Abstract
Animals have evolved diverse behaviors that serve the purpose of finding food in the environment. We investigated the food seeking strategy of the soil bacteria-eating nematode Caenorhabditis elegans. C. elegans bacterial food varies in quality: some species are easy to eat and support worm growth well, while others do not. We show that worms exhibit dietary choice: they hunt for high quality food and leave hard-to-eat bacteria. This food seeking behavior is enhanced in animals that have already experienced good food. When hunting for good food, worms alternate between two modes of locomotion, known as dwelling: movement with frequent stops and reversals; and roaming: straight rapid movement. On good food, roaming is very rare, while on bad food it is common. Using laser ablations and mutant analysis, we show that the AIY neurons serve to extend roaming periods, and are essential for efficient food seeking.
Keywords: appetite, preference, food-seeking, learning, C. elegans
Introduction
Most natural environments offer a variety of food sources to animals that inhabit them. Therefore, feeding usually involves making choices between foods. Animals such as rats (Osborne and Mendel, 1918; Young, 1932) and human infants (Davis, 1928), when offered a selection of foods, will chose the diet that provides optimal balance of nutrients for growth. If a certain element is missing from the diet, a drive state commonly known as appetite develops that drives a change in feeding behavior. Vitamin B-deprived rats, for example, develop a strong appetite for it (Richter et al., 1937); and adrenectomized rats, which lose more sodium in their urine, have an increased appetite for salt (Richter, 1936).
In mammalian paradigms, food acts as a reward that has a powerful effect on behavior. Mammals learn to associate the availability of food with an otherwise irrelevant stimulus (Pavlov, 1927), to prefer the flavor of nutritive food (Elizalde and Sclafani, 1990), and to associate the presence of food with a location (Schechter and Calcagnetti, 1993). Animals learn and perform actions to get food (Skinner, 1938). In contrast, experiencing poisonous food results in learned aversion (Garcia et al., 1968). That food nutritive content regulates food intake in mammals was best shown by intragastric (IG) infusion experiments. If rats are given a choice of water from two tubes, drinking from one of which results in the IG infusion of sugar or fat, while drinking from the other only results in water infusion, animals develop a 90% preference for a tube pared with a nutrient (Elizalde and Sclafani, 1990; Perez et al., 1999). Therefore, mammals can learn to choose the food source solely based on its nutritive content, even when no taste cues are available. Food rich in fats and carbohydrates is a particularly strong stimulant of feeding in mammals including humans. Rats given unlimited access to such a ‘cafeteria’ diet overeat and develop obesity (Sclafani, 1989).
However, it is not well understood how the biological value of food, its nutritive content and ability to support growth, link to behavior. We chose as a model system the bacteria-eating roundworm Caenorhabditis elegans to study this question. Almost all known C. elegans behaviors are affected by food, as usually measured by the presence versus absence of the standard worm food, Escherichia coli strain OP50 (Avery and Horvitz, 1990; Chalfie and White, 1988; Croll, 1975; de Bono et al., 2002; Gray et al., 2005; Hedgecock and Russell, 1975; Sawin et al., 2000; Tsalik and Hobert, 2003). In research on C. elegans, the bacterial food has been treated mainly as a sensory stimulus. The action of food as a reinforcement that provides feedback after it is eaten has not been directly addressed. We show that C. elegans exhibits food seeking behavior and hunts for easier-to-eat food that best supports growth. For this behavior, the balance of two locomotory states, known as roaming and dwelling, is crucial, and AIY neurons function to extend food seeking periods.
Materials and methods
Worms
For routine purposes, worms were grown at 18°C on modified nematode growth medium (NGMSR; Davis et al., 1995). Plates were seeded with E. coli strain HB101. The strains used were: wild-type Bristol strain N2, Hawaiian strain CB4856, DA465 eat-2(ad465) II, DA1402 eat-5(ad1402) I, PR811 osm-6(p811) V, OH161 ttx-3(ot22) X, FK134 ttx-3(ks5) X, MT1073 egl-4(n1073) IV, PR808 osm-1(p808) X, CB1033 che-2(e1033) X, PR802 osm-3(p802) IV, CB1124 che-3(e1124) I, PR767 ttx-1(p767) V, CX2304 odr-2(n2154) V, CX2205 odr-3(n2150) V, CX4 odr-7(ky4) X, PR679 che-1(p679) I, PR671 tax-2(p671) II, MT3564 osm-7(n1515) III, CX10 osm-9(ky10) IV, CB1338 mec-3(e1338) IV, XA406 ncs-1(qa406) X, JC2154 hen-1(tm501) X, DA609 npr-1(ad609) X, GR1321 tph-1(mg280) II, MT7988 bas-1(ad446) III, CB193 unc-29(e193) I, CB55 unc-2(e55) X, CB251 unc-36(e251) III, osm-6(p811) V, ttx-3(ot22) X, egl-4(n1073) IV, osm-6(p811) V, egl-4(n1073) IV, ttx-3(ot22) X, OH99 mgIs18[ttx-3p::GFP] (a gift from Dr Oliver Hobert; Hobert et al., 1997) was used for AIY laser kills.
Bacteria
Bacterial strains were described by Avery and Shtonda (2003) with one exception: here, a spontaneous sporulation-deficient variety of Bacillus megaterium was used. The original B. megaterium isolate produces sporulation mutants at rather high incidence, so the bacterial culture is heterogeneous. We isolated and used the sporulation mutant in this work, because it grows to a homogeneous bacterial lawn. E. coli strains were HB101 (Boyer and Roulland-Dussoix, 1969) and DA837 (Davis et al., 1995).
Growth rate measurements
Growth rates were measured as described by Avery and Shtonda (2003), except that all bacteria were grown on standard NGM (Sulston and Hodgkin, 1988) plates. The growth rate was calculated as the inverse of the number of days it took for animals to reach adulthood. The experiment shown in Fig. S1 in supplementary material was done over many days, and the results were averaged. As mentioned in the legend to that figure, in three cases (eat-5 on DA837, eat-2 on Bacillus megaterium and eat-5 on B. megaterium) the growth rate variability between animals was too high to determine the growth rate by looking at the population of worms, so the same assay was done with one larva on each plate, and the results were averaged.
Food choice assays
For experiments in Figs 1, 3A and 5E (‘unbiased’ food preference assays), bacteria were grown overnight at 37°C and then used within 5 ·days. Assays were done on 50 mm polystyrene plates filled with NGM medium from which the bactopeptone was omitted to slow bacterial growth during the experiment. Bacterial food was placed on assay plates in three different arrangements with a worm pick, as shown on Fig. 1A. Distances were controlled with an ocular micrometer. Plates were kept at 22–25°C and experiments were done the next day. L1 larvae were prepared by egg synchronization: hermaphrodites were lysed in 40% bleach (Chlorox), 0.1 mol l−1 NaOH until fragmented, and eggs were incubated overnight (14–18·h) at 18°C to allow larvae to hatch (Emmons et al., 1979). Larvae were washed once in M9 and 70–150 worms were placed in the center of each assay plate in a 1–1.5 μl drop of M9 buffer. (The number of worms could not be strictly controlled. We found, however, that the exact number of worms in this range did not affect food choice.) Assays were done at 18°C in a temperature-controlled room. At time points shown in Fig. 1A, worms were killed by inverting the plates over chloroform, and the number of worms in each food and outside of the bacteria was counted. Data for all colonies of the same type on a single plate were pooled together, so each data point represents one plate. For Fig. 1A–C, experiments done over many days were averaged.
Fig. 1.
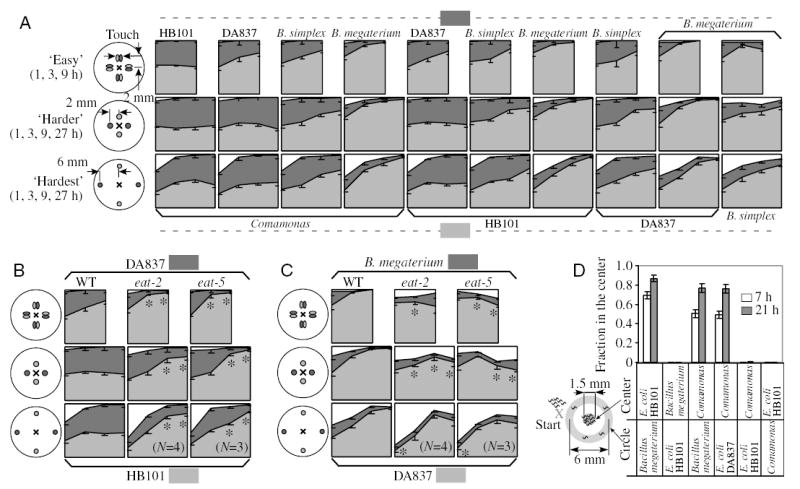
Food choice behavior. (A) Food choice of wild-type L1 larvae. There are three arrangements of bacterial food, detailed on the left. In the ‘easy’ arrangement, colonies of different food touch each other; in the ‘harder’ and ‘hardest’ arrangements they are separate and 2 mm and 6 mm from the center of the plate, respectively. At time point 0, worms were placed in the center of the plate (marked with ×) at equal distance from the bacterial colonies. Worms were killed and counted at the indicated time points. (Right) Each area diagram represents a time course of the food choice between two bacterial species. Light gray and dark gray areas depict the fraction of worms in each food, and the white area depicts the fraction of worms outside the bacteria. The two bacterial species in each test are listed beneath and above the panels. In almost all cases where choice develops, it is in favor of the bacteria that better support growth. (B) Food choice of the eat mutants eat-2 and eat-5 between two Escherichia coli strains, HB101 and DA837. In all arrangements of bacteria the preference of eat mutants for the better food, HB101, is stronger than that of the wild type, consistent with DA837 being far worse food for both eat mutants (Fig. S1 in supplementary material). (C) Food choice of eat mutants between DA837 and Bacillus megaterium. While the wild type shows clear preference for DA837, the choice of eat mutants is not as strong and does not show a distinct trend in the easy and the harder arrangements, consistent with both DA837 and B. megaterium being bad foods for eat mutants. Mean ± s.e.m., N=5, except four cases with N=3 and 4, as indicated. Each data point is derived from a distribution of 70–150 worms on one assay plate. *Different from wild-type on the same food combination, P<0.05, Student’s t-test. (D) Food choice of wild-type L1 larvae in the biased food preference assay. At time point 0, worms were placed outside the circle, as shown by the ×. With time, animals crossed the circle and located the food in the center (y-axis of the plot). At indicated times, worms were killed and their distribution was counted. Results are expressed as a fraction of animals in the center. Worms migrate to the central colony only if the central colony is good food, Comamonas or E. coli HB101, whereas the circle is mediocre food, B. megaterium or E. coli DA837. Values are means ± s.e.m. Numbers of assays are 6–18 for different pairs of food.
Fig. 3.
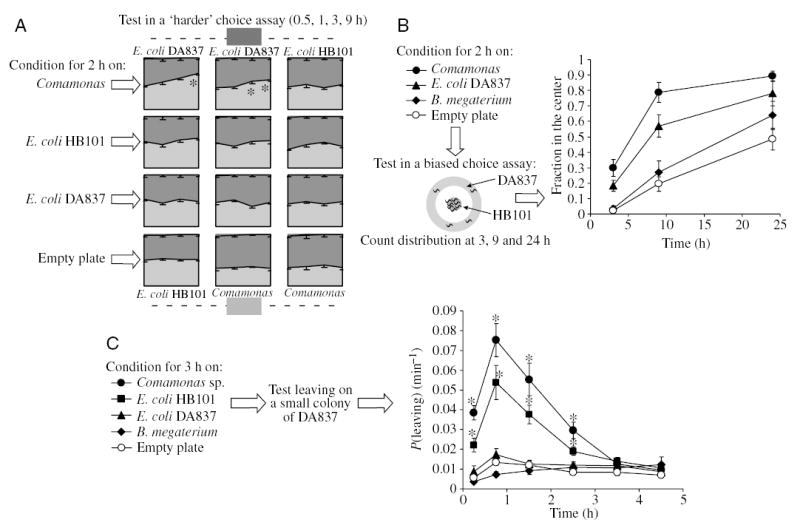
Effect of dietary experience on food seeking behavior. (A,B) Effect of experience on food choice. (A) Wild-type L1 larvae were kept for 2·h in one of the four conditions, and then each group was tested in the ‘harder’ choice assay with three food combinations. The only two cases where the consistent time course of the food choice was observed were groups conditioned on Comamonas and tested on pairs of E. coli DA837 vs E. coli HB101, and E. coli DA837 vs Comamonas; in both cases worms chose better food. The effect of experience seems mild, but note that without conditioning, no preference develops with these pairs in a harder arrangement (Fig. 1A). Values are means ± s.e.m. (N=5). *Different from worms conditioned on E. coli DA837 and empty plate (P<0.02; Student’s t-test). (B) Wild-type larvae were exposed to one of the listed conditions for 2·h and then tested in a biased choice assay with a circle of DA837 surrounding a central colony of HB101. Worms that have experienced the high quality food, Comamonas and E. coli HB101, show the strongest food choice. Values are means ± s.d. (N=20; 10 plates with two circle assays on each). At all time points groups conditioned on food are different from those conditioned on the control empty plate (P<0.01; Student’s t-test). (C) Effect of food experience on leaving behavior. 50–80 naive eat-2 L1 larvae were conditioned in one of the five indicated conditions for 3·h, washed and transferred to another plate for a leaving assay. The time when the first worm entered the colony is time point 0. The x-axis shows the time intervals within which leaving probability was determined: 0–30·min, 30·min–1·h, 1–2·h, and in 1-h increments thereafter. After exposure to high quality food, Comamonas or HB101, leaving behavior was increased as compared to conditioning on the same food, worse food, or without food (empty plate). Values are means ± s.e.m. (N=6). *Different from worms conditioned in any of the other conditions (P<0.05; Student’s t-test).
Fig. 5.
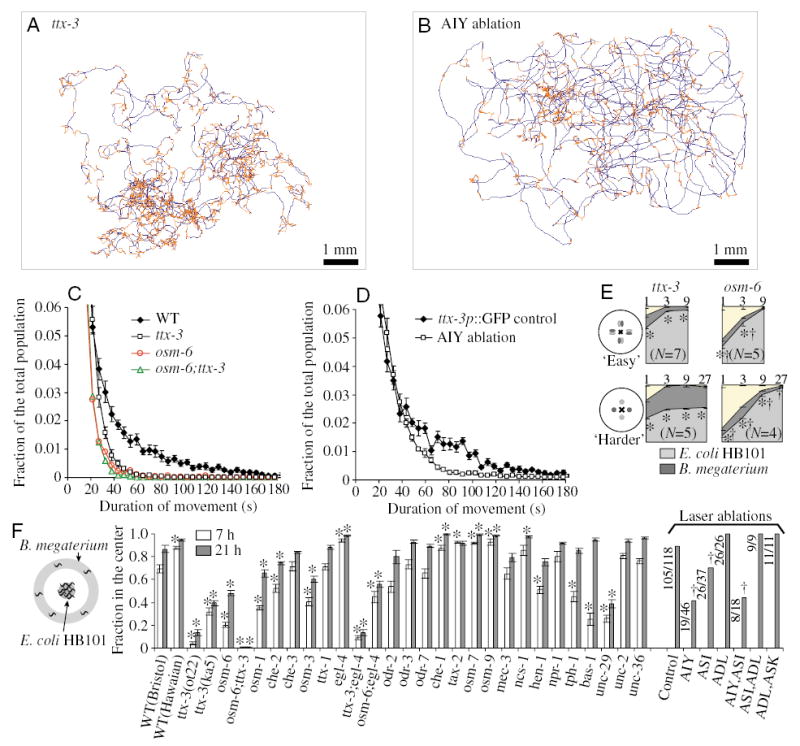
AIY neurons function to extend food-seeking periods. Trajectory on mediocre food, E. coli DA837, of (A) a ttx-3 mutant and (B) an animal whose AIY neurons have been killed. Compare to wild-type in Fig. 4C. ttx-3 mutant trajectories did not span the whole lawn; and there were far fewer long straight roaming events. Trajectories of AIY worms also had fewer straight long movements than wild-type controls. (C,D) Movement duration distribution of (C) wild type, ttx-3, osm-6, osm-6;ttx-3 and (D) AIY-ablated animals, all tested on E. coli DA837 food. N=10 for WT, 10 for ttx-3, 6 for osm-6, 6 for osm-6;ttx-3, 10 for AIY ablations and 8 for ttx-3p::GFP controls. (E) ttx-3 was defective in the food preference behavior if bacterial foods were located at a small distance from each other. By 3·h, all ttx-3 worms found food, but there was no preference in the harder arrangement. In contrast to ttx-3, osm-6 animals took longer to discriminate between good and bad food, but they finally managed to make the right choice even if foods were located at a distance. Values are means ± s.e.m. *Different from the wild type (P<0.01); †different from ttx-3 (P<0.01; Student’s t-test). (F) Biased food preference for E. coli HB101 over B. megaterium of mutants and animals with laser-ablated neurons. The fraction of animals that reached the central colony of good food, E. coli HB101, was determined. ttx-3 mutants and AIY-ablated animals performed worse than controls. In laser ablation experiments, worms were counted after 20·h. For tests on mutants, the number of assays is 18 for WT, 15–17 for ttx-3 alleles and 6–15 for various mutants tested. For laser ablations, number of worms found in the center and the total number of worms tested is indicated next to the bars. Values are means ± s.e.m. *Different from the wild type (P<0.01; Student’s t-test); †Different from the ablation control (P<0.01; χ2 test of independence).
For the experiments shown in Figs 1D, 3B and 5F (‘biased’ food choice assay), the bacterial source plates were the same as described above, but the assay plates were normal NGM plates (with bactopeptone). The outer circle of bacterial food was made with the back end of a Pasteur pipette. These circles were grown overnight at 37°C. Next day, chunks of another bacterial food were placed in the center of the circle with a worm pick to obtain the arrangement shown in Fig. 3A. On each plate, two such circles were made, except for the assays on laser ablated worms (Fig. 5F) which had one circle per plate. Egg-synchronized L1 larvae were plated outside the circle, as shown in Fig. 1D, followed by incubation at 18°C. At given time points, worms were killed by chloroform vapor, stored at 4°C and counted. Each data point represents one circle. For Fig. 5F, experiments done over many days were averaged.
For testing the effect of food experience on food choice (Fig. 3A,B), naive synchronized L1s were placed in M9 on different conditioning plates, either seeded with bacterial food or unseeded (empty), as described in the legend. After 2·h, worms were washed off with 1 ml of M9 and washed twice with 1 ml of M9, with each wash followed by a 15·s spin at 270 g (2000 r.p.m.). After the final spin, larvae were transferred onto assay plates in 1–1.5 μl of M9 for the food choice assay. For Fig. 3A,B, an assay was done on a single day with the same batch of worms and assay plates in order to reduce variability, and all conditionings for a given time point were done in parallel.
Leaving assay
For a population leaving assay (Figs 2A and 3C), assay plates were standard NGM plates. Chunks of bacteria were transferred from bacterial food plates onto assay plates with a worm pick and shaped into a small, roughly ellipsoid colony with the smaller dimension of >0.5 mm and the bigger dimension of <1 mm, as measured with an ocular micrometer. One colony was made on each plate; plates were immediately used in the assay. 50–80 egg-synchronized larvae were placed in a 1–1.5 μl drop of M9 as close as possible to the food colony. Plates were placed on a video recording apparatus (see below) for recording. Video recordings were analyzed using Adobe Premiere 6.5 software. The moment at which the first worm entered the colony was time point 0. Then, the number of worms entering and leaving the colony each minute was counted. The leaving probability, P(leaving), in each minute of the recording was determined as the ratio of the number of worms that left the colony during this minute to the total number of worms in the colony at the start of that minute. For Fig. 2A, this per-minute probability was averaged between 1 and 2h of the recording (i.e. for minutes 61–120); for Fig. 3C P(leaving) was averaged in several intervals as described in the legend to this figure. Leaving assays on single worms (Fig. 3B–D) were done on NGM plates from which the bactopeptone was omitted and (for B and C only) agar replaced with agarose to limit the growth of the colony during the experiment. In single worm assays, a late stage (pretzel) embryo was placed close to the colony. The larva usually hatched within the next 3·h, and time point 0 was the time at which it first entered the colony. The experiment in Fig. 2A was done over many days, and results were averaged.
Fig. 2.
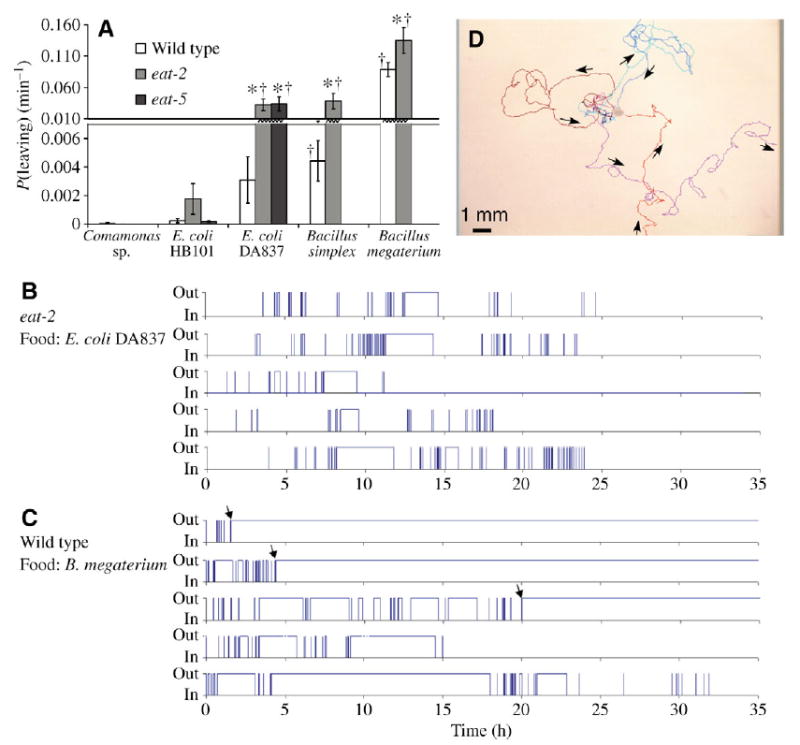
Leaving behavior. (A) Leaving frequency of the wild type and two eat mutants, eat-2 and eat-5, in a population leaving assay (eat-5 was tested on E. coli foods only). Worms were placed near the small chunk of bacteria and allowed to enter it; the time when the first worm entered the colony was time 0. The plot shows mean leaving frequency between 1 and 2h after the start. On poor quality food, leaving is more active, and leaving behavior of eat-2 and eat-5 is more active than that of the wild type. Values are means ± s.e.m. (N=8). *Different from the wild type on the same bacteria (P<0.05). †Different from P(leaving) of the same worm strain on good food, HB101 and Comamonas (P<0.05). All comparisons are by Student’s t-test. (B) Leaving behavior of five individual eat-2 worms on E. coli DA837. A late-stage egg was placed near the colony. Time 0 is when the hatched larva first entered the colony. On the y-axis, ‘in’ is the time spent in the bacterial colony, while ‘out’ is the time spent outside the colony. (C) Leaving behavior of five individual wild-type worms on Bacillus megaterium; procedure as in B. Three out of five worms at some point, marked with arrows, left the colony and never returned. (D) Sample leaving trajectories of an individual wild-type worm on B. megaterium. Five typical segments of the animal’s trajectory are shown with different colors, and the direction of movement is shown with arrows.
For the experiments in Fig. 3C larvae were prepared in the same way, but incubated for exactly 15·h. This experiment was done on six consecutive days, and each time all five conditions were done in parallel. Naive synchronized L1s were placed in M9 on different conditioning plates, seeded with bacterial food or unseeded (empty) as described in the legend. After 3·h, worms were washed off with 1 ml of M9 and washed three more times with 1 ml of M9, with each wash followed by a 1 min spin at 70 g (1000 r.p.m.). After the final spin, larvae were transferred onto assay plates in a 1–1.5 μl of M9 for the leaving assay, 50–70 larvae per assay. All incubations and video recordings were done at 18°C.
Laser ablations
Laser ablations of neurons were performed as described by Bargmann and Avery (1995). AIY neurons were identified by GFP epifluorescence in the mgIs18[ttx-3p::GFP] strain OH99. The ADL, ASI, ASK and ASH neurons were identified by staining with the fluorescent dye DiI (Starich et al., 1995). For the food preference assay (Fig. 5F), egg-synchronized L1s were incubated in 0.01 mg ml−1 DiI in M9 for 2·h. Then animals were destained for 1·h on an unseeded NGM plate and transferred onto 3% agarose pads containing 3 mmol l−1 NaN3 for the laser ablation. Ablations were monitored by GFP or DiI bleaching by the laser beam, and confirmed after the assay. All laser ablated groups shown in Fig. 5F were treated the same, except that in the control group lasering was omitted. After ablation, worms were transferred in M9 onto an empty NGM plates for a 2·h recovery and then plated on food preference assay plates, with one assay per plate. On each plate, two to four worms were assayed (usually from one ablation batch). After 20·h, plates were scored. Experiments were done over many days, and results were averaged.
For the trajectory analysis on AIY-ablated animals (Fig. 5B,D), eggs were obtained by bleaching hermaphrodites as described above. After 1·h, newly hatched larvae were picked for ablations. Ablations were performed as described above, except that DiO staining was omitted (AIY neurons were identified by GFP in the ttx3::GFP strain). Controls were treated the same, but neurons were not ablated. After a 2·h recovery on an empty NGM plate, single worms were transferred onto assay plates for trajectory recordings (see below).
Video recordings
For the leaving assay and trajectory recordings, a nine-worm recording station was built. Nine PC23C (Supercircuits, TX, USA) monochrome cameras were mounted on a custom-made rack and connected to a 16 port GV-600 recording board (Geovision, Taiwan), which was installed in a Pentium 4 computer running Windows 2000. The light sources were also custom built. Recordings were made using the Geovision software at a rate of approximately one frame per second (on average, every 1.07·s); video files were automatically saved every 5 min. These files were then compiled into one large continuous file using Adobe Premiere 6.5 and analyzed as described below. All recording experiments were done at 18°C.
Trajectory recordings and analysis
For the trajectory assay (Figs 4, 5), roughly 9 mm × 4 mm rectangular lawns of bacteria were streaked on NGM plates and incubated for 3.5–5·h at 37°C to produce a thin smooth bacterial lawn. Different bacteria were found to grow at different rates: B. megaterium was the fastest; E. coli DA837 was intermediate and Comamonas and E. coli HB101 were the slowest, so the incubation times were adjusted correspondingly to produce lawns of about equal thickness. (The time of the lawn growth and its thickness had no detectable effect on behavior.) Eggs were obtained by bleaching, and L1 larvae hatched within 1·h and were then transferred in a small (1–1.5 μl) drop of M9 onto the trajectory assay plates. The video field for the trajectory recording was 10.3 mm wide, which was found to be the maximal size to allow automated tracking of an L1 larva. Video recordings were made using the apparatus described above.
Fig. 4.
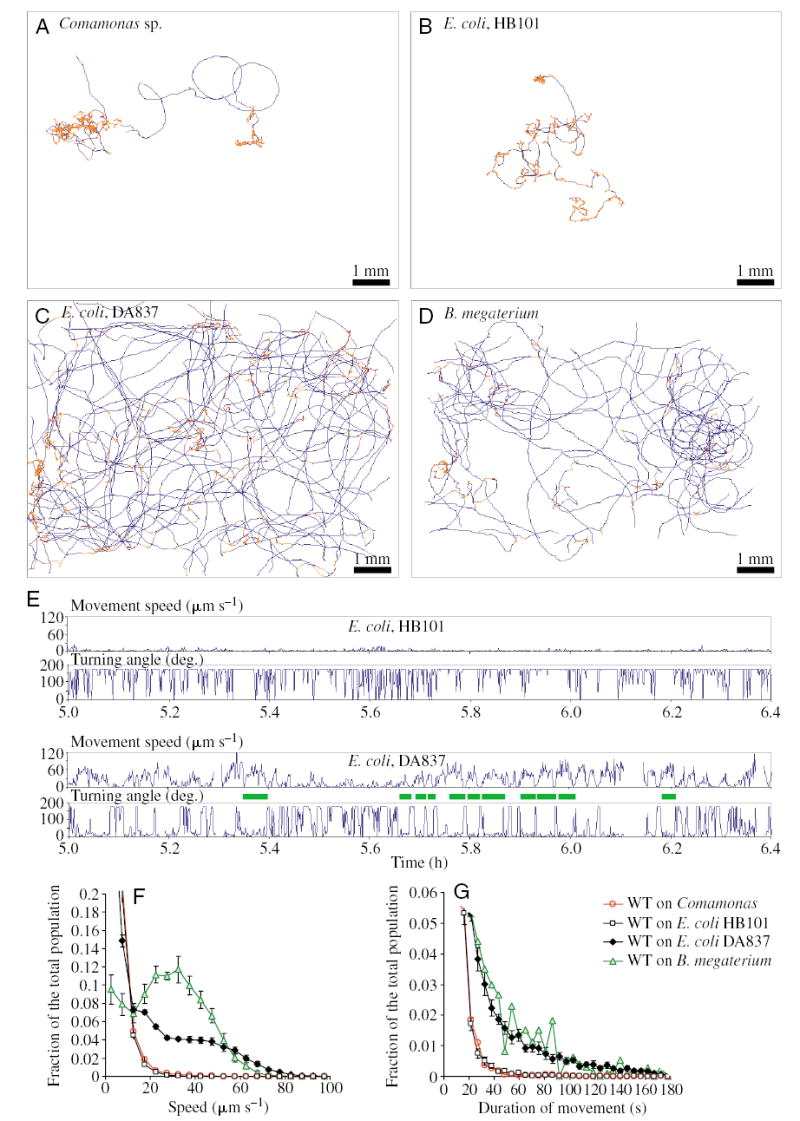
Trajectories of wild-type worms on different bacterial food sources. (A) on Comamonas, (B) on E. coli HB101, (C) on E. coli DA837 and (D) on Bacillus megaterium. A single L1 larva was placed on a roughly rectangular bacterial lawn, and its movement trajectory during the subsequent 10–15·h was recorded. (Here, trajectories during the interval from 2–10·h of the experiment are shown.) The width of the field of view is approximately 10.3 mm; the bacterial lawn fits into the video field. The trajectory on B. megaterium is fragmented because of poor contrast; this problem was most severe at the edges, where bacteria tended to be the thickest. Roaming periods are shown in blue, dwelling in orange; there was much more roaming on mediocre food. Note that, since by definition the worms move slowly or not at all during dwelling, the extent of the orange traces doesn’t reflect the proportion of time spent dwelling. (E) Sample speed of locomotion and turning angle (direction of movement change) traces of the wild type on E. coli foods HB101 and DA837. Roaming periods (green bars), when the turning angle stays low and the speed is high, are common on mediocre food, E. coli DA837, but very rare on good food, E. coli HB101. (F) Speed of locomotion and (G) movement duration distributions. On mediocre foods, the speed of locomotion and the movement duration is increased. In F and G, data from 10 worms on E. coli DA837, 11 on E. coli HB101, eight on Comamonas sp. and five on B. megaterium were averaged; trajectories in the interval from 2 to 10·h from the start of the recording were analyzed for each animal. Data are expressed as mean ± s.e.m. between individual animals. In G, error bars for E. coli foods only are shown. Because the trajectory on B. megaterium is fragmented (D), the population of long events is artificially decreased. The increased roaming duration on B. megaterium is still obvious, but the real effect is even bigger, as suggested by the speed analysis (F).
Video files were analyzed using custom worm tracking software written with Microsoft Visual C++ 6. In every frame, worm coordinates were recorded. For the locomotion analysis, the trajectory of the worm between 2 to 10·h from the start of the recording was used. These regions were run through a custom-written Labview 6 (National Instruments) program to extract movement segments using the segmentation procedure described by Pierce-Shimomura et al. (1999). Worm coordinates in every fifth frame (on average, every 5.37·s) were used to calculate the movement speed and turning angles. Every turn of more than 50° was considered a direction change and the start of the new movement segment. Unlike previous workers (Fujiwara et al., 2002; Pierce-Shimomura et al., 1999), we found that on good food, wild-type larvae exhibit extended periods of no movement. This could not be due to sickness because it was found in almost all experiments, and, most importantly, the behavior was very different on mediocre food (Fig. 4). During periods of no movement, direction change (turning angle) is undefined. We assigned 180° to the value of turning angle during these periods, because no movement is a behavior totally opposite to the rapid straight locomotion, when the turning angle stays low, close to 0. Moreover, periods of no movement are indistinguishable from very small movements at our video and time resolution and from noise resulting from brightness and contrast variations. Therefore, the dwelling mode of locomotion (Fujiwara et al., 2002) in our analysis includes both periods of small movement and frequent direction change, as well as periods of no detectable movement.
On B. megaterium lawns the contrast was so poor that the L1 could not be seen for a large fraction of the time, so the trajectory is fragmented. This creates a bias in favor of short movements, because the long ones are often interrupted (interrupted segments were always discarded). Trajectories on B. megaterium were poorly suited for analysis, and most mutant and laser ablation analyses were done on HB101 and DA837 strains of E. coli.
Data presentation and statistics
Data are presented as mean ± standard deviation (s.d.) or standard error of the mean (s.e.m.), as stated in figure legends. Significance was determined using Student’s two-tailed heteroscedastic t-test or the Chi-squared test of independence.
Results
Bacterial food as a variable
C. elegans is a bacteria-eating soil dwelling nematode that swallows bacteria with the large pump-like organ called the pharynx. Soil is inhabited by various species of bacteria, and we reasoned that not all of those might be good foods for C. elegans. Escherichia coli, a mammalian intestinal symbiotic bacterium, has been used for decades to grow C. elegans in the laboratory (Sulston and Hodgkin, 1988). For obvious reasons, E. coli is unlikely to be the main natural C. elegans food, although occasional encounters are certainly possible.
Previously, we identified a number of species of soil bacteria that vary in their ability to support C. elegans growth and reproduction (Avery and Shtonda, 2003). Here, we chose five bacterial strains and defined their ability to support growth as the food quality (this is not to be confused with digestibility or nutrient content). We measured the growth rate of wild-type C. elegans and two feeding-deficient eat mutants on these bacteria and confirmed that Comamonas sp. and E. coli strain HB101 are high quality food, E. coli DA837 and B. simplex are mediocre, and B. megaterium is poor food (see Fig. S1 in supplementary material).
The two eat mutants used here, eat-2 and eat-5, are of different molecular nature, but they both interfere with swallowing of bacteria and therefore reduce food flux to the intestine. eat-2 is a non-alpha subunit of a pharyngeal acetylcholine receptor required for rapid pharyngeal pumping (McKay et al., 2004; Raizen et al., 1995). eat-5 encodes an invertebrate gap junction (innexin) subunit (Starich et al., 1996). In eat-5 mutants, contractions of two pharyngeal compartments, called the corpus and the terminal bulb, are not synchronized, and the rate of terminal bulb contractions is reduced.
While it is not critical for the conclusions of our study, it is interesting to know what makes bad foods bad. One possible explanation of why worms grow slower on E. coli DA837, B. simplex and B. megaterium is that these bacteria are toxic or pathogenic. Indeed, some bacteria are known to be pathogenic for C. elegans (Garsin et al., 2001; Pujol et al., 2001). If, however, the poor foods limited growth because they are toxic, we would not expect to see differences in growth rates between wild-type worms and eat mutants, since food flux to the intestine would not be limiting growth. That eat mutants grow slower than the wild-type suggests that the function of the pharynx is likely to be limiting for growth on these foods. Consistent with this explanation, the difference between the wild type and eat mutants is particularly large when fed on B. megaterium, which is an extremely bad food, even for the wild-type C. elegans. (In this study, we used a sporulation-deficient variety of B. megaterium, see Materials and methods.)
One could still argue that eat mutants cause defects outside the pharynx that weaken the worm’s immunity, causing an increased susceptibility to pathogenic bacteria and a further growth delay. This is possible, but unlikely, for eat-5 and especially eat-2, which is expressed in a single cell in the worm, and localizes to a single synapse that the motor neuron MC makes on pharyngeal muscle (McKay et al., 2004). We also observed the worms’ morphology and behavior on poor food. Except for the expected Eat (starved) phenotype, worms did not show any noticeable behavioral or anatomical abnormalities on E. coli DA837, B. simplex and B. megaterium. Worms grown on known pathogens, in contrast, have obvious defects (Garsin et al., 2001).
These results suggest that foods on which worms grow slower, E. coli DA837, B. simplex and B. megaterium, are not toxic, but hard-to-eat. In experiments described below, eat mutants show behavioral differences from the wild type, which further support this hypothesis.
All experiments described below were done on L1 larvae, newly hatched worms. The critical advantage of using newly hatched L1 larvae is that they are naive: they have never experienced food. Obviously, it is impossible to grow worms to any stage beyond L1 without letting them experience some food, and we found that food experience does affect subsequent behavior (see below). In addition, food quality selection is probably most critical for young animals with the small pharynx, as suggested by our previous observation that smaller bacteria are better food for C. elegans (Avery and Shtonda, 2003).
Dietary choice behavior
In a simplified laboratory environment, the roundworm Caenorhabditis elegans is routinely fed E. coli. In the wild, worms are probably exposed to a variety of bacterial food. How they deal with this choice is unknown. To test whether C. elegans can select better quality food, we gave worms a choice between two types of bacteria (Fig. 1A). In nine out of ten tested pairs, worms chose the higher quality food, the one that better supports growth. The exception is a weak preference for E. coli DA837 in the DA837/B. simplex pair; these foods are about the same in quality for the wild type (Fig. S1 in supplementary material). In other cases when there is no difference in growth rate on two different foods, the choice is hardly detectable (such as Comamonas vs HB101). In contrast, in all cases when there is a difference in the food quality, worms choose the food that better supports growth. For example, for wild-type worms B. megaterium is the worst food, and the preference against B. megaterium is particularly strong, whatever the other food is.
By varying the distance between foods, the sensitivity of this assay could be adjusted. We used three different arrangements. In the ‘easy’ configuration, when two foods are located very close to each other (so close that it is enough for an animal to stick its head out of one colony to get into the other) the choice is stronger: for example, there is a weak preference for Comamonas and HB101 when the other choice is E. coli DA837 (Fig. 1A). In the ‘harder’ and ‘hardest’ arrangements, no preference is seen for either food. In most cases food preference develops with time, suggesting that worms need to try the food to make a decision. This is similar to observations on rats, which show that animals taste both foods before making a choice, and the preference also exhibits a trend (Young, 1933; Young, 1941).
One could argue that the food choices observed in Fig. 1 are the result of the worms’ varying degree of chemoattraction to different bacteria. In a chemotaxis assay, worms are placed equidistant between sources of attractant, and their accumulation at the sources is measured after a relatively short time – the chemotaxis develops to a maximum within 1·h on a standard 50 mm plate (Bargmann et al., 1993). The 1·h time points of the ‘harder’ and ‘hardest’ food arrangements are therefore equivalent to chemotaxis assays, and we observed that worms did not show preferential chemotaxis towards any food (Fig. 1). The only possible exception is B. megaterium: even at early time points, worms showed a weak bias against it. However, the initial bias against B. megaterium was far weaker than the bias that developed over time, and this is unlikely to be explained only by differential chemotaxis. If the innate preference for the smell of particular bacteria were the main guidance cue in food choice, animals would migrate to the better-smelling bacteria from the start, and the ratio of worms in one food to another would not change with time. That this ratio changes may have two nonexclusive explanations: (1) chemotaxis rates to different bacteria have divergent time dependences, and, (2) worms not only find bacteria, but also leave them – animals that have initially entered less preferred food at some point leave that colony and go to the other one. The next section describes experiments in which we tested the latter possibility.
Given a choice of two E. coli strains, DA837 and HB101, eat mutants show a much stronger preference than the wild type (Fig. 1B). For example, in the ‘harder’ and ‘hardest’ arrangements, the wild-type does not show preference, whereas both eat mutants do show a robust, ~90%, preference. It is very unlikely that C. elegans has evolved an ability to discriminate between two E. coli strains by their smell. Even if this were true, it would not explain why eat mutants show a stronger preference for the high quality food than the wild type. The most plausible explanation for this observation is that food consumption provides feedback that drives food choice. In feeding-limited eat mutants, the food consumption is altered, and their appetitive behavior is adjusted to compensate.
Given a choice of mediocre (E. coli DA837) and bad (B. megaterium) foods, the behavior of eat mutants is also different for that of the wild type, but in the other direction: their preference for DA837 in this pair is slightly less robust than the preference of the wild type (Fig. 1C), and the preference time course is shallower. For eat mutants, DA837 is mediocre or bad food, especially for eat-5 mutants, and B. megaterium is very bad food (Fig. S1 in supplementary material). Probably, animals are confused by having to make a choice between bad and very bad. In agreement with this explanation, the fraction of eat mutants that are searching (outside of bacteria) stays very high throughout the experiment, whereas for the wild type, it drops to almost zero by 9·h. The increased fraction of eat mutants observed outside of food in Fig. 1C even at late, 27-h time points is unlikely to be explained by their impaired ability to find food. As seen in Fig. 1B, eat mutants find food as efficiently as wild type, if at least one good food (E. coli HB101) is present. The most likely explanation of these results is that if both foods on the plate are bad for eat mutants because of the impaired swallowing ability of the mutants, eat mutants repeatedly and actively leave both foods and explore the plate.
The choice assay described in Fig. 1A–C did not distinguish between two choice possibilities: (1) animals locate the good food from the start, and (2) animals locate the bad food first, but then switch to the good one. If the food finding rates are equal, 50% of animals would be expected to find the good food from the start simply by chance, because the two foods are equally accessible. We wanted to design an assay that discriminates against this uninteresting possibility and only measures the ‘switching’ events. In the food choice assay shown in Fig. 1D, one food was surrounded by a circle of another food. Worms were placed outside of the circle, and accumulation of worms in the central colony with time was measured. The range of this assay is from 0 (no worms reach the center) to 1 (all worms reach the center) – only the meaningful ‘switching’ events are detected. Because all animals were forced to experience one food first, we called this assay a ‘biased choice assay’. It is somewhat similar to the stimulus integration assay (Ishihara et al., 2002), where an attractive stimulus is surrounded by a circle of repellent. In our assay, however, the circle did not repel animals, but fooled them. In agreement with results obtained in the ‘unbiased’ food choice assay described above, worms went to the center only if the mediocre food surrounded the good food, such as when B. megaterium or E. coli DA837 surrounded Comamonas or E. coli HB101 (Fig. 1D). If good food encircled either another species of good food or bad food, almost all worms stayed in the circle. Indeed, because only worms that cross the circle and migrate to the center are counted, the observed preferences ranged from 0 to 0.9.
Leaving behavior
The food choice experiments imply that worms leave hard-to-eat food. We tested this directly (Fig. 2). In the experiment shown in Fig. 2A, the frequency of leaving the small colony of bacteria was measured. Between 1 and 2h after the first worm has entered the bacterial colony, wild-type larvae leave B. megaterium with a probability of about 9% per minute. They leave DA837 and B. simplex much less actively: 0.3 and 0.4% min−1, and do not detectably leave the good foods Comamonas and HB101. Consistent with the food choice experiments, eat mutants leave mediocre food more actively: with P(leaving) about 3.5% min−1 for DA837 and B. simplex and 13.5% min−1 for B. megaterium (Fig.·2A). These results explain why eat mutants exhibit much stronger choice in the pair of two E. coli strains, HB101 and DA837 (Fig. 1B) and are consistent with the hypothesis that it is the differential leaving, not differential finding of food that determines the food preference.
Recordings of individual worms (Fig. 2B–D) showed that animals leave food, explore, and return multiple times. Leaving behavior was a stochastic all-or-none phenomenon (Fig. 2B,C); there was no obvious stimulus causing every single event. The pattern of leaving activity was interesting. Most leaving events were very short and lasted just a few minutes, but very infrequently, worms spent 2–5·h outside of food and then still returned. In some cases, a worm left food and never returned within the observation period; it probably crawled off the plate and dehydrated (Fig. 2C).
Effect of previous food experience
Since previous experience is known to affect appetitive behaviors in other animals, we tested the effect of prior food experience in C. elegans in two assays: leaving behavior and food choice behavior.
We found that after experiencing the highest quality food, Comamonas, wild-type worms showed food choice in conditions where untrained animals exhibit no choice (Fig. 3A). In these experiments, naive L1s were conditioned for 2·h on good food (Comamonas or E. coli HB101), mediocre food (E. coli DA837), or a plate without food, and then their food choice was tested in the ‘harder’ arrangement with pairs of E. coli DA837 versus E. coli HB101, DA837 vs Comamonas and Comamonas vs HB101. On the DA837/HB101 pair, animals conditioned on Comamonas showed a consistent preference trend from 46% at 0.5·h to 71% at 9·h. On the DA837/Comamonas pair, the effect was less robust, 42%–58%, but still significantly different from groups conditioned on E. coli DA837 or empty plate. With both of these pairs in the ‘harder’ arrangement, naive animals show no choice (Fig. 1A). In these experiments we found that animals showed a positive bias for both E. coli strains over Comamonas after being conditioned on an empty plate. This preference, however, does not show any change in time, indicating that it is determined by the initial food finding rates.
In the ‘biased’ food choice assay the effect of experiencing good food was more pronounced, which is expected since even naive animals exhibit strong choice in these conditions (Fig. 3B, compare with Fig. 1D). Wild-type naive L1s were conditioned for 2·h on good food (Comamonas), mediocre food (E. coli DA837), bad food (B. megaterium), or a plate without food, then their food choice was tested on a circle of E. coli DA837 surrounding an E. coli HB101 colony. At 9·h, only about 20% of worms that had experienced an empty plate reached the center, compared with 80% of worms that had experienced Comamonas (Fig. 3B).
Note that in both food choice assays, the high quality food used during conditioning did not have to be the same as the high quality food used during the test. The biggest effects were in fact observed using Comamonas for conditioning and E. coli DA837 vs E. coli HB101 pair for the test. Thus, the worms did not necessarily seek exactly the same food, by following its odor, for example. More probably, their exploratory activity was increased, so any good food could be found faster.
Next, we tested the effect of experience on leaving behavior. Naive larvae were conditioned for 3·h on either high quality food, E. coli HB101 or Comamonas, or mediocre food, E. coli DA837 or B. megaterium, and then their leaving behavior was tested on a small colony of mediocre food, E. coli DA837 (Fig. 3C). The results were consistent with the effect of dietary experience in food choice assays: the better the food was during conditioning, the more active leaving behavior was during the test. Worms that had experienced high quality food, such as E. coli HB101 or Comamonas, left the mediocre food E. coli DA837 much more actively than worms conditioned on the same food (DA837), bad food (B. megaterium), or a plate without food. In this experiment eat-2 mutants were used instead of the wild type because the wild-type frequency of leaving DA837 is too low to be measured accurately (Fig. 2A).
Regulation of C. elegans locomotion in response to food quality
On standard food, the E. coli strain OP50 (the E. coli from which DA837 was derived), C. elegans exhibits two modes of locomotion, called roaming and dwelling (Fujiwara et al., 2002). Similarly, worms are able to locate chemoattractants by alternating rapid ‘running’ and ‘pirouettes’ (Pierce-Shimomura et al., 1999). Roaming or running is a rapid straight movement; dwelling consists of short movements with frequent reversals and turns. On OP50, wild-type adult worms spend about 75% of the time in the dwelling mode and 25% in the roaming mode (Fujiwara et al., 2002).
We hypothesized that at least one role of roaming is exploration of the environment that allows worms to leave poor quality food and find high quality food. Therefore, we predicted that worms would regulate locomotion in response to the food quality. To test this, we videorecorded locomotion of wild-type naive L1s on different foods (Fig. 4A–D). We found that on good food, such as E. coli HB101 (Fig. 4A) or Comamonas (Fig. 4B), the worm trajectory was very compact; there were very few periods of straight movement. In fact, most of the time larvae did not move at all. In contrast, on mediocre food, E. coli DA837 (Fig. 4C) and B. megaterium (Fig. 4D), the animals’ trajectory spanned the whole field of view, and worms traversed the bacterial lawn dozens of times during the 10-hour experiment. Some periods of movement culminated in leaving food.
The way food quality affects locomotion was also apparent when the speed of movement and the turning angle (change of direction) were plotted against time (Fig. 4E). On the good food E. coli HB101, the speed stayed low and the turning angle high, indicating frequent direction changes or no net movement (see Materials and methods). On mediocre food, E. coli DA837, periods of rapid movement in which the speed reached 80 μm s−1 and the turning angle stayed low, were very common. The effect of food on the speed of locomotion is summarized in a histogram (Fig. 4F).
To quantify the roaming event duration, we algorithmically split the trajectory into movement segments, each new segment starting when the direction change exceeds 50° (Pierce-Shimomura et al., 1999). The movement duration histogram shows that the population of longer straight movements was significant on poor food, while it was much smaller on good food (Fig. 4G).
AIY neurons function to extend food-seeking periods
To better understand C. elegans food-seeking strategy we tested the effect of various perturbations on this behavior. A number of mutations had a measurable affect on the food choice behavior (Fig. 5F).
Among locomotion-defective mutants, severely paralyzed unc-29 showed an impairment. Two other sluggish unc mutants, unc-2 and unc-36, were normal, suggesting that even severe locomotion defects may not necessarily cause a problem in food preference behavior. Mutants with anatomically defective sensory organs (amphids), such as osm-1, osm-6 and osm-3 showed up to a 50% decrease in food preference. Mutations in amphid anatomy probably cause a variety of defects: osm-6, for example, is expressed in 56 neurons, including 24 amphid neurons, which is more than a quarter of the worms’ nervous system (Collet et al., 1998). Osm-3 is expressed in 26 neurons with sensory cilia (Tabish et al., 1995). However, mutants more specifically defective in odortaxis – odr-2, odr-3, odr-7 (Bargmann et al., 1993), showed normal food preference, as did tax-2, which is partially defective in chemo- and thermotaxis (Coburn and Bargmann, 1996),
There might be two nonexclusive explanations for these results. First, it is likely that worms sense a multitude of stimuli emitted by bacteria, so deficiencies in the ability to sense some chemicals are compensated by the normal perception of others. Second, some additional defects may be present in worms with defective amphids in addition to their impaired chemosensation. We hypothesized that these are previously described defects in the control of locomotion, and tested this hypothesis (see below).
The strongest food preference deficiency was found in the ttx-3 mutant, and it was also the most intriguing finding since ttx-3 was initially found in screens for mutants defective in thermotaxis (Hedgecock and Russell, 1975). That ttx-3 is defective in food seeking has not been previously described. The gene ttx-3 encodes a LIM homeodomain transcription factor required for the differentiation of AIY thermosensory interneurons (Altun-Gultekin et al., 2001; Hobert et al., 1997). It is also expressed in three other neuronal types: AIA, ADL, ASI (Altun-Gultekin et al., 2001), and possibly ADF (Tsalik and Hobert, 2003), where its role is unknown. AIY is required for C. elegans thermotaxis, a learned association of temperature with food (Mori and Ohshima, 1995). Wild-type worms, after having been conditioned with food and transferred to an empty plate, migrate to the cultivation temperature and move in straight trajectories (isothermal tracking; Hedgecock and Russell, 1975). ttx-3 mutants or worms in which AIY is ablated with a laser are incapable of isothermal tracking and migrate to cooler temperatures (cryophilic phenotype; Mori and Ohshima, 1995). Yet it is unlikely that the food-preference defect of ttx-3 is caused only by the altered temperature sensation, because another cryophilic mutant, ttx-1, is normal (Fig. 5F).
We have shown that wild-type C. elegans activates roaming behavior, which is a rapid straight movement, in response to mediocre food (Fig. 4). In addition, amphid-defective mutants were previously found to be biased toward dwelling (Fujiwara et al., 2002), which is consistent with the osm-6 defect in food preference. This led us to hypothesize that the ttx-3 mutant is defective in roaming on poor food, which disrupted its food seeking behavior. Using video recordings of locomotion, we tested this hypothesis.
The ttx-3 trajectory on mediocre food was different from that of the wild type: the duration of roaming periods was greatly reduced (Fig. 5A), and there were far fewer of them. This is quantified in the histogram in Fig.·5C. A similar phenotype was observed in osm-6, and the defect was further enhanced in the osm-6; ttx-3 double mutant (Fig. 5C). osm-6; ttx-3 also showed no food preference (Fig. 5F). A mutation in egl-4, which encodes a cGMP-dependent protein kinase, causes increased roaming (Fujiwara et al., 2002), but did not rescue the ttx-3 food preference phenotype (Fig. 5F).
The locomotion and food preference phenotypes of ttx-3 mutants were partially reproduced by laser ablation of AIY neurons. There were fewer longer movements in AIY-ablated animals (Fig. 5B and the corresponding histogram in D). In the biased food preference assay, only 40% of AIY-ablated animals concentrated in the E. coli HB101 center after 20·h, compared with 90% in control (Fig. 5F). Ablation of the ASIs, another chemosensory neuron pair in which ttx-3 might be expressed, also resulted in a food choice defect, although milder than the effect of AIY ablation. ASI, AIY double ablation had the same effect as AIY. The simplest interpretation of these results is that AIY normally inhibits the transition from roaming to dwelling, causing an increase in roaming event duration, which is essential for the animals’ ability to leave poor food and find good.
However, if the food choice task was made very easy by putting two foods very close to each other (the ‘easiest’ arrangement in Fig. 1), ttx-3 mutants could still correctly find the good food almost as efficiently as the wild type (Fig. 5E, upper left panel). But in the ‘harder’ food arrangement, when bacterial colonies were only 2.8 mm from each other (Fig. 1D), ttx-3 mutants did not show preference (Fig. 5E, bottom left panel). Note that ttx-3 mutant worms were finding food normally: by 3·h all worms were in one or another food in both arrangements. osm-6, by contrast, clearly had difficulties finding food; but despite that, it eventually made the correct food choice in both arrangements (Fig. 5E, right panels). That osm-6 was defective in finding bacteria was expected, since its taste and olfactory senses are severely compromised (Tabish et al., 1995), but it was surprising, given its sensory defects and very broad expression pattern, that it outperformed ttx-3 mutants in food preference tests.
Our results suggests that the control of the equilibrium between two modes of locomotion is essentially the C. elegans food seeking strategy; and it is more critical for efficient food seeking than chemo- and odor-sensation. Only sensory mutations that are also deficient in roaming, such as osm-6, result in food preference problems, ones with more restricted sensory defects, such as tax-2, were normal. A mutant specifically defective in roaming, ttx-3, showed the worst performance. Our results suggest that TTX-3 works in AIY to extend food-seeking periods, and this is critical for the ability of C. elegans to find the high-quality bacterial foods in diverse environments.
Discussion
C. elegans dietary choice behavior
Here, we describe novel behavioral paradigms in C. elegans, food seeking and food preference. We have identified five worm bacterial foods to establish a range of food quality as measured by the food’s ability to support growth (see Fig. S1 in supplementary material). Remarkably, an animal as simple as C. elegans can exhibit dietary choice (Fig. 1). Worms preferred high quality food, i.e. that better supported growth. This choice developed with time, suggesting that animals needed to try the food to make a decision. Using eat mutants, we showed that this choice requires food assessment via feeding. This is similar to mammals, which are also capable of selecting food that better supports growth, and try foods before making a choice (Osborne and Mendel, 1918; Young, 1941).
Next, we showed that C. elegans left hard-to-eat bacteria (Fig. 2), and, like food preference, this behavior required food quality assessment. Previously, it was generally thought that once C. elegans finds food, it stays there and eats until death or until the source is exhausted, although Lipton et al. (2004) have found recently that adult males leave food in search of hermaphrodites. Leaving experiments showed that even after food is found, the animal could decide to stay in the food or leave, and this decision was based on the assessment of whether the food was good or bad. Leaving behavior is a compromise: on the one hand, the worm risks losing the food that has already been found and ending up in an adverse environment, or, on the other hand, there is a chance of finding even better food.
Leaving behavior that we describe here is somewhat related to the known phenomenon of adaptation to a volatile or soluble attractant. Upon extended exposure to an odor (typically, 1·h or more) in the absence of food, odortaxis to this odor dwindles (Colbert and Bargmann, 1995; Colbert and Bargmann, 1997). Likewise, attraction to a soluble chemical switches to avoidance after 3–4·h exposure (Saeki et al., 2001) in the absence of food. In view of our results, adaptation is an increase of a food-seeking behavior because of the lack of reinforcement. Consistent with this, Nuttley et al. (2002) have shown that in the presence of food, chemoattraction is suppressed. Also, aerotaxis fades in the presence of food (Gray et al., 2004). And, if animals are conditioned to an odor or taste in the presence of food, no adaptation occurs. If worms are adapted to the stimulus in the absence of food, but then briefly exposed to food, chemoattraction robustly revives (Nuttley et al., 2002; Saeki et al., 2001). These and our data suggest the food feeds back on behavior after it is eaten and acts as a reinforcer in C. elegans.
Effect of previous dietary experience
If bacterial food was switched from good to mediocre, C. elegans appetitive behavior was increased compared to animals that had not experienced good food (Fig. 3). Previous experience of good food made worms more risk-loving, more willing to explore.
In the leaving experiment (Fig. 3C), the time course of the effect of experience could be observed: the enhancement of leaving was not high at the very beginning, but reached a maximum after 0.5–1·h. This indicates that time was needed to assess new conditions, followed by comparison and output. If a worm is taken off food, there is a period of about half an hour of area-restricted search with frequent reversals and turns, followed by active ‘running’, when reversals and turns are suppressed (Gray et al., 2005; Hills et al., 2004). This time, however, is much shorter than that required to deplete fat stores, which is about 6·h (McKay et al., 2003). Probably for worms, which feed continuously throughout life to support a 3-day life cycle, even brief food deprivation or brief decline in food quality is an alert signal that motivates them to explore the environment.
One might propose a trivial explanation for the results in Fig. 3: well-fed worms are simply healthier and explore the environment more actively than unfed ones, which strive to save energy. We think this is unlikely, because in the continuous presence of good food, both leaving behavior (Fig. 2) and exploratory activity (Fig. 4) were suppressed, while in the continuous presence of poor food, worms were very active. Therefore, it was the switch from good to bad that causes an increase in exploratory behavior.
At least two other explanations are possible. First, experiencing different quality foods changes worms’ satiation (or hunger) status, and that, in turn, affects their food choice and leaving behaviors. More hungry animals tend to accept any first food they encounter, thus their leaving behavior and food choice is less pronounced. Less hungry (more satiated) animals, on the contrary, tend to be more particular about food and their exploratory behavior is more active. Another explanation invokes memory: worms may learn that previous conditions were associated with a different satiation status and compare those with the new conditions. These two mechanisms are not mutually exclusive and may function in parallel.
In humans, powerful diet preferences can form, especially for nutritious fat- and carbohydrate-rich foods, such as sodas, desserts, pizza, etc. These feeding habits are hard to change. Dieting often results in ‘food craving’, ‘carbohydrate craving’, and ‘binge eating’, which eventually lead to even further increase of food consumption (Capaldi, 1996). These phenotypes are analogous to the increased food-seeking behavior when the food is switched from good to bad in C. elegans. (Of course, the time scale has to be normalized to the life span.) These behaviors are adaptive in the wild, where good food is usually scarce, but in developed countries, where high quality food is always easily available, they contribute to overeating and obesity.
The C. elegans food-seeking strategy
C. elegans locomotion, in particular the equilibrium between roaming, rapid straight movement, and dwelling, slow movement with frequent reversals and stops, was affected by the food source (Fig. 4). On poor food, straight rapid movement, called roaming, was drastically increased, while dwelling predominated on high quality food.
In previous studies, it has been shown that the speed of C. elegans locomotion is increased in the absence of food, and reversals and turns are suppressed after about 30·min in the absence of food (Gray et al., 2005; Hills et al., 2004). Here, we showed that this also happened on food, if the food was hard to eat. This suggests that it is not the mere presence of food that is decisive in regulating C. elegans locomotion, but food quality.
We identified mutants defective in food preference behavior; and two of them, osm-6 and ttx-3 were also defective in roaming. TTX-3 is a transcription factor required for the differentiation of the AIY thermosensory interneuron. The defects of the ttx-3 mutant were partially reproduced by killing AIY. The latter also caused a decrease in duration of roaming periods, suggesting that AIY functions to suppress the roaming-to-dwelling transition and to extend the food-seeking periods. This is consistent with, and extends, the results of other reports, which demonstrated that AIY suppresses reversals and turns (Gray et al., 2005; Tsalik and Hobert, 2003).
Supplementary Material
Acknowledgments
We thank the C. elegans Genetics Center and Oliver Hobert for providing strains. This work was supported by research grant HL46154 from the US Public Health. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
Footnotes
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/209/01/89/DC1
References
- Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 2001;128:1951–1969. doi: 10.1242/dev.128.11.1951. [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Avery L, Shtonda BB. Food transport in the C. elegans pharynx. J Exp Biol. 2003;206:2441–2457. doi: 10.1242/jeb.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 1995;48:225–250. doi: 10.1016/s0091-679x(08)61390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Capaldi, E. (1996). Why We Eat What We Eat: The Psychology of Eating Washington, DC: American Psychological Association.
- Chalfie, M. and White, J. (1988). The nervous system. In The Nematode C. elegans (ed. W. Wood), pp. 337–391. New York: Cold Spring Harbor Laboratory Press.
- Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995;14:803–812. doi: 10.1016/0896-6273(95)90224-4. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans. Learn Mem. 1997;4:179–191. doi: 10.1101/lm.4.2.179. [DOI] [PubMed] [Google Scholar]
- Collet J, Spike CA, Lundquist EA, Shaw JE, Herman RK. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics. 1998;148:187–200. doi: 10.1093/genetics/148.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll NA. Components and patterns in the behavior of the nematode Caenorhabditis elegans. J Zool. 1975;176:159–176. [Google Scholar]
- Davis CM. Self-selection of diet by newly weaned infants. Am J Dis Child. 1928;36:651–679. [Google Scholar]
- Davis MW, Somerville D, Lee RY, Lockery S, Avery L, Fambrough DM. Mutations in the Caenorhabditis elegans Na,K-ATPase alpha-subunit gene, eat-6, disrupt excitable cell function. J Neurosci. 1995;15:8408–8418. doi: 10.1523/JNEUROSCI.15-12-08408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric polycose infusions: a detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47:63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- Emmons SW, Klass MR, Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 1979;76:1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Sengupta P, McIntire SL. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron. 2002;36:1091–1102. doi: 10.1016/s0896-6273(02)01093-0. [DOI] [PubMed] [Google Scholar]
- Garcia J, McGowan BK, Ervin FR, Koelling RA. Cues: their relative effectiveness as a function of the reinforcer. Science. 1968;160:794–795. doi: 10.1126/science.160.3829.794. [DOI] [PubMed] [Google Scholar]
- Garsin DA, Sifri CD, Mylonakis E, Qin X, Singh KV, Murray BE, Calderwood SB, Ausubel FM. A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci USA. 2001;98:10892–10897. doi: 10.1073/pnas.191378698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2005;102:3181–3183. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;24:1217–1225. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O, Mori I, Yamashita Y, Honda H, Ohshima Y, Liu Y, Ruvkun G. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Iino Y, Mohri A, Mori I, Gengyo-Ando K, Mitani S, Katsura I. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell. 2002;109:639–649. doi: 10.1016/s0092-8674(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW. Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J Neurosci. 2004;24:7427–7434. doi: 10.1523/JNEUROSCI.1746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JP, Raizen DM, Gottschalk A, Schafer WR, Avery L. eat-2 and eat-18 are required for nicotinic neurotransmission in the Caenorhabditis elegans pharynx. Genetics. 2004;166:161–169. doi: 10.1534/genetics.166.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay RM, McKay JP, Avery L, Graff JM. C. elegans: a model for exploring the genetics of fat storage. Dev Cell. 2003;4:131–142. doi: 10.1016/s1534-5807(02)00411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- Nuttley WM, Atkinson-Leadbeater KP, Van Der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:12449–12454. doi: 10.1073/pnas.192101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne TB, Mendel LB. The choice between adequate and inadequate diets, as made by rats. J Biol Chem. 1918;35:19–27. [Google Scholar]
- Pavlov, I. P. (1927). Conditioned Reflexes Oxford: Oxford University Press.
- Perez C, Fanizza LJ, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in rats fed chow or a cafeteria diet. Appetite. 1999;32:155–170. doi: 10.1006/appe.1998.0182. [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19:9557–9569. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol N, Link EM, Liu LX, Kurz CL, Alloing G, Tan MW, Ray KP, Solari R, Johnson CD, Ewbank JJ. A reverse genetic analysis of components of the Toll signaling pathway in Caenorhabditis elegans. Curr Biol. 2001;11:809–821. doi: 10.1016/s0960-9822(01)00241-x. [DOI] [PubMed] [Google Scholar]
- Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141:1365–1382. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP. Increased salt appetite in adrenalectomized rats. Endocrinology. 1936;115:155–167. [Google Scholar]
- Richter CP, Holt LE, Barelare B. Vitamin B1 craving in rats. Science. 1937;86:354–355. doi: 10.1126/science.86.2233.354-a. [DOI] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol. 2001;204:1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Calcagnetti DJ. Trends in place preference conditioning with a cross-indexed bibliography; 1957–1991. Neurosci Biobehav Rev. 1993;17:21–41. doi: 10.1016/s0149-7634(05)80228-3. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Dietary-induced overeating. Ann New York Acad Sci. 1989;575:281–289. doi: 10.1111/j.1749-6632.1989.tb53250.x. discussion 290–291. [DOI] [PubMed] [Google Scholar]
- Skinner, B. F. (1938). The Behavior of Organisms; an Experimental Analysis. New York, London: D. Appleton-Century Company.
- Starich TA, Herman RK, Kari CK, Yeh WH, Schackwitz WS, Schuyler MW, Collet J, Thomas JH, Riddle DL. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics. 1995;139:171–188. doi: 10.1093/genetics/139.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Lee RY, Panzarella C, Avery L, Shaw JE. eat-5 and unc-7 represent a multigene family in Caenorhabditis elegans involved in cell-cell coupling. J Cell Biol. 1996;134:537–548. doi: 10.1083/jcb.134.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J. and Hodgkin, J. (1988). Methods. In The Nematode C. elegans (ed. W. Wood), pp. 587–606. New York: Cold Spring Harbor Laboratory.
- Tabish M, Siddiqui ZK, Nishikawa K, Siddiqui SS. Exclusive expression of C. elegans osm-3 kinesin gene in chemosensory neurons open to the external environment. J Mol Biol. 1995;247:377–389. doi: 10.1006/jmbi.1994.0146. [DOI] [PubMed] [Google Scholar]
- Tsalik EL, Hobert O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol. 2003;56:178–197. doi: 10.1002/neu.10245. [DOI] [PubMed] [Google Scholar]
- Young PT. Relative food preferences of the white rat. J Comp Psychol. 1932;14:297–319. [Google Scholar]
- Young PT. Relative food preferences of the white rat. II. J Comp Psychol. 1933;15:149–165. [Google Scholar]
- Young PT. The experimental analysis of appetite. Psychol Bull. 1941;38:129–164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


