Abstract
Cyclic AMP plays an important role in regulating sperm motility and acrosome reaction through activation of cAMP-dependent protein kinase A (PKA). Phosphodiesterases (PDEs) modulate the levels of cyclic nucleotides by catalyzing their degradation. Although PDE inhibitors specific to PDE1 and PDE4 are known to alter sperm motility and capacitation in humans, little is known about the role or subcellular distribution of PDEs in spermatozoa. The localization of PKA is regulated by A-kinase anchoring proteins (AKAPs), which may also control the intracellular distribution of PDE. The present study was undertaken to investigate the role and localization of PDE4 during sperm capacitation. Addition of Rolipram or RS25344, PDE4-specific inhibitors significantly increased the progressive motility of bovine spermatozoa. Immunolocalization techniques detected both PDE4A and AKAP3 (formerly known as AKAP110) in the principal piece of bovine spermatozoa. The PDE4A5 isoform was detected primarily in the Triton X-100-soluble fraction of caudal epididymal spermatozoa. However, in ejaculated spermatozoa it was seen primarily in the SDS-soluble fraction, indicating a shift in PDE4A5 localization into insoluble organelles during sperm capacitation. AKAP3 was detected only in the SDS-soluble fraction of both caudal and ejaculated sperm. Immunoprecipitation experiments using COS cells cotransfected with AKAP3 and either Pde4a5 or Pde4d provide evidence that PDE4A5 but not PDE4D interacts with AKAP3. Pulldown assays using sperm cell lysates confirm this interaction in vitro. These data suggest that AKAP3 binds both PKA and PDE4A and functions as a scaffolding protein in spermatozoa to regulate local cAMP concentrations and modulate sperm functions.
Keywords: phosphodiesterases, signal transduction, sperm, sperm motility, transport
INTRODUCTION
Cyclic AMP is a key second messenger that regulates a variety of cell functions including the acrosome reaction and motility of spermatozoa [1–3]. Activation of cAMP-dependent protein kinase (PKA) by cAMP initiates and maintains flagellar motility. Demembranated spermatozoa regain motility in the presence of cAMP [4] and cAMP restores motility in intact spermatozoa from Sacy (soluble adenylyl cyclase) or Scl9a10 (Na-H exchanger) null mice [5, 6]. The accumulation of cAMP is tightly and dynamically regulated by two major enzymes, adenylyl cyclase (AC) and phosphodiesterase (PDE), which catalyze the synthesis or degradation of cAMP, respectively. Several researchers have reported a rapid increase of cAMP levels in spermatozoa incubated in capacitating medium [7–9]. The duration of this increase is usually brief, with the concentration of cyclic nucleotide returning to basal levels in approximately 1 min. To efficiently produce this rapid change in cAMP levels, the regulating enzymes need to be in close proximity to each other. Anchoring proteins and targeting subunits provide a molecular framework that groups signaling enzymes with particular substrates. These protein-protein interactions not only lead to phosphorylation of localized substrate by different kinases but also spatially segregate the different cAMP signaling pathways.
PDEs degrade cyclic nucleotides to the respective nucleotide monophosphates by hydrolysis of the phosphodiester bond. There are many different isoforms of PDEs that differ with respect to amino acid composition, substrate specificity and affinity, their selectivity to activators and inhibitors, subcellular distribution, and expression in various cells, tissues, and organs. Presently there are 11 known members in the PDE family, each with subtypes and different splice variants [10, 11]. Many isoforms of PDE are targeted to distinct subcellular locations in spermatozoa [12]. Among these, PDE4 (cAMP-specific) and PDE1 (calmodulin-dependent PDE) are well known for their roles in sperm functions. Inhibitors specific to these PDEs affect sperm motility, tyrosine phosphorylation, and acrosome reaction [3, 12–14].
In polarized cells like spermatozoa, PDEs play an important role in controlling cAMP diffusion and its access to different PKAs. PKA is directed to specific locations through its interaction with A-kinase anchoring proteins (AKAPs). Spermatozoa contain several AKAPs including AKAP3 and AKAP4 [15, 16]. Both AKAP3 and AKAP4 are present in the fibrous sheath [15, 17], and both increase their level of tyrosine phosphorylation during capacitation [18].
Feedback regulation of cAMP can be achieved through PKA-dependent activation of PDE activity or inhibition of AC activity in somatic cells [19, 20]. Spermatozoa from PKA Cα null mice exhibit increased basal cAMP levels, which was attributed to the lack of PKA-mediated feedback inhibition of sAC [7]. This effect can be replicated pharmacologically in wild-type but not null mice by addition of H89 and bicarbonate. The ability of a PDE to interact with AKAPs offers the potential for controlling PKA activity in this module. We postulate, similar to somatic cells, that activation of PDE4s in spermatozoa is also dependent on PKA. It has been shown that PDE4D3 localizes with AKAP6 (previously known as mAKAP) [21] in muscle cells and AKAP 9 (previously known as AKAP450) in the Sertoli cells in testes [22]. Additionally, the long PDE4D3 form can be phosphorylated by PKA, which causes marked activation of the enzyme [23]. Adding to the complexity, ERK mitogen-activated kinase (MAPK1) phosphorylation of PDE limits its activity and hence activates PKA [24]. This creates a feedback loop in which PKA activates PDE, resulting in a drop in cAMP levels.
The cAMP-specific PDE4 enzymes are encoded by four genes designated as PDE4A, B, C, and D. Each gene exhibits multiple splice variants with common catalytic and C-terminal regions but is distinguished by its unique N-terminal region. The long PDE4 isozymes are characterized by two upstream conserved regions 1 and 2 (UCR1 and UCR2, respectively) [25]. The N-terminal and UCR regions are known to have regulatory properties associated with intracellular targeting [24, 26]. Among the various long PDE4A variants, PDE4A5 and PDE4A8 are the predominant forms expressed during the latter stages of spermatogenesis, starting in round spermatids, and their expression level remains high in mature rat spermatozoa [27, 28].
The compartmentalization of PDE4A5 in spermatozoa is undoubtedly crucial to the functioning of this isoform. However, not much is known about its localization in spermatozoa. The specific PDE4 inhibitor RS23544 alters human sperm motility, suggesting PDE4 may be present in the flagellum [3]. The objective of the present study was to determine the location of PDE4A5 during sperm maturation and to determine if it interacts with AKAPs located in the principal piece of the spermatozoa.
MATERIALS AND METHODS
Chemicals, Equipment, and Sources
Bovine serum albumin (BSA) Fraction V, Rolipram, 0.01% poly-l-lysine, Triton X-100, 2-chloro-2N-deoxyadenosine, protease inhibitor mixture (mammalian), and lectin from Pisum sativum (lyophilized powder, FITC conjugate) were obtained from Sigma-Aldrich (St. Louis, MO). RS-25344 was from Roche-Syntex (Palo Alto, CA). H89 was purchased from BIOMOL (Plymouth Meeting, PA), and Staurosporine was from Calbiochem (La Jolla, CA). Paraformaldehyde was obtained from Electron Microscopy Sciences (Hatfield, PA) and the antiquenching buffer (Slowfade light Antifade kit) from Molecular Probes (Eugene, OR). Glutathione Sepharose fusion protein beads were from Amersham Biosciences (Uppsala, Sweden). S-protein agarose and S-protein HRP conjugate were from Novagen (Madison, WI). Immobilon-P PVDF membrane was purchased from Millipore Corporation (Bedford, MA). Eight-well slides were custom made by CellPoint Scientific (Gaithersburg, MD).
Phospho-(Ser/Thr) PKA substrate antibody (monoclonal) was purchased from Cell Signaling (Beverly, MA). Production of α-AKAP3 antibody (rabbit, polyclonal) is described previously in Vijayaraghavan et al. [29]. Blocking peptide (amino acids 187–208 of AKAP3) was used to produce this antibody. α-PDE4A (AC55, rabbit polyclonal), PDE4A-GST fusion protein, and α-PDE4D (M3S1, mouse monoclonal) have also been described previously in Iona et al. [30]. Rabbit IgG was purchased from BD Pharmingen (San Diego, CA), and α-GFP antibody (BD Living Colors A.V. Monoclonal Antibody JL-8) was from BD Clontech (Mountain View, CA). Goat anti-rabbit and goat antimouse HRP-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Western Lightning chemiluminescence and [3H]cAMP were purchased from Perkin Elmer (Boston, MA).
Bovine testes came from Carlton Farms (Carlton, OR), and ejaculated spermatozoa (cryopreserved) were purchased from Select Sires, Inc. (Plain City, OH). COS-7 cells were obtained from ATCC (Manassas, VA), maintained with DMEM (low glucose) containing 10% fetal bovine serum, and transfected with Lipofectamine 2000 using OPTI-MEM I Reduced Serum Media (all Life Technologies, Gaithersburg, MD).
Zeiss Axiphot epifluorescence microscope and digital camera were obtained from Carl Zeiss, AxioCam (Germany). Beckman LS5000 TA Liquid Scintillation Counter was purchased from Beckman (Fullerton, CA), and the Branson Sonifier 450 came from Branson (Danbury, CT).
Motility Studies
Bovine caudal epididymal spermatozoa were incubated with CESD buffer (10 mM Tris-HCl [pH 7.2] containing 120 mM NaCl, 10 mM KCl, and 5 mM MgSO4) with the addition of 10 mM glucose and 5 mg/ml BSA at 37°C for 15 min with or without Rolipram (10, 50, or 100 μM), RS23544 (3 or 5 μM), or 2- chloro-2N-deoxyadenosine (20 μM). Motility and motion parameters were assessed in a computer-assisted motion analyzer (Hamilton Thorn v 12.2 g, Hamilton Thorne Biosciences, Inc., Beverly, MA) using a 20-μm-deep chamber (Petroff Hausser Counter, Hausser Scientific, Horsham, PA) at 37°C. Settings: frames, 30; frame rate, 60 Hz; minimum contrast, 55; minimum cell size, 8 pixels; VAP, 50 μ/sec with a cutoff 30 μ/sec; LIN, 70%. The studied motion parameters can be defined as follows: curvilinear velocity (VCL) is the time-average velocity of a sperm head along its actual curvilinear path as perceived in two dimensions in the microscope; straight-line velocity (VSL) is the time-average velocity of a sperm head along the straight line between its first detected position and its last; amplitude of lateral head displacement (ALH) is the magnitude of lateral displacement of a sperm head about its average path; and linearity of a curvilinear path (LIN) is reported as a ratio of VSL to VCL.
Phosphorylation
Bovine caudal spermatozoa for all experiments were obtained by removing the cauda from the testis, inserting a needle into the vas deferens, filling the cauda with PBS, making a small cut into the tubule, and backflushing to extract. Spermatozoa for phosphorylation experiments (4.0 × 107) were incubated in buffer A (120 mM NaCl, 10 mM KCl, 10 mM Tris-HCl pH 7.4, 10 mM glucose, and 10μg/ml BSA) at 37°C in a 95% air/5% CO2 incubator for 15, 30, or 60 min with or without 50 μM Rolipram. Sperm were washed twice with PBS (58 mM Na2HPO4, 17 mM NaH2PO4, 68 mM NaCl, pH 7.4) then lysed in SDS-buffer (125 mM Tris/HCl pH 7.5, 4% SDS, 1 mM sodium Vanadate, 1 mM sodium fluoride, 5ng/ml leupeptin, 1 mM AEBSF, 20 ug/ml Aprotinin) and boiled. SDS-sample buffer (0.5M Tris-HCl 12.5%, pH 6.8, glycerol 0.1%, 10% SDS w/v 0.2%, 2-mercaptoethanol 0.05%, 1% bromophenol blue [0.05% final concentration]) was added, and samples were separated by 10% SDS-PAGE and transferred to Immobilon-P. After 1 h blocking in blotto (TBS with 0.1% BSA and 5% nonfat milk), Western blots were performed using a Phospho-(Ser/Thr) PKA substrate antibody at a 1:500 dilution in 2.5% BSA in TTBS (10 mM Tris pH 7.5, 150 mM NaCl, 0.05% Tween-20). (Phospho-[Ser/Thr] PKA substrate antibody detects peptides and proteins containing a phospho-serine/threonine residue with arginine at the −3 position. It is a useful tool in identifying substrates of AGC family kinases, including PKA and PKC. It does not cross-react with the nonphosphorylated PKA substrate motif.)
Acrosome Reaction
Bovine caudal spermatozoa were treated with Rolipram as described previously for phosphorylation experiments, then washed once in PBS. Spermatozoa (100 μl of 10 × 106/ml) were placed in 100 μl of host medium (75 mM fructose, 25 mM sodium citrate), incubated 39°C in a 95% air/5% CO2 incubator for 30 min, spun for 10 min at 400 × g, and resuspended in 100 μl of medium 0.1% BSA in PBS. Ten microliters of spermatozoa were spotted onto eight-well slides and allowed to air-dry, then fixed in ice-cold methanol for 1 min. Slides were washed with PBS + 0.1% BSA and air-dried. FITC conjugated lectin from Pisum sativum (FITC-PSA, 10 μg/ml) was added to each well (10 μl/well), and slides were incubated in a dark, moist container for 30 min. Slides were washed twice with PBS and air-dried. Slides were mounted with antiquenching buffer and checked in a Zeiss Axiphot epifluorescence microscope. In each well, 200 spermatozoa were counted per well for each experiment, and ratios of caps (staining over the entire acrosomal region), molted (patchy staining over the acrosomal region), bars (staining of the equatorial region), and negatives (no staining) were recorded.
Immunocytochemistry
Murine sperm (strain C57BL/6J) were obtained by flushing caudal epididymis with PBS. Bovine and murine (both wild-type and Pde4a knockout) caudal epididymal spermatozoa were washed once in 1 ml PBS (phosphate buffer saline) + 0.1% BSA and spun down at 1000 × g. PBS was removed, and a 1:50 dilution of 0.01% poly-l-lysine was added to a final concentration of 100 000 sperm per microliter, and 10 μl (1 000 000 spermatozoa) of the sample were spotted onto each of eight wells per slide. After a 1 h incubation at room temperature in a moist chamber, the slides were washed with PBS + 0.1% BSA. Sperm were fixed with 4% formaldehyde and 0.25% glutaraldehyde for 60 min, further fixed and permeabilized for 10 min with ice-cold acetone, washed twice in PBS + 0.1% BSA, and allowed to dry. To ensure complete permeabilization, slides were then incubated for 10 min with 10 μl 0.1% Triton X-100, washed, and dried as described previously. Sperm spots were subsequently incubated with primary antibody for 1 h, washed with PBS + 0.1% BSA twice, and further incubated with anti-rabbit or anti-goat IgG-FITC or IgG-Texas Red for 1 h at room temperature. Primary antibodies were α-AKAP3 (rabbit, polyclonal; production described previously in Vijayaraghavan et al. [16]; 1:4 dilution in PBS + 0.1% BSA), α-PDE4A (AC55, rabbit polyclonal; 1:10 dilution in PBS + 0.1% BSA [31]), or α-PDE4D (M3S1, mouse monoclonal; 1:4 dilution in PBS + 0.1% BSA [31]). For blocking experiments, α-AKAP3 or α-PDE4A antibodies were preincubated for 30 min at a 3:1 (blocker:antibody) concentration using either a blocking peptide (amino acids 187–208 of AKAP3) or a PDE4A-GST fusion protein (fusion protein, AC55 and M3S1 antibodies described in Morena et al. [31]). The blocked antibodies were then diluted and incubated with the sperm as described previously. After incubation with the appropriate secondary antibody, slides were washed twice in PBS + 0.1% BSA, allowed to dry thoroughly, then mounted in antiquenching buffer and checked in a Zeiss Axiphot epifluorescence microscope connected to a digital camera. All investigations were conducted in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching.
Sperm Fractionation
Bovine caudal and cryopreserved ejaculated spermatozoa (5.0 × 108) were incubated at 39°C in a 95% air/5% CO2 incubator for 0, 15, or 30 min or for 60 min with or without 50 μM H-89 (BIOMOL, Plymouth Meeting, PA) or 3 μM Staurosporine (Calbiochem, La Jolla, CA) in Talp-HEPES buffer (10 mM HEPES, 136 mM NaCl, 2.7 mM KCl, 0.5 mM sodium biphosphate, 12 mM sodium bicarbonate, 1 mM MgCl2, 2.0 mM CaCl2, 3 mg/ml BSA, 10 μg/ml heparin). Sperm were washed twice with PBS and then lysed in 0.5% Triton X-100 at 4°C in a shaker for 20 min. The sample was centrifuged at 13 000 × g for 10 min and the supernatant fraction saved as fraction 1. The pellet was lysed in low-ionic-strength buffer (250 mM sucrose and 5 mM Tris-HCl, pH 7.6) with 2 mM dithiothreitol (DTT; Sigma-Aldrich, St. Louis, MO) and sonicated at maximum setting for 30 sec. The samples were centrifuged and the supernatant saved as fraction 2. The pellet was further lysed in 4% SDS and 0.1% NP40 and centrifuged at high speed for 30 sec and the supernatant saved as fraction 3. Each lysis buffer was supplemented with 5 ng/ml of leupeptin, 1 mM of AEBSF, and 20 μg/ml of Aprotinin. Bovine caudal epididymal and ejaculated (cryopreserved) spermatozoa were washed in PBS and directly fractionated for comparison as described previously (see Fig. 8, A and B).
FIG. 8.
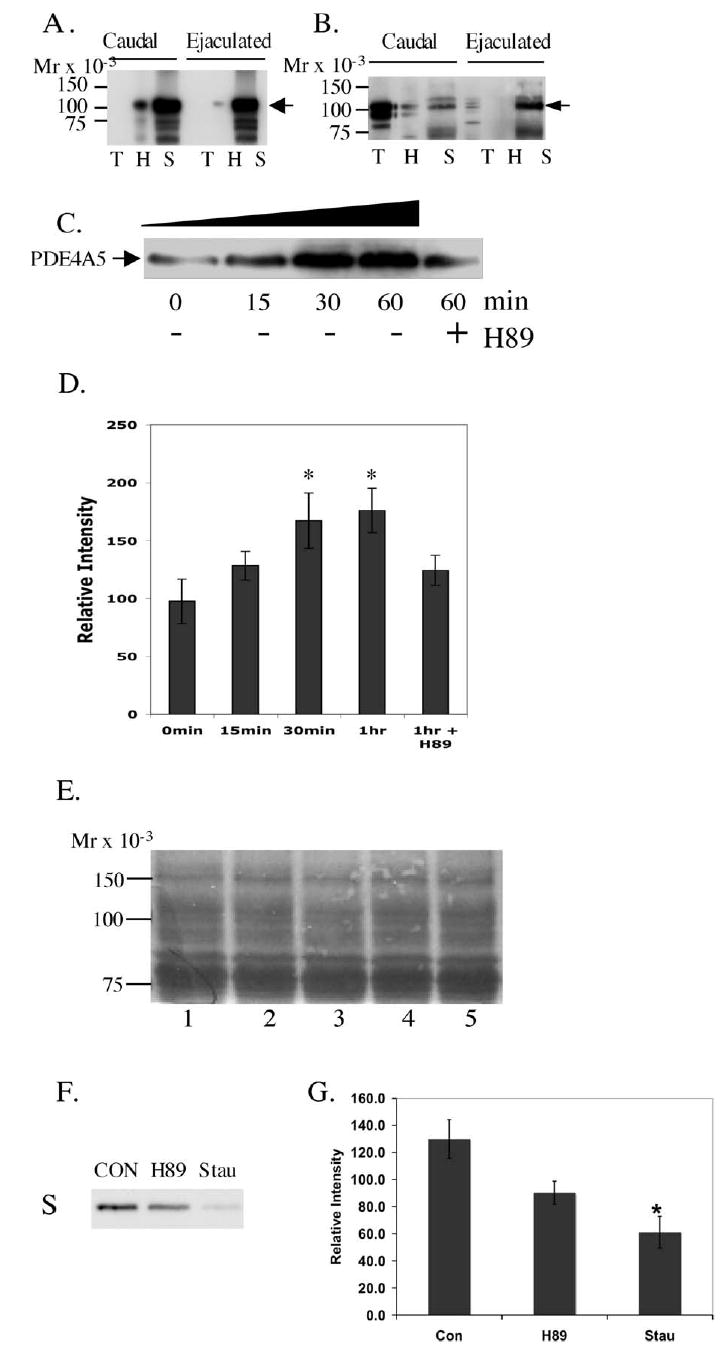
Differential solubilization of AKAP3 and PDE4A5 in spermatozoa. Caudal and ejaculated spermatozoa were solubilized sequentially in a series of buffers (see Materials and Methods for details). Lane T indicates the Triton X-100-solubilized fraction, lane H is the hypoosmotic buffer fraction, and lane S is the SDSsolubilized fraction. The proteins were then separated in 10% SDS-PAGE and transferred to Immobilon-P and analyzed by Western blotting using anti-AKAP3 (A) and anti- PDE4A (B). Caudal epididymal sperm were incubated in a capacitating medium for 0– 60 min at 39°C in 5% CO2. The intensity of immunoblots for the SDS fraction (C), probed with anti-PDE4A5, is indicated in panel D. A significant (P < 0.05) difference in 1 h or 30 min is denoted by an asterisk versus 0 min. Equal loading of samples was accessed by Coomassie stain (E). Incubation with kinases inhibitors H89 and Staurosporine (H89 is fairly specific for PKA but at higher concentrations will also inhibit other kinases; Staurosporin is a very potent broadspectrum kinase inhibitor) under capacitating conditions for 60 min is compared to the control with no inhibitors (F and G). This figure is representative of 10 experiments.
Immunoprecipitation in COS Cells
COS-7 cells (Cercophitecus aethiops, SV40 tranformed) were purchased from ATCC (Manassas, VA) and maintained in culture with DMEM (low glucose; 1000 mg/L d-glucose, l-glutamine, pyridoxine HCl, and 110 mg/L sodium pyruvate) and 10% fetal bovine serum at 37°C in a 95% air/5% CO2 incubator. Cells were plated at approximately 120 000/ml (350 000 total) in 60-mm culture dishes the day before transfection. When cells were approximately 90%–95% confluent, they were transfected using Lipofectamine 2000. Briefly, 2.6 μg of a mouse Pde4a-pCMV construct and 5.25 μg of a human AKAP3-GFPC2 construct (full-length AKAP3 cut from a human AKAP3-pGEX construct and put in GFPC2 using EcoRI and SalI) were diluted in 50 μl of OPTI-MEM I Reduced Serum Media. Lipofectamine 2000 was diluted at 2.5 μl/100 μl OPTI-MEM I and incubated at room temperature for 5 min. The reagent mixture (100 μl) was added to the DNA and incubated for 30 min at room temperature before addition to the cells. Transfections were incubated for 24 h before collection. Human AKAP3-GFPC2 and rat Pde4d-pcDNA were cotransfected as described previously using 7.8 μg of human AKAP3-GFPC2 and 5.25 μg rat Pde4d-pcDNA.
Cells from each 60-mm plate were lysed in 1.1 ml lysis buffer (1% Triton X-100, 150 mM NaCl, 20 mM Tris-HCl pH 7.5, 2 mM EDTA, 2 mM EGTA, 1/100 protease inhibitor mixture, 1 mM benzamidine, and 10 μg/ml soybean trypsin inhibitor) and incubated with rotation at 4°C for 30 min. The mixtures were centrifuged at 13 000 × g for 15 min at 4°C, and the supernatant was precleared with 1 μg rabbit IgG for 15 min and an additional 30 min at 4°C with 30 μl of 50% protein A-sepharose slurry made in lysis buffer. For AKAP3 immunoprecipitation, the precleared supernatants (lysate) were incubated for 1 h at 4°C with 8 μg of rabbit IgG, 8 μg of α-AKAP3 antibody, or 8 μg of α-AKAP3 antibody preincubated for 30 min with 24 μg of blocking peptide. After adding 50 μl of 50% Protein-A slurry, the mixtures were rotated for 30 min at 4°C. The beads were washed four times with lysis buffer by centrifugation at 13 000 × g for 4 min at 4°C and then suspended in 280 μl of water and divided into five tubes of 50 μl for PDE assay and one tube of 30 μl for SDS-PAGE and Western blotting. SDS-PAGE samples were spun down for 4 min at 4°C, and beads were boiled for 5 min in SDS-sample buffer. PDE4A immunoprecipitation was performed similarly using 15 μg of rabbit IgG, 1.5 μl of AC55, or 1.5 μl AC55 preincubated for 30 min with a PDE4AGST fusion protein. Since IPs were not analyzed by PDE assay, beads were boiled in SDS-sample buffer as described previously directly after washing in lysis buffer. Samples were separated by 10% SDS-PAGE and transferred to Immobilon-P. After 1 h blocking in blotto (TBS with 0.1% BSA and 5% nonfat milk), Western blots were performed using AC55, M3S1, α-AKAP3 antibody, or a monoclonal mouse α-GFP antibody. AC55 and α-AKAP3 antibodies were diluted 1:2000 in 2.5% BSA in TTBS. Secondary antibody was goat antirabbit, HRP conjugated, diluted 1:5000. M3S1 antibody was diluted 1:500 in 2.5% BSA in TTBS, α-GFP antibody was diluted 1:1000 in TTBS, and the goat anti-mouse HRP conjugated secondary was diluted 1:5000. Signals were detected by chemiluminescence.
PDE Assay
To assay for PDE activity, immunoprecipitations with α-AKAP3 were performed as outlined in the IP section. The beads were washed and then used for the PDE activity assay using 1 μM cAMP as a substrate according to the method described by Beavo et al. [32], except the time of incubation was 20 min instead of 30 min. This change was a result of a time course assay (data not shown) that indicated that 20 min was the optimal incubation time for our assay. Samples were assayed in 50 μl of PDE buffer A (100 mM MOPS pH 7.5, 4 mM EGTA, 1 mg/ml BSA) and 50 μl of PDE buffer B (100 mM MOPS pH 7.5, 75 mM magnesium acetate, 100 000 c.p.m. of [3H]cAMP) with or without 50 μM Rolipram in a total volume of 250 μl. The reaction was terminated by boiling and subsequently adding 10 μl of snake venom (2.5 mg/ml). The samples were then transferred to an ion exchange column, and [3H]nucleoside was eluted into scintillation vials. Scintillation fluid was added to the eluted samples, mixed, and counted in a Beckman LS5000 TA Liquid Scintillation Counter.
Pulldown Assay
Pulldown assays using bacterial extracts as previously described [33]. Briefly, full-length human AKAP3 was subcloned into a pGEX 5X-1 plasmid and transformed into BL21 (LysS) DE3 cells, and expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG). Full-length mouse Pde4a was subcloned into a pET30a vector, which was also induced with IPTG after transformation into BL21 (LysS) DE3. Extracts were prepared from bacterial pellets thawed on ice, resuspended in 500 μl cold bacterial lysis buffer (50 mM Tris pH 8.0, 2 mM EDTA + 0.05% Aprotinin [5 mg/ml], 0.5% soybean trypsin inhibitor [5 mg/ml], 1 mM AEBSF, 0.05 mM Leupeptin) and sonicated 3 × 20 bursts at 3.5% power. The sonicated mixture was combined with equal volume (500 μl) of bacterial lysis buffer and 2% Triton X-100. Samples were rotated at 4°C for 30 min, then centrifuged at 13 000 × g for 15 min. GST-tagged AKAP3 was immobilized onto glutathione Sepharose fusion protein beads by rotating the supernatant with beads for 1 h at 4°C (glutathione Sepharose beads were previously washed 5× in cold PBS, spun at 1000 × g, 1 min). The beads were then centrifuged at 1000 × g for 1 min, the supernatant was discarded, and the beads were washed 5× with cold PBS (centrifuged 1000 × g, 1 min each wash). The bacterial supernatant containing S-tagged PDE4A plus an amount of blotto (TBS with 0.1% BSA and 5% nonfat milk) equal to half the volume of the supernatant was then rotated with the beads for 2 h at 4°C. Nonspecific binding was removed by washing with PBS as described previously. To control for nonspecific binding of PDE4A to the glutathione beads or to the GST-tag, beads were also incubated with PBS alone or with bacterially expressed pGEX 5X-1 (extracts prepared as described previously, except sonication was 1 × 15 bursts). Proteins were eluted from the beads by boiling in SDS gel loading buffer. The eluates were separated by 10% SDS-PAGE and transferred and blocked as with IPs, and the interaction was detected using S-protein HRP conjugate by chemiluminescence.
For pulldown assays using a combination of bacterial extracts and caudal sperm lysates, full-length human AKAP3 was subcloned into pET30a, transformed, induced, sonicated, and lysed as described previously. This Stagged AKAP3 was immobilized on S-protein agarose by rotating the supernatant with the beads for 1 h at 4°C (S-beads were previously washed 5× in cold PBS, spun at 1000 × g, 1 min). The beads were then centrifuged at 1000 × g for 1 min, the supernatant was discarded, and the beads were washed 5× with cold PBS (centrifuged 1000 × g, 1 min each wash). Bovine caudal sperm (2 × 109) were washed twice in cold PBS. Triton lysis buffer (1 ml of 0.1% Triton X-100, 50 mM Tris-HCl, + 0.05% Aprotinin [5 mg/ml], 0.5% soybean trypsin inhibitor [5 mg/ml], 1 mM AEBSF, 0.05 mM Leupeptin) was added, and sperm were triturated and left on ice for 20 min and then sonicated 3 × 20 bursts at 8.0% power. Samples were rotated for 30 min before centrifugation at 13 000 × g at 4°C for 15 min. Eight hundred microliters of this supernatant plus 200 μl of blotto were rotated with the beads for 2 h at 4°C. To control for nonspecific binding of sperm proteins to the S-beads or to the Histag, beads were also incubated with PBS alone or with bacterially expressed pET30a (bacterial extract prepared as described previously) before addition of the sperm lysate. Proteins were eluted, separated, transferred, and blocked as described previously. Interaction was detected using AC55 diluted 1:1000 in 2.5% BSA in TTBS, goat anti-rabbit secondary at 1:5000 in TTBS, and chemiluminescence.
Statistical Analysis
All statistical analysis was performed using ANOVA with significance defined as P < 0.05. If the main effect of the treatment was significant, then a multiple comparison analysis was performed using the Tukey analysis. These analyses were performed using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA).
RESULTS
Role of PDE4 in Sperm Motility Regulation
To investigate the role of PDE4 in regulating motility and the acrosome reaction, PDE4-specific inhibitors, Rolipram and RS-23544 [34], were added to bovine caudal epididymal sperm and changes in motility parameters were measured after incubating for 15 min in CESD buffer. Both inhibitors caused a significant increase in both motility and progressive motility (Fig. 1). 2-chloro-2N-deoxyadenosine, a potent motility stimulator [29], was added as a positive control. Curvilinear velocity (VCL), progressive velocity (VSL), and amplitude of lateral heads displacement (ALH) increased slightly with Rolipram and RS23544 compared to untreated control. However, the linearity (LIN) had no significant increase with either compound. No change was seen in the acrosome reaction with either PDE4 inhibitor when compared with positive control (addition of calcium ionophore) or negative control (sample prepared without PDE inhibitor) (data not shown). These data suggest that PDE4 isoforms are present and involved in regulating bovine sperm motility.
FIG. 1.
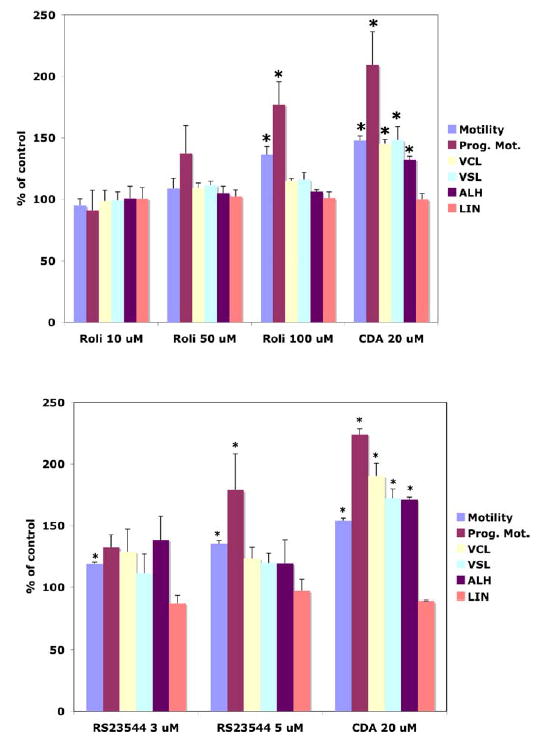
Effect of PDE4-specific inhibitors (Rolipram and RS23544) on sperm motion parameters. Bovine caudal epididymal spermatozoa were incubated for 15 min at 37°C with Rolpiram, RS23544, 2-chloroadenosine, or vehicle control. Data have been normalized against baseline preincubation values (equal to 100%). VCL, Curvilinear velocity, VSL, progressive velocity, ALH, amplitude of lateral head displacement, LIN, linearity. Asterisks indicate significance difference from baseline value (100%) by P < 0.05, (n = 3).
Localization of PDE4 and AKAP3 in Spermatozoa
Immunofluorescence staining experiments were performed to determine the subcellular locations of PDE4A and AKAP3 (previously known as AKAP110). Using polyclonal antibody AC55, PDE4A staining was detected in the principal piece of the flagellum and in the anterior and equatorial segments of the acrosomal region of bovine caudal spermatozoa (Fig. 2A). There was no difference in staining pattern in caudal and ejaculated sperm (data not shown). AKAP3 staining was similar to that seen for PDE4A, except that staining of the equatorial segment was less pronounced (Fig. 2B [16]). Incubation of the PDE4 or AKAP3 antibodies with a fusion protein or antigenic peptide completely blocked the PDE4 and AKAP3 staining (Fig. 2, A and B, inset). The specificity of PDE4A antibody was also tested using wild-type and Pde4a knockout mice, where AC55 staining of wild-type sperm was detected in the apical segment of the acrosome (Fig. 2C). No staining was detected in the knockout mice (Fig. 2C, inset). The different staining patterns detected in the bovine and mouse is most likely due to species specificity of antibody recognition or species variation in PDE4A expression.
FIG. 2.
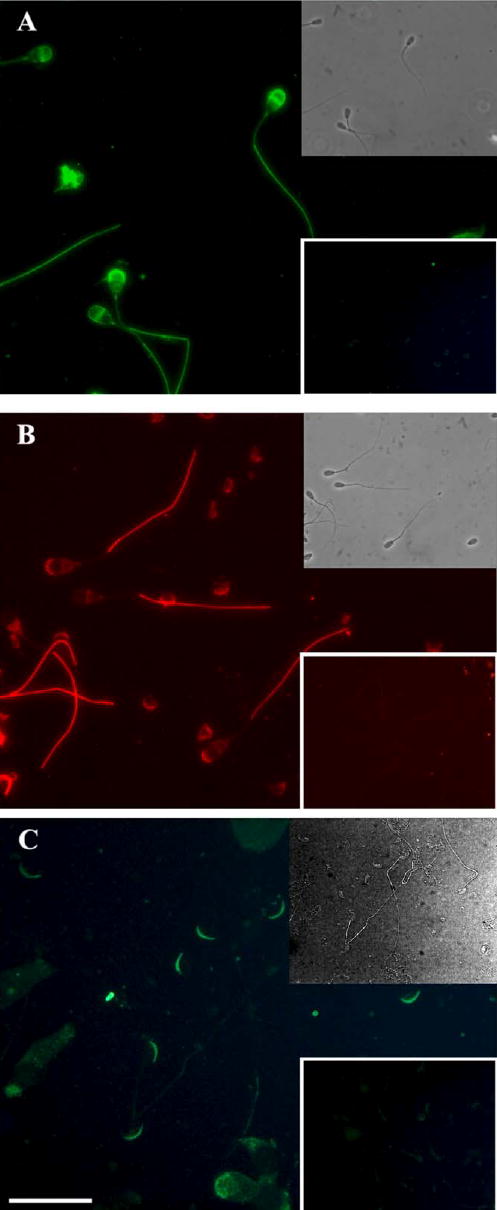
Immunolocalization of PDE4A and AKAP3 in caudal epididymal spermatozoa. Matched blocked antibody (insert) with the fluorescence photomicrograph of formalin-fixed, acetone, and Triton X-100-permeabilized bovine caudal epididymal spermatozoa immunostained with antibodies to PDE4A (A) or AKAP3 (B) and of murine caudal epididymal spermatozoa with antibodies to PDE4A in wild-type (C) and knockout mice (insert). No difference in staining was observed between caudal epididymal and ejaculated spermatozoa. Bar = 10 μm. Micrographs are representative from three experiments; however, the inserts (blocking peptides and knockout mice) were performed only once.
PDE-Dependent Phosphorylation of Sperm Proteins
If PDE4 interacts with AKAPs in a complex that also includes PKA, it is likely to alter the availability of cAMP near this complex and thus affect PKA activity. Phospho-serine/threonine PKA substrate antibodies were used to determine if specific inhibitors of PDE4 affect phosphorylation of specific substrates by PKA. Of particular interest was the phosphorylation of AKAPs. Ficarro and coworkers have shown that both AKAP3 (AKAP110) and AKAP4 (AKAP82) are phosphorylated on serine residues [18]. Incubation of intact bovine caudal epididymal spermatozoa with Rolipram for 15–30 min produced an increase in the serine/threonine phosphorylation of an 82-kDa protein compared to control (Fig. 3; compare lanes 2, 4, and 6 with lanes 1, 3, and 5). No other proteins demonstrate consistent changes in phosphorylation on incubation with Rolipram. No significant differences in phosphorylation levels were detectable between untreated ejaculated samples and those treated with Rolipram (data not shown), as the control samples were already phosphorylated extensively and small changes in phosphorylation were thus undetectable. These data indicate that PDE4 is involved in regulating cAMP levels and phosphorylation of proteins in sperm.
FIG. 3.
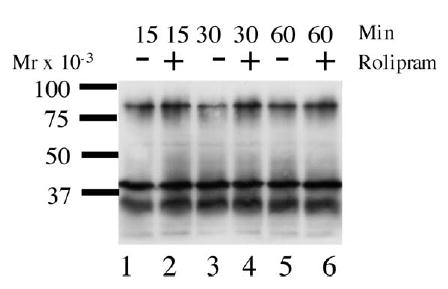
PKA phosphorylation of caudal epididymal spermatozoa with or without Rolipram. Approximately 50 μg protein from the SDS-solubilized sperm pellet was separated by SDS-gel electrophoresis and subjected to Western blot analysis with anti-phospho-serine/threonine antibody. The sperm samples were incubated for 15, 30, or 60 min with or without Rolipram at 39°C in 5% CO2. This result is one representative blot of three independent experiments.
Role of AKAPs in PDE Localization
Because of low solubility of the proteins in spermatozoa, an in vitro system using COS cells was used to determine if PDE4 was capable of interacting with AKAP3. We performed immunoprecipitations (IPs) on COS cells cotransfected with Pde4a5-pCMV or Pde4d3-pCDNA and AKAP3-GFP. PDE4A did coprecipitate with AKAP3 when IPs were performed with anti-AKAP3 (polyclonal), but no band of appropriate size for PDE4A was detected when IPs were performed using anti- AKAP3 plus blocking peptide or IgG (Fig. 4A; compare lane 2 with lanes 3 and 4 [lane 1 is the lysate]). This interaction was also confirmed by doing a reverse IP, using antibodies for PDE4A (polyclonal antibody AC55) and an IB with anti-GFP (monoclonal) to detect the presence of AKAP3-GFP (Fig. 4B, lane 2). Again, the blocking peptide and IgG controls produced no detectable bands (Fig. 4B, lanes 3 and 4). To further examine the specificity of the interaction between AKAP3 and PDE4 isoforms, the interaction of PDE4D with AKAP3 was also analyzed. Cells cotransfected with Pde4d3-pcDNA and AKAP3-GFP were immunoprecipitated with anti-AKAP3 antibody and then analyzed by PDE4D immunoblot but produced no detectable bands (Fig. 4C, lane 2). IgG control IP also produced no detectable bands (Fig. 4C, lane 3). A control IB using anti-GFP antibody clearly indicates the presence of AKAP3 in the IP (Fig. 4D, lane 2). Thus, AKAP3-GFP and PDE4D3 do not show any sign of interaction when analyzed under the same conditions as AKAP3/PDE4A.
FIG. 4.
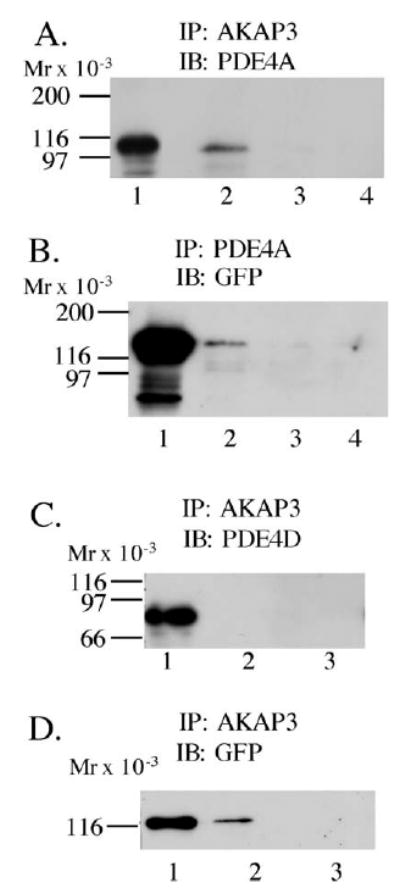
PDE4A5, but not PDE4D3, coprecipitates with AKAP3 in COS7 cells. COS cells transfected with AKAP3-GFP and Pde4a5-pCMV (A and B) were immunoprecipitated with (A) anti-AKAP3 (lane 2), anti-AKAP3 + blocking peptide (lane 3), or IgG (lane 4) with lysate (precleared supernatant) in lane 1, followed by IB with anti PDE4A; reverse IP (B) was done with anti-PDE4A (lane 2), anti-PDE4A + blocking peptide (lane 3), or IgG (lane 4) with lysate in lane 1, followed by IB with anti-GFP; COS cells transfected with AKAP3-GFP and Pde4d3-pcDNA (C and D) were immunoprecipitated with anti-AKAP3 (lane 2) or IgG (lane 3) with lysate in lane 1, followed by IB with anti-PDE4D (C) or anti-GFP (D) to confirm that AKAP3 was immunoprecipitated. These results are representative of three independent experiments.
A direct interaction between AKAP3 and PDE4A was also observed with bacterially expressed protein lysates using a pulldown technique. PDE4A5-S-tag and AKAP3-GST fusion protein were expressed in BL21 LysS bacterial cells and lysed. The resulting samples were subjected to SDS-PAGE followed by S-tag Western analysis. PDE4A5-S-tag was pulled down with AKAP3-GST immobilized on GST beads but not with GST-tag (pGEX expression protein) coupled with GST beads or with noncoupled GST beads alone (Fig. 5; compare lane 4 with lanes 1–3). Equal loading of AKAP3-GST and vector GST was assessed using anti-GST IB (data not shown). The in vitro data using pulldowns provide additional evidence that AKAP3 interacts with PDE4A5.
FIG. 5.
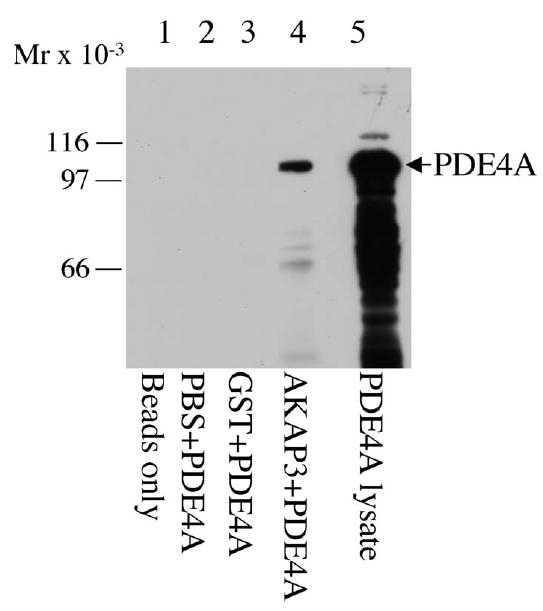
AKAP3 interacts with PDE in pulldown assays. Pulldown assays using glutathione sepharose (GST) beads were performed. PDE4A5-S protein bacterial lysate was incubated with GST beads (lane 2), GST beads bound to GST bacterial lysates (lane 3), and AKAP3-GST bacterial lysates (lane 4). A lane of PDE4A-S protein bacterial lysate was analyzed to determine the size of the recombinant fusion protein (lane 5). The samples were separated by SDS-PAGE, transferred to immobilon membranes, and immunoblotted with S-protein-HRP conjugate antibody. This result is one representative blot of three independent experiments.
PDE Activity Associated with AKAP3
To determine if PDE activity is associated with AKAP3, we analyzed IP samples from COS cells cotransfected with AKAP3 and Pde4a. Cell lysates were immunoprecipitated with an antibody to AKAP3, and PDE activity of the IP samples was analyzed with or without PDE4-specific inhibitor Rolipram (50 μM). The AKAP3-immunoprecipitated sample showed significantly more PDE activity compared to samples immunoprecipitated with IgG control antibody or AKAP3 antibody in presence of antigenic peptide (blocking peptide) (Fig. 6). In the presence of 50 μM Rolipram, PDE activity in AKAP3 IP sample showed complete reduction, indicating that the PDE activity is specific to PDE4 (Fig. 6).
FIG. 6.
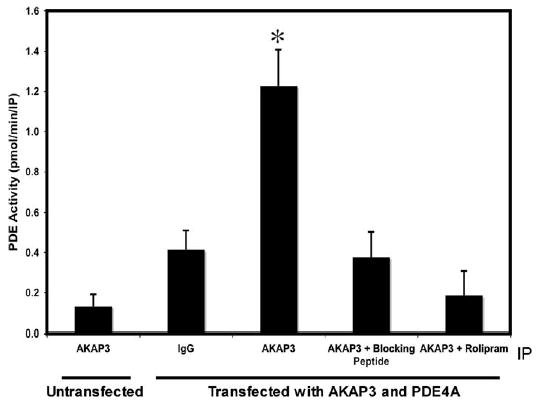
PDE4 activity associated with AKAP3. COS cells, untransfected or transfected with AKAP3 and Pde4a, were immunoprecipitated with IgG, anti-AKAP3, and anti-AKAP3 blocked with antigenic protein, then assayed for PDE activity in the presence or absence of Rolipram. PDE activity is presented as mean ± SEM of three independent experiments. Data was analyzed by ANOVA followed by Tukey multiple comparison. A significant (P < 0.05) difference in PDE activity is denoted by an asterisk compared to all other treatments.
Interaction of AKAP3 with Endogenous PDE4A5, PDE4A8 but Not with PDE4D
A pulldown technique was performed using bacterially expressed AKAP3 and endogenously expressed PDE4s from caudal spermatozoa, where PDE4A is soluble. AKAP3-S-Tag fusion protein from bacterial lysate was immobilized on beads and used to pull down any endogenous sperm proteins that bind to AKAP3. The PDE4A isoforms that bound to the AKAP were detected using an antibody specific for PDE4A (AC55). Western analysis of the caudal sperm lysate detects two bands of Mr 110 000 and 98 000 (corresponding to PDE4A5 and PDE4A8, respectively) (Fig. 7, lane 3). Both of these bands are also detected in the AKAP3 pulldown but not in the vector control (vector-S-Tag lacking the AKAP3 fusion protein) (Fig. 7; compare lanes 2 and 1). These data suggest the AKAP3 is capable of binding two endogenous isoforms of sperm PDE4A.
FIG. 7.

AKAP3 interacts with PDE4A5 in sperm pulldown assays. Sperm proteins were pulled down with S-tagged-AKAP3 (lane 2) or S-tag fusion partner alone (lane 1) bound to agarose beads. The interaction was detected by an anti-PDE4A Western blot analysis. A lane of sperm lysate was analyzed to confirm the presence of PDE4A (lane 3). This result is one representative blot of three independent experiments.
Differential Solubilization of AKAP3 and PDE4A
The principal piece of spermatozoa consists of the fibrous sheath, outer dense fiber, and the axoneme. Depending on where the proteins are located in the principal piece, the solubilization of the protein may differ. Proteins embedded in the cytoskeletal structures are most difficult to solubilize. If two proteins are associated with each other, it is logical to assume that they will be found in the same solubilized fraction, although it is possible that those loosely associated may end up in different fractions. To determine if AKAP3 and PDE4A have similar solubilization properties, bovine spermatozoa were solubilized in a series of buffers as described by various investigators [35, 36]. For fraction I, a Triton X-100 buffer was used to solubilize the membrane fraction and cytosolic fraction of the sperm (Fig. 8, A and B, lane T). For fraction II, lowionic-strength buffer with DTT was used to solubilize the peripheral sperm proteins (Fig. 8, A and B, lane H). For fraction III, the remaining fibrous sheath was solubilized using a 4% SDS buffer (SDS is presumed to solubilize cytoskeletal proteins) (Fig. 8, A and B, lane S), which has been shown to solubilize AKAP3 [37–40] (details in Materials and Methods). The three fractions were analyzed for the presence of AKAP3 (panel A) and PDE4A (panel B). Distribution of the two proteins was compared between caudal and ejaculated spermatozoa in the three respective fractions (Fig. 8, A and B). AKAP3 was detected primarily in the SDS-solubilized fraction of both caudal and ejaculated sperm (Fig. 8A). However, PDE4A5 showed a dramatic difference in solubilization properties when isolated from caudal versus ejaculated sperm. It was detected mostly in the Triton X-100 solubilized fraction of caudal sperm but mostly in the SDS-solubilized fraction of ejaculated sperm (Fig. 8B; compare lanes T and S between caudal and ejaculated spermatozoa). This difference in the solubility of PDE4A5 suggests that it associates with different cytoskeletal structures in caudal and ejaculated spermatozoa. Since the previously described trend was observed in caudal sperm that had not been incubated in capacitating media, we wanted to ascertain whether maturation of sperm with capacitation could change the solubility of PDE4A. To determine this, caudal sperm were incubated in capacitating medium at different time intervals at 39°C in 5% CO2. Under these conditions, the amount of PDE4A detected in the SDS-solubilized fraction steadily and significantly increased with time (Fig. 8, C and D). Equal loading of all samples was analyzed by Coomassie staining (Fig. 8E). Under the same conditions, no significant increase in PDE4A was detected in ejaculated sperm where the PDE4A was already found predominantly in the particulate or SDS fraction (data not shown). Capacitation of caudal sperm had no effect on localization of AKAP3 (data not shown).
The process of capacitation is accompanied by increase in phosphorylation of many proteins [41, 42]. To determine the role of kinases in differential localization of the PDE4A, protein kinase inhibitors were added to the capacitating media. Addition of the PKA inhibitor H89 (50 μM) or the broad-range kinase inhibitor Staurosporin (3 μM) to the caudal sperm for 1 h decreased the amount of PDE4A detected in the SDS fraction compared to the control incubated for 1 h under similar conditions (Fig. 8, F and G), suggesting that kinase activity facilitates the association of PDE4A to a nonsoluble structure. These data are consistent with a model in which PDE4A increases its association with AKAP3 as the sperm matures and that phosphorylation of AKAP3 by PKA or other kinases [18] may play a role in regulating this interaction.
DISCUSSION
PDEs modulate the concentration of cAMP, which in turn regulates the activity of PKA. To function efficiently, these two enzymes need to be located in close proximity to each other. Evidence supporting this concept has been reported for both muscle and Sertoli cells, where PKA and PDE4Ds have been shown to bind simultaneously to AKAPs, forming a threeprotein complex [21, 22]. Though there are many isoforms of PDE, only a few subtypes have been characterized in spermatozoa, the two major subtypes being PDE1 and PDE4. PDE1 regulates the acrosome reaction, while PDE4 regulates motility without affecting the acrosome reaction [3, 12, 43, 44]. The present study was designed to explore the role of PDE4 and its interaction with sperm AKAPs, specifically AKAP3.
The addition of two different PDE4 specific inhibitors to bovine caudal epididymal spermatozoa caused a significant increase in motility, especially progressive motility (Fig. 1). The concentration of inhibitors needed to stimulate motility were higher than those needed to inhibit the PDE4 enzyme in a broken cell prep [3], but this may be due to the poor permeability of these inhibitors in intact spermatozoa. Progressive motility is a key parameter in determining the sperm’s ability to penetrate the cervical mucus [45]. Thus, these data suggest that PDE4 is a key component of the biochemical machinery that determines the fertilizing potential of the sperm. No changes in the acrosome reaction were observed with either Rolipram or RS-23544 (data not shown). Therefore, PDE4 appears to only be involved in regulating motility functions, as previously suggested for human spermatozoa [3]. This specificity is consistent with a need for specific subcellular localization and compartmentalization of this enzyme in spermatozoa.
To determine the localization of different PDE4 isoforms, immunocytochemistry analysis of bovine spermatozoa was performed using antibodies specific for PDE4A and PDE4D. Staining for PDE4A (Fig. 2A) but not PDE4D (data not shown) was detected in the principal piece, supporting a role for PDE4A in regulating motility. Staining for both PDE4A and PDE4D was detected in the acrosome of the head (Fig. 2, A and C; data not shown). However, PDE4 specific inhibitors had no effect on the acrosome reaction. It is possible that PDE4A or PDE4D may play a role in other head-associated functions such as chemotaxis. Their location in the acrosomal membrane is similar to that of adenylyl cyclase III (ACIII), thus placing them in a key position to control the cAMP levels implicated in the regulation of sperm chemotaxis during fertilization [3, 46–48]. Supporting this hypothesis, the PDE4A that is expressed mainly in odorant receptor neurons of olfactory neuroepithelium is also expressed in spermatids [27] and spermatozoa [49].
Immunofluorescence analysis detects AKAP3 in the principal piece and the acrosomal region of the head (Fig. 2B), similar to the staining pattern of the PDE4 isoforms. Previous studies at the ultrastructural level by Mandal and colleagues [17] has detected AKAP3 associated with the ribs of the fibrous sheath with no label associated with the outer dense fibers or the axoneme. The data in this paper showing an interaction between AKAP3 and PDE4A suggest that PDE4A would also be present in the ribs of the fibrous sheath, although it might also be present in other parts of the principal piece. The binding of the AKAP3 with PDE4A and PDE4D was explored using immunoprecipitation assays of COS cells cotransfected with Pde4d3 or Pde4a5 and AKAP3. Data from these experiments suggest that PDE4A but not PDE4D binds to AKAP3 (Figs. 4 and 6). The lack of association between AKAP3 and PDE4D3 could be due to the lack of AKAP3 in the head [17]. The binding of both bacterially expressed and endogenously expressed sperm PDE4A5 with bacterially expressed AKAP3 also provides evidence for an interaction between the two proteins (Figs. 5 and 7).
Compartmentalization of PKA and PDE allows spatially distinct pools to be differentially activated. This should allow subcellular levels of cAMP to be maintained by isoform-specific PDE interactions with different AKAPs in spermatozoa, similar to that seen in somatic cells [21, 22, 33]. Thus, the activity of a specific isoform of PDE may modulate the phosphorylation of a specific PKA substrate. Incubation of bovine spermatozoa with Rolipram produced a significant increase in motility and also produced a significant increase in the phosphorylation of an 82-kDa protein, without detectable changes in other phosphoproteins (Fig. 3). These data are consistent with a model in which the inhibition of PDE4 activates only PKA molecules that are colocalized with the 82-kDa protein. As AKAP3 and AKAP4 have been shown to interact in the fibrous sheath [50] and to be phosphorylated on serine residues during capacitation [18], we suspect that the 82-kDa phosphoprotein is AKAP4 (AKAP82). It is interesting that we did not detect a phosphoband at 110 kDa, corresponding to AKAP3 (AKAP110). This might simply be due to the sensitivity of our assay. Nearly half the protein in fibrous sheath isolated from mouse sperm is AKAP4 (AKAP82) [51]. Thus, it is possible we are not detecting changes in the phosphorylation levels of AKAP3 simply because it is less abundant.
Previous studies have shown that PDE4D3 associates with AKAP6 (previously known as mAKAP) through a region upstream of the UCR1 interaction site [21] and with AKAP9 (previously known as AKAP450) through the UCR2 domain [22]. It has also been shown that these domains have phosphorylation sites for specific kinases that may influence the targeting and localization of PDE4A5 in a cell [52, 53]. Recently, PKA phosphorylation of PDE4D3 has been shown to increase its affinity for AKAP6 [54]. To determine the role of phosphorylation in PDE4A5 targeting and its interaction with AKAP3, caudal sperm were incubated in capacitating media with the PKA inhibitor H89, the broad-specificity kinase inhibitor Staurosporin, or without inhibitor (Fig. 8F). The shift in solubility of PDE4A5 during capacitation/maturation to the less soluble SDS fraction most likely reflects its increased interaction with insoluble fibrous sheath proteins such as AKAP3 (Fig. 8, A and B). It is interesting to note that we did not observe any changes in the location of PDE4A in caudal versus ejaculated spermatozoa using immunocytochemical analysis, but this may simply be due to the sensitivity of our detection system. The addition of both H89 and Staurosporin lowered the amount of PDE4A5 detected in the insoluble fraction, suggesting that kinase activity is required for PDE4A5 to associate with the insoluble proteins (Fig. 8, C–G). These data are consistent with a model where capacitation promotes increased kinase activity, which in turn promotes the association of PDE4A with insoluble proteins in the fibrous sheath, such as AKAP3, thus regulating the concentration of cAMP/PKA in this compartment. It is interesting to note that the overall activity of PDE does not change during capacitation of ejaculated bovine spermatozoa [55]. Our data raise the possibility that a change in location of certain PDE isoforms during this process could have a dramatic effect on localized concentrations of cAMP but not be reflected in the overall activity of PDE.
In summary, we have demonstrated that PDE4A and AKAP3 are colocalized in bovine spermatozoa and that they interact at least in vitro. Additionally, the use of specific inhibitors indicates that PDE4A plays a role in progressive motility and also affects the phosphorylation of an 82-kDa protein, perhaps AKAP4. Finally, decreased solubility of PDE4A following capacitation suggests increased interaction with fibrous sheath proteins, underscoring the concept that PDE targeting is an important event in sperm function.
Footnotes
Supported by NIH Grants HD36408 (D.W.C.) and VA Merit Award (D.W.C.), NIH Grant HD38520 (S.V.), and HD31544 (M.C.).
References
- 1.Tash JS. Role of cAMP, calcium and protein phosphorylation in sperm motility. In: Gagnon C, (ed.),Controls of Sperm Motility: Biological and Clinical Aspects. Boca Raton, FL: CRC Press; 1990:229–241.
- 2.De Jonge CJ, Han HL, Lawrie H, Mack SR, Zaneveld LJ. Modulation of the human sperm acrosome reaction by effectors of the adenylate cyclase/cyclic AMP second-messenger pathway. J Exp Zool. 1991;258:113–125. doi: 10.1002/jez.1402580113. [DOI] [PubMed] [Google Scholar]
- 3.Fisch JD, Behr B, Conti M. Enhancement of motility and acrosome reaction in human spermatozoa: differential activation by type-specific phosphodiesterase inhibitors. Hum Reprod. 1998;13:1248–1254. doi: 10.1093/humrep/13.5.1248. [DOI] [PubMed] [Google Scholar]
- 4.Lindemann CB. A cAMP-induced increase in the motility of demembranated bull sperm models. Cell. 1978;13:9–18. doi: 10.1016/0092-8674(78)90133-2. [DOI] [PubMed] [Google Scholar]
- 5.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003;5:1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 7.Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A. 2004;101:13483–13488. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morisawa M, Ishida K. Short-term changes in levels of cyclic AMP, adenylate cyclase, and phosphodiesterase during the initiation of sperm motility in rainbow trout. J Exp Zool. 1987;242:199–204. doi: 10.1002/jez.1402420211. [DOI] [PubMed] [Google Scholar]
- 9.Cascieri M, Amann RP, Hammerstedt RH. Adenine nucleotide changes at initiation of bull sperm motility. J Biol Chem. 1976;251:787–793. [PubMed] [Google Scholar]
- 10.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 11.Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 12.Lefievre L, de Lamirande E, Gagnon C. Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3A in human spermatozoa. Biol Reprod. 2002;67:423–430. doi: 10.1095/biolreprod67.2.423. [DOI] [PubMed] [Google Scholar]
- 13.Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMPdependent pathway. Development. 1995;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 14.Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–692. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- 15.Carrera A, Gerton GL, Moss SB. The major fibrous sheath polypeptide of mouse sperm: structural and functional similarities to the A-kinase anchoring proteins. Dev Biol. 1994;165:272–284. doi: 10.1006/dbio.1994.1252. [DOI] [PubMed] [Google Scholar]
- 16.Vijayaraghavan S, Liberty GA, Mohan J, Winfrey VP, Olson GE, Carr DW. Isolation and molecular characterization of AKAP110, a novel, sperm-specific protein kinase A-anchoring protein. Mol Endocrinol. 1999;13:705–717. doi: 10.1210/mend.13.5.0278. [DOI] [PubMed] [Google Scholar]
- 17.Mandal A, Naaby-Hansen S, Wolkowicz MJ, Klotz K, Shetty J, Retief JD, Coonrod SA, Kinter M, Sherman N, Cesar F, Flickinger CJ, Herr JC. FSP95, a testis-specific 95-kilodalton fibrous sheath antigen that undergoes tyrosine phosphorylation in capacitated human spermatozoa. Biol Reprod. 1999;61:1184–1197. doi: 10.1095/biolreprod61.5.1184. [DOI] [PubMed] [Google Scholar]
- 18.Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, Kalab P, Marto JA, Shabanowitz J, Herr JC, Hunt DF, Visconti PE. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem. 2003;278:11579–11589. doi: 10.1074/jbc.M202325200. [DOI] [PubMed] [Google Scholar]
- 19.Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 20.Macphee CH, Reifsnyder DH, Moore TA, Lerea KM, Beavo JA. Phosphorylation results in activation of a cAMP phosphodiesterase in human platelets. J Biol Chem. 1988;263:10353–10358. [PubMed] [Google Scholar]
- 21.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tasken KA, Collas P, Kemmner WA, Witczak O, Conti M, Tasken K. Phosphodiesterase 4D and protein kinase a type II constitute a signaling unit in the centrosomal area. J Biol Chem. 2001;276:21999–22002. doi: 10.1074/jbc.C000911200. [DOI] [PubMed] [Google Scholar]
- 23.MacKenzie SJ, Baillie GS, McPhee I, MacKenzie C, Seamons R, McSorley T, Millen J, Beard MB, van Heeke G, Houslay MD. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1) Br J Pharmacol. 2002;136:421–433. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann R, Baillie GS, MacKenzie SJ, Yarwood SJ, Houslay MD. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. EMBO J. 1999;18:893–903. doi: 10.1093/emboj/18.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolger GB. Molecular biology of the cyclic AMP-specific cyclic nucleotide phosphodiesterases: a diverse family of regulatory enzymes. Cell Signal. 1994;6:851–859. doi: 10.1016/0898-6568(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 26.Houslay MD, Sullivan M, Bolger GB. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and antidepressant actions. Adv Pharmacol. 1998;44:225– 342. doi: 10.1016/s1054-3589(08)60128-3. [DOI] [PubMed] [Google Scholar]
- 27.Naro F, Zhang R, Conti M. Developmental regulation of unique adenosine 3′,5′-monophosphate-specific phosphodiesterase variants during rat spermatogenesis. Endocrinology. 1996;137:2464–2472. doi: 10.1210/endo.137.6.8641200. [DOI] [PubMed] [Google Scholar]
- 28.Salanova M, Chun SY, Iona S, Puri C, Stefanini M, Conti M. Type 4 cyclic adenosine monophosphate-specific phosphodiesterases are expressed in discrete subcellular compartments during rat spermiogenesis. Endocrinology. 1999;140:2297–2306. doi: 10.1210/endo.140.5.6686. [DOI] [PubMed] [Google Scholar]
- 29.Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J Biol Chem. 1997;272:4747–4752. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]
- 30.Iona S, Cuomo M, Bushnik T, Naro F, Sette C, Hess M, Shelton ER, Conti M. Characterization of the rolipram-sensitive, cyclic AMP-specific phosphodiesterases: identification and differential expression of immunologically distinct forms in the rat brain. Mol Pharmacol. 1998;53:23–32. doi: 10.1124/mol.53.1.23. [DOI] [PubMed] [Google Scholar]
- 31.Morena AR, Boitani C, de Grossi S, Stefanini M, Conti M. Stage and cell-specific expression of the adenosine 3′,5′ monophosphate-phosphodiesterase genes in the rat seminiferous epithelium. Endocrinology. 1995;136:687–695. doi: 10.1210/endo.136.2.7835302. [DOI] [PubMed] [Google Scholar]
- 32.Beavo JA, Bechtel PJ, Krebs EG. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- 33.Asirvatham AL, Galligan SG, Schillace RV, Davey MP, Vasta V, Beavo JA, Carr DW. A-kinase anchoring proteins interact with phosphodiesterases in T lymphocyte cell lines. J Immunol. 2004;173:4806–4814. doi: 10.4049/jimmunol.173.8.4806. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez R, Sette C, Yang D, Eglen RM, Wilhelm R, Shelton ER, Conti M. Activation and selective inhibition of a cyclic AMP-specific phosphodiesterase, PDE-4D3. Mol Pharmacol. 1995;48:616–622. [PubMed] [Google Scholar]
- 35.Uma Devi K, Jha K, Patil SB, Padma P, Shivaji S. Inhibition of motility of hamster spermatozoa by protein tyrosine kinase inhibitors. Andrologia. 2000;32:95–106. doi: 10.1046/j.1439-0272.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 36.Shetty J, Diekman AB, Jayes FC, Sherman NE, Naaby-Hansen S, Flickinger CJ, Herr JC. Differential extraction and enrichment of human sperm surface proteins in a proteome: identification of immunocontraceptive candidates. Electrophoresis. 2001;22:3053–3066. doi: 10.1002/1522-2683(200108)22:14<3053::AID-ELPS3053>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Garbi M, Rubinstein S, Lax Y, Breitbart H. Activation of protein kinase calpha in the lysophosphatidic acid-induced bovine sperm acrosome reaction and phospholipase D1 regulation. Biol Reprod. 2000;63:1271– 1277. doi: 10.1095/biolreprod63.5.1271. [DOI] [PubMed] [Google Scholar]
- 38.Luconi M, Porazzi I, Ferruzzi P, Marchiani S, Forti G, Baldi E. Tyrosine phosphorylation of the a kinase anchoring protein 3 (AKAP3) and soluble adenylate cyclase are involved in the increase of human sperm motility by bicarbonate. Biol Reprod. 2005;72:22–32. doi: 10.1095/biolreprod.104.032490. [DOI] [PubMed] [Google Scholar]
- 39.Jassim A, Gillott DJ, al-Zuhdi Y, Gray A, Foxon R, Bottazzo GF. Isolation and biochemical characterization of the human sperm tail fibrous sheath. Hum Reprod. 1992;7:86–94. doi: 10.1093/oxfordjournals.humrep.a137566. [DOI] [PubMed] [Google Scholar]
- 40.Ricci M, Breed WG. Isolation and partial characterization of the outer dense fibres and fibrous sheath from the sperm tail of a marsupial: the brushtail possum (Trichosurus vulpecula) Reproduction. 2001;121:373–388. doi: 10.1530/rep.0.1210373. [DOI] [PubMed] [Google Scholar]
- 41.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 42.Bajpai M, Asin S, Doncel GF. Effect of tyrosine kinase inhibitors on tyrosine phosphorylation and motility parameters in human sperm. Arch Androl. 2003;49:229–246. doi: 10.1080/01485010390196715. [DOI] [PubMed] [Google Scholar]
- 43.Fournier V, Leclerc P, Cormier N, Bailey JL. Implication of calmodulin-dependent phosphodiesterase type 1 during bovine sperm capacitation. J Androl. 2003;24:104–112. [PubMed] [Google Scholar]
- 44.Yan C, Zhao AZ, Sonnenburg WK, Beavo JA. Stage and cell-specific expression of calmodulin-dependent phosphodiesterases in mouse testis. Biol Reprod. 2001;64:1746–1754. doi: 10.1095/biolreprod64.6.1746. [DOI] [PubMed] [Google Scholar]
- 45.Engel S, Petzoldt R. Human sperm penetration in different media. Andrologia. 1999;31:233–239. doi: 10.1046/j.1439-0272.1999.00149.x. [DOI] [PubMed] [Google Scholar]
- 46.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 47.Vanderhaeghen P, Schurmans S, Vassart G, Parmentier M. Specific repertoire of olfactory receptor genes in the male germ cells of several mammalian species. Genomics. 1997;39:239–246. doi: 10.1006/geno.1996.4490. [DOI] [PubMed] [Google Scholar]
- 48.Cherry JA, Pho V. Characterization of cAMP degradation by phosphodiesterases in the accessory olfactory system. Chem Senses. 2002;27:643–652. doi: 10.1093/chemse/27.7.643. [DOI] [PubMed] [Google Scholar]
- 49.Richter W, Dettmer D, Glander H. Detection of mRNA transcripts of cyclic nucleotide phosphodiesterase subtypes in ejaculated human spermatozoa. Mol Hum Reprod. 1999;5:732–736. doi: 10.1093/molehr/5.8.732. [DOI] [PubMed] [Google Scholar]
- 50.Brown PR, Miki K, Harper DB, Eddy EM. A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol Reprod. 2003;68:2241–2248. doi: 10.1095/biolreprod.102.013466. [DOI] [PubMed] [Google Scholar]
- 51.Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–115. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 52.Beard MB, Huston E, Campbell L, Gall I, McPhee I, Yarwood S, Scotland G, Houslay MD. In addition to the SH3 binding region, multiple regions within the N-terminal noncatalytic portion of the cAMP-specific phosphodiesterase, PDE4A5, contribute to its intracellular targeting. Cell Signal. 2002;14:453–465. doi: 10.1016/s0898-6568(01)00264-9. [DOI] [PubMed] [Google Scholar]
- 53.Baillie GS, MacKenzie SJ, McPhee I, Houslay MD. Sub-family selective actions in the ability of Erk2 MAP kinase to phosphorylate and regulate the activity of PDE4 cyclic AMP-specific phosphodiesterases. Br J Pharmacol. 2000;131:811–819. doi: 10.1038/sj.bjp.0703636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlisle Michel JJ, Dodge KL, Wong W, Mayer NC, Langeberg LK, Scott JD. PKA-phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signalling complex. Biochem J. 2004;381:587–592. doi: 10.1042/BJ20040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galantino-Homer HL, Florman HM, Storey BT, Dobrinski I, Kopf GS. Bovine sperm capacitation: assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol Reprod Dev. 2004;67:487–500. doi: 10.1002/mrd.20034. [DOI] [PubMed] [Google Scholar]


