Abstract
The presence of Salmonella and Campylobacter spp. in rodents and insectivores (n = 282) was investigated on organic farms. Infections were encountered in house mice (8 of 83 Campylobacter positive and 1 of 83 Salmonella sp. strain Livingstone positive) and brown rats (1 of 8 Campylobacter positive) but not in other species. No shared Campylobacter genotypes were found between rodent and pig manure isolates. Effective on-farm rodent management is recommended.
Salmonella and Campylobacter spp. are the most important causes of bacterial gastroenteritis in humans and are responsible for 24% of food-borne diseases caused by known pathogens in the United States (11, 15). Food from animal origin is one of the main sources of infection (15). Prevention of the introduction of zoonotic agents in the primary production is strongly dependent on the level of biosecurity. Wild rodents may spread zoonotic bacteria between farms (5, 6, 10, 14). This risk may be even greater in organic production, where contact with livestock is more likely and rodenticides are used less often.
Rodents and insectivores were trapped on ten organic farms (nine pig farms, one broiler farm) by using live-traps between August and October 2004. Farms were subdivided into five areas: feeding passage (near the feed trough), storage (inside the stable), outdoor area (next to the stable, solid/slatted concrete floor, sometimes roofed), pasture, and “nature area” (further from stable, wild vegetation). After CO2 euthanasia, trapped animals were taken to the laboratory within hours. Upon arrival, their ceca and colons were processed.
Pig manure samples were collected on 6 of 10 farms in October 2004. Four farms were omitted because the livestock batch had meanwhile changed. On each farm, five fresh manure samples (10 g) were taken throughout the stables. These samples were pooled and analyzed for the presence of Salmonella and Campylobacter.
Rodent intestinal contents or pig manure were directly streaked on CCDA Campylobacter-selective agar plates (Oxoid CM739 + SR155) by using cotton swabs. These swabs were subsequently transferred to 5 ml of Preston enrichment broth (Oxoid CM67 + SR84 + SR204) without blood (8). Enrichment broth was incubated for 24 h at 41.5°C under microaerobic conditions and plated onto CCDA plates. All CCDA plates were incubated for 48 h at 41.5°C under microaerobic conditions and examined for presence of characteristic colonies of Campylobacter. Confirmation was performed by checking morphology and motility microscopically. The amplified fragment length polymorphism (AFLP) method was used for species identification and genotyping (3, 4).
For Salmonella detection, a swab containing 0.1 to 1 g of rodent intestinal content or pig manure was added to 9 ml of nonselective preenrichment medium, buffered peptone water (Biotrading K168B009). After preenrichment for 18 h at 37°C, 3 drops were transferred to Modified Semi-Solid Rappaport Vassiliadis agar plates (MSRV; Lab M, Lab150). MSRV plates were incubated at 41.5°C for 24 h. Growth on MSRV plates suspected to be Salmonella sp. was streaked on Brilliant Green Agar (Oxoid CM329) and examined for presence of suspected colonies after incubation for 18 to 24 h at 37°C. A suspected colony was then agglutinated with polyvalent anti-specific O antisera (Oxoid) and specific flagellar H antisera. Each isolate was biochemically confirmed (triple sugar iron [Oxoid] agar, urease, and lysine decarboxylase).
In total, 282 animals were examined, and 9 were positive for Campylobacter spp. (8 of 83 house mice, 1 of 8 brown rats). One Salmonella sp. strain Livingstone-positive house mouse was detected. Positive animals were only found in the storage, feeding passage and outdoor areas (Table 1). On three farms (A, B, and C), three single rodent isolates were obtained. On farm A, 1 of 6 (16.7%) house mice was positive (Campylobacter coli). On farm B, 1 of 8 (12.5%) brown rats caught was positive (C. coli). On farm C, 1 of 30 (3.3%) house mice was positive (Campylobacter spp.). This isolate was lost in follow-up and could not be speciated. On farm D, 1 C. hyointestinalis, 2 C. coli, and 3 C. jejuni strains were isolated from 6 of 15 (40%) house mice that were caught. The Salmonella sp. strain Livingstone-positive house mouse (also positive for C. jejuni) was caught here. Salmonella and Campylobacter spp. were not isolated from the pig manure at this farm. Overall, Campylobacter strains was isolated from pig manure on three of six farms (farms A, B, and E), but Salmonella strains were not encountered.
TABLE 1.
Cross-tabulation of animal species (n = 283), their numbers, and the area in which they were encountered
| Species | Latin name | Total no. | No. found in (area of farm)a:
|
||||
|---|---|---|---|---|---|---|---|
| Feed passage | Storage | Outdoor area | Pasture | Nature area | |||
| White-toothed shrew | Crocidura russula | 119 | 4 | 20 | 29 | 23 | 43 |
| Common shrew | Sorex araneus | 10 | 0 | 0 | 0 | 2 | 8 |
| House mouse | Mus musculus | 83 | 37 (2*) | 21 (1*) | 14 (5*, 1†) | 7 | 4 |
| Common vole | Microtus arvalis | 31 | 1 | 2 | 2 | 12 | 14 |
| Wood mouse | Apodemus sylvaticus | 19 | 0 | 1 | 0 | 3 | 15 |
| Brown rat | Rattus norvegicus | 8 | 8 (1*) | 0 | 0 | 0 | 0 |
| Harvest mouse | Micromys minutus | 6 | 0 | 0 | 0 | 0 | 6 |
| Field vole | Microtus agrestis | 3 | 0 | 0 | 0 | 2 | 1 |
| Bank vole | Clethrionomys glareolus | 3 | 0 | 0 | 0 | 2 | 1 |
| Northern voleb | Microtus oeconomus | 1 | 0 | 0 | 0 | 0 | 1 |
Numbers indicate number of animals captured; numbers of positives are between parentheses (*, Campylobacter positive; †, Salmonella positive).
This animal was released again because of its endangered status in The Netherlands.
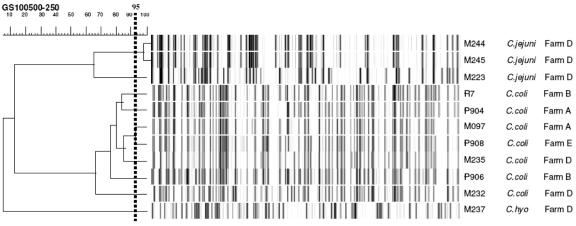
AFLP analysis revealed no direct shared genotypes of Campylobacter isolates from rodents and those from pigs at farms A and B (Fig. 1; a cutoff point of 95% similarity to define identical isolates was chosen). Campylobacter strains in two house mice caught at the same time at exactly the same trapping point on farm D were indistinguishable (Fig. 1, numbers 244 and 245). Further examination of this particular farm showed that almost all (five of six) Campylobacter-positive animals were found in one pig stable. The sixth infected animal was trapped in the feeding passage of another stable, only 25 meters away. No infections were encountered elsewhere.
FIG. 1.
AFLP analysis of Campylobacter strains identified in the study, linked to farms of origin. M, house mouse; R, rat; P, pig.
Although we realize the limitations concerning the number of herds and their microbiological status, our study shows that on Dutch organic farms two rodent species were found to be carriers of Salmonella and Campylobacter strains. Salmonella prevalence was limited, a finding similar to a previous study (13) in which fecal pellets from house mice caught on-farm were analyzed (0 of 222 animals infected). However, Salmonella prevalence may increase when rodents are caught near a Salmonella sp.-positive herd or flock. The Campylobacter species found in rodents in the present study were C. jejuni, C. coli, and C. hyointestinalis subsp. hyointestinalis.
Although no shared Campylobacter genotypes in rodents and pigs were found, horizontal transmission by infected rodents cannot be excluded. Pigs can carry multiple Campylobacter strains (mixed infections), a phenomenon also observed in poultry (1, 7). In turn, this phenomenon may facilitate genetic exchange between different Campylobacter strains in the pig gastrointestinal tract under normal farm conditions. Such genetic instability under natural conditions could undermine the application of genetic subtyping (2). Hypothetically, infected rodents and pigs can acquire their infection from the same source or by infecting each other after having acquired the infection from some other source. For example, this could result from the consumption of an infected rodent by a pig or by a mouse foraging through infected pig manure.
Understanding of epidemiology of pathogens is important to ensure livestock health and food safety. Here, house mice and brown rats were found to carry Salmonella and Campylobacter strains, but rodents can also transmit other pathogens, such as Toxoplasma gondii (9). Transmission risks might be limited, but a reliable assessment is necessary to quantify the risks posed by rodents. Organic farmers should apply effective rodent management that is in line with organic principles to protect livestock and human health (12).
Acknowledgments
This study was supported by grants from the Dutch Ministry for Agriculture, Nature, and Food Quality and the European Union Integrated Project Quality Low Input Food.
We thank the participating farmers, the pig production group of Biologica, the Dutch Society for the Study and Conservation of Mammals, Frans-Freddy Putirulan, Arie Hoogendoorn, Jan Cornelissen, Albert de Boer, Nico Bolder, Fimme Jan van der Wal, Bryan Jones, and Davy Duijsings.
REFERENCES
- 1.Bang, D. D., E. M. Nielsen, K. Knudsen, and M. Madsen. 2003. A one-year study of campylobacter carriage by individual Danish broiler chickens as the basis for selection of Campylobacter spp. strains for a chicken infection model. Epidemiol. Infect. 130:323-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Boer, P., J. A. Wagenaar, R. P. Achterberg, J. P. M. Van Putten, L. M. Schouls, and B. Duim. 2002. Generation of Campylobacter jejuni genetic diversity in vivo. Mol. Microbiol. 44:351-359. [DOI] [PubMed] [Google Scholar]
- 3.Duim, B., P. A. R. Verdamme, A. Rigter, S. Laevens, J. R. Dijkstra, and J. A. Wagenaar. 2001. Differentiation of Campylobacter species by AFLP fingerprinting. Microbiology 147:2729-2737. [DOI] [PubMed] [Google Scholar]
- 4.Duim, B., T. M. Wassenaar, A. Rigter, and J. A. Wagenaar. 1999. High-resolution genotyping of Campylobacter strains isolated from poultry and humans with amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 65:2369-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gratz, N. G. 1994. Rodents as carriers of disease, p. 85-108. In A. P. Buckle and R. H. Smith (ed.), Rodent pests and their control. CAB International, Oxford, England.
- 6.Hiett, K. L., N. J. Stern, P. Fedorka-Cray, N. A. Cox, M. T. Musgrove, and S. Ladely. 2002. Molecular subtype analyses of Campylobacter spp. from Arkansas and California poultry operations. Appl. Environ. Microbiol. 68:6220-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs-Reitsma, W. F., H. M. Maas, and W. H. Jansen. 1995. Penner serotyping of Campylobacter isolates from poultry, with absorbed pooled antisera. J. Appl. Bacteriol. 79:286-291. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs-Reitsma, W. F., M. Van der Wal, R. Achterberg, and J. A. Wagenaar. 2003. Comparative studies on Campylobacter isolation methods from fresh poultry products. 12th International Workshop on Campylobacter, Helicobacter, and Related Organisms. Int. J. Med. Microbiol. Aarhus 293:6-7. [Google Scholar]
- 9.Kijlstra, A., B. G. Meerburg, and M. F. Mul. 2004. Animal-friendly production systems may cause re-emergence of Toxoplasma gondii. NJAS-Wag J. Life Sci. 52:119-132. [Google Scholar]
- 10.Leirs, H., J. Lodal, and M. Knorr. 2004. Factors correlated with the presence of rodents on outdoor pig farms in Denmark and suggestions for management strategies. NJAS-Wag J. Life Sci. 52:133-143. [Google Scholar]
- 11.Mead, P., L. Slutsker, V. Dietz, L. McCaig, J. Bresee, C. Shapiro, P. Griffin, and R. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meerburg, B. G., M. Bonde, F. W. A. Brom, S. Endepols, A. N. Jensen, H. Leirs, J. Lodal, G. R. Singleton, H.-J. Pelz, T. B. Rodenburg, and A. Kijlstra. 2004. Towards sustainable management of rodents in organic animal husbandry. NJAS-Wag J. Life Sci. 52:195-205. [Google Scholar]
- 13.Pocock, M. J. O., J. B. Searle, W. B. Betts, and P. C. L. White. 2001. Patterns of infection by Salmonella and Yersinia spp. in commensal house mice (Mus musculus domesticus) populations. J. Appl. Microbiol. 90:755-760. [DOI] [PubMed] [Google Scholar]
- 14.Stern, N. J., M. P. Hernandez, L. Blankenship, K. E. Deibel, S. Doores, M. P. Doyle, M. D. Pierson, J. N. Sofos, W. H. Sveum, and D. C. Westhoff. 1985. Prevalence and distribution of Campylobacter jejuni and Campylobacter coli in retail meats. J. Food Prot. 48:595-599. [DOI] [PubMed] [Google Scholar]
- 15.Tauxe, R. 2002. Emerging food-borne pathogens. Int. J. Food Microbiol. 78:31-41. [DOI] [PubMed] [Google Scholar]