Abstract
DNA microarrays were used to examine the effect of an insertional mutation in the Shewanella oneidensis etrA (electron transport regulator) locus on gene expression under anaerobic conditions. The mRNA levels of 69 genes with documented functions in energy and carbon metabolism, regulation, transport, and other cellular processes displayed significant alterations in transcript abundance in an etrA-mutant genetic background. This is the first microarray study indicating a possible involvement of EtrA in the regulation of gene expression in S. oneidensis MR-1.
In recent years, it has become apparent that microorganisms have developed complex regulatory mechanisms for controlling gene expression in response to alterations in growth conditions (3, 23, 26). For example, Escherichia coli Fnr (fumarate-nitrate reduction regulator) mediates global changes in gene expression during transitions between aerobic and anaerobic growth (12). Fnr homologs have been described for a number of bacterial species (7, 16, 22, 29), including Shewanella oneidensis MR-1 (20) (formerly Shewanella putrefaciens MR-1 [28]), a facultatively anaerobic metal-reducing bacterium (19). The predicted S. oneidensis EtrA (electron transport regulator) protein (20) shares a high degree of amino acid sequence identity with E. coli Fnr and with the analogous Anr (anaerobic regulator of arginine deiminase and nitrate reductase) protein from Pseudomonas aeruginosa (50.8 and 73.6% identity, respectively), thus suggesting the possibility that etrA is involved in regulating anaerobic energy metabolism in MR-1 (20). Subsequent experiments, however, demonstrated that insertional inactivation of the etrA gene had no significant physiological effect on the respiratory growth of S. oneidensis under anaerobic conditions (17). In this study, we used partial DNA microarrays to examine the transcriptional effects of an insertional disruption in the chromosomal etrA locus under fumarate- and nitrate-reducing conditions. Our results indicated that etrA mutation affects the mRNA levels of various functionally grouped genes involved in energy metabolism, transcriptional regulation, biosynthesis, and other cellular functions, although, as shown previously (17), the presence of etrA is not essential for anaerobic growth and reduction of electron acceptors by S. oneidensis.
Generation and phenotype analysis of the etrA mutant strain.
S. oneidensis and E. coli strains were grown as described previously (18, 21). S. oneidensis strain DSP-10, a spontaneous rifampin-resistant derivative of the wild type, was used as a parental strain to generate an etrA null allele by integrative disruption with the suicide plasmid pKNOCK-Kmr (1). Briefly, an internal fragment (247 bp) of the etrA gene was amplified by PCR with primers 5398IM-F (5′-AGGTGATGAACAGATCACAGG-3′) and 5398IM-R (5′-TGCGTTTTTCTTACTCAGTAGC-3′) and cloned into the EcoRV site of plasmid pKNOCK-Kmr. The resulting construct was propagated in E. coli S17-1/λpir and subsequently transferred into S. oneidensis DSP-10 by conjugation, essentially as described elsewhere (27). Integration of the plasmid into the etrA locus was verified by PCR amplification with external primers 5398F (5′-GCCGCTAGTGGG TGTGCAAT-3′) and 5398R (5′-TCCTAGCATTACCCGCCAAGAGA-3′), which are complementary to sequences flanking the S. oneidensis etrA gene. As expected, a product of approximately 709 bp in length was amplified from the parental DSP-10 DNA, whereas a 3-kb product was amplified from etrA::pKNOCK-Kmr DNA (data not shown), verifying disruption of the etrA gene.
The resulting etrA mutant strain, designated ETRA1, was compared to DSP-10 for anaerobic utilization of different electron acceptors as described elsewhere (4, 18). While no significant differences were found between the mutant and DSP-10 strains in their abilities to reduce and/or grow on MnO2, Fe(OH)3, Fe(III) citrate, thiosulfate, sulfite, dimethyl sulfoxide (DMSO), and trimethylamine-N-oxide (TMAO), the ETRA1 cells displayed a decrease of up to 30% in initial growth rates when fumarate or nitrate was used as an electron acceptor (data not shown). The DSP-10 strain displayed a higher initial growth rate when fumarate rather than nitrate was used as an electron acceptor (0.76 ± 0.03 versus 0.53 ± 0.01 h−1), while the ETRA1 strain grew at similar rates in the presence of either fumarate or nitrate (0.51 ± 0.04 and 0.47 ± 0.04 h−1, respectively). The results of physiological studies agreed with previous findings (17), further supporting the observation that EtrA is not essential for anaerobic growth and respiration of S. oneidensis MR-1.
Gene expression profiling.
To examine the functional role of EtrA in the regulation of anaerobic metabolism in S. oneidensis MR-1, transcription profiles of DSP-10 and ETRA1 strains under fumarate- and nitrate-reducing conditions were compared by using partial DNA microarrays. A complete list of the MR-1 genes represented on the microarray is available online (http://www.esd.ornl.gov/facilities/genomics/partial_microarrays.html). Sample preparation, probe synthesis, and microarray procedures were performed as described previously (27). For each condition, a total of four independent hybridization experiments were performed, including two biological replicate experiments with fluorescent dye reversal.
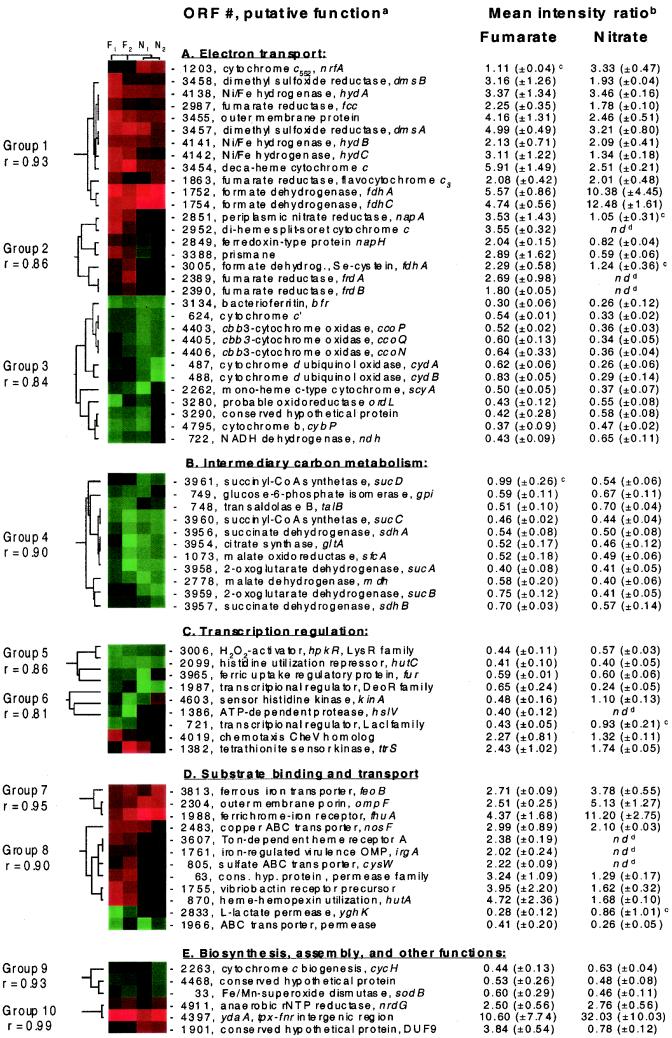
Following signal intensity quantification and normalization, 69 genes showed significant alterations in mRNA abundance, as defined previously (27), in the ETRA1 mutant under fumarate- and/or nitrate-reducing conditions (Fig. 1 ). Based on the genome sequencing results (The Institute for Genomic Research, unpublished data), responsive genes fell into five putative functional categories: (i) electron transport, (ii) intermediary carbon metabolism, (iii) transcription regulation, (iv) substrate transport and binding, and (v) biosynthesis, assembly, and other cellular processes.
FIG.1.
Pairwise average-linkage clustering analysis of the 69 S. oneidensis MR-1 genes exhibiting altered mRNA levels in the ETRA1 mutant. Hierarchical clustering, based on pairwise correlations across all experimental points, groups together genes of known similar function (A to E) and also provides a means of obtaining leads as to the function of unknown or poorly characterized genes (9). Each column indicates a separate biological replicate (F1 and F2 refer to fumarate-reducing conditions; N1 and N2 refer to nitrate-reducing conditions). Red and green colors indicate genes that are induced and repressed, respectively, in the presence of etrA. The Pearson correlation coefficients (r) are displayed to the left of the nodes. a, sequence information provided as a courtesy by The Institute for Genomic Research (unpublished results). The ORF numbers provided here are for the purpose of tracking gene locations and sequence information; they may change once the complete version of S. oneidensis MR-1 genome sequence is released. b, relative mRNA levels presented as the ratio of the dye intensity of the parental strain to that of the etrA mutant averaged across two biological replicate experiments. Values in parentheses indicate the standard deviation for each mean expression ratio. c, value not significantly different from 1 at a P of 0.05 based on a one-tailed t test. d, unable to determine expression ratio for this ORF due to the very low levels of hybridization intensities in both DSP-10 and ETRA1 strains (nd).
The upstream regulatory regions of all genes displaying altered mRNA levels in ETRA1 were analyzed for the presence of Fnr consensus sequences (TTGATN4ATCAA). Previous studies have shown that the etrA gene of S. oneidensis MR-1 is able to complement an fnr mutant of E. coli (20), suggesting that the cellular functions and the specific DNA-binding recognition sites for both proteins are highly similar, possibly identical. Based on the information about the Fnr motif conservation in E. coli, which was obtained from the RegulonDB database (http://www.cifn.unam.mx/Computational_Genomics/regulondb), a percent identity cutoff equal to or greater than 70% was selected. The search results identified 36 potential Fnr-binding sites located in the putative regulatory regions of 26 of 52 responsive operons or genes that were affected by the disruption of etrA (Table 1).
TABLE 1.
Putative Fnr motifs located upstream of the genes affected by the etrA mutation
| Gene or operon and product (ORF) | Putative Fnr boxa | Position (bp)b |
|---|---|---|
| nrfA, cytochrome c552, nitrite reductase (1203) | TTGAT . . . . cgCAA | 83 |
| frdA, fumarate reductase, flavocytochrome c3 (1863) | aTGAa . . . . ATCAA | 272 |
| Diheme split-Soret cytochrome c (2952) | TcGAT . . . . AcCAA | 192 |
| fccA, fumarate reductase (2987) | gTGAT . . . . tTCAA | 210 |
| fdhA, selenocysteine formate dehydrogenase (3005) | cTGAT . . . . ATaAA | 222 |
| Prismane, 6Fe-6S protein (3388) | gTGAT . . . . cTCtA | 119 |
| fdhABC, formate dehydrogenase (1752) | cTGtT . . . . ATCAA | 55 |
| frdABC, fumarate reductase (2389) | TTGAT . . . . cTCAg | 105 |
| napDAHGB, nitrate reductase (2848-2852) | TTGAT . . . . ATCgA | 119 |
| dmsAB, DMSO reductase (3454-3458) | gTGAT . . . . tTCAA | 169 |
| hydABC, Ni-Fe hydrogenase (4138-4142) | TTGAT . . . . ATCAA | 100 |
| cydAB, cytochrome d ubiquinol-oxidase (487 and 488) | TTGAT . . . . ATCAA | 338 |
| scyA, monoheme c cytochrome (2262) | TTGAT . . . . tTCcA | 170 |
| bfr, bacterioferritin (3134) | TTGAT . . . . ATCAA | 43 |
| ordL, probable oxidoreductase (3280) | TaGAT . . . . AaCAg | 200 |
| ccoPOQN, cytochrome cbb3 oxidase (4403) | TTGAT . . . . ATCAA | 212 |
| ccoPOQN, cytochrome cbb3 oxidase (4404) | TTGAc . . . . cTCAA | 169 |
| ccoPOQN, cytochrome cbb3 oxidase (4405) | TTGAc . . . . ATCAA | 106 |
| ccoPOQN, cytochrome cbb3 oxidase (4406) | TTGAa . . . . cgCAA | 163 |
| fur, ferric uptake regulatory protein (3965) | TTGAa . . . . cgCAA | 163 |
| kinA, sensor histidine kinase (4603) | TTGAc . . . . AgCAA | 176 |
| Ferrichrome-iron receptor (1988) | TTGAT . . . . ATCAc | 103 |
| ompF, outer membrane porin (2304) | gTGAT . . . . AatAA | 166 |
| feoB, ferrous iron transporter (3813) | TTGAT . . . . ATtAg | 369 |
| TTGAT . . . . gTaAA | 87 | |
| nrdG, anaerobic ribonucleotide reductase (4911) | tTGAT . . . . ATCAt | 121 |
| TTGtT . . . . ATCAA | 63 | |
| sodB, Fe-Mn superoxide dismutase (33) | aTGAT . . . . ATCAc | 87 |
| cycH, cytochrome c biogenesis (2263) | TTGAT . . . . AaagA | 344 |
| ydaA, tpx-fnr intergenic region (4397) | TTGAT . . . . AaCAA | 36 |
| Conserved hypothetical protein (4468) | gTGAT . . . . ATCAc | 314 |
Nucleotides matching those of the E. coli fnr consensus sequence (TTGATNNNNATCAA) are shown in uppercase letters. The selected identity cutoff is 70%.
The position of the putative fnr box x is given as the distance from the 5′ end of the motif to the presumed translation start codon.
Of these 52 genes, 31 encoded putative components of the electron transport chain, including a predicted formate dehydrogenase (open reading frames [ORFs] 1752, 1754, and 3005), DMSO reductase (ORFs 3454, 3455, 3457, and 3458), and three fumarate reductases (ORFs 1863, 2389, 2390, and 2987). While the transcription levels of frdC and fccA were consistently repressed in ETRA1 under both fumarate- and nitrate-reducing conditions, the mRNA levels of the frdAB operon decreased slightly only in the presence of fumarate (Fig. 1A). Analysis of hybridization signal intensities suggested that the transcription levels of the frdAB operon were strongly repressed in both the DSP-10 and ETRA1 strains under nitrate-reducing conditions. A similar expression pattern was observed for ORF 2952, which encodes a homolog of the Desulfovibrio desulfuricans diheme split-Soret cytochrome c that was previously implicated in sulfate and thiosulfate reduction (8) (Fig. 1A). Genes encoding a periplasmic nitrate reductase (napAH; ORFs 2849 and 2851) and prismane (ORF 3388) showed an approximately two- to threefold increase in mRNA levels in an etrA+ background under fumarate-reducing conditions, while the expression of these same genes under nitrate-reducing conditions was either unaffected or exhibited marginal decreases. Previous microarray analysis of the wild-type S. oneidensis MR-1 indicated that mRNA levels of the nitrate reductase-encoding operon napBHGA and a prismane-encoding gene increased substantially (8- to 56-fold) in response to nitrate relative to levels found with growth on fumarate (5). Together, these data suggest that expression of the S. oneidensis nap gene cluster and the prismane-encoding gene may be subject to two levels of global control.
Inactivation of the etrA locus did not alter the transcription levels of the tor operon, which encodes the putative TMAO reductase, or of a second DMSO reductase gene cluster present in the genome of S. oneidensis. These findings are in accordance with the physiological evidence indicating that DMSO and TMAO respiration in S. oneidensis is not affected by etrA inactivation. It also suggests that gene duplication in MR-1 may contribute to the wild-type levels of DMSO reduction in the ETRA1 strain.
Genes exhibiting increased mRNA levels in the ETRA1 mutant were largely those associated with aerobic respiration, including those encoding NADH dehydrogenase, cytochrome cbb3 and d oxidases, and tricarboxylic acid cycle components (Fig. 1A and B). In addition, genes for a conserved hypothetical protein (ORF 3290) and a putative oxidoreductase (ORF 3280) of unknown function exhibited a twofold decrease in mRNA levels under fumarate- and nitrate-reducing conditions. Sequence analysis of the conserved hypothetical protein revealed 76% identity to another hypothetical protein (GenBank accession no. PA2776) from P. aeruginosa PAO1 and 67% identity to a putative OrdL oxidoreductase (GenBank accession no. U38543) from E. coli. The deduced amino acid sequence of the putative oxidoreductase gene was 59% identical to that of the ordL gene product (GenBank accession no. PA5309) from P. aeruginosa PAO1.
Putative Fnr motifs were located upstream of all 11 operons which displayed decreased mRNA levels in ETRA1 and which are putatively involved in anaerobic respiration and the reduction of DMSO, fumarate, and nitrate. Highly conserved Fnr motifs were found upstream of the hydABC, frdA, napDAHGB, cydAB, bfr, and ccoNOQP operons (Table 1). These findings are consistent with previous promoter studies indicating that the same orthologous genes in E. coli are directly regulated by Fnr (10, 14, 24).
The etrA mutation affected the transcription levels of 9 putative regulatory genes and 12 genes involved in substrate transport and binding (Fig. 1C and D). Of these effects, the most notable was the approximately twofold increase in mRNA abundance that was observed for the ferric uptake regulator (fur) gene in the ETRA1 strain. Fur, a classic iron-responsive repressor, has been shown to negatively control the expression of siderophore biosynthesis and other iron acquisition genes in a number of organisms (2), including S. oneidensis (27). Putative iron acquisition and siderophore-binding genes (ORFs 870, 1755, 1761, 1988, 2304, 3607, and 3813) exhibited a 2- to 11-fold decrease in mRNA levels under fumarate- and nitrate-reducing conditions. In addition, a putative Fnr-binding motif was found upstream of the fur regulatory gene (Table 1). These observations suggest that EtrA influences the transcription of other regulatory genes; however, it is unclear whether a mechanism of coordinate regulation is operating in S. oneidensis to control anaerobic respiration.
A significant decrease in mRNA levels (10- to 30-fold) was displayed by the putative ydaA gene, whose predicted product is distantly related to a family of universal stress proteins. The ydaA gene is located 90 bp downstream of the etrA stop codon and is transcribed in the same orientation as etrA. Within the intragenic etrA-ydaA region, we identified a conserved Fnr-binding site (TTGAT N4 AACAA) that was located 36 bp upstream of the predicted translational start for ydaA and which displayed a 90% identity to the consensus Fnr-binding site sequence (TTGAT N4 ATCAA). Although a potential rho-independent site was identified downstream of etrA, we cannot exclude the possibility that the etrA mutation has a polar effect on the transcription of ydaA.
Concluding remarks.
The S. oneidensis MR-1 etrA gene encodes a putative DNA-binding protein that shares a high degree of sequence identity with E. coli Fnr. Despite the absence of a discernible physiological effect for ETRA1, the microarray data indicated that disruption of the chromosomal etrA locus affects the mRNA levels of 69 S. oneidensis genes (Fig. 1). The results also suggest that EtrA is functional and that it participates, either directly or indirectly, in gene regulation under anaerobic conditions. One possible explanation for the observed ETRA1 phenotype could be the presence of other regulatory genes that encode proteins with similar functions. It was previously suggested that another gene similar to etrA is present in MR-1 (20), but no genes exhibiting high sequence identity to those for either EtrA or Fnr were identified in the S. oneidensis genome. Sequence analysis, however, revealed a putative cyclic nucleotide-binding protein that shows 88 and 44% identity at the amino acid level to the E. coli Crp and Bacillus subtilis Fnr regulators, respectively. Although comparison of the deduced amino acid sequences of the S. oneidensis crp and etrA genes did not reveal any substantial degree of homology (21% identity and 45% similarity), the products of both of these genes belong to the same family of transcriptional regulators (15). Previous studies of Fnr- and Crp-activated promoters have shown that Fnr and Crp are structurally and mechanistically similar (11). Also, evidence exists demonstrating a reciprocal recognition of binding sites where the Crp regulator can bind and activate transcription from natural Fnr-binding sites (25). Based on this information, we cannot rule out the possibility that the presence of a Crp homolog or other regulator(s) of energy metabolism in S. oneidensis alleviates the effect of etrA inactivation. Previous studies of genes involved in anaerobic metabolism in E. coli have demonstrated that gene expression is often controlled by several regulatory elements such as ArcA, Fnr, Crp, and NarL (6, 13, 25). Given the metabolic diversity and genome plasticity of S. oneidensis, it is possible that coordinate regulation by multiple transcription factors may alleviate the effects of the etrA inactivation. Further studies are necessary to elucidate the complex regulatory networks and mechanisms controlling energy metabolism in S. oneidensis MR-1.
Acknowledgments
We thank Guangshan Li for PCR amplification of S. oneidensis MR-1 ORFs, John Heidelberg for S. oneidensis MR-1 genome sequence information, Craig Brandt for help with statistical analysis of the microarray data, and Allison Murray for advice on microarray data analysis.
This research was supported by the Microbial Cell Project, the Microbial Genome Program, the Natural and Accelerated Bioremediation Research Program, Office of Biological and Environmental Research, Office of Science, the U.S. Department of Energy. Oak Ridge National Laboratory is managed by the University of Tennessee—Battelle under contract DE-AC05-00OR22464.
REFERENCES
- 1.Alexeyev, M. F. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. BioTechniques 26:824-828. [DOI] [PubMed] [Google Scholar]
- 2.Bagg, A., and J. B. Neilands. 1987. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol. Rev. 51:509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, C. E., S. Elsen, and T. H. Bird. 1999. Mechanisms for redox control of gene expression. Annu. Rev. Microbiol. 53:495-523. [DOI] [PubMed] [Google Scholar]
- 4.Beliaev, A. S., and D. A. Saffarini. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J. Bacteriol. 180:6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beliaev, A. S., D. K. Thompson, T. Khare, H. Lim, C. C. Brandt, G. Li, A. Murray, J. F. Heidelberg, C. S. Giometti, K. H. Nealson, J. M. Tiedji, and J. Zhou. 2002. Gene and protein expression profiles of Shewanella oneidensis during anaerobic growth with different electron acceptors. OMICS 6:39-60. [DOI] [PubMed] [Google Scholar]
- 6.Cotter, P. A., S. B. Melville, J. A. Albrecht, and R. P. Gunsalus. 1997. Aerobic regulation of cytochrome d oxidase (cydAB) operon expression in Escherichia coli: roles of FNR and ArcA in repression and activation. Mol. Microbiol. 25:605-616. [DOI] [PubMed] [Google Scholar]
- 7.Cuypers, H., and W. G. Zumft. 1993. Anaerobic control of denitrification in Pseudomonas stutzeri escapes mutagenesis of an fnr-like gene. J. Bacteriol. 175:7236-7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devreese, B., C. Costa, H. Demol, V. Papaefthymiou, I. Moura, J. J. Moura, and J. Van Beeumen. 1997. The primary structure of the split-Soret cytochrome c from Desulfovibrio desulfuricans ATCC 27774 reveals an unusual type of diheme cytochrome c. Eur. J. Biochem. 248:445-451. [DOI] [PubMed] [Google Scholar]
- 9.Eisen, M. B., P.T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgiou, C. D., T. J. Dueweke, and R. B. Gennis. 1988. Regulation of expression of the cytochrome d terminal oxidase in Escherichia coli is transcriptional. J. Bacteriol. 170:961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green, J., A. Irvine, W. Meng, and J. Guest. 1996. FNR-DNA interactions at natural and semi-synthetic promoters. Mol. Microbiol. 19:125-137. [DOI] [PubMed] [Google Scholar]
- 12.Guest, J., J. Green, A. Irvine, and S. Spiro. 1996. The FNR modulon and FNR-regulated gene expression, p. 317-342. In E. C. Lin and A. S. Lynch (ed.), Regulation of gene expression in Escherichia coli. R. G. Landes Company, Austin, Tex.
- 13.Hassan, H. M., and H. C. Sun. 1992. Regulatory roles of Fnr, Fur, and Arc in expression of manganese-containing superoxide dismutase in Escherichia coli. Proc. Natl. Acad. Sci. USA 89:3217-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iuchi, S., D. R. Kuritzkes, and E. C. Lin. 1985. Escherichia coli mutant with altered respiratory control of the frd operon. J. Bacteriol. 161:1023-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiley, P. J., and H. Beinert. 1998. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol. Rev. 22:341-352. [DOI] [PubMed] [Google Scholar]
- 16.MacInnes, J. I., J. E. Kim, C. Lian, and G. A. Soltes. 1990. Actinobacillus pleuropneumoniae hlyX gene homology with the fnr gene of Escherichia coli. J. Bacteriol. 172:4587-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maier, T. M., and C. R. Myers. 2001. Isolation and characterization of a Shewanella putrefaciens MR-1 electron transport regulator etrA mutant: reassessment of the role of EtrA. J. Bacteriol. 183:4918-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers, C. R., and J. M. Myers. 1993. Ferric reductase is associated with the membranes of anaerobically grown Shewanella putrefaciens MR-1. FEMS Microbiol. Lett. 108:15-22. [Google Scholar]
- 19.Nealson, K. H., and D. A. Saffarini. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 48:311-343. [DOI] [PubMed] [Google Scholar]
- 20.Saffarini, D. A., and K. H. Nealson. 1993. Sequence and genetic characterization of etrA, an fnr analog that regulates anaerobic respiration in Shewanella putrefaciens MR-1. J. Bacteriol. 175:7938-7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Sawers, R. G. 1991. Identification and molecular characterization of a transcriptional regulator from Pseudomonas aeruginosa PAO1 exhibiting structural and functional similarity to the FNR protein of Escherichia coli. Mol. Microbiol. 5:1469-1481. [DOI] [PubMed] [Google Scholar]
- 23.Sawers, R. G. 1999. The aerobic/anaerobic interface. Curr. Opin. Microbiol. 2:181-187. [DOI] [PubMed] [Google Scholar]
- 24.Sawers, R. G., S. P. Ballantine, and D. H. Boxer. 1985. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J. Bacteriol. 164:1324-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawers, R. G., M. Kaiser, A. Sirko, and M. Freundlich. 1997. Transcriptional activation by FNR and CRP: reciprocity of binding-site recognition. Mol. Microbiol. 23:835-845. [DOI] [PubMed] [Google Scholar]
- 26.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63:479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson, D. K., A. Beliaev, C. S. Giometti, D. P. Lies, K. H. Nealson, H. Lim, J. Yates, III, J. Tiedje, and J. Zhou. 2002. Transcription and proteomic analysis of a ferric uptake regulator (Fur) mutant of Shewanella oneidensis: possible involvement of Fur in energy metabolism, regulation, and oxidative stress. Appl. Environ. Microbiol. 68:881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkateswaran, K., D. Moser, M. Dollhopf, D. Lies, D. Saffarini, B. MacGregor, D. Ringelberg, D. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]
- 29.Zeilstra-Ryalls, J. H., and S. Kaplan. 1995. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: the role of the fnrL gene. J. Bacteriol. 177:6422-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]