Abstract
The abundance of aerobic anoxygenic phototrophic (AAP) bacteria, cyanobacteria, and heterotrophs was examined in the Mid-Atlantic Bight and the central North Pacific Gyre using infrared fluorescence microscopy coupled with image analysis and flow cytometry. AAP bacteria comprised 5% to 16% of total prokaryotes in the Atlantic Ocean but only 5% or less in the Pacific Ocean. In the Atlantic, AAP bacterial abundance was as much as 2-fold higher than that of Prochlorococcus spp. and 10-fold higher than that of Synechococcus spp. In contrast, Prochlorococcus spp. outnumbered AAP bacteria 5- to 50-fold in the Pacific. In both oceans, subsurface abundance maxima occurred within the photic zone, and AAP bacteria were least abundant below the 1% light depth. The abundance of AAP bacteria rivaled some groups of strictly heterotrophic bacteria and was often higher than the abundance of known AAP bacterial genera (Erythrobacter and Roseobacter spp.). Concentrations of bacteriochlorophyll a (BChl a) were low (∼1%) compared to those of chlorophyll a in the North Atlantic. Although the BChl a content of AAP bacteria per cell was typically 20- to 250-fold lower than the divinyl-chlorophyll a content of Prochlorococcus, the pigment content of AAP bacteria approached that of Prochlorococcus in shelf break water. Our results suggest that AAP bacteria can be quite abundant in some oceanic regimes and that their distribution in the water column is consistent with phototrophy.
Prokaryotic microbes play a central role in carbon cycling and food web dynamics in the ocean. Much has been learned about the contribution to primary production by autotrophic prokaryotes (15, 20) and the central role of heterotrophic prokaryotes in the consumption of dissolved organic materials (DOM) (35) and degradation of sinking particles in the ocean (25). However, less is known about photoheterotrophic bacteria, such as the aerobic anoxygenic phototrophic (AAP) bacteria and proteorhodopsin-containing bacteria (1), which probably have phototrophic as well as heterotrophic metabolisms. These bacteria may have unique impacts on carbon cycling.
Kolber et al. (19) obtained the first evidence that AAP bacteria may be more abundant in the ocean than implied by the low concentrations of bacteriochlorophyll a (BChl a) measured in seawater (14). Direct counts of infrared (IR) fluorescing bacteria suggested that AAP bacteria could comprise as much as 10% of the total microbial community (19). However, Schwalbach and Fuhrman (27) pointed out that this estimate may be too high because of problems in distinguishing cyanobacteria from AAP bacteria. Their direct-count and quantitative-PCR data indicate that AAP bacteria were usually a small fraction of total prokaryotic abundance in surface waters of several marine environments, but surface waters of the Chesapeake Bay and Long Island Sound did have relatively high proportions of AAP bacteria (10% to 18%). In addition to uncertainty about surface waters, it is not clear how AAP bacteria vary with depth in the oceans, except for two depth profiles in the Pacific Ocean (19, 27). Furthermore, no study has compared AAP bacteria with the abundance of Prochlorococcus, Synechococcus, and heterotrophic bacterial groups.
In this study we used pigment analysis to assess the phototrophic potential of AAP bacteria and infrared fluorescence microscopy, flow cytometry, and fluorescence in situ hybridization (FISH) to compare the abundance of AAP bacteria to that of cyanobacteria and heterotrophic bacteria in the Mid-Atlantic Bight and the central North Pacific Gyre. The abundance of heterotrophic bacterial groups has been useful in assessing their contribution to bacterial production (8). Similarly, abundance data may provide insight into the biogeochemical importance of AAP bacteria. Other characteristics of AAP bacteria, such as the concentration of BChl a per cell, may reveal the importance of phototrophy to their metabolism. Data on the abundance of AAP bacteria suggest that the contribution of AAP bacteria to bacterioplankton metabolism is comparable to that of recognized groups of heterotrophic bacteria.
MATERIALS AND METHODS
Environmental sampling.
Seawater was collected from the Mid-Atlantic Bight in August 2003 and from the central North Pacific Gyre in February 2004. Samples for FISH and AAP bacterial abundance were preserved with 2% paraformaldehyde for 18 h at 4°C. The FISH samples were then filtered onto 0.2-μm-pore-size white polycarbonate filters, rinsed with deionized water, and stored at −20°C. The AAP bacterial samples were filtered onto 0.2-μm-pore-size black polycarbonate filters and were not rinsed. The FISH sample filters were stored at −20°C, and the AAP bacteria and Prochlorococcus filters were stored at −20°C for a few days until back in the lab, where they were then stored at −80°C for up to 2 months prior to analysis.
Oceanographic parameters.
Bacterial production was measured by using the 3H-leucine method (17). Samples were incubated with 20 nM leucine for 1 h at the in situ temperature in the dark. Incubations were terminated by the addition of 5% trichloroacetic acid. Macromolecules were precipitated by trichloroacetic acid extraction, collected by centrifugation (28), rinsed with 80% ethyl alcohol, and radioassayed. Bacterial production was calculated assuming a ratio of 1.5 kg C per mol of leucine incorporated.
Samples for chlorophyll a (Chl a) and BChl a analysis were collected by filtering 10 liters of seawater onto GF/F glass fiber filters (Whatman), which were then stored at −80°C until analysis. Pigments were extracted in 95% acetone using a 1-min sonication step followed by 4 h of incubation at −20°C (7). Pigments were analyzed by reverse-phase high-performance liquid chromatography using an Agilent Technologies 1100 series system fitted with a Zorbax Eclipse XDB-C8 high-performance liquid chromatography column. The mobile phase consisted of a binary gradient that went from a 70:30 mixture of methanol (95%) and tetrabutylammonium acetate buffer (28 mM) to 100% methanol (31). Pigment absorbance was monitored at 665 nm and 770 nm to quantify chlorophyll a and bacteriochlorophyll a, respectively. Pigments were quantified by using chlorophyll a and bacteriochlorophyll a standards (Sigma-Aldrich).
Seawater samples for nutrient analyses were frozen on dry ice and stored at −20°C until analysis. Concentrations of NO3+NO2, PO4, and NH4+ were determined by automated, segmented flow colorimetric analysis, using a Flo-Solution IV analyzer (O/I Analytical, College Station, TX).
Fluorescence in situ hybridization.
The abundance of selected bacteria was determined by FISH using probe Alf968 for alpha-proteobacteria (13), CF319a for Cytophaga-like bacteria (22), Ros537 for Roseobacter spp. (9), and Ery731 (TAA CTG TCC AGT GAG TCG) for Erythrobacter spp. (this study). A slice of the filter was placed on a 30-μl drop of hybridization solution containing 75 ng of Cy3-labeled oligonucleotide probe and incubated for 18 h at 46°C. The hybridization solution contained 0.9 M NaCl, 20 mM Tris-HCl (pH 7.4), 0.01% sodium dodecyl sulfate, and the concentration of formamide determined to achieve specificity for the targeted bacteria (13, 22). The Ery731 probe was used with a formamide concentration of 35%. The specificity of the Erythrobacter FISH probe, Ery731, was assessed using Erythrobacter longus (ATCC 33941) and various negative controls, including Roseobacter litoralis (ATCC 49566) and Vibrio harveyi (ATCC 700106), marine alpha-proteobacterial strains O21 and E37 and Silicobacter pomeroyi (16), and Cytophaga-like bacterial strains IRI113 and IRI26 isolated from Delaware coastal waters. FISH samples were analyzed using image analysis as described previously (8).
AAP bacterial abundance.
Samples for AAP bacterial abundance were stained with a solution containing 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) in 2X phosphate-buffered saline (1× phosphate-buffered saline contains 8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, and 0.24 g KH2PO4 in 800 ml water [pH 7.4]) for 5 min. After removing excess stain, the sample was then mounted on a glass slide with a coverslip using an antifade mountant comprised of Citifluor (Ted Pela) and Vectashield (Vector Labs) mixed in a ratio of 4:1 by volume. Stained samples were counted immediately or stored at −80°C and counted within 24 h.
AAP bacteria were counted using an Olympus Provis AX70 microscope and image analysis software (ImagePro Plus, Media Cybernetics) to identify cells having DAPI and IR fluorescence but not Chl a or phycoerythrin (PE) fluorescence. A series of four images was acquired for each field of view using the following optical filter sets: DAPI (excitation, 360 ± 40; emission, 460 ± 50), IR (excitation, 390 ± 100; emission, 750 long pass), Chl a (excitation, 480 ± 30; emission, 660 ± 50), and PE (excitation, 545 ± 30, emission, 610 ± 75) (Chroma). Images were captured using a charge-coupled-device camera (Intensified Retiga Extended Blue; Q Imaging) with the following exposure times: DAPI, 40 ms; IR, 200 ms; Chl 1,500 ms; PE, 50 ms. Focus was adjusted by approximately 0.8 μm between the DAPI and IR images using a computer-controlled z-axis controller (Prior Instruments) to correct for chromatic aberration. Cells were identified by detecting edges with Laplacian and Gaussian filters applied in series (23). The filtered images were segmented into binary format and then overlaid to identify cells with DAPI and IR fluorescence but not Chl or PE fluorescence.
The method for counting AAP bacteria was tested using Erythrobacter longus (ATCC 33941), which has BChl a. We also tested microbes with other photosynthetic pigments, including the cyanobacteria Prochlorococcus marinus (CCMP1375) and Synechococcus strain WH7803 (CCMP1334), and the picoeukaryotic alga Aureococcus anophagefferens (CCMP1706). A seawater sample collected from a depth of 2,000 m in the Arctic Ocean was also examined.
Cyanobacterial abundance.
Seawater samples for counting Prochlorococcus and Synechococcus spp. by flow cytometry were preserved with 2% paraformaldehyde, frozen in liquid nitrogen, and stored at −80°C until analysis. Samples for microscopic analysis were collected on black 0.2-μm-pore-size polycarbonate filters. Flow cytometry was performed with a Becton-Dickinson FACSCalibur using 488-nm laser excitation and seawater sheath fluid passed through a 0.2-μm-pore-size filter. Synechococcus and Prochlorococcus spp. were identified in scatter plots of red (>640-nm-wavelength) versus orange (560- to 640-nm-wavelength) fluorescence (4). Counts were calibrated using fluorescent beads (F-8823; Molecular Probes), which were counted by fluorescence microscopy and added to the sample. Microscopic counts of Synechococcus were performed using PE fluorescence (21). Prochlorococcus spp. were enumerated using a modification of the image overlay approach described above for counting AAP bacteria by identifying cells having DAPI and Chl a fluorescence. The data for Prochlorococcus abundance are based on flow cytometry, whereas the data on Synechococcus abundance are from microscopic analyses.
RESULTS
Automated microscopic counting of AAP bacteria and Prochlorococcus.
AAP bacteria were readily distinguished from microbes with photosynthetic pigments other than BChl a. The percentage of cells that were scored as AAP positive in an Erythrobacter longus culture was 84% ± 10% (Table 1). AAP bacterial abundance was not significantly different from zero in a Prochlorococcus culture, although some cells (0.3% ± 0.3%) were AAP positive. No cells were AAP positive in control cultures of Synechococcus and Aureococcus anophagefferens. The percentage of cells scoring AAP positive in a sample from a depth of 2,000 m in the Arctic Ocean was 0.3% ± 0.3%.
TABLE 1.
Testing the infrared microscopic method for counting AAP bacteria
| Sample or source | Description | BChl aa | % AAP positive | nb |
|---|---|---|---|---|
| Erythrobacter longus | AAP bacterium | + | 84 ± 10 | 162 |
| Prochlorococcus marinus | Cyanobacterium | − | 0.3 ± 0.3 | 220 |
| Aureococcus anophagefferens | Picoeukaryote | − | 0 ± 0 | 3,062 |
| Synechococcus sp. | Cyanobacterium | − | 0 ± 0 | 900 |
| Arctic Ocean at 2,000 m | Deep-sea prokaryotic community | ND | 0.3 ± 0.3 | 10,531 |
+, present; −, not present; ND, not determined.
Number of cells examined.
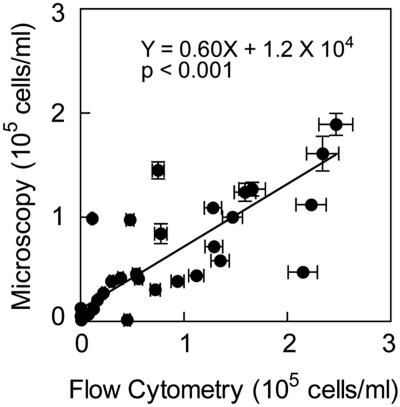
There was a significant correlation between microscopic counts and abundances determined by flow cytometry of Synechococcus (data not shown) and Prochlorococcus (Fig. 1) in the central North Pacific. However, model II regression analysis of the Prochlorococcus data suggest that microscopic counts are lower than the flow cytometric estimates of Prochlorococcus abundance (Y = 0.60X + 1.2 × 104; P < 0.001). Data from flow cytometry are reported here for Prochlorococcus abundance, whereas the data on Synechococcus abundance are from microscopic analyses.
FIG. 1.
Abundance of Prochlorococcus determined by microscopy and flow cytometry in the central North Pacific Gyre. Error bars indicate standard errors (SE).
Environmental setting.
In the Mid-Atlantic Bight, several biogeochemical parameters indicated that the shelf break was mesotrophic, whereas the coastal and Gulf Stream waters were more oligotrophic. Concentrations of Chl a in surface water were two- to sixfold higher at the shelf break than in the Gulf Stream and coastal waters due to higher concentrations of phosphate and nitrate plus nitrite (Table 2). However, integrated primary production was almost 10-fold higher in the Gulf Stream than at the shelf break, in part due to the deeper photic zone in the Gulf Stream (Table 2). In contrast, integrated bacterial production was twofold higher at the shelf break than in the Gulf Stream (Table 2).
TABLE 2.
Characteristics of waters sampled in the Mid-Atlantic Bight in August 2003 and in the central North Pacific in February 2004a
| Water sampled | Geographic location | Photic zoneb (m) | PO4c (μmol liter−1) | NO3 + NO2c (μmol liter−1) | Bacterial productivity (mg C m−2 day−1) | Primary productivity (mg C m−2 day−1) | Chl ag (μg−1) liter |
|---|---|---|---|---|---|---|---|
| Mid-Atlantic Bight | |||||||
| Gulf stream | 35°55′N, 73°58′W | 49 | 0.08-0.39 | 0.18-0.73 | 73 (4) | 2,266 | 0.13-0.25 |
| Gulf stream | 36°00′N, 72°60′W | 65 | 0.07-0.17 | 0.41-2.7 | 38 (2) | 900 | 0.04-0.11 |
| Coast | 36°49′N, 73°36′W | 65 | 0.07-0.81 | 0.27-14.1 | 66 (16) | 279 | 0.04-0.88 |
| Shelf break | 38°00′N, 74°26′W | 35 | 0.37-0.76 | 1.48-8.0 | 96 (4) | 259 | 0.23-0.83 |
| Central North Pacific | |||||||
| N of Oahud | 22°45′N, 157°60′W | 117e | 0.11-0.11 | 0.17-0.52 | ND | 466e | 0.35-1.56 |
| NW of Oahu | 22°27′N, 158°5′W | ND | ND | ND | ND | ND | ND |
| W of Oahu | 22°10′N, 158°10′W | ND | 0.15-0.30 | 0.23-2.62 | ND | ND | 0.46-1.8 |
| SW of Oahu | 21°53′N, 158°14′W | 109f | 0.12-0.12 | 0.20-0.15 | ND | ND | 0.53-2.48 |
| SW of Oahu | 21°21′N, 158°16′W | 100f | 0.15-0.23 | 0.38-1.75 | ND | ND | 0.37-1.3 |
Abbreviations: N, north; NW, northwest; W, west; SW, southwest; ND, not determined. Standard errors are given in parentheses.
Depth to which 1% of surface irradiance reached.
Range of concentrations between the surface and the bottom of the photic zone.
Hawaii ocean time-series station Aloha (http://hahana.soest.hawaii.edu/hot/hot.html).
Hawaii ocean time-series, 24 to 28 February 2003 (http://hahana.soest.hawaii.edu/hot/hot-dogs/interface.html).
Hawaii ocean time-series, 23 to 26 February 2004.
Range of concentrations between the surface and subsurface maximum Chl a concentrations.
Concentrations of inorganic nutrients and Chl a were low in the Pacific, indicative of oligotrophic conditions. The concentrations of nitrate plus nitrite in the Pacific were comparable to concentrations in the Mid-Atlantic Bight, but phosphate in the Pacific was about twofold higher than in the Mid-Atlantic Bight (Table 2). Chl a in the Pacific was about fourfold higher than in the Atlantic, and the photic zone was approximately twice as deep.
Standing stocks of AAP bacteria.
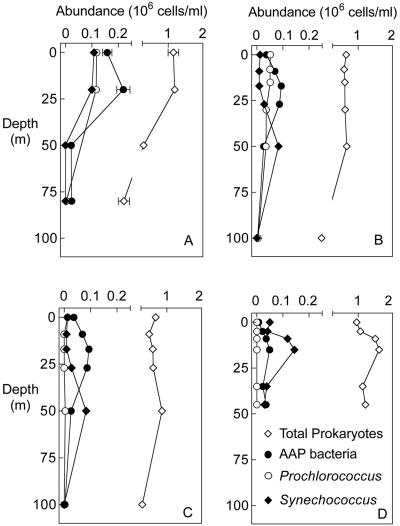
The abundance of AAP bacteria varied across regimes and with depth. In surface waters of the Mid-Atlantic Bight, AAP bacteria were most abundant in coastal waters (5.0 × 104 cell ml−1) and the Gulf Stream (1.5 × 105 cells ml−1) and less abundant at the shelf break (6.9 × 103 cells ml−1) (Fig. 2A, 2B, and 2C). AAP abundance varied more with depth than with location. AAP bacterial abundance was higher in the photic zone than at the surface and lowest below the photic zone. For example, at the shelf break the abundance of AAP bacteria at 5 m was sevenfold higher than at the surface (Fig. 2D). In the Gulf Stream and coastal waters, AAP bacterial abundance below the photic zone at 100 m was 3.0 × 103 cells ml−1 and 1.0 × 105 cells ml−1 at 20 m (Fig. 2B and 2C).
FIG. 2.
Abundance of AAP bacteria, Prochlorococcus, and Synechococcus at Gulf Stream (A, B), coastal (C), and shelf break (D) sampling sites in the Mid-Atlantic Bight in August 2003. Error bars indicate SE.
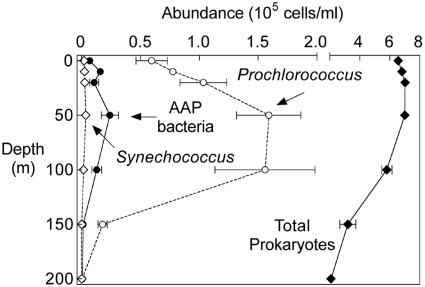
AAP bacteria were less abundant in the central North Pacific (Fig. 3) than in the Mid-Atlantic Bight. The number of AAP bacteria in the surface waters sampled near Oahu was 10- to 100-fold lower than in the Gulf Stream and coastal waters of the Atlantic (Fig. 2). However, subsurface maxima in the Pacific were similar to those in the Atlantic. In the Pacific, AAP bacterial abundances were highest at depths ranging from 20 m to 100 m, and these subsurface maxima were 2- to 100-fold higher than the surface values (Fig. 3). Other similarities between the Atlantic and the Pacific were the low abundance of AAP bacteria at the 1% light depth and the even lower numbers below the photic zone (Fig. 3).
FIG. 3.
Abundance of AAP bacteria, Prochlorococcus, Synechococcus, and total prokaryotes in the central North Pacific Gyre in February 2004. The error bars indicate the variation (standard deviation) in abundance among sampling sites located near Oahu.
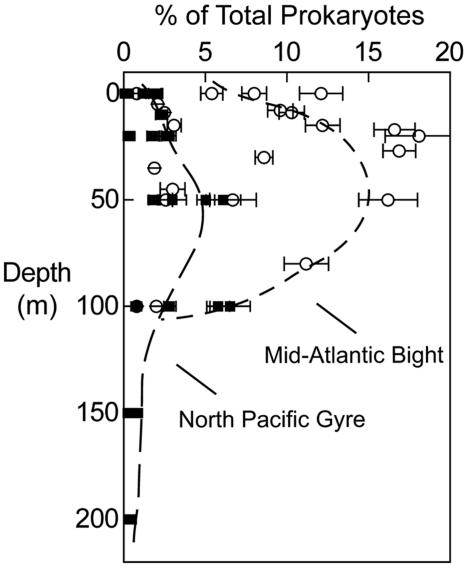
AAP bacteria comprised a substantially larger fraction of the total prokaryotic community in the mid-Atlantic Bight than in the central North Pacific Ocean. The maximum abundance of AAP bacteria in the Pacific was only 5% of the total prokaryotic community (Fig. 4), whereas AAP bacteria comprised from 5% to 15% of total prokaryotes in the mid-Atlantic Bight (Fig. 4). AAP bacteria in the surface waters of the Atlantic made up 5% to 10% of the total community compared to 3% in the Pacific. At depths below 100 m AAP bacteria made up 2% or less of the total community in the Atlantic and Pacific.
FIG. 4.
Contribution of AAP bacteria to community structure in the Mid-Atlantic Bight in August 2003 (filled symbols) and the central North Pacific Gyre in February 2004 (open symbols). The dashed lines were drawn by hand. Error bars indicate SE.
AAP bacteria versus cyanobacteria.
In the Gulf Stream and Atlantic coastal waters, AAP bacteria were as much as 2-fold more abundant than Prochlorococcus and 10-fold more abundant than Synechococcus at depths shallower than 25 m (Fig. 2A, 2B, and 2C). In contrast, at the bottom of the photic zone Synechococcus outnumbered AAP bacteria by twofold (Fig. 2B and 2C). At the shelf break Synechococcus was the most abundant phototroph and outnumbered AAP bacteria by 2- to 10-fold (Fig. 2D).
In contrast, in the central North Pacific Prochlorococcus outnumbered AAP bacteria and Synechococcus at all depths. Prochlorococcus was 5- to 50-fold more numerous than AAP bacteria at depths from the surface to 100 m (Fig. 3). However, as in the Atlantic, AAP bacteria were more abundant than Synechococcus in the central North Pacific Gyre. In surface waters, the abundance of AAP bacteria was equal to or as much as fivefold higher than that of Synechococcus. At depths ranging from 20 m to 200 m AAP bacteria outnumbered Synechococcus by 10- to 20-fold.
Contribution of AAP bacteria to prokaryotic community structure.
We compared the abundance of AAP bacteria to the major groups of bacteria known to be active in DOM consumption. Alpha-proteobacteria and Cytophaga-like bacteria were major components of the bacterial communities in the Mid-Atlantic Bight as determined by FISH. At the shelf break alpha-proteobacteria and Cytophaga-like bacteria (comprising on average 27% and 23% of the prokaryotic community) outnumbered AAP bacteria by 10-fold (Table 3). In the Gulf Stream alpha-proteobacteria and Cytophaga-like bacteria on average made up just 6% to 12% of the total prokaryotes, a proportion which was similar to the average abundance of AAP bacteria (about 10%). However, the FISH estimates are probably conservative estimates due to limitations of FISH, since in the Gulf Stream only 40% to 60% of the total prokaryotes were detected by a general FISH probe (Eub338) for all bacteria.
TABLE 3.
Abundance of select bacteria in the Mid-Atlantic Bight in August 2003
| Source | Depth (m) | % of total prokaryotes from indicated sourcea
|
||||||
|---|---|---|---|---|---|---|---|---|
| Eub338-positiveb | α-Proteobacteria | SAR11 | Cytophaga-like | Roseobacter | Erythrobacter | AAP bacteria | ||
| Gulf Stream | 0 | 58 (4.2) | 14 (1.5) | 34 (3.8) | 11 (2.6) | 7.0 (2.3) | 3.7 (1.3) | 12 (1.3) |
| 7 | 63 (3.8) | 7.5 (2.2) | 32 (6.3) | 14 (12) | 7.2 (1.9) | 3.7 (2.8) | ND | |
| 13 | 60 (3.8) | 14 (12.2) | 28 (6.8) | 16 (1.5) | 4.9 (1.6) | 3.8 (1.5) | ND | |
| 20 | 62 (4.6) | 9.8 (2.4) | 38 (5.2) | 9.6 (1.3) | 4.2 (1.0) | 2.6 (0.9) | 18 (2.1) | |
| 50 | NDc | ND | 26 (4.9) | ND | ND | ND | ND | |
| 80 | ND | ND | 28 (9.9) | ND | ND | ND | 11 (1.4) | |
| Gulf Stream | 0 | 36 (5.5) | 21 (7.6) | 26 (5.4) | 3.5 (0.9) | 3.1 (1.5) | 5.6 (1.9) | 8.0 (0.8) |
| 8 | 56 (4.9) | 8.2 (2.3) | 20 (4.8) | 8 (23) | 4.3 (0.7) | 1.6 (0.6) | 9.6 (0.8) | |
| 15 | 50 (4.3) | 7.7 (1.2) | 24 (4.4) | 7.7 (1.2) | 8.1 (12) | 1.2 (1.3) | 12 (1.1) | |
| 30 | 38 (9.9) | 1.9 (0.9) | 19 (3.9) | 1.8 (0.9) | 3.3 (12) | 0.9 (0.8) | 8.6 (0.5) | |
| 50 | 35 (11.0) | 6.8 (1.9) | 22 (4.7) | 6.8 (1.9) | 1.5 (1.1) | 1.3 (1.2) | 16 (1.8) | |
| 100 | 23 (2.7) | 0.7 (0.7) | 11 (7.2) | 0.5 (0.8) | 1.0 (0.6) | 0.4 (1.2) | 2.0 (0.4) | |
| Coast | 0 | 60 (9.1) | 6.4 (3.0) | 27 (5.5) | 17 (4.2) | 3.5 (1.1) | 1.4 (1.3) | 5.4 (0.7) |
| 9 | 73 (15.7) | 30 (12) | 28 (3.8) | 24 (12) | 2.8 (1.2) | 0.6 (0.5) | 10 (0.8) | |
| 17 | 69 (12.6) | 18 (4.5) | 29 (6.9) | 39 (4.8) | 3.4 (1.6) | 0.9 (0.8) | 17 (1.3) | |
| 27 | 63 (9.5) | 26 (13) | 22 (4.4) | 48 (14) | 5.2 (2.0) | 1.5 (0.9) | 17 (1.0) | |
| 50 | 37 (8.8) | 5.4 (2.5) | 20 (4.4) | 16 (6.5) | 6.0 (2.1) | 2.9 (0.8) | 2.6 (0.4) | |
| 100 | 41 (11.1) | 6.8 (3.6) | 14 (5.4) | 46 (18) | 5.0 (4.2) | 1.0 (1.5) | 0.8 (0.2) | |
| Shelf break | 0 | 74 (3.8) | 40 (6.1) | 31 (3.7) | 25 (4.3) | 5.6 (0.9) | 2.9 (1.5) | 0.8 (0.1) |
| 5 | 73 (3.4) | 32 (6.0) | 32 (4.2) | 22 (4.7) | 4.3 (1.2) | 1.8 (0.9) | 2.1 (0.2) | |
| 9 | 75 (8.2) | 30 (6.8) | 28 (5.0) | 24 (2.8) | 7.7 (1.5) | 1.7 (0.6) | 2.5 (0.2) | |
| 15 | 61 (6.5) | 27 (6.1) | 34 (6.2) | 18 (6.4) | 18 (6.4) | 2.2 (1.1) | 3.1 (0.4) | |
| 35 | 59 (6.1) | 20 (5.7) | 20 (4.0) | 20 (4.8) | 4.2 (2.1) | 1.3 (0.8) | 1.9 (0.3) | |
| 45 | ND | 16 (4.3) | 27 (4.7) | 31 (5.6) | 3.9 (1.2) | 1.4 (1.0) | 3.0 (0.8) | |
Values are averages for 10 fields of view. Standard errors are given in parentheses.
Eub338 is the general bacterial probe. The average negative-control FISH probe was 3% of total prokaryotes.
ND, not determined.
We also compared AAP bacterial abundance to more narrowly defined bacterial groups, such as SAR11, a type of alpha-proteobacterium (24). Overall, the abundance of AAP bacteria was about one-third that of SAR11, which accounted for 20% to 30% of the prokaryotic community (Table 3). The relationship between AAP bacteria and Roseobacter, which is another type of alpha-proteobacterium with some cultured representatives that carry out AAP metabolism (37), varied between regimes and depths in the water column. In coastal and shelf break water Roseobacter comprised 3% to 6% of the total prokaryotic community regardless of depth, whereas AAP bacterial abundance did vary with depth. In the photic zone of coastal waters AAP bacteria were threefold more abundant than Roseobacter, but in surface water and below the photic zone the abundance of AAP bacteria and Roseobacter were about equal (<1% to 5% of total prokaryotes) (Table 3). In contrast, at the shelf break throughout the water column Roseobacter were on average three times as abundant as AAP bacteria (4% to 18%) (Table 3).
AAP bacteria were always more abundant than Erythrobacter spp., which is another α-proteobacterial group potentially involved in aerobic anoxygenic photosynthesis (18). In the Gulf Stream waters where AAP bacteria were most abundant, Erythrobacter comprised 1% to 6% of total prokaryotes while AAP bacteria comprised 5% to 15% (Table 3 and Fig. 4). In contrast, in coastal waters and at the shelf break the abundance of Erythrobacter usually was not distinguishable from that of the negative control.
Photosynthetic pigments of AAP bacteria and primary producers.
Similar to the variation in AAP bacterial abundance, the concentration of BChl a was highest at depths ranging from 15 m to 30 m within the photic zone. BChl a was not detected (limit of 0.05 ng liter−1) below the photic zone in the Gulf Stream and Atlantic coastal waters (Table 4). In contrast, the horizontal distribution of BChl a was different from the pattern in AAP bacterial abundance. BChl a concentrations were highest at the mesotrophic shelf break (up to 6 ng liter−1) and decreased offshore to their lowest concentrations (<2 ng liter−1) in the Gulf Stream (Table 4). At all sites in the Mid-Atlantic Bight the concentration of BChl a was low compared to the Chl a concentration (0.3% to 2.6%) (Table 4).
TABLE 4.
Concentrations of photosynthetic pigments in the Mid-Atlantic Bight in August 2003a
| Source | Depth (m) | Chl a (μg/liter) | Div-Chl a (ng/liter) | Div-Chl a (fg/cell) | BChl a (ng/liter) | BChl a/Chl a (%) | BChl a (fg/cell) |
|---|---|---|---|---|---|---|---|
| Gulf stream | 10 | 0.130 | 58.9 | 0.50 | 0.33 | 0.3 | 0.002 |
| 20 | 0.148 | 78.2 | 0.67 | 1.56 | 1.1 | 0.007 | |
| 50 | 0.248 | 36.4 | ND | 0.76 | 0.3 | ND | |
| 80 | 0.050 | 7.2 | 2.33 | 0.00 | ND | ND | |
| Gulf stream | 0 | 0.042 | 18.2 | 0.35 | 0.42 | 1.0 | 0.008 |
| 8 | 0.118 | 23.1 | 0.46 | 1.26 | 1.1 | 0.021 | |
| 15 | 0.063 | 22.1 | 0.43 | 0.40 | 0.6 | 0.005 | |
| 30 | 0.058 | 25.3 | 0.71 | 1.52 | 2.6 | 0.028 | |
| 50 | 0.106 | 85.7 | 2.45 | 0.69 | 0.7 | 0.006 | |
| 100 | 0.044 | 11.5 | 2.50 | 0.00 | ND | ND | |
| Coast | 0 | 0.044 | 32.4 | 53.3 | 0.60 | 1.3 | 0.016 |
| 9 | 0.051 | 31.7 | 22.5 | 0.59 | 1.2 | 0.009 | |
| 17 | 0.058 | 34.1 | 14.8 | 0.45 | 0.8 | 0.005 | |
| 27 | 0.211 | 60.5 | 5.55 | 1.26 | 0.6 | 0.015 | |
| 50 | 0.885 | 17.5 | 1.29 | 2.15 | 2.4 | 0.084 | |
| 100 | 0.017 | 1.1 | 5.77 | 0.00 | ND | ND | |
| Shelf break | 0 | 0.227 | 4.3 | 0.43 | 1.65 | 0.7 | 0.241 |
| 5 | 0.371 | 5.9 | 0.45 | 2.23 | 0.6 | 0.105 | |
| 9 | 0.510 | 6.9 | 0.32 | 4.87 | 1.0 | 0.136 | |
| 15 | 0.825 | 19.6 | 1.19 | 5.84 | 0.7 | 0.121 | |
| 35 | 0.653 | 2.4 | 0.47 | 2.01 | 0.3 | 0.089 | |
| 45 | 0.457 | 3.3 | 1.62 | 1.88 | 0.4 | 0.054 |
ND, not determined.
Estimates of the amount of BChl a per cell in AAP bacteria varied substantially among depths and sampling sites as well. BChl a per cell at the shelf break site was 0.24 fg cell−1 at the surface and decreased almost fivefold to 0.054 fg cell−1 at the bottom of the photic zone (Table 4). In Atlantic coastal waters and in the Gulf Stream, pigment concentrations per cell were typically 10-fold lower than at the shelf break (Table 4).
Concentrations of divinyl-chlorophyll a (div-Chl a) were much higher than those of BChl a. Offshore, the average concentration of div-Chl a was 34 ng liter−1 versus 0.7 ng liter−1 for BChl a (Table 4). However, at the shelf break the concentrations of the two pigments were similar (about 5 ng liter−1). Cellular concentrations of div-Chl a were also typically higher than those of BChl a. In the Gulf Stream, the amount of div-Chl a per Prochlorococcus cell was 20- to 250-fold higher than the BChl a content of AAP bacteria. In contrast, at the shelf break, the photosynthetic pigment content of Prochlorococcus was only 2- to 10-fold higher than in AAP bacteria (Table 4).
DISCUSSION
We examined AAP bacteria in mesotrophic and oligotrophic regimes in the Mid-Atlantic Bight and in the oligotrophic central North Pacific Ocean to assess their abundance and contribution to bacterial community structure. We hypothesized that AAP bacteria would be an abundant component of oceanic bacterial communities because the selective pressures for efficient DOM utilization by bacteria in the ocean would be substantial when bacterial growth is limited by the availability of DOM (3). Photoheterotrophic bacteria that use DOM and light could be more efficient than strictly heterotrophic bacteria because they supplement their energy requirements with light. Evidence for direct effects of sunlight on bacterial growth (6) and on community structure (32) suggests that phototrophic metabolism may be prevalent in marine bacteria. Large numbers of proteorhodopsin genes uncovered by whole-genome sequencing of Sargasso Sea bacteria also suggests an important role for photoheterotrophy (33).
Our data indicate that AAP bacteria are widespread in the Mid-Atlantic Bight and the oligotrophic central North Pacific Gyre and make up from 1% to 10% of the total prokaryotic community. The possibility of Prochlorococcus contamination of AAP bacterial counts must be taken seriously when cyanobacteria are abundant, because Chl a is visible in the infrared (27). However, two lines of evidence indicate that our measurements of AAP bacterial abundance do not include cyanobacteria. Only 0.3% ± 0.3% of cells were scored AAP positive in a Prochlorococcus culture, indicating that removing Chl a fluorescing cells from the infrared image was highly effective at excluding Prochlorococcus and Synechococcus from the AAP bacterial counts. In the central North Pacific, even though Prochlorococcus abundance was high our estimates of AAP bacterial abundance were low, averaging only about 4% of the Prochlorococcus abundance. The inclusion of Prochlorococcus in AAP bacterial counts was less problematic in the Mid-Atlantic Bight, where Prochlorococcus was not as abundant and in some samples was outnumbered 25-fold by AAP bacteria.
The ecology of AAP bacteria and their role in microbial food webs is potentially complex because they probably are both phototrophic and heterotrophic. Cultivated AAP bacteria are capable of purely heterotrophic growth in the dark but grow more rapidly when exposed to a light-dark cycle (39). Previous measurements of infrared fluorescence transients suggest that AAP bacteria in the ocean are photosynthetically competent (19). Our data on the depth distribution of AAP bacteria is consistent with higher growth rates in the light since the abundance of AAP bacteria was higher in the photic zone than below the sunlit layers of the water column. AAP bacteria were distributed in the water column like other phototrophs whether they were abundant, as in the Mid-Atlantic Bight, or rare, as in the central North Pacific Ocean. Abundance of AAP bacteria did not appear to vary in the water column like that of heterotrophic bacteria.
A previous study in the Northeast Pacific also reported that the abundance of AAP bacteria was highest in the photic zone (19). Kolber et al. (19) found that the maximum abundance of IR-fluorescing cells (about 10% of total prokaryotes) occurred at 25 m, which is similar to the depth distribution of AAP bacteria in the Pacific near Oahu and in the Mid-Atlantic Bight. In another study in the Pacific, AAP bacterial abundance in the San Pedro Channel off the California coast was also highest below the surface within the photic zone (27).
Variation in the abundance of AAP bacteria in different environmental settings may also illuminate the role of phototrophy in their ecology. A recent survey revealed substantial variation in AAP bacterial abundance in surface waters ranging from the tropical waters of the Atlantic to the high-latitude waters of the Weddell Sea and included coastal environments such as the estuarine waters of Chesapeake Bay and Long Island Sound. The abundance of AAP bacteria estimated using quantitative PCR analysis of pufM genes ranged from 0.01% to 2% in oceanic surface waters (27). Our measurements of AAP bacterial abundance ranged from 0.1% to 2% of total prokaryote abundance in the surface waters of the Pacific and from 0.8% to 17% in the Atlantic; the abundance was highest in coastal water. AAP bacterial abundances higher than 10% of total prokaryotes have been reported for only the shelf break waters of the Mid-Atlantic Bight, the Chesapeake Bay estuary (11%), and Long Island Sound (19%). These high AAP bacterial abundances in estuaries and coastal waters run counter to the hypothesis that photoheterotrophy provides AAP bacteria with an advantage over strict heterotrophs in oligotrophic waters.
Our data on BChl a and divinyl-Chl a can be used to explore further the importance of phototrophy in these bacteria. BChl a is the main photosynthetic pigment in AAP bacteria and serves both in light harvesting and in reaction centers. Although carotenoids are abundant in AAP bacteria, they play only a minor role in harvesting light energy in culture (38) and in the ocean (19). The concentrations of BChl a per cell in coastal water and in the Gulf Stream were typically 20-fold lower than the divinyl-Chl a content of Prochlorococcus. Such low pigment content suggests that phototrophy is probably a smaller part of the metabolism of AAP bacteria than in Prochlorococcus, which is generally recognized as relying purely on autotrophy, although Prochlorococcus may have some heterotrophic activity (6, 40). However, at the shelf break concentrations of BChl a per cell were 20-fold higher than offshore and about 2-fold higher than has been reported for cultured AAP bacteria (38). These values approached the concentrations of photosynthetic pigments in Prochlorococcus, which range from 0.22 to 1.83 fg cell−1 (11). These data suggest that reliance on phototrophy varies between the Gulf Stream, coastal, and shelf break waters.
Some BChl a-producing bacteria are not AAP bacteria, but these physiologically distinct bacteria are probably not abundant in the ocean. Some aerobic methylotrophic bacteria and Rhizobia spp. as well as the beta-proteobacterium Roseateles depolymerans (29) are capable of BChl a synthesis (10, 30, 36). However, Rhizobia spp. are not abundant in marine systems (12) and a Roseateles sp. occurs in freshwater (29). In addition, infrared direct counts and quantitative PCR using the pufM gene gave similar estimates of AAP bacterial abundance in the San Pedro Channel (27).
Cultivated marine AAP bacteria are restricted to just two genera of α-proteobacteria, Roseobacter spp. and Erythrobacter spp. (26), and recently cultivated oligotrophic gamma-proteobacteria (5), but the actual diversity of AAP bacteria in the ocean appears to be much greater. Our FISH and microscopic IR data indicate that the bulk of the AAP bacteria are not members of the Erythrobacter group. Furthermore, AAP bacteria were often more abundant than Erythrobacter and Roseobacter combined, indicating that the diversity of AAP bacteria extends beyond these two groups. Analysis of pufM sequences obtained from the Sargasso Sea also indicates that the diversity of AAP bacteria in the ocean is not limited to Roseobacter spp. and Erythrobacter spp. (33). In addition, analysis of pufM genes in large-insert clones containing environmental DNA from the Pacific Ocean (2) and the Delaware River (34) revealed novel AAP bacteria related to alpha-, beta-, and gamma-proteobacteria (2).
Our study revealed that AAP bacteria are an abundant component of bacterial communities in the Mid-Atlantic Bight. The abundance of AAP bacteria was higher further offshore in the Gulf Stream than at the shelf break, which was consistent with the hypothesis that phototrophy provides AAP bacteria with an advantage in oligotrophic environments. However, our data suggest that the role of phototrophy may not be as simple as originally hypothesized. Pigment content was lowest furthest offshore, and AAP bacteria were not abundant in the central North Pacific. Phototrophy probably provides some advantage though, as reflected in the distribution of AAP bacteria in the water column. However, it is not clear how phototrophy can explain the high abundance of AAP bacteria in estuarine environments (27). The abundance of AAP bacteria revealed by our study suggests a potentially important impact on DOM cycling that may vary under different environmental conditions. Additional information from experiments utilizing genomics, cultivation, and in situ analysis will be necessary for assessing the role of phototrophy versus heterotrophy in determining the success of AAP bacteria in the ocean.
Acknowledgments
This study was supported by grants from the U.S. Department of Energy (BIOMP DFFG02-97 ER 62479) and the National Science Foundation (Biocomplexity program 430119).
We thank Rex Malmstrom and Paul Jones for their assistance with sample collection in the Mid-Atlantic Bight aboard the R/V Cape Henlopen and Kenia Whitehead for her support as chief scientist during sample collection in the Pacific aboard the R/V Kilo Moana. Michael Koblizek kindly provided the BChl a-containing Erythrobacter culture.
REFERENCES
- 1.Beja, O., E. N. Spudich, J. L. Spudich, M. Leclerc, and E. F. DeLong. 2001. Proteorhodopsin phototrophy in the ocean. Nature 411:786-789. [DOI] [PubMed] [Google Scholar]
- 2.Beja, O., M. T. Suzuki, J. F. Heidelberg, W. C. Nelson, C. M. Preston, T. Hamada, J. A. Eisen, C. M. Fraser, and E. F. DeLong. 2002. Unsuspected diversity among marine aerobic anoxygenic phototrophs. Nature 415:630-633. [DOI] [PubMed] [Google Scholar]
- 3.Billen, G., P. Servais, and S. Becquevort. 1990. Dynamics of bacterioplankton in oligotrophic and eutrophic aquatic environments—bottom-up or top-down control. Hydrobiologia 207:37-42. [Google Scholar]
- 4.Campbell, L. 2001. Flow cytometric analysis of autotrophic picoplankton, p. 317-343. In J. H. Paul (ed.), Methods in microbiology, vol. 30. Academic Press, San Diego, Calif. [Google Scholar]
- 5.Cho, J. C., and S. J. Giovannoni. 2004. Cultivation and growth characteristics of a diverse group of oligotrophic marine gammaproteobacteria. Appl. Environ. Microbiol. 70:432-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Church, M. J., H. W. Ducklow, and D. A. Karl. 2004. Light dependence of 3H-leucine incorporation in the oligotrophic North Pacific ocean. Appl. Environ. Microbiol. 70:4079-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claustre, H., S. B. Hooker, L. Van Heukelem, J. F. Berthon, R. Barlow, J. Ras, H. Sessions, C. Targa, C. S. Thomas, D. van der Linde, and J. C. Marty. 2004. An intercomparison of HPLC phytoplankton pigment methods using in situ samples: application to remote sensing and database activities. Mar. Chem. 85:41-61. [Google Scholar]
- 8.Cottrell, M. T., and D. L. Kirchman. 2003. Contribution of major bacterial groups to bacterial biomass production (thymidine and leucine incorporation) in the Delaware estuary. Limnol. Oceanogr. 48:168-178. [Google Scholar]
- 9.Eilers, H., J. Pernthaler, J. Peplies, F. O. Glockner, G. Gerdts, and R. Amann. 2001. Isolation of novel pelagic bacteria from the German bight and their seasonal contributions to surface picoplankton. Appl. Environ. Microbiol. 67:5134-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans, W. R., D. E. Fleischman, H. E. Calvert, P. V. Pyati, G. M. Alter, and N. S. S. Rao. 1990. Bacteriochlorophyll and photosynthetic reaction centers in Rhizobium strain BTAI-1. Appl. Environ. Microbiol. 56:3445-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibb, S. W., D. G. Cummings, X. Irigoien, R. G. Barlow, R. Fauzi, and C. Mantoura. 2001. Phytoplankton pigment chemotaxonomy of the northeastern Atlantic. Deep-Sea Res. Part II Top. Stud. Oceanogr. 48:795-823. [Google Scholar]
- 12.Giovannoni, S. J., and M. S. Rappé. 2000. Evolution, diversity and molecular ecology of marine prokaryotes, p. 47-84. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, New York, N.Y.
- 13.Glöckner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goericke, R. 2002. Bacteriochlorophyll a in the ocean: is anoxygenic bacterial photosynthesis important? Limnol. Oceanogr. 47:290-295. [Google Scholar]
- 15.Goericke, R., and N. A. Welschmeyer. 1993. The marine prochlorophyte Prochlorococcus contributes significantly to phytoplankton biomass and primary production in the Sargasso Sea. Deep-Sea Res. Part I Oceanogr. Res. Pap. 40:2283-2294. [Google Scholar]
- 16.Gonzalez, J. M., and M. A. Moran. 1997. Numerical dominance of a group of marine bacteria in the alpha-subclass of the class Proteobacteria in coastal seawater. Appl. Environ. Microbiol. 63:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchman, D. L. 2001. Measuring bacterial biomass production and growth rates from leucine incorporation in natural aquatic environments, p. 227-237. In J. H. Paul (ed.), Methods in microbiology, vol. 30. Academic Press, San Diego, Calif. [Google Scholar]
- 18.Koblizek, M., O. Beja, R. R. Bidigare, S. Christensen, B. Benitez-Nelson, C. Vetriani, M. K. Kolber, P. G. Falkowski, and Z. S. Kolber. 2003. Isolation and characterization of Erythrobacter sp. strains from the upper ocean. Arch. Microbiol. 180:327-338. [DOI] [PubMed] [Google Scholar]
- 19.Kolber, Z. S., F. G. Plumley, A. S. Lang, J. T. Beatty, R. E. Blankenship, C. L. VanDover, C. Vetriani, M. Koblizek, C. Rathgeber, and P. G. Falkowski. 2001. Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292:2492-2495. [DOI] [PubMed] [Google Scholar]
- 20.Liu, H. B., H. A. Nolla, and L. Campbell. 1997. Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquat. Microb. Ecol. 12:39-47. [Google Scholar]
- 21.MacIsaac, E. A., and J. G. Stockner. 1993. Enumeration of phototrophic picoplankton by autofluorescence microscopy, p. 187-197. In P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (ed.), Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, Fla.
- 22.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K. H. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 23.Massana, R., J. M. Gasol, P. K. Bjornsen, N. Blackburn, A. Hagstrom, S. Hietanen, B. H. Hygum, J. Kuparinen, and C. PedrosAlio. 1997. Measurement of bacterial size via image analysis of epifluorescence preparations: description of an inexpensive system and solutions to some of the most common problems. Sci. Mar. 61:397-407. [Google Scholar]
- 24.Morris, R. M., M. S. Rappe, S. A. Connon, K. L. Vergin, W. A. Siebold, C. A. Carlson, and S. J. Giovannoni. 2002. SAR11 clade dominates ocean surface bacterioplankton communities. Nature 420:806-810. [DOI] [PubMed] [Google Scholar]
- 25.Nagata, T., H. Fukuda, R. Fukuda, and I. Koike. 2000. Bacterioplankton distribution and production in deep Pacific waters: large-scale geographic variations and possible coupling with sinking particle fluxes. Limnol. Oceanogr. 45:426-435. [Google Scholar]
- 26.Rathgeber, C., J. T. Beatty, and V. Yurkov. 2004. Aerobic phototrophic bacteria: new evidence for the diversity, ecological importance and applied potential of this previously overlooked group. Photosynth. Res. 81:113-128. [Google Scholar]
- 27.Schwalbach, M. S., and J. A. Fuhrman. 2005. Wide-ranging abundances of aerobic anoxygenic phototrophic bacteria in the world ocean revealed by epifluorescence microscopy and quantitative PCR. Limnol. Oceanogr. 50:620-628. [Google Scholar]
- 28.Smith, D. C., and F. Azam. 1992. A simple, economical method for measuring bacterial protein synthesis rates in sea water using 3H-leucine. Mar. Microb. Food Webs 6:107-114. [Google Scholar]
- 29.Suyama, T., T. Shigematsu, S. Takaichi, Y. Nodasaka, S. Fujikawa, H. Hosoya, Y. Tokiwa, T. Kanagawa, and S. Hanada. 1999. Roseateles depolymerans gen. nov., sp. nov., a new bacteriochlorophyll a-containing obligate aerobe belonging to the beta-subclass of the Proteobacteria. Int. J. Syst. Bacteriol. 49:449-457. [DOI] [PubMed] [Google Scholar]
- 30.Urakami, T., and K. Komagata. 1984. Protomonas, a new genus of facultatively methylotrophic bacteria. Int. J. Syst. Bacteriol. 34:188-201. [Google Scholar]
- 31.Van Heukelem, L., and C. S. Thomas. 2001. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A. 910:31-49. [DOI] [PubMed] [Google Scholar]
- 32.Van Mooy, B., A. H. Devol, and R. G. Keil. 2004. Relationship between bacterial community structure, light, and carbon cycling in the eastern subarctic North Pacific. Limnol. Oceanogr. 49:1056-1062. [Google Scholar]
- 33.Venter, J. C., K. Remington, J. F. Heidelberg, A. L. Halpern, D. Rusch, J. A. Eisen, D. Y. Wu, I. Paulsen, K. E. Nelson, W. Nelson, D. E. Fouts, S. Levy, A. H. Knap, M. W. Lomas, K. Nealson, O. White, J. Peterson, J. Hoffman, R. Parsons, H. Baden-Tillson, C. Pfannkoch, Y. H. Rogers, and H. O. Smith. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66-74. [DOI] [PubMed] [Google Scholar]
- 34.Waidner, L. A., and D. L. Kirchman. 2005. Aerobic anoxygenic photosynthesis genes and operons in uncultured bacteria in the Delaware River. Environ. Microbiol. 7:1896-1908. [DOI] [PubMed] [Google Scholar]
- 35.Williams, P. J. L. 2000. Heterotrophic bacteria and the dynamics of dissolved organic material, p. 153-200. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, New York, N.Y.
- 36.Young, J. P. W., H. L. Downer, and B. D. Eardly. 1991. Phylogeny of the phototrophic Rhizobium strain BTAI1 by polymerase chain reaction-based sequencing of a 16S ribosomal-RNA gene segment. J. Bacteriol. 173:2271-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yurkov, V. 2001. Aerobic phototrophic proteobacteria. .In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes: an evolving electronic resource for the microbiological community. Springer-Verlag, New York, N.Y. [Online.] http://141.150.157.117:8080/prokPUB/index.htm.
- 38.Yurkov, V. V., and J. T. Beatty. 1998. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 62:695-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yurkov, V. V., and H. van Gemerden. 1993. Impact of light dark regimen on growth rate, biomass formation and bacteriochlorophyll synthesis in Erythromicrobium hydrolyticum. Arch. Microbiol. 159:84-89. [Google Scholar]
- 40.Zubkov, M. V., B. M. Fuchs, G. A. Tarran, P. H. Burkill, and R. Amann. 2003. High rate of uptake of organic nitrogen compounds by Prochlorococcus cyanobacteria as a key to their dominance in oligotrophic oceanic waters. Appl. Environ. Microbiol. 69:1299-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]