Abstract
The spore coat protein CotA from Bacillus subtilis was previously identified as a laccase. We have now found that CotA also shows strong bilirubin oxidase activity and markedly higher affinity for bilirubin than conventional bilirubin oxidase. This is the first characterization of bilirubin oxidase activity in a bacterial protein.
Bilirubin oxidase (BOD; EC 1.3.3.5) catalyzes the oxidation of bilirubin to biliverdin (2) and is used clinically to determine the levels of total and conjugated bilirubin in serum (3, 12, 14). It is distributed mainly in fungi, and BODs from Myrothecium verrucaria (20) and Trachyderma tsunodae K-2593 (6) have been purified and characterized. BOD is thought to be a member of the multicopper oxidase family because it has been shown to bind a type I (blue) copper ion, a paramagnetic (electron paramagnetic resonance) type II copper ion, and a pair of diamagnetic type III copper ions (6, 16). Moreover, strong sequence identity between BOD and the other multicopper oxidases, including laccase (EC 1.10.3.2), ascorbate oxidase (EC 1.10.3.3), and ceruloplasmin (EC 1.16.3.1), particularly at potential copper coordination sites (8) (Fig. 1), has been widely reported.
FIG. 1.
Comparison of the amino acid sequences of potential copper coordination sites in multicopper oxidases. CotA, B. subtilis CotA (NCBI entry NP_388511); BOD, M. verrucaria BOD (NCBI entry Q12737); CLA, Coprinus cinereus laccase (NCBI entry 1HFUA); TLA, Trametes villosa laccase (NCBI entry AAB47735); MLA, Melanocarpus albomyces laccase (NCBI entry CAE00180). The numbers 1, 2, and 3 indicate the potential coordination sites for type 1, 2, and 3 copper ions, respectively. The amino acid residues presumed to be involved in binding copper are boxed. The amino acid residues of CotA closely related to those of BOD are indicated by arrowheads.
On the other hand, BOD differs from other multicopper oxidases in that it catalyzes reactions involving substances comprised of tetrapyrroles, especially bilirubin (8, 21). It is known that BOD oxidizes some laccase substrates (20, 22); however, laccase, ascorbate oxidase, and ceruloplasmin show little or no BOD activity (1, 21), though one exception is the alkaliphilic laccase from Myrothecium verrucaria 24G-4 (18). It has been proposed that these differences in substrate specificity reflect the heterogeneity of the amino acid sequences within the consensus domains (8). X-ray crystallographic analysis of the structure of Coprinus cinereus laccase showed that the type I copper center of the enzyme contains His396, Cys452, and His457 as the copper ligands and a nonligating Leu462 at a position axial to the ligand (4). In addition, Kumar et al. (11) reported that most, but not all, fungal laccases carry a Leu or a Phe at this axial position 10 residues downstream from Cys452 (Fig. 1), though several other multicopper oxidases, including BOD, feature a Met at this position. Finally, Koikeda et al. (8) showed that Leu442 and Asp504, within the C-terminal consensus domain of M. verrucaria BOD, are not present in the other multicopper oxidases (Fig. 1).
Recently, the Bacillus subtilis endospore component CotA was identified as a bacterial laccase (7, 13), and the crystal structure of the protein was solved (5). This is the first bacterial laccase that has been both functionally and structurally characterized in detail. CotA shows relatively high (19.7 to 22.4%) amino acid sequence identity with multicopper oxidases (13) and contains the four consensus copper-binding sites (17). We also analyzed the consensus domains of CotA and found that the amino acid sequences around the copper coordination sites are closely related to those of BOD in terms of the aforementioned features; that is, CotA has a Met at the position corresponding to Leu462 in C. cinereus laccase and Leu/Asp at the positions corresponding to the Leu442/Asp504 in M. verrucaria BOD (Fig. 1). Notably, when we expressed the CotA gene in Escherichia coli and examined characteristics of the product, we found that in addition to the previously identified laccase activity, the enzyme also shows strong BOD activity. Here we describe the characteristics of this first identified bacterial BOD. In addition, we describe our application of this enzyme for the determination of bilirubin levels in human serum.
Production and purification of recombinant CotA.
It was previously reported that recombinant CotA protein was mainly produced as a precipitate when pET21a(+) and E. coli AH3517 were used for the gene expression and that attempts to recover the soluble protein from the precipitate were unsuccessful (13). This means that the gene product was produced as an inclusion body in the cell extract. To improve the protein's solubility, we evaluated its production by use of several combinations of expression vectors, host cells, and cultivation conditions. We found that E. coli JM109 transformed with pUC119 carrying the CotA gene produces large amounts of the soluble protein under low-temperature conditions. For construction of the expression vector, the oligonucleotide primers used to amplify the CotA gene fragment by PCR were 5′-ATAAAAGCTTATGAAATGACACTTGAAAAATT-3′, which contains a unique HindIII restriction site overlapping the 5′ initial codon (italics), and 5′-TCATGTGGATCCTGTGTGAGCATAAAAGCTCC-3′, which contains a unique BamHI restriction site proximal to the 3′ end of the termination codon (italics). Chromosomal B. subtilis DNA isolated as described previously (15) served as the template. The amplified 1.5 kbp fragment was digested with HindIII and BamHI and ligated with pUC119 vector linearized with the same restriction enzymes to generate pUC119BOX8. E. coli JM109 was then transformed with pUC119BOX8 and cultivated in Luria-Bertani medium containing 0.25 mM CuSO4 and 50 μg/ml ampicillin. With this protocol, the yield of soluble CotA was dependent on the cultivation temperature (Fig. 2). At 21°C there was very little production, and beyond 31°C soluble protein accounted for less than 5% of the total CotA protein. But at 24°C approximately 50% of the CotA protein was detected in the soluble fraction.
FIG. 2.

Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of B. subtilis CotA heterologously produced in E. coli. Insoluble fractions of crude extract from pUC119BOX8 cultivated at 21, 24, and 31°C were loaded onto lanes 1, 3, and 5, respectively; the soluble fractions were loaded onto lanes 2, 4, and 6, respectively. The arrow indicates the position of CotA.
So cultivation of the recombinant E. coli was carried out at 24°C for 20 h, after which the cells were harvested by centrifugation, suspended in 10 mM potassium phosphate buffer (pH 7.0) containing 1 mM CuSO4, and disrupted by ultrasonication. The entire operation was done at room temperature (∼25°C), and the fractions containing CotA were checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis at each purification step. The crude extract was heated at 65°C for 40 min, and the denatured protein was then removed by centrifugation. The remaining protein solution was then loaded onto a Q Sepharose Big Beads column (Amersham Biosciences) (50 by 300 mm) equilibrated with 10 mM potassium phosphate buffer (pH 7.0), and after the column was washed with the same buffer, the protein was eluted with a linear gradient of 0 to 0.5 M KCl in the same buffer. The fractions containing CotA were collected, and solid (NH4)2SO4 was added to achieve a 25% (wt/vol) concentration. The protein solution was then loaded onto a phenyl Sepharose 6 Fast Flow column (Amersham Biosciences) (50 by 300 mm) equilibrated with 10 mM potassium phosphate buffer (pH 7.0) containing 25% (NH4)2SO4, and after the column was washed with the same buffer, the protein was eluted with a linear gradient of 25% to 0% (NH4)2SO4 in the same buffer. The CotA-containing fractions were collected and dialyzed against 10 mM potassium phosphate buffer (pH 7.0), after which the protein solution was loaded onto a DEAE Sepharose Fast Flow column (Amersham Biosciences) (50 by 300 mm) equilibrated with 10 mM potassium phosphate buffer (pH 7.0). After the column was washed with the same buffer, the protein was eluted with a linear gradient of 0 to 0.5 M NaCl in the same buffer. The fractions containing CotA were pooled, concentrated by ultra filtration, and used as the purified preparation. Because the concentrated enzyme (19 mg/ml) was easily precipitated when NaCl was removed from the buffer by dialysis, we ordinarily used buffer containing 0.6 M NaCl as the enzyme solution. About 2 g of purified enzyme was prepared from the cells obtained from a 38-liter culture.
Activities and enzymatic properties.
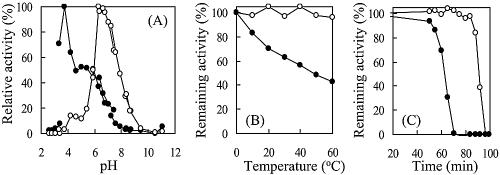
Conjugated bilirubin is known to be a better indicator of liver function than total bilirubin (3, 12, 14), and ditaurobilirubin is widely utilized as the standard for conjugated bilirubin. For that reason, we assayed the enzyme activity of the isolated CotA by use of both bilirubin and ditaurobilirubin (solubilized materials; Interference Check A) (Sysmex, Kobe, Japan) as substrates. The oxidation of bilirubin or ditaurobilirubin at 37°C was monitored spectrophotometrically at 450 nm (ɛ450 = 32 and 74 mM−1 cm−1 for bilirubin and ditaurobilirubin, respectively). Unless otherwise specified, the reaction mixture contained 100 mM potassium phosphate (pH 6.0), 20 μg of substrate, and 1 to 3 mU of enzyme in a total volume of 1 ml. The reaction was started by the addition of the enzyme. The optimum pH of the enzyme was determined by running the standard assay at 37°C in the following 40 mM buffer systems: glycine-HCl (pH 2.6 to 3.4), acetate-NaOH (pH 3.4 to 5.9), citrate-NaOH (pH 5.9 to 6.8), potassium phosphate (pH 6.3 to 7.4), Tris-HCl (pH 7.4 to 8.7), and glycine-NaOH (pH 8.4 to 11.0). The optimum pH for bilirubin oxidation was found to be about pH 7.0 whereas that for ditaurobilirubin oxidation was about pH 4.0 (Fig. 3A). M. verrucaria BOD also reportedly oxidizes ditaurobilirubin at acidic pH, which proved useful for selective measurement of conjugated bilirubin at about pH 5.5 (10). Thus, the large difference in the optimum pHs for CotA-catalyzed oxidation of bilirubin and ditaurobilirubin may be advantageous for selective determination of conjugated bilirubin levels in serum.
FIG. 3.
Optimum pH and thermostability. (A) Effects of pH on CotA-catalyzed oxidation of bilirubin (open circles) and ditaurobilirubin (closed circles). (B) Residual BOD activity after incubation at the indicated temperatures for 30 min. (C) Effect of incubation time at 65°C on BOD activity. In panels B and C, open circles and closed circles represent B. subtilis CotA and T. tsunodae K-2593 BOD, respectively.
We next compared the thermostability of CotA with that of T. tsunodae K-2593 BOD (Takara Shuzo Co., Ltd.) (Fig. 3B and C) and, as reported previously (13), found CotA to be highly thermostable. To determine its thermostability, the enzyme (10 mg/ml) was incubated in 50 mM potassium phosphate buffer (pH 7.0) containing 0.1% Triton X-100 at various temperatures, and the residual activity was determined using the standard assay described above. We found that CotA had lost none of its BOD activity after being heated at 84°C for 30 min, whereas the activity of the T. tsunodae enzyme was completely lost (Fig. 3B). CotA also retained its full activity after heating at 65°C for at least 60 min, whereas T. tsunodae BOD showed a half-life of inactivation of about 40 min at the same temperature (Fig. 3C). Thermostability has so far been reported only for M. verrucaria BOD, which rapidly inactivates at 70°C (20). CotA thus appears to be the most thermostable BOD identified to date.
The Michaelis constants were determined from double-reciprocal plots of the initial oxidation rates and concentrations of bilirubin or ditaurobilirubin at 37°C and pH 7.0. CotA showed typical Michaelis-Menten kinetics for both bilirubin and ditaurobilirubin. With bilirubin as the substrate, the apparent Km and Vmax values were 0.0080 mM and 28 μmol/min/mg, respectively. With ditaurobilirubin as the substrate, these constants were 0.015 mM and 10 μmol/min/mg, respectively. This observed Km value of CotA for bilirubin is extremely low, and the Vmax/Km ratio is more than 20 times higher than that obtained with M. verrucaria BOD (Table 1), which is frequently utilized for determining serum bilirubin levels.
TABLE 1.
Kinetic constants for bilirubin and ditaurobilirubin
Serum bilirubin assay.
We assayed total bilirubin in serum by use of CotA according to the method described by Otsuji et al. (14) and compared the results to those obtained using the commercially available Iatro T-Bil kit (Mitsubishi Kagaku Iatron, Inc., Tokyo, Japan). Sera were obtained from 51 healthy individuals and from 19 patients with hepatobiliary disease. Figure 4 shows the correlation between the results obtained with the two assays. The correlation coefficient was 0.994 over a wide range of measurements, and the slope of the linear regression equation was approximately 1.08, showing that CotA could be used for accurate determination of serum bilirubin levels.
FIG. 4.

Correlation of total bilirubin levels measured in 70 samples by use of CotA (x axis) and an Iatro T-Bil kit (y axis). The regression line was defined by the equation Y = 1.08X + 0.27; R2 = 0.994.
The genes encoding laccases have been cloned for a number of species, but in most cases the proteins are produced at levels too low to be used for commercial purposes (9, 19). In the present study, however, we were able to develop an effective protocol for the high-level production of soluble CotA in E. coli. The isolated enzyme showed markedly higher affinity for bilirubin than conventional BOD and also exhibited greater stability. CotA thus shows great potential for use as a diagnostic reagent and may serve as the basis for a useful and practical method of bilirubin determination.
A possible link between laccase activity of CotA and production of a melanin-like pigment which appears to protect spores against UV light or hydrogen peroxide has been suggested (5, 13). Although the physiological significance of the BOD activity is currently unknown, the significantly high affinity for bilirubin shows that CotA may have additional biological function beyond the laccase activity.
REFERENCES
- 1.Brodersen, R., and P. Bartels. 1969. Enzymatic oxidation of bilirubin. Eur. J. Biochem. 10:468-473. [DOI] [PubMed] [Google Scholar]
- 2.Cardenas-Vazquez, R., O. Yokosuka, and B. H. Billing. 1986. Enzymic oxidation of unconjugated bilirubin by rat liver. Biochem. J. 236:625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doumas, B. T., F. Yein, B. Perry, B. Jendrzejczak, and A. Kessner. 1999. Determination of the sum of bilirubin sugar conjugates in plasma by bilirubin oxidase. Clin. Chem. 45:1255-1260. [PubMed] [Google Scholar]
- 4.Ducros, V., A. M. Brzozowski, K. S. Wilson, S. H. Brown, P. Ostergaard, P. Schneider, D. S. Yaver, A. H. Pedersen, and G. J. Davies. 1998. Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat. Struct. Biol. 5:310-316. [DOI] [PubMed] [Google Scholar]
- 5.Enguita, F. J., L. O. Martins, A. O. Henriques, and M. A. Carrondo. 2003. Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. J. Biol. Chem. 278:19416-19425. [DOI] [PubMed] [Google Scholar]
- 6.Hiromi, K., Y. Yamaguchi, Y. Sugiura, H. Iwamoto, and J. Hirose. 1992. Bilirubin oxidase from Trachyderma tsunodae K-2593, a multi-copper enzyme. Biosci. Biotechnol. Biochem. 56:1349-1350. [Google Scholar]
- 7.Hullo, M. F., I. Moszer, A. Danchin, and I. Martin-Verstraete. 2001. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 183:5426-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koikeda, S., K. Ando, H. Kaji, T. Inoue, S. Murao, K. Takeuchi, and T. Samejima. 1993. Molecular cloning of the gene for bilirubin oxidase from Myrothecium verrucaria and its expression in yeast. J. Biol. Chem. 268:18801-18809. [PubMed] [Google Scholar]
- 9.Kojima, Y., Y. Tsukuda, Y. Kawai, A. Tsukamoto, J. Sugiura, M. Sakaino, and Y. Kita. 1990. Cloning, sequence analysis, and expression of ligninolytic phenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J. Biol. Chem. 265:15224-15230. [PubMed] [Google Scholar]
- 10.Kosaka, A., C. Yamamoto, Y. Morishita, and K. Nakane. 1987. Enzymatic determination of bilirubin fractions in serum. Clin. Biochem. 20:451-458. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, S. V., P. S. Phale, S. Durani, and P. P. Wangikar. 2003. Combined sequence and structure analysis of the fungal laccase family. Biotechnol. Bioeng. 83:386-394. [DOI] [PubMed] [Google Scholar]
- 12.Kurosaka, K., S. Senba, H. Tsubota, and H. Kondo. 1998. A new enzymatic assay for selectively measuring conjugated bilirubin concentration in serum with use of bilirubin oxidase. Clin. Chim. Acta 269:125-136. [DOI] [PubMed] [Google Scholar]
- 13.Martins, L. O., C. M. Soares, M. M. Pereira, M. Teixeira, T. Costa, G. H. Jones, and A. O. Henriques. 2002. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 277:18849-18859. [DOI] [PubMed] [Google Scholar]
- 14.Otsuji, S., K. Mizuno, S. Ito, S. Kawahara, and M. Kai. 1988. A new enzymatic approach for estimating total and direct bilirubin. Clin. Biochem. 21:33-38. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p. 9.14-9.23. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Shimizu, A., J. H. Kwon, T. Sasakis, T. Satoh, N. Sakurai, T. Sakurai, S. Yamaguchi, and T. Samejima. 1999. Myrothecium verrucaria bilirubin oxidase and its mutants for potential copper ligands. Biochemistry 38:3034-3042. [DOI] [PubMed] [Google Scholar]
- 17.Solomon, E. I., U. M. Sundaram, and T. E. Machonkin. 1996. Multicopper oxidases and oxygenases. Chem. Rev. 96:2563-2606. [DOI] [PubMed] [Google Scholar]
- 18.Sulistyaningdyah, W. T., J. Ogawa, H. Tanaka, C. Maeda, and S. Shimizu. 2004. Characterization of alkaliphilic laccase activity in the culture supernatant of Myrothecium verrucaria 24G-4 in comparison with bilirubin oxidase. FEMS Microbiol. Lett. 230:209-214. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki, T., K. Endo, M. Ito, H. Tsujibo, K. Miyamoto, and Y. Inamori. 2003. A thermostable laccase from Streptomyces lavendulae REN-7: purification, characterization, nucleotide sequence, and expression. Biosci. Biotechnol. Biochem. 67:2167-2175. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka, N., and S. Murao. 1982. Purification and some properties of bilirubin oxidase from Myrothecium verrucaria MT-1. Agric. Biol. Chem. 46:2499-2503. [Google Scholar]
- 21.Tanaka, N., and S. Murao. 1983. Difference between various copper-containing enzymes (Polyporus laccase, mushroom tyrosinase and cucumber ascorbate oxidase) and bilirubin oxidase. Agric. Biol. Chem. 47:1627-1628. [Google Scholar]
- 22.Xu, F., W. Shin, S. H. Brown, J. A. Wahleithner, U. M. Sundaram, and E. I. Solomon. 1996. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim. Biophys. Acta 1292:303-311. [DOI] [PubMed] [Google Scholar]