF | 1 mM | 101 ± 15 (3) |
| TLCK | 0.1 mM | 91 ± 3 (3) |
| TPCK | 0.1 mM | 92 ± 11 (3) |
| Aprotinin | 0.8 μM | 81 ± 17 (3) |
| Leupeptin | 20 μM | 117 ± 18 (3) |
| Antipain | 16.5 μM | 83 ± 8 (3) |
| EDTA | 2 mM | 6 ± 3 (3)*** |
| EGTA | 2 mM | 16 ± 1 (3)** |
| o-Phenanthroline | 2 mM | 107 ± 14 (3) |
| Pepstatin A | 10 μM | 102 ± 7 (5) |
| Bestatin | 50 μM | 118 ± 17 (3) |
| E-64 | 15 μM | 113 ± 18 (3) |
| pCMB | 0.1 mM | 83 ± 2 (3)** |
| pHMBb | 0.1 mM | 73 ± 6 (6) |
| Cocktail | 1% (vol/vol) | 106 ± 9 (3) |
Determined at pH 10.5 with 50 pmol Cry1Aa/mg of membrane protein. Data are means±SEM of the number of experiments indicated in parentheses. Statistical analyses are for comparisons with the control values obtained in the absence of inhibitor. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
pHMB, p-hydroxymercuribenzoate.
PMSF significantly promoted membrane permeabilization by Cry1Aa (Fig. 2A), while EDTA clearly abolished this permeabilization (Fig. 2B). Although the effect of PMSF suggests that a membrane protease could inhibit pore formation, all other serine protease inhibitors tested (AEBSF, TLCK, TPCK, aprotinin, leupeptin, and antipain) were ineffective (Table 1). The efficacy of TLCK and TPCK is maximal around neutral pH values and decreased rapidly under alkaline conditions (53, 56). The effect of these two inhibitors on Cry1Aa pore formation was therefore also tested at pH 7.5. At this pH, the osmotic swelling rates were 96% ± 4% (based on the results of three experiments) and 99% ± 8% (three experiments) control activity in the presence of TLCK and TPCK, respectively, thus confirming that these two inhibitors had no effect on the pore-forming activity of the toxin.
Like EDTA, EGTA also inhibited Cry1Aa activity, but o-phenanthroline had no effect (Table 1). Both zinc-dependent metalloproteases and some calcium-stabilized proteases from other classes could be inactivated by the first two chelating agents. o-Phenanthroline is usually preferred as an inhibitor of metalloproteases, since it has a much higher stability constant for zinc than for calcium (51). Considering the fact that the solubility of most divalent cations is very low at pH 10.5, EDTA and EGTA were also tested at pH 7.5. At this pH, EDTA inhibited pore formation (16.5% ± 0.1% of control activity; three experiments; P = 0.003), but EGTA had no effect (105% ± 8% of control activity; three experiments; P = 0.7). The lack of effect of EGTA at pH 7.5 and of o-phenanthroline clearly indicates that a metalloprotease is not essential for pore formation.
Among the cysteine protease inhibitors tested (E-64, pCMB, and p-hydroxymercuribenzoate) only pCMB reduced significantly, although slightly, the activity of Cry1Aa (Table 1). The aspartate protease inhibitors tested (antipain and pepstatin A) and an aminopeptidase inhibitor (bestatin) had no significant effect (Table 1). Since several types of membrane proteases could potentially act at the same time, a cocktail that inhibits aminopeptidases, as well as serine, cysteine, and aspartate proteases, was also tested. However, it had no effect on the activity of Cry1Aa (Table 1). Furthermore, to ensure that the apparent lack of activity of membrane proteases on the pore-forming ability of Cry1Aa did not result from the use of a Cry1Aa preparation that was already cleaved within domain I, the N-terminal end was sequenced. It corresponded exactly to the beginning of the activated toxin (29IETGY33).
Effect of PMSF on the pore-forming activity of Cry1 toxins.
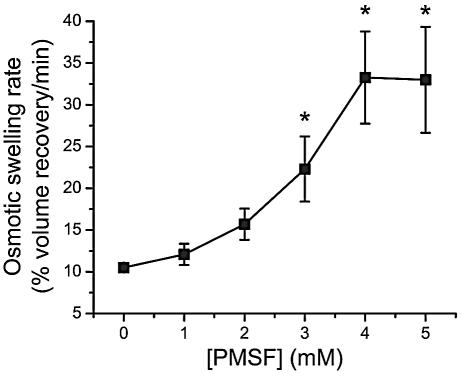
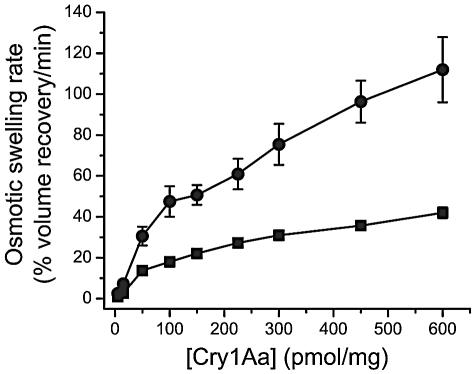
PMSF significantly enhanced the rate of osmotic swelling induced by Cry1Aa, with a maximum effect reached at about 4 mM (Fig. 3). Osmotic swelling rates increased more rapidly as a function of toxin concentration in the presence of PMSF than in its absence (Fig. 4). Nevertheless, swelling rates never reached a constant value indicative of receptor saturation for toxin concentrations up to 600 pmol/mg of membrane protein. PMSF also promoted membrane permeabilization by Cry1Ab, Cry1Ac, Cry1Ca, and Cry1Ea (Table 2). Since Cry1Ca causes a much smaller osmotic swelling rate at pH 10.5 than the other toxins tested (59), experiments with this toxin were performed with a higher toxin concentration (150 pmol/mg of membrane protein). The stimulatory effect of PMSF on the pore-forming activity of Cry1Aa, Cry1Ac, and Cry1Ca was similar. However, this effect was significantly (P < 0.05) stronger for Cry1Ea and weaker for Cry1Ab than for Cry1Aa.
FIG. 3.
Effect of PMSF concentration on the rate of pore formation by Cry1Aa in brush border membrane vesicles. Vesicles were mixed with a solution containing 150 mM KCl, 10 mM CAPS-KOH (pH 10.5), 1-mg/ml bovine serum albumin, and 50 pmol Cry1Aa/mg of membrane protein. Data are means ± SEM of three experiments. Asterisks indicate a significant difference (P < 0.05) relative to controls (0 mM PMSF).
FIG. 4.
Effect of PMSF on the rate of pore formation by Cry1Aa. Vesicles were mixed with a solution containing 150 mM KCl, 10 mM CAPS-KOH (pH 10.5), 1-mg/ml bovine serum albumin, and the indicated concentration of Cry1Aa (in picomoles of toxin per milligram of membrane protein). The experiments were performed without PMSF (▪) and with 4 mM PMSF (•). Data are means ± SEM of four (without PMSF) or five (with PMSF) experiments.
TABLE 2.
Effect of PMSF on the activity of Cry1 toxins
| Toxin | Osmotic swelling ratea (% control activity) |
|---|---|
| Cry1Aa | 317 ± 53 (3)* |
| Cry1Ab | 152 ± 9 (3)* |
| Cry1Ac | 321 ± 27 (3)** |
| Cry1Ca | 458 ± 138 (5)* |
| Cry1Ea | 531 ± 25 (3)*** |
Determined in the presence of 4 mM PMSF at pH 10.5 with 50 pmol toxin/mg of membrane protein for Cry1Aa, Cry1Ab, Cry1Ac, and Cry1Ea and with 150 pmol toxin/mg of membrane protein for Cry1Ca. Data are means ± SEM of the number of experiments indicated in parentheses. Statistical analyses are for comparisons with the control values obtained, for each toxin, in the absence of PMSF. *, P < 0.06; **, P < 0.01; ***, P < 0.001.
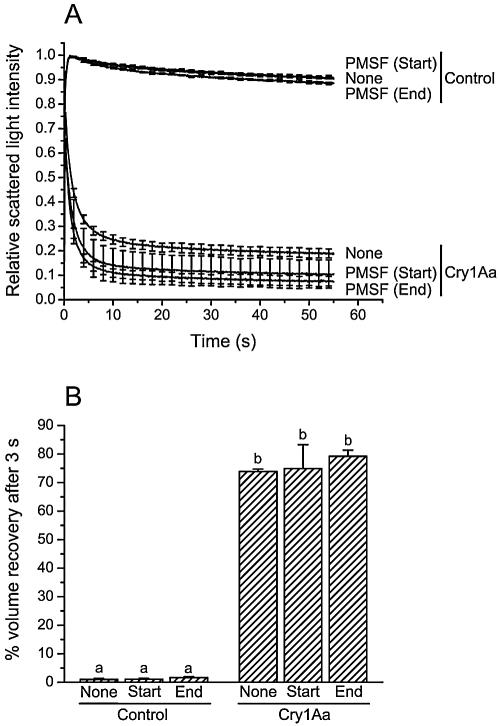
To investigate whether PMSF acts on the toxin molecule or on the vesicles, toxin and vesicles concentrated 30 and 10 fold, respectively, were incubated overnight with 4 mM PMSF (Table 3). The toxin and vesicles were then diluted to their usual final concentration immediately before the onset of the osmotic swelling experiments. This dilution and the short half-life of PMSF in water (about 1 h) (28) ensure that the active concentration of PMSF becomes negligible during the osmotic swelling part of the experiment. Overnight incubation of vesicles, toxin, or both with PMSF or ethanol (the solvent in which PMSF was solubilized) had no significant effect on the activity of the toxin (Table 3). According to these results, the effect of PMSF could have been due to a modification of the toxin molecule on a serine residue (22) that was only accessible after binding of the toxin to its receptor. The effect of PMSF (Fig. 3) could also be due to a modification of pore properties. To test this hypothesis, vesicles were incubated for 60 min with or without 50 pmol of toxin/mg of membrane protein. PMSF was added to the vesicle suspension and to the KCl solution 10 min before the addition of the toxin or for 10 min at the end of the incubation period (Fig. 5). PMSF had no effect on the control experiments performed without toxin and no effect on Cry1Aa-induced permeability, whether it was added at the beginning or at the end of the incubation period. Previous results show that during incubation experiments performed in the absence of PMSF, volume recovery induced by Cry1Aa reaches a constant value between 50 to 100 pmol of toxin/mg of membrane protein (60). However, the lack of activity of PMSF does not appear to be due to the use of a saturating toxin concentration, since experiments performed with a lower toxin concentration (15 pmol of toxin/mg of membrane protein) yielded similar results (data not shown).
TABLE 3.
Effect of overnight incubation of Cry1Aa and brush border membrane vesicles with ethanol and PMSF
| Concn added to brush border membrane vesiclesa
|
Toxinb
|
Osmotic swelling ratec (% control activity) | ||
|---|---|---|---|---|
| Ethanol (%) | PMSF (mM) | Ethanol (%) | PMSF (mM) | |
| 2 | 101 ± 11 | |||
| 4 | 120 ± 7 | |||
| 2 | 105 ± 11 | |||
| 4 | 93 ± 12 | |||
| 2 | 2 | 101 ± 2 | ||
| 4 | 4 | 109 ± 3 | ||
Brush border membrane vesicles were incubated overnight at 4 mg of membrane protein/ml in 10 mM CAPS-KOH (pH 10.5) and the indicated amounts of PMSF and ethanol. Prior to the experiments, the vesicles were diluted to a final concentration of 0.4 mg of membrane protein/ml with 10 mM CAPS-KOH and enough bovine serum albumin to achieve a final concentration of 1 mg/ml.
Cry1Aa was incubated overnight at 0.04 mg/ml in 150 mM KCl, 10 mM CAPS-KOH (pH 10.5) and the indicated amount of PMSF or ethanol and added in the KCl solution before the osmotic swelling experiments.
Determined at pH 10.5 with 50 pmol Cry1Aa/mg of membrane protein. Data are means ± SEM of three experiments.
FIG. 5.
Effect of PMSF additions during incubation of the vesicles with Cry1Aa. Vesicles were incubated for 60 min and mixed with a solution containing 150 mM KCl, 10 mM CAPS-KOH (pH 10.5), and 1-mg/ml bovine serum albumin. Vesicles were incubated either without (Control) or with 50 pmol Cry1Aa/mg of membrane protein. Ethanol (2%) (None) or PMSF (4 mM) was added at the beginning of the incubation period, 10 min before the addition of the toxin (Start), or after an incubation of 50 min with the toxin (End). Percent volume recovery at 3 s (B) was derived from the experimental curves shown in panel A. Data are means ± SEM of three experiments. Bars labeled with the same letter are not significantly different (P > 0.05).
Effect of EDTA and EGTA on the pore-forming activity of Cry1 toxins.
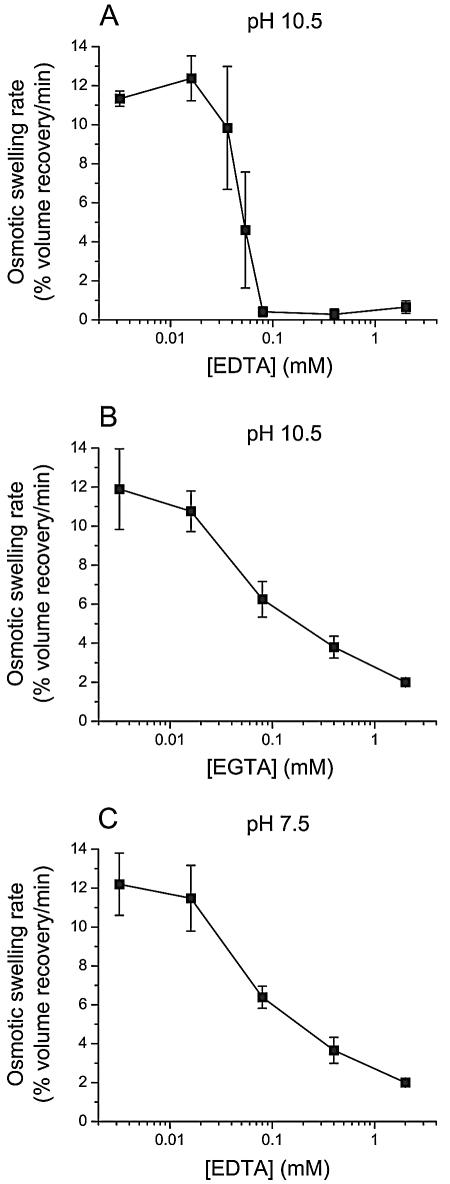
The rate of pore formation by Cry1Aa was first examined at pH 10.5 in the presence of various concentrations of EDTA and EGTA (Fig. 6A and B). Since EGTA did not inhibit pore formation by Cry1Aa at pH 7.5, as mentioned above, a dose-response experiment was only performed for EDTA at this pH (Fig. 6C). At pH 10.5, the inhibition curve was much steeper for EDTA (Fig. 6A) than for EGTA (Fig. 6B); for EDTA, the curve was much steeper at pH 10.5 (Fig. 6A) than at pH 7.5 (Fig. 6C).
FIG. 6.
Effect of EDTA and EGTA on the rate of pore formation by Cry1Aa. Vesicles were mixed with a solution containing 150 mM KCl and 10 mM CAPS-KOH (pH 10.5) (A and B) or 10 mM HEPES-KOH (pH 7.5) (C), 1-mg/ml bovine serum albumin, and 50 pmol Cry1Aa/mg of membrane protein. The vesicle suspension and the KCl solution contained the indicated concentration of EDTA (A and C) or EGTA (B). Data are means ± SEM of three experiments.
The addition of an excess of divalent cations after a 10-min incubation of the vesicles with EDTA was used to test the reversibility of the inhibitory effect of EDTA (Table 4). Based on the dose-response curves of Fig. 6, vesicles and toxin were first incubated for 10 min with 0.4 mM EDTA at pH 7.5 or with 0.2 mM EDTA at pH 10.5. Then, 2 mM CaCl2, MgCl2, or BaCl2 (pH 7.5) or 1 mM CaCl2 (pH 10.5) was added before the osmotic swelling experiment. Because Mg2+ and Ba2+ form insoluble hydroxides at high pH, MgCl2 and BaCl2 were only tested at pH 7.5. At both pH values, addition of divalent cations reversed the inhibition of Cry1Aa pore-forming activity by EDTA (Table 4). However, the addition of 2 mM CaCl2 at pH 7.5 significantly reduced the pore-forming activity of the toxin by approximately 25% in both the presence and absence of EDTA.
TABLE 4.
Reversibility of the effect of EDTA by an excess of divalent cations
| pH | [EDTA]a (mM) | [CaCl2] (mM) | [MgCl2] (mM) | [BaCl2] (mM) | Osmotic swelling rateb (% control activity) |
|---|---|---|---|---|---|
| 7.5 | 0.4 | 38 ± 5*** | |||
| 0.4 | 2 | 74 ± 1* | |||
| 0.4 | 2 | 119 ± 5 | |||
| 0.4 | 2 | 78 ± 6 | |||
| 2 | 75 ± 3* | ||||
| 2 | 88 ± 5 | ||||
| 2 | 79 ± 4 | ||||
| 10.5 | 0.2 | 8 ± 5*** | |||
| 0.2 | 1 | 92 ± 10 | |||
| 1 | 96 ± 3 |
EDTA was added to both the vesicle suspension and the KCl solution 10 min before the addition of CaCl2, MgCl2, or BaCl2.
Determined with 50 pmol Cry1Aa/mg of membrane protein. Data are means±SEM of three experiments. Statistical analyses are for comparisons with the control values obtained in the absence of inhibitor and added divalent cation. *, P < 0.05; ***, P < 0.001.
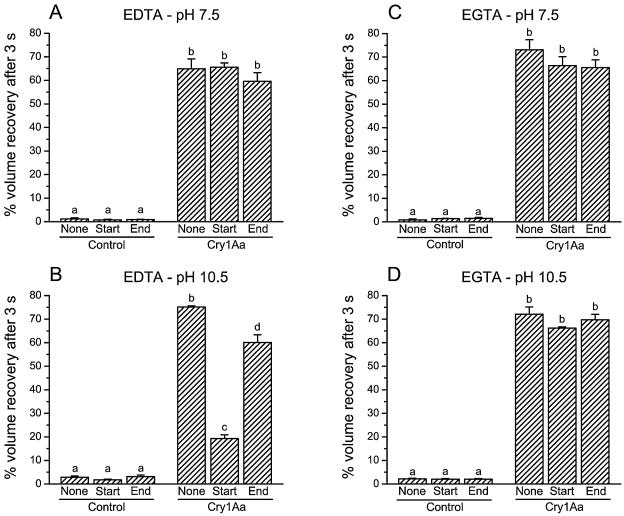
The effect of EDTA was also tested on other Cry1 toxins at pH 10.5. At 2 mM, EDTA inhibited pore formation by Cry1Ab, Cry1Ac, and Cry1Ea but had no effect on the activity of Cry1Ca (Table 5). The effect of EDTA and EGTA could be due to an inhibition of pore formation or to a modification of the properties of the pores. To distinguish between these two possibilities, incubation experiments were performed with EDTA (Fig. 7A and B) and EGTA (Fig. 7C and D). Vesicles were incubated for 60 min with or without 50 pmol toxin/mg of membrane protein. EDTA or EGTA was added to the vesicle suspension and the KCl solution 10 min before the toxin or for 10 min at the end of the incubation period. These experiments were carried out at pH 7.5 (Fig. 7A and C) and pH 10.5 (Fig. 7B and D). At pH 7.5, neither EDTA (Fig. 7A) nor EGTA (Fig. 7C) affected membrane permeability in the presence or absence of toxin when added at the beginning or at the end of the incubation period. At pH 10.5, EDTA (Fig. 7B) reduced membrane permeabilization by Cry1Aa, but EGTA (Fig. 7D) had no detectable effect. The effect of EDTA was considerably more pronounced when added at the beginning of the incubation period.
TABLE 5.
Effect of EDTA on the activity of Cry1 toxins
| Toxin | Osmotic swelling ratea (% control activity) |
|---|---|
| Cry1Aa | 6 ± 3*** |
| Cry1Ab | 8 ± 8*** |
| Cry1Ac | 0.7 ± 0.4*** |
| Cry1Ca | 101 ± 2 |
| Cry1Ea | 5 ± 2*** |
Determined at pH 10.5 in the presence of 2 mM EDTA with 50 pmol toxin/mg of membrane protein for Cry1Aa, Cry1Ab, Cry1Ac, and Cry1Ea and with 150 pmol toxin/mg of membrane protein for Cry1Ca. Data are means ± SEM of three experiments. Statistical analyses are for comparisons with the control values obtained in the absence of inhibitor. ***, P < 0.001.
FIG. 7.
Effect of EDTA and EGTA additions during incubation of the vesicles with Cry1Aa. Vesicles were incubated for 60 min and mixed with a solution containing 150 mM KCl and 10 mM HEPES-KOH (pH 7.5) (A and C) or CAPS-KOH (pH 10.5) (B and D) and 1-mg/ml bovine serum albumin. Vesicles were incubated either without (Control) or with 50 pmol Cry1Aa/mg of membrane protein. EDTA (A and B) or EGTA (C and D) were added at a final concentration of 2 mM at the beginning of the incubation period, 10 min before the addition of the toxin (Start), or after an incubation of 50 min with the toxin (End). Data are means ± SEM of three experiments. Bars labeled with the same letter are not significantly different (P > 0.05).
DISCUSSION
To test the hypothesis that membrane proteases could play a role in the mode of action of B. thuringiensis toxins, the effect of a variety of protease inhibitors on the pore-forming activity of Cry1Aa was tested with brush border membrane vesicles. In this assay, the only proteases present are those located at the surface of the vesicles. Commonly used inhibitors of each class of proteases were chosen. Since the principal proteases found in the lepidopteran midgut are serine proteases, mainly trypsins and chymotrypsins (58), almost half of the inhibitors tested were active against this type of proteases. PMSF promoted Cry1Aa pore formation in the kinetic experiments, but the other six serine protease inhibitors tested, including AEBSF, another sulfonyl fluoride inhibitor, were ineffective. Thus, PMSF clearly increased the rate of pore formation, but this does not appear to be due to the inhibition of a membrane protease that could inhibit pore formation by degrading toxin molecules. This interpretation is further supported by the fact that stimulation of toxin activity requires substantially higher concentrations of PMSF than commonly used to inhibit proteases. Unfortunately, AEBSF could not be used at concentrations at which PMSF was stimulatory (3 to 5 mM) because under these conditions the solutions became turbid in the stopped-flow apparatus. Apparently, this was caused by an interaction of AEBSF with the silicone grease used to lubricate the syringes. Synergistic effects between B. thuringiensis toxins and serine protease inhibitors have nevertheless been documented (39, 55). For instance, MacIntosh et al. (39) reported that micromolar amounts of the soybean serine protease inhibitors Bowman-Birk and Kunitz potentiate (2 to 40 fold) the insecticidal activity of B. thuringiensis toxins against Heliothis virescens, Heliothis zea, Leptinotarsa decemlineata, M. sexta, and Trichoplusia ni; it was suggested that such enhancement could be partly the result of reduced toxin degradation when midgut juice proteases are inhibited (47). However, Tabashnik et al. (57) found that these two inhibitors failed to synergize toxin activity in Plutella xylostella.
The lack of effect of PMSF, when added at the beginning or at the end of the incubation experiments, shows that PMSF does not change the maximum number of pores that can be formed in the vesicles and does not alter the biophysical properties of the pores. Furthermore, pretreatment of the vesicles and toxin molecules with PMSF does not increase the rate of pore formation in the kinetic experiments, suggesting that PMSF acts specifically during the process of pore formation. The effect of PMSF could possibly be explained by a modification of the toxin molecule on a serine residue (22) that only becomes accessible after binding of the toxin to the receptor. This modification could enhance toxin activity by adding one or several hydrophobic groups on the toxin molecule that could accelerate toxin insertion into the membrane. PMSF not only stimulated Cry1Aa but also enhanced the rate of pore formation by Cry1Ab, Cry1Ac, Cry1Ca, and Cry1Ea. However, the fact that PMSF promotes pore formation only when added simultaneously with the toxin and vesicles complicates its potential use to enhance the activity of commercial B. thuringiensis-based products, since PMSF could also inhibit midgut proteases that are essential for toxin activation. Furthermore, PMSF is relatively toxic and not very stable in water (28, 51).
EDTA and EGTA reduced the rate of pore formation by Cry1Aa at pH 10.5, but only EDTA was inhibitory at pH 7.5. EDTA and EGTA did not prevent pore formation during a long (60-min) incubation period with the toxin and did not affect the properties of the pores already formed. At pH 10.5, the rate of pore formation by Cry1Ab, Cry1Ac, and Cry1Ea was also reduced by EDTA, but this chelator did not affect that of Cry1Ca. Since under certain conditions, pore formation was not affected by divalent cation chelators, the effect of EDTA and EGTA on toxin activity does not correlate with the inhibition of a proteolytic activity that would be essential for toxin activity. This conclusion is supported by the lack of effect of o-phenanthroline, which has a much higher stability constant for heavy metal ions (essential cofactors for the activity of metalloproteases) than for calcium (51). Furthermore, the effect of EDTA and EGTA can be reversed in a nonspecific way by the addition of an excess of calcium, magnesium, or barium. In contrast with earlier suggestions (16, 38) but in agreement with other experiments using brush border membrane vesicles (25, 30, 63), calcium and barium do not block the pores formed by the toxin. The slight reduction in the activity of Cry1Aa observed in the presence of 2 mM CaCl2 at pH 7.5 can be explained by an effect of the increased ionic strength of the KCl solution (M. Fortier, V. Vachon, M. Kirouac, J.-L. Schwartz, and R. Laprade, submitted for publication).
The best-characterized receptors for Cry1Aa and other B. thuringiensis Cry toxins are aminopeptidase N and cadherin-like proteins (52). EDTA and EGTA could possibly influence the enzymatic activity or the stability of these receptors. Aminopeptidase N from Bombyx mori (27), for example, is a zinc enzyme that is well inhibited by o-phenanthroline and bestatin but poorly inhibited by EDTA. In the present study, the pore-forming activity of Cry1Aa was not affected by o-phenanthroline or bestatin, indicating that the role of aminopeptidase N as a toxin receptor is independent of its enzymatic activity. This conclusion was already suggested by Lorence et al. (37), although the reliability of the fluorometric assay that was used to monitor pore formation (37) has been questioned (31). However, it is in contradiction with the interpretation recently formulated by Hossain et al. (26) according to whom the activity of aminopeptidase N can modify the N terminus of Cry toxins to expose a recognition site for receptor binding. On the other hand, chelation of calcium by EDTA and EGTA favors cleavage of the cadherin-like toxin receptor BT-R1 (9, 40). However, the major degradation products appear to retain full capacity to bind Cry1Ab in ligand blot experiments, even after prolonged (24-h) incubation (9, 40). It is nevertheless not clear whether binding of the toxin to a partially degraded receptor leads to pore formation. Calcium-dependent structural integrity of the cadherins does not to appear to be important for toxin activity, since EGTA had no effect at pH 7.5 and Cry1Aa always formed pores following a 60-min incubation in the presence of either Ca2+ chelator.
Cultured High Five insect cells have recently been shown to become susceptible to Cry1Ab when expressing the cadherin receptor BT-R1 (69). Interestingly, Cry1Ab toxicity was abolished by EDTA but not by EGTA. Based on this observation and the fact that EGTA chelates Ca2+ much better than Mg2+, it was suggested that toxicity could involve the activation of an Mg2+-dependent intracellular signaling pathway (69). The inhibition by EDTA of pore formation observed in the present study, however, contradicts this hypothesis, since signaling pathways clearly cannot be operational in isolated brush border membrane vesicles. The observation that EGTA had a detectable effect on toxin activity in osmotic swelling experiments but was ineffective in experiments performed with cultured cells is consistent with the use of a higher pH in the former experiments and the fact that EGTA chelates Mg2+ more efficiently as pH is increased (54). The effects of EDTA and EGTA could therefore be better explained by the chelation of divalent cations involved elsewhere in the mechanism of pore formation, at the level of the membrane, such as the oligomerization of toxin molecules. It should be pointed out that the observation that EDTA is more strongly inhibitory than EGTA does not necessarily imply that Mg2+ is required for pore formation. The possibility that EDTA inhibits toxin activity because of its ability to chelate efficiently most divalent cations cannot be excluded. The observed effects of EDTA and EGTA nevertheless contrast with bioassays, demonstrating a synergistic effect of B. thuringiensis toxins and EDTA, which reduces 50% lethal concentrations of B. thuringiensis toxins by 5 fold against P. xylostella (36), 10 fold against Agrotis ipsilon (50), and 100 fold against Plodia interpunctella (20). The mechanism by which these chelators enhance toxicity thus appears to differ from a simple depletion of divalent cations in the midguts of the larvae.
The effect of PMSF, EDTA, and EGTA does not correlate with the inhibition of the activity of a membrane protease; all the other protease inhibitors tested had little effect on pore formation by Cry1Aa. Thus, in contrast with earlier suggestions based on experiments carried out with Cry1Ab (8, 23), a cleavage in domain I by membrane proteases does not appear to be a necessary step in the mode of action of B. thuringiensis toxins. Nevertheless, we cannot exclude the possibility that brush border membrane vesicles contain a membrane protease that could not be inhibited by any of the compounds used in this study and that this unusual protease plays a critical role in the activity of the toxin. It also remains possible that a proteolytic cleavage within domain I of the receptor-bound toxin stimulates pore formation (35). However, brush border membrane proteases do not appear to be sufficient to allow such a cleavage, at least after vesicles are prepared. The possible involvement of soluble proteases from the insect midgut remains to be studied. Since we have observed that brush border membrane vesicles are destabilized by midgut juice, another experimental system will be required to address this question. Preliminary results indicate that this can be achieved by the electrophysiological technique developed in our laboratory (49).
Acknowledgments
We thank Dominique Michaud, Université Laval, Quebec, Canada, for his help in preparing toxin samples for sequencing.
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada, the Fonds québécois de la recherche sur la nature et les technologies (FQRNT), and Valorisation-Recherche Québec. M. Kirouac received a graduate scholarship from the FQRNT.
REFERENCES
- 1.Andrews, R. E., Jr., M. M. Bibilos, and L. A. Bulla, Jr. 1985. Protease activation of the entomocidal protoxin of Bacillus thuringiensis subsp. kurstaki. Appl. Environ. Microbiol. 50:737-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angsuthanasombat, C., N. Crickmore, and D. J. Ellar. 1993. Effects on toxicity of eliminating a cleavage site in a predicted interhelical loop in Bacillus thuringiensis CryIVB δ-endotoxin. FEMS Microbiol. Lett. 111:255-262. [DOI] [PubMed] [Google Scholar]
- 3.Audtho, M., A. P. Valaitis, O. Alzate, and D. H. Dean. 1999. Production of chymotrypsin-resistant Bacillus thuringiensis Cry2Aa1 δ-endotoxin by protein engineering. Appl. Environ. Microbiol. 65:4601-4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bah, A., K. van Frankenhuyzen, R. Brousseau, and L. Masson. 2004. The Bacillus thuringiensis Cry1Aa toxin: effects of trypsin and chymotrypsin site mutations on toxicity and stability. J. Invertebr. Pathol. 85:120-127. [DOI] [PubMed] [Google Scholar]
- 5.Barrett, A. J. 1994. Classification of peptidases. Methods Enzymol. 244:1-15. [DOI] [PubMed] [Google Scholar]
- 6.Bietlot, H., P. R. Carey, C. Choma, H. Kaplan, T. Lessard, and M. Pozsgay. 1989. Facile preparation and characterization of the toxin from Bacillus thuringiensis var. kurstaki. Biochem. J. 260:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonserm, P., P. Davis, D. J. Ellar, and J. Li. 2005. Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications. J. Mol. Biol. 348:363-382. [DOI] [PubMed] [Google Scholar]
- 8.Bravo, A., I. Gómez, J. Conde, C. Muñoz-Garay, J. Sánchez, R. Miranda, M. Zhuang, S. S. Gill, and M. Soberón. 2004. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta 1667:38-46. [DOI] [PubMed] [Google Scholar]
- 9.Candas, M., B. R. Francis, N. B. Griko, E. G. Midboe, and L. A. Bulla, Jr. 2002. Proteolytic cleavage of the developmentally important cadherin BT-R1 in the midgut epithelium of Manduca sexta. Biochemistry 41:13717-13724. [DOI] [PubMed] [Google Scholar]
- 10.Carroll, J., D. Convents, J. Van Damme, A. Boets, J. Van Rie, and D. J. Ellar. 1997. Intramolecular proteolytic cleavage of Bacillus thuringiensis Cry3A δ-endotoxin may facilitate its coleopteran toxicity. J. Invertebr. Pathol. 70:41-49. [DOI] [PubMed] [Google Scholar]
- 11.Carroll, J., and D. J. Ellar. 1993. An analysis of Bacillus thuringiensis δ-endotoxin action on insect-midgut-membrane permeability using a light-scattering assay. Eur. J. Biochem. 214:771-778. [DOI] [PubMed] [Google Scholar]
- 12.Carroll, J., J. Li, and D. J. Ellar. 1989. Proteolytic processing of a coleopteran-specific δ-endotoxin produced by Bacillus thuringiensis var. tenebrionis. Biochem. J. 261:99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choma, C. T., W. K. Surewicz, P. R. Carey, M. Pozsgay, T. Raynor, and H. Kaplan. 1990. Unusual proteolysis of the protoxin and toxin from Bacillus thuringiensis. Structural implications. Eur. J. Biochem. 189:523-527. [DOI] [PubMed] [Google Scholar]
- 14.Convents, D., M. Cherlet, J. Van Damme, I. Lasters, and M. Lauwereys. 1991. Two structural domains as a general fold of the toxic fragment of the Bacillus thuringiensis δ-endotoxins. Eur. J. Biochem. 195:631-635. [DOI] [PubMed] [Google Scholar]
- 15.Coux, F., V. Vachon, C. Rang, K. Moozar, L. Masson, M. Royer, M. Bes, S. Rivest, R. Brousseau, J.-L. Schwartz, R. Laprade, and R. Frutos. 2001. Role of interdomain salt bridges in the pore-forming ability of the Bacillus thuringiensis toxins Cry1Aa and Cry1Ac. J. Biol. Chem. 276:35546-35551. [DOI] [PubMed] [Google Scholar]
- 16.Crawford, D. N., and W. R. Harvey. 1988. Barium and calcium block Bacillus thuringiensis subspecies kurstaki δ-endotoxin inhibition of potassium current across isolated midgut of larval Manduca sexta. J. Exp. Biol. 137:277-286. [DOI] [PubMed] [Google Scholar]
- 17.Dai, S.-M., and S. S. Gill. 1993. In vitro and in vivo proteolysis of the Bacillus thuringiensis subsp. israelensis CryIVD protein by Culex quinquefasciatus larval midgut proteases. Insect Biochem. Mol. Biol. 23:273-283. [DOI] [PubMed] [Google Scholar]
- 18.Dow, J. A. T. 1984. Extremely high pH in biological systems: a model for carbonate transport. Am. J. Physiol. 246:R633-R635. [DOI] [PubMed] [Google Scholar]
- 19.Dow, J. A. T. 1992. pH gradients in lepidopteran midgut. J. Exp. Biol. 172:355-375. [DOI] [PubMed] [Google Scholar]
- 20.El-Moursy, A., R. Aboul-Ela, H. S. Salama, and A. Abdel-Razek. 1992. Chemical additives that affect the potency of endotoxin of Bacillus thuringiensis against Plodia interpunctella. Insect Sci. Applic. 13:775-779. [Google Scholar]
- 21.Galitsky, N., V. Cody, A. Wojtczak, D. Ghosh, J. R. Luft, W. Pangporn, and L. English. 2001. Structure of the insecticidal bacterial δ-endotoxin Cry3Bb1 of Bacillus thuringiensis. Acta Crystallogr. D Biol. Crystallogr. 57:1101-1109. [DOI] [PubMed] [Google Scholar]
- 22.Gold, A. M. 1965. Sulfonyl fluorides as inhibitors of esterases. III. Identification of serine as the site of sulfonylation in phenylmethanesulfonyl α-chymotrypsin. Biochemistry 4:897-901. [DOI] [PubMed] [Google Scholar]
- 23.Gómez, I., J. Sánchez, R. Miranda, A. Bravo, and M. Soberón. 2002. Cadherin-like receptor binding facilitates proteolytic cleavage of helix α-1 in domain I and oligomer pre-pore formation of Bacillus thuringiensis Cry1Ab toxin. FEBS Lett. 513:242-246. [DOI] [PubMed] [Google Scholar]
- 24.Grochulski, P., L. Masson, S. Borisova, M. Pusztai-Carey, J.-L. Schwartz, R. Brousseau, and M. Cygler. 1995. Bacillus thuringiensis CryIA(a) insecticidal toxin: crystal structure and channel formation. J. Mol. Biol. 254:447-464. [DOI] [PubMed] [Google Scholar]
- 25.Hendrickx, K., A. De Loof, and H. Van Mellaert. 1990. Effects of Bacillus thuringiensis delta-endotoxin on the permeability of brush border membrane vesicles from tobacco hornworm (Manduca sexta) midgut. Comp. Biochem. Physiol. 95C:241-245. [DOI] [PubMed] [Google Scholar]
- 26.Hossain, D. M., Y Shitomi, K. Moriyama, M. Higuchi, T. Hayakawa, T. Mitsui, R. Sato, and H. Hori. 2004. Characterization of a novel plasma membrane protein, expressed in the midgut epithelia of Bombyx mori, that binds to Cry1A toxins. Appl. Environ. Microbiol. 70:4604-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hua, G., K. Tsukamoto, R. Taguchi, M. Tomita, S. Miyajima, and H. Ikezawa. 1998. Characterization of aminopeptidase N from the brush border membrane of the larvae midgut of silkworm, Bombyx mori as a zinc enzyme. Biochim. Biophys. Acta 1383:301-310. [DOI] [PubMed] [Google Scholar]
- 28.James, G. T. 1978. Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers. Anal. Biochem. 86:574-579. [DOI] [PubMed] [Google Scholar]
- 29.Keller, M., B. Sneh, N. Strizhov, E. Prudovsky, A. Regev, C. Koncz, J. Schell, and A. Zilberstein. 1996. Digestion of δ-entotoxin by gut proteases may explain reduced sensitivity of advanced instar larvae of Spodoptera littoralis to Cry1C. Insect Biochem. Mol. Biol. 26:365-373. [DOI] [PubMed] [Google Scholar]
- 30.Kirouac, M., V. Vachon, J.-F. Noël, F. Girard, J.-L. Schwartz, and R. Laprade. 2002. Amino acid and divalent ion permeability of the pores formed by the Bacillus thuringiensis toxins Cry1Aa and Cry1Ac in insect midgut brush border membrane vesicles. Biochim. Biophys. Acta 1561:171-179. [DOI] [PubMed] [Google Scholar]
- 31.Kirouac, M., V. Vachon, S. Rivest, J.-L. Schwartz, and R. Laprade. 2003. Analysis of the properties of Bacillus thuringiensis insecticidal toxins using a potential-sensitive fluorescent probe. J. Membr. Biol. 196:51-59. [DOI] [PubMed] [Google Scholar]
- 32.Lambert, B., L. Buysse, C. Decock, S. Jansens, C. Piens, B. Saey, J. Seurinck, K. Van Audenhove, J. Van Rie, A. Van Vliet, and M. Peferoen. 1996. A Bacillus thuringiensis insecticidal crystal protein with a high activity against members of the family Noctuidae. Appl. Environ. Microbiol. 62:80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, J., J. Carroll, and D. J. Ellar. 1991. Crystal structure of insecticidal δ-endotoxin from Bacillus thuringiensis at 2.5 Å resolution. Nature 353:815-821. [DOI] [PubMed] [Google Scholar]
- 34.Li, J., D. J. Derbyshire, B. Promdonkoy, and D. J. Ellar. 2001. Structural implications for the transformation of the Bacillus thuringiensis δ-endotoxins from water-soluble to membrane-inserted forms. Biochem. Soc. Trans. 29:571-577. [DOI] [PubMed] [Google Scholar]
- 35.Lightwood, D. J., D. J. Ellar, and P. Jarrett. 2000. Role of proteolysis in determining potency of Bacillus thuringiensis Cry1Ac δ-endotoxin. Appl. Environ. Microbiol. 66:5174-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, Y.-B., and B. E. Tabashnik. 1997. Synergism of Bacillus thuringiensis by ethylenediamine tetraacetate in susceptible and resistant larvae of diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 90:287-292. [Google Scholar]
- 37.Lorence, A., A. Darszon, and A. Bravo. 1997. Aminopeptidase dependent pore formation of Bacillus thuringiensis Cry1Ac toxin on Trichoplusia ni membranes. FEBS Lett. 414:303-307. [DOI] [PubMed] [Google Scholar]
- 38.Lorence, A., A. Darszon, C. Díaz, A. Liévano, R. Quintero, and A. Bravo. 1995. δ-Endotoxins induce cation channels in Spodoptera frugiperda brush border membranes in suspension and in planar lipid bilayers. FEBS Lett. 360:217-222. [DOI] [PubMed] [Google Scholar]
- 39.MacIntosh, S. C., G. M. Kishore, F. J. Perlak, P. G. Marrone, T. B. Stone, S. R. Sims, and R. L. Fuchs. 1990. Potentiation of Bacillus thuringiensis insecticidal activity by serine protease inhibitors. J. Agric. Food Chem. 38:1145-1152. [Google Scholar]
- 40.Martínez-Ramírez, A. C., S. González-Nebauer, B. Escriche, and M. D. Real. 1994. Ligand blot identification of a Manduca sexta midgut binding protein specific to three Bacillus thuringiensis CryIA-type ICPs. Biochem. Biophys. Res. Commun. 201:782-787. [DOI] [PubMed] [Google Scholar]
- 41.Masson, L., A. Mazza, L. Gringorten, D. Baines, V. Aneliunas, and R. Brousseau. 1994. Specificity domain localization of Bacillus thuringiensis insecticidal toxins is highly dependent on the bioassay system. Mol. Microbiol. 14:851-860. [DOI] [PubMed] [Google Scholar]
- 42.Masson, L., G. Préfontaine, L. Péloquin, P. C. K. Lau, and R. Brousseau. 1989. Comparative analysis of the individual protoxin components in P1 crystals of Bacillus thuringiensis subsp. kurstaki isolates NRD-12 and HD-1. Biochem. J. 269:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miranda, R., F. Z. Zamudio, and A. Bravo. 2001. Processing of Cry1Ab δ-endotoxin from Bacillus thuringiensis by Manduca sexta and Spodoptera frugiperda midgut proteases: role in protoxin activation and toxin inactivation. Insect Biochem. Mol. Biol. 31:1155-1163. [DOI] [PubMed] [Google Scholar]
- 44.Morse, R. J., T. Yamamoto, and R. M. Stroud. 2001. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure 9:409-417. [DOI] [PubMed] [Google Scholar]
- 45.Nichols, C. N., W. Ahmad, and D. J. Ellar. 1989. Evidence for two different types of insecticidal P2 toxins with dual specificity in Bacillus thuringiensis subspecies. J. Bacteriol. 171:5141-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogiwara, K., L. S. Indrasith, S. Asano, and H. Hori. 1992. Processing of δ-endotoxin from Bacillus thuringiensis subsp. kurstaki HD-1 and HD-73 by gut juices of various insect larvae. J. Invertebr. Pathol. 60:121-126. [DOI] [PubMed] [Google Scholar]
- 47.Pang, A. S. D., and J. L. Gringorten. 1998. Degradation of Bacillus thuringiensis δ-endotoxin in host insect gut juice. FEMS Microbiol. Lett. 167:281-285. [Google Scholar]
- 48.Pang, A. S. D., J. L. Gringorten, and C. Bai. 1999. Activation and fragmentation of Bacillus thuringiensis δ-endotoxin by high concentrations of proteolytic enzymes. Can. J. Microbiol. 45:816-825. [DOI] [PubMed] [Google Scholar]
- 49.Peyronnet, O., V. Vachon, R. Brousseau, D. Baines, J.-L. Schwartz, and R. Laprade. 1997. Effect of Bacillus thuringiensis toxins on the membrane potential of lepidopteran insect midgut cells. Appl. Environ. Microbiol. 63:1679-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salama, H. S., M. S. Foda, and A. Sharaby. 1989. Potentiation of Bacillus thuringiensis endotoxin against the greasy cutworm Agrotis ypsilon. J. Appl. Entomol. 108:372-380. [Google Scholar]
- 51.Salvesen, G., and H. Nagase. 1989. Inhibition of proteolytic enzymes, pp. 83-104. In R. J. Beynon and J. S. Bond (ed.), Proteolytic enzymes: a practical approach. IRL Press, Oxford, United Kingdom.
- 52.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoellmann, G., and E. Shaw. 1963. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry 2:252-255. [DOI] [PubMed] [Google Scholar]
- 54.Schwarzenbach, G., and H. Flaschka. 1969. Complexometric titrations, 2nd ed., p. 3-30. Methuen & Co., Ltd., London, United Kingdom.
- 55.Shao, Z., Y. Cui, X. Liu, H. Yi, J. Ji, and Z. Yu. 1998. Processing of δ-endotoxin of Bacillus thuringiensis subsp. kurstaki HD-1 in Heliothis armigera midgut juice and the effects of protease inhibitors. J. Invertebr. Pathol. 72:73-81. [DOI] [PubMed] [Google Scholar]
- 56.Shaw, E., M. Mares-Guia, and W. Cohen. 1965. Evidence for an active-center histidine in trypsin through use of a specific reagent, 1-chloro-3-tosylamido-7-amino-2-heptanone, the chloromethyl ketone derived from Nα-tosyl-l-lysine. Biochemistry 4:2219-2224. [Google Scholar]
- 57.Tabashnik, B. E., N. Finson, and M. W. Johnson. 1992. Two protease inhibitors fail to synergize Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 85:2082-2087. [Google Scholar]
- 58.Terra, W. R., and C. Ferreira. 1994. Insect digestive enzymes: properties, compartmentalization and function. Comp. Biochem. Physiol. 109B:1-62. [Google Scholar]
- 59.Tran, L. B., V. Vachon, J.-L. Schwartz, and R. Laprade. 2001. Differential effects of pH on the pore-forming properties of Bacillus thuringiensis insecticidal toxins. Appl. Environ. Microbiol. 67:4488-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vachon, V., G. Préfontaine, F. Coux, C. Rang, L. Marceau, L. Masson, R. Brousseau, R. Frutos, J.-L. Schwartz, and R. Laprade. 2002. Role of helix 3 in pore formation by the Bacillus thuringiensis insecticidal toxin Cry1Aa. Biochemistry 41:6178-6184. [DOI] [PubMed] [Google Scholar]
- 61.Visser, B., E. Munsterman, A. Stoker, and W. G. Dirkse. 1990. A novel Bacillus thuringiensis gene encoding a Spodoptera exigua-specific crystal protein. J. Bacteriol. 172:6783-6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Visser, B., T. Van der Salm, W. Van den Brink, and G. Folkers. 1988. Genes from Bacillus thuringiensis entomocidus 60.5 coding for insect-specific crystal proteins. Mol. Gen. Genet. 212:219-224. [Google Scholar]
- 63.Wolfersberger, M. G. 1989. Neither barium nor calcium prevents the inhibition by Bacillus thuringiensis δ-endotoxin of sodium- or potassium gradient-dependent amino acid accumulation by tobacco hornworm midgut brush border membrane vesicles. Arch. Insect Biochem. Physiol. 12:267-277. [Google Scholar]
- 64.Wolfersberger, M., P. Luethy, A. Maurer, P. Parenti, F. V. Sacchi, B. Giordana, and G. M. Hanozet. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86A:301-308. [Google Scholar]
- 65.Wolfson, J. L., and L. L. Murdock. 1990. Diversity in digestive proteinase activity among insects. J. Chem. Ecol. 16:1089-1102. [DOI] [PubMed] [Google Scholar]
- 66.Yamagiwa, M., M. Esaki, K. Otake, M. Inagaki, T. Komano, T. Amachi, and H. Sakai. 1999. Activation process of dipteran-specific insecticidal protein produced by Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 65:3464-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamagiwa, M., R. Ogawa, K. Yasuda, H. Natsuyama, K. Sen, and H. Sakai. 2002. Active form of dipteran-specific insecticidal protein Cry11A produced by Bacillus thuringiensis subsp. israelensis. Biosci. Biotechnol. Biochem. 66:516-522. [DOI] [PubMed] [Google Scholar]
- 68.Zalunin, I. A., L. P. Revina, L. I. Kostina, G. G. Chestukhina, and V. M. Stepanov. 1998. Limited proteolysis of Bacillus thuringiensis Cry1G and CryIVB δ-endotoxins leads to formation of active fragments that do not coincide with the structural domains. J. Protein Chem. 17:463-471. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, X., M. Candas, N. B. Griko, L. Rose-Young, and L. A. Bulla, Jr. 2005. Cytotoxicity of Bacillus thuringiensis Cry1Ab toxin depends on specific binding of the toxin to the cadherin receptor BT-R1 expressed in insect cells. Cell Death Differ. 12:1407-1416. [DOI] [PubMed] [Google Scholar]