Abstract
The nptA gene of Vibrio cholerae has significant protein sequence homology with type II sodium-dependent phosphate (Pi) cotransporters found in animals but not previously identified in prokaryotes. The phylogeny of known type II cotransporter sequences indicates that nptA may be either an ancestral gene or a gene acquired from a higher eukaryotic source. The gene was cloned into an expression vector under the control of an inducible promoter and expressed in Escherichia coli. The results demonstrate that nptA encodes a functional protein with activity similar to that of the animal enzyme, catalyzing high-affinity, sodium-dependent Pi uptake with comparable affinities for both sodium and phosphate ions. Furthermore, the activity of NptA is influenced by pH, again in a manner similar to that of the NaPi-2a subtype of the animal enzyme, although it lacks the corresponding REK motif thought to be responsible for this phenomenon. Pi uptake activity, a component of which appeared to be sodium dependent, was increased in V. cholerae by phosphate starvation. However, it appears from the use of a reporter gene expressed from the nptA promoter that none of this activity is attributable to the induction of expression from nptA. It is thus proposed that the physiological function of NptA protein may be the rapid uptake of Pi in preparation for rapid growth in nutrient-rich environments and that it may therefore play a role in establishing infection.
Vibrio cholerae is a gram-negative bacterium that can be isolated from coastal waters around the world. It is a highly varied species, with over 150 identified serotypes. Many strains are pathogenic in humans, usually causing diarrhea. However, only two serotypes (O1 and O139) are known to cause the severe diarrheal disease of epidemic cholera. Owing to the severe and widespread health problems caused by V. cholerae, most investigations have centered upon mechanisms of pathogenicity and efforts to develop an effective vaccine against cholera. Relatively little is known about adaptations of these bacteria to their free-living environment.
In marine and estuarine environments, sodium concentrations are relatively high whereas other essential nutrients can be limiting. One such limitation can be the availability of inorganic phosphate (Pi), since phosphorus, although relatively abundant in the earth's crust, often occurs in insoluble forms. Phosphate, however, is an essential nutrient, and therefore bacteria have generally evolved elaborate systems to regulate its uptake and metabolism (36). Two systems are present in bacteria. The Pit system is driven by proton motive force and is characterized by low-affinity, high-capacity uptake. It is active in the presence of normal or increased external phosphate concentrations. The Pst system is activated when the external phosphate level falls below 20 mM (29). Phosphate starvation in Escherichia coli results in the induction of the Pho regulon, which encodes a large number of genes that are distributed around the chromosome and whose expression is controlled by phoB-phoR. PhoR is a histidine kinase which phosphorylates the response regulator PhoB, and this then activates transcription by binding to a specific 18-base consensus sequence which is part of the promoter of genes in the Pho regulon (22, 23). The Pst operon is a part of the Pho regulon that has been identified both in E. coli and other bacterial species, including V. cholerae (27, 33, 14). It encodes a series of proteins structurally similar to ATP-dependent ABC transporters (2), whose function is the slow but high-affinity uptake of inorganic phosphate (37). The regulon does not, however, contain any genes similar to those for type II sodium-phosphate cotransporters that are central to certain aspects of eukaryote phosphate metabolism or, indeed, any other known sodium-phosphate cotransporters.
The type II cotransporters, first identified in rabbit kidney by expression cloning (39), are encoded by the Npt-2a and Npt-2b genes. The mammalian cotransporter is a transmembrane symporter of approximately 640 amino acids that catalyzes the cellular import of phosphate by utilizing the sodium gradient present across the cytoplasmic membrane. The stoichiometry of transport is approximately three sodium ions per ion of inorganic phosphate. For reviews of mammalian type II cotransporters structure and function see references 5, 8, and 38.
In the present study we demonstrate the presence of a gene immediately downstream of the thyA locus of V. cholerae (GenBank accession no. AJ006514) that encodes a protein involved in sodium-dependent phosphate uptake from the environment that is similar to the type II sodium-phosphate cotransporters described above. Although such proteins exist in various organs of higher eukaryotes, including vertebrates, until now no equivalent system has been described for prokaryotes. The results suggest either that the vertebrate gene is considerably older than previously thought and may be a development of an ancestral bacterial gene involved in phosphate metabolism or that V. cholerae has acquired a eukaryotic gene by horizontal transfer from a host organism. Whatever its source, V. cholerae has an additional functional phosphate uptake mechanism distinct from that of other prokaryotes so far studied.
MATERIALS AND METHODS
Bacteria and plasmids.
The V. cholerae strain used throughout was JS1569 (32). This strain is a rifampin-resistant derivative of CVD103, which in turn was derived from the O1 classical strain 569B by deletion of the gene encoding the A subunit of cholera toxin (19).
Cultures of V. cholerae were maintained on agar plates containing a minimal growth medium which was essentially M9 salts (31) supplemented with FeCl2 · 6H2O (5 mg/liter) and MnCl2 · 4H2O (4 mg/liter) in which the NaCl concentration was increased to 0.5%. The carbon source was glucose, which was added to a final concentration of 0.2%. The same medium was used in liquid form to grow cultures for use in phosphate uptake experiments.
The E. coli strains used in the present study were HB101 (6) and XL1-Blue (Stratagene, San Diego, Calif.). E. coli strains were maintained on Luria-Bertani agar plates supplemented when necessary with the appropriate antibiotic (ampicillin, 100 μg/ml).
V. cholerae strains tested by PCR for the presence of nptA included a range of clinical and environmental isolates that were both O1 and non-O1 serotypes and toxigenic and nontoxigenic. Strains of other species of Vibrio were taken from the Culture Collection of the University of Göteborg (CCUG). These were Vibrio sp. (non-V. cholerae) strain CCUG 537, V. metschinikovii CCUG 7490, V. mimicus CCUG 13624, V. alginolyticus CCUG 4990, V. parahaemolyticus CCUG 15657, V. proteus CCUG 3280, V. vulnificus CCUG 13448, and V. fluvalis CCUG 13622.
DNA manipulation and sequencing.
DNA manipulations, including restriction enzyme analysis, ligation, and transformations, were done by standard methods as outlined by Ausubel et al. (3) or according to the instructions supplied by the manufacturers of the reagents used.
The Expand High Fidelity PCR system (Roche Diagnostics Scandinavia AB, Bromma, Sweden) kit was used for generation of the nptA gene with EcoRI and SpeI ends. Chromosomal DNA from V. cholerae JS1569 served as the template for the reaction. The primers used for the PCR of the nptA gene were NAP-1 (5′-CTC TCT ACC ATC AGC CTC GAA TTC-3′) and NAP-2 (5′-GCG CGG ACT AGT CGG TAT GGC TTG ATG GGT-3′). An additional forward primer (nptA1, 5′-GGGGGGGATCCAAATTGCTCAAGCCAAACATACGATCAGCG-3′) was used in conjunction with NAP-2 to amplify a larger fragment carrying the nptA gene and its promoter region. Primers used for detection of nptA in other V. cholerae strains or other Vibrio species were nptA3 (5′-CAA GGC TCG GTA ACC AAA GCG G-3′) and nptA4 (5′-GCC ACT AAA CCA ATC ACA ATC CTG-3′).
All oligonucleotides were synthesized by Innovagen AB (Lund, Sweden). DNA sequencing was performed using an Applied Biosystems 373 automated sequencer with Thermo Sequenase dye terminator cycle sequencing (Amersham Pharmacia Biotech, Solna, Sweden).
Computer search and similarity algorithms.
Routine computer-aided analysis of DNA sequences was done using the DNA Strider software version 1.3 (24). DNA sequences were assembled in AutoAssembler version 1.4 (Perkin-Elmer Corp., Foster City, Calif.).
DNA database searches were done using the BLAST algorithms (1) and databases available from online services provided by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). The deduced NptA amino acid sequence was aligned with all known sodium-phosphate cotransporter type II sequences by using the online ClustalW (15) service at the European Bioinformatics Institute (www2.ebi.ac.uk/clustalw). Phylogenetic trees were constructed from this alignment by using the Macintosh application TreeView (28).
Transmembrane regions and protein topology of NptA and human NaPi-3a (21) were predicted using online services. NptA was analyzed by TopPred2 (34) in the prokaryotic mode at the Department of Biochemistry, Stockholm University, Stockholm, Sweden (http://www.biokemi.su.se/), and NaPi-3a was analyzed by TopPred2 in the eukaryotic mode. TopPred2 did not detect the membrane-associated hinge region HII, but the presence of this region was inferred from existing models and predicted by Tmpred (Expasy [www.expasy.ch]).
Statistics and curve fitting were performed using the Prism software package (GraphPad, San Diego, Calif.).
Sodium-dependent phosphate uptake by V. cholerae and E. coli.
V. cholerae strain JS1569 was grown overnight at 30°C in minimal medium in the presence of phosphate. The culture was divided into two equal aliquots. The cells were spun down (6,000 × g for 20 min), and half of the pellet was resuspended in an equal volume of fresh medium containing phosphate. The other half was resuspended in medium buffered with Tris-HCl (20 mM) containing no phosphate. The two cultures were then incubated with shaking at 30°C for a further 3 h. The cells were collected by centrifugation, washed twice, and finally resuspended in 1 ml of buffer (10 mM Tris-HCl, pH 7.5) containing 1 mM MgCl2 and 1 mM CaCl2. Volumes were finally adjusted so that the optical densities of different cell suspensions used in each experiment were the same.
Cells were incubated at 30°C in 10 mM Tris-HCl (pH 7.5) with 10 μCi of 32P-labeled inorganic phosphate (Amersham) per ml adjusted with cold K2HPO4 to a final concentration in the reaction mixture of 1 mM, in the presence and absence of NaCl which was added to a final concentration of 100 mM. When NaCl was absent, the osmolarity was preserved by addition of choline chloride to the same concentration. The cells were removed from the reaction mixture by centrifugation through a nonaqueous layer containing a 4:1 mixture of n-butyl phthalate and corn oil (18). The pellet was resuspended in a small volume of 10 mM Tris-HCl (pH 7.5) containing 1 mM MgCl2 and 1 mM CaCl2, and the amount of 32P-labeled phosphate accumulated in the pelleted cells was determined in a scintillation counter.
Cultures of E. coli strain HB101 carrying the nptA gene on a recombinant plasmid were treated in essentially the same manner after harvesting. However, the cells were grown up overnight in Luria-Bertani broth at 37°C. Five hundred microliters of the overnight culture was used to inoculate 50 ml of fresh medium, and the new culture was grown at 37°C for 2 to 2 1/2 h. The culture was then divided into two 25-ml aliquots, and IPTG (isopropyl-β-d-thiogalactopyranoside) was then added to one aliquot to a final concentration of 1 mM. The second aliquot was used as an noninduced control. Incubation was continued for a further 2 1/2 to 3 h before the cells were harvested by centrifugation, washed, and resuspended in 10 mM Tris-HCl (pH 7.5) containing 1 mM CaCl2 and 1 mM MgCl2.
For kinetic studies on NptA protein expressed in E. coli, essentially the same methods were used. However, the amount of phosphate in the reactions was varied by varying the amount of cold Pi added, whereas the amount of labeled Pi was kept constant. In reactions where the influence of Na+ concentration on Pi uptake was measured, the amount of Pi was kept constant (1 mM) and the amount of Na+ added to the reaction mixtures was varied, with the addition of choline chloride to preserve osmolarity.
To determine the activity of NptA at different pH values, similar assays were done in which the reactions were buffered in the range from pH 6.5 to 9.0.
To assess the dependence of NptA activity on the maintenance of a sodium concentration gradient across the cell membrane, the sodium ionophore monensin was added to the uptake assay mixture described above in the concentration range from 0 to 100 μM.
Reactions in all cases were run for a constant time (4 min). All reactions were done in quadruplicate.
Protein concentrations were determined using the MicroBCA kit (Pierce) and used to normalize isotope uptake measurements in the different samples.
C23O assays.
Assays of catechol-2,3-dioxygenase (C23O) were done essentially by the spectrophotometric assay described by Ingram et al. (17). Cells in which activity was measured were harvested by centrifugation and resuspended in a volume of cold 100 mM sodium-phosphate buffer (pH 7.5) containing 10% (vol/vol) acetone, such that the cells were concentrated at least 10 times with respect to the original culture volume. All subsequent procedures were done at 4°C or on ice. The cells were disrupted by sonication, and the resulting extract was centrifuged at 13,000 × g for 10 min in order to remove cell debris. Extracts were used immediately. Assays were done at room temperature in a standard 3-ml reaction volume containing 100 μl of cell extract and 0.2 mM catechol (Sigma) in 100 mM sodium-phosphate buffer (pH 7.5). Reactions were started after a 1-min equilibration period by the addition of catechol (30 μl of a freshly made 20 mM stock solution). The change in absorbance at 375 nm resulting from the formation of 2-hydroxymuconic semialdehyde was monitored for 3 min. Activities were expressed as milliunits per milligram of protein as described by Sala-Trepat and Evans (30). The protein concentrations in the samples were determined as described above.
Nucleotide sequence accession number.
The sequence of the nptA gene has been deposited in the EMBL database under accession number AJ010968.
RESULTS
Cloning of the nptA gene, encoding the sodium-dependent phosphate pump of V. cholerae.
As a part of the characterization of the lgt-thyA locus of V. cholerae, 600 bp of DNA downstream of the thyA gene was sequenced. A large, incomplete open reading frame reading in the opposite orientation to thyA was detected, which, when compared with sequences in the GenBank database, was found to have significant homology with a class of renal sodium-dependent phosphate transport proteins (NaPi-2) from a variety of vertebrates, including humans. In order to obtain the missing amino-terminal sequence of the protein, a HindIII digest of V. cholerae chromosomal DNA was hybridized against a PstI/HindIII probe derived from the sequence already obtained. A single 1.5-kb band appeared, and this was subsequently isolated and cloned in pBR322.
The cloned fragment was sequenced, and a single open reading frame encoding a protein of 382 amino acids was identified. The TAA stop signal is located 18 bases upstream of the 18-base perfect inverted repeat that we had previously proposed as the transcription terminator for the thyA gene, which is transcribed in the opposite direction (GenBank accession no. AJ006514). Owing to its homology with vertebrate type II sodium-dependent phosphate transport protein (NaPi-2) genes, the gene was named Na+-dependent phosphate transporter A, or nptA. Sequence similarity searches using the BLAST program revealed that the DNA immediately upstream of the nptA gene carries a homologue of the nhaR gene, encoding the Na+/H+ antiporter-affecting protein (NhaA) regulator reading in the opposite direction (41). The position of the gene relative to thyA and nhaR is shown in Fig. 1.
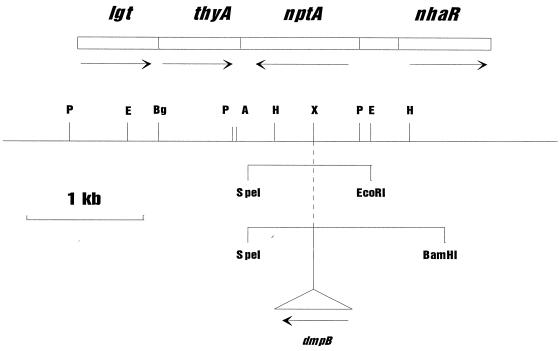
FIG. 1.
Physical map of the nptA locus on the V. cholerae chromosome, showing the relative positions of lgt-thyA and nhaR. Restriction sites used in the cloning and mapping procedures: P, PstI; E, EcoRI; Bg, BglII; A, AatII; H, HindIII; and X, XbaI. The short lines below the main restriction map indicate the DNA amplified from the V. cholerae chromosome for insertion into the expression vector pML-tac1. The EcoRI site at the 5′ end of nptA occurs naturally immediately upstream of the ribosome binding site; the SpeI site at the 3′ end was generated by the PCR to allow directional insertion into the vector. The dmpB reporter gene was inserted into the unique XbaI site within the nptA structural gene in a fragment carrying the entire nptA promoter region and part of nhaR. This was inserted into a p15A-based plasmid for analysis of expression of nptA in V. cholerae (see text).
Occurrence of nptA in Vibrio species.
In order to determine how widespread the occurrence of the nptA gene is within Vibrio species, strains of V. cholerae from different clinical and environmental sources and samples of other different Vibrio species were subjected to PCR amplification with nptA-specific primers (see Materials and Methods). Within V. cholerae, comparison of our deposited sequence (from an O1 classical strain) and that of the entire genome (from an O1 El Tor strain) showed not only that the gene is present but also that it is highly conserved. PCR analysis of toxigenic and nontoxigenic strains of V. cholerae from different serotypes confirmed that the gene was present in all of the strains tested, giving an amplified band of 279 bp. A range of other Vibrio species was tested with the same primers. All except one (identified only as a non-V. cholerae Vibrio species, strain CCUG 537) gave negative results, demonstrating that the gene may not be widely distributed in other Vibrio species (data not shown).
Structural comparison with other type II sodium-phosphate cotransporters.
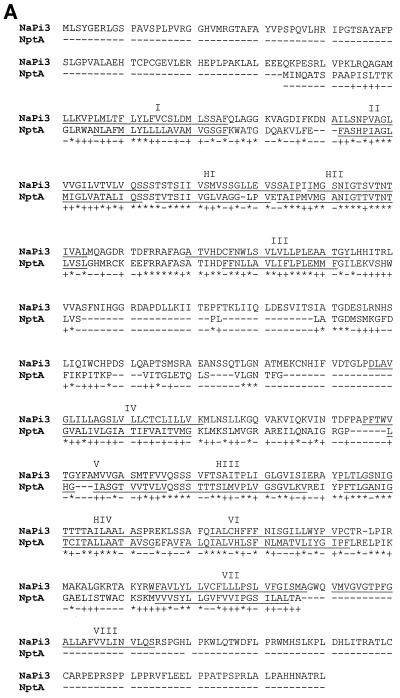
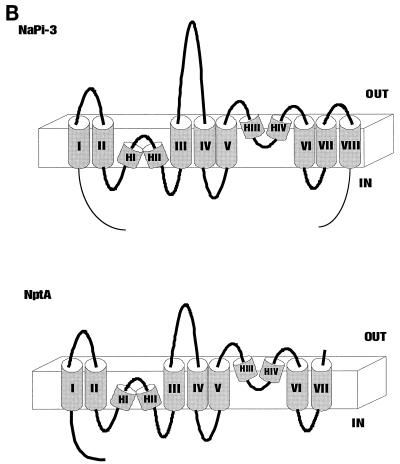
Alignment of the deduced NptA amino acid sequence with known eukaryotic sequences showed a high degree of sequence similarity between NptA and previously identified NaPi-2 cotransporters. An alignment of the deduced NptA amino acid sequence with the NaPi-3 (human) sequence is shown in Fig. 2A. NptA and mammalian proteins have a similar topology with the exception of deletions in the N terminus, the central loop of the protein, and the C terminus. The absence of the last transmembrane region from the NptA sequence suggests that the bacterial C terminus is located extracellularly, as opposed to intracellularly in animal transporters (Fig. 2B).
FIG. 2.
Comparison between the sequence of the human NaPi-3a protein (the human homologue of NaPi-2a; GenBank accession no. AAA36354) and the deduced sequence of the nptA gene product (GenBank accession no. CAA09443). (A) Alignment of deduced amino acid sequences. ∗, identity; +, conservative substitution; -, deletion or nonconservative substitution. Underlined sequences I to VIII represent predicted transmembrane helices in the eight-helix model proposed by Murer et al. (25), and HI to HIV represent additional predicted hydrophobic membrane-associated hinge regions (38). Corresponding predicted transmembrane helix regions are underlined in each sequence. (B) Schematic diagram of proposed NaPi-3a and NptA structures based on the transmembrane helix predictions shown in panel A (based on the model in reference 38). Note the deletion in the large extracellular loop and the absence of transmembrane helix VIII in NptA.
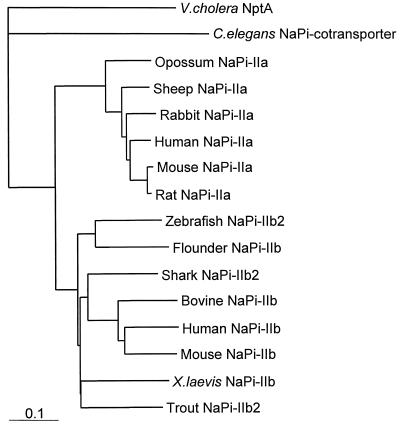
A phylogenetic analysis of representative type II cotransporter sequences is shown as a rectangular cladogram in Fig. 3. It can be seen that NptA occupies a separate branch in the phylogenetic tree, suggesting a significant evolutionary distance between it and other known NaPi-2 sequences, and the divergence of NptA and other type II cotransporters probably predates the divergence of type IIa and type IIb cotransporters.
FIG. 3.
Phylogenetic tree of representative NaPi-II cotransporter sequences. The sequences used were V. cholerae NptA (accession no. CAA09443), Caenorhabditis elegans NaPi cotransporter (AAA81148), human NaPi-IIa and NaPi-IIb (AAA36354 and AAC98695, respectively), mouse NaPi-IIa and NaPi-IIb (AAC42026 and AAC80007, respectively), rat NaPi-IIa (AAC37608), rabbit NaPi-IIa (I46534), sheep NaPi-IIa (CAA04715), opossum NaPi-IIa (AAA30978), bovine NaPi-IIb (CAA57345), Xenopus laevis NaPi-IIb (AAF21134), zebrafish NaPi-IIb2 (AF297180), flounder NaPi-IIb (AAB16821), trout NaPi-IIb2 (AF297186), and shark NaPi-IIb2 (AF297182).
Enhanced sodium-dependent Pi uptake in V. cholerae is induced by phosphate starvation.
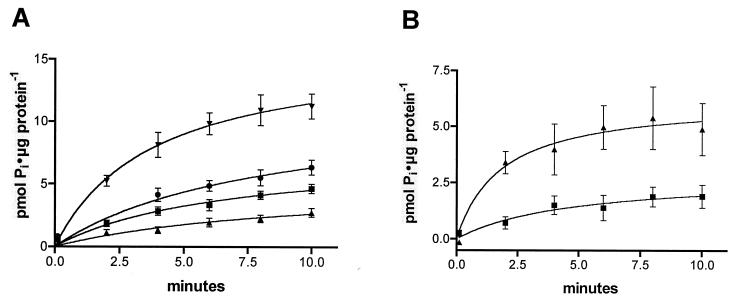
V. cholerae cultures were shown to be able to take up 32P-labeled phosphate significantly more rapidly and to a larger extent after incubation under conditions of phosphate starvation than after incubation in the presence of phosphate. It is thus clear that there are inducible phosphate uptake mechanisms in V. cholerae. Despite increased sodium-independent phosphate uptake, suggesting the presence of more than one inducible system in the cells, uptake was shown to be significantly enhanced by the addition of sodium to cells grown in the absence of phosphate, compared to cells grown with phosphate (Fig. 4).
FIG. 4.
Phosphate uptake in V. cholerae cells. Phosphate uptake was measured in 1 mM Pi with 10 μCi of 32Pi per ml and 100 mM NaCl (or 100 mM choline chloride in the absence of Na+), pH 7.5. Means ± SEMs are shown (n = 4). (A) ▪, Pi uptake in the presence of sodium; ▴, Pi uptake in the absence of sodium; ▾, Pi uptake in the presence of sodium after phosphate starvation; •, Pi uptake in the absence of sodium after phosphate starvation. (B) Sodium-dependent component of the V. cholerae phosphate uptake shown in panel A (Pi uptake in the presence of sodium − Pi uptake in the absence of sodium). ▪, sodium-dependent Pi uptake of V. cholerae cells grown in minimal medium; ▴, sodium-dependent Pi uptake of V. cholerae cells after phosphate starvation. Sodium-dependent Pi uptake is enhanced 2.5 times after Pi starvation.
nptA expression is not induced by Pi limitation.
In order to determine whether any of the observed increase in Pi uptake could be attributed to nptA expression, a low-copy-number plasmid based on the p15A origin of replication was constructed, which carried the nptA gene together with the entire intergenic region between nhaR and nptA, which is assumed to carry the nptA promoter. A promoterless dmpB gene, encoding C23O (4), was inserted into the XbaI site within the nptA gene such that it was under the control of the nptA promoter (Fig. 1). The resulting plasmid (pMT-nptA::C23O) also carried the oriT region of plasmid RP4 and could consequently be transferred from E. coli into V. cholerae strain JS1569 by conjugation using a helper plasmid. The resulting strain was then subjected to growth on minimal medium in the presence and absence of Pi as described in Materials and Methods. The cells were then harvested, disrupted by sonication, and assayed for dmpB expression as indicated by the presence of C23O as described in Materials and Methods. Following growth under these conditions, no C23O activity could be detected regardless of the concentration of Pi in the growth medium. The presence of the plasmid was confirmed by plasmid isolation from cultures used in the assays. This suggests that nptA is not induced in response to alterations in external Pi concentration and that the observed increases in Pi uptake resulting from Pi starvation are not attributable to nptA expression.
Activity of the cloned nptA gene product in E. coli.
In order to confirm the activity of the nptA gene product, chromosomal DNA from V. cholerae was amplified by PCR and cloned between the EcoRI and SpeI sites in the expression vector pMT-tac1. In this plasmid the expression of nptA was placed under the control of the tac promoter and regulated by the lacIq gene carried on the same plasmid. The resulting plasmid was pML-NPTtac. Effective suppression of expression from the tac promoter due to the lacIq repressor was confirmed by insertion of the dmpB gene into the unique XbaI site in the nptA gene. In the presence of IPTG in the medium, high levels of expression of C23O could be detected, whereas in the absence of inducer, expression was undetectable (data not shown).
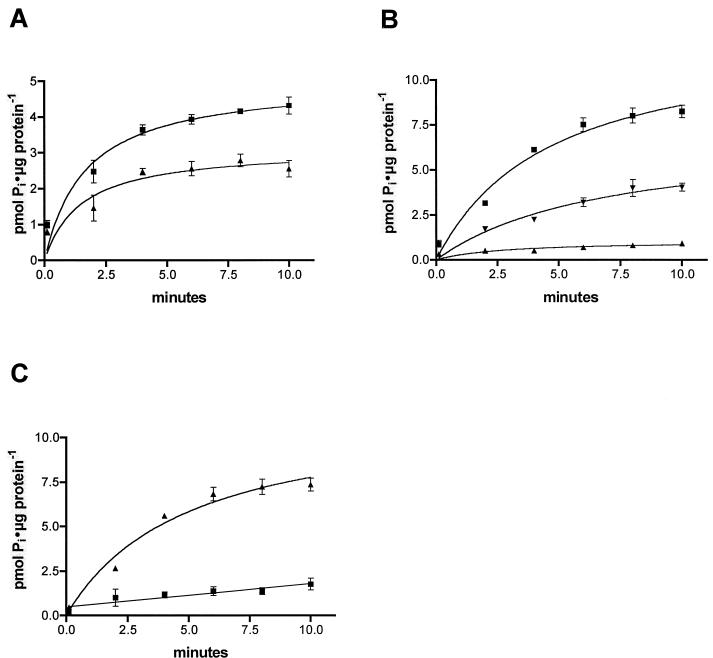
Phosphate uptake experiments were done with E. coli strain HB101(pML-NPTtac) in the presence and absence of IPTG according to the procedure described in Materials and Methods. From the results it is clear that nptA cloned from V. cholerae confers the ability to take up phosphate in the presence of sodium on an E. coli strain carrying the gene on a recombinant plasmid. The specificity of the enzyme for sodium was confirmed by the lack of activity when NaCl was replaced by choline hydrochloride in the assay reactions. Furthermore, the induction of expression of the cloned nptA gene by the addition of IPTG appears to repress the expression of other host-encoded phosphate uptake mechanisms that result in the uptake of phosphate in noninduced cells (Fig. 5A and B). In the uninduced cell the sodium-dependent component of the phosphate uptake was low and linear, suggesting background unspecific uptake or absorption of Pi to the cells (Fig. 5C). After induction, the sodium-dependent component was dramatically increased and showed saturation behavior (Fig. 5C), suggesting an induction of NptA activity and repression of host-encoded sodium-independent Pi uptake systems. These observations suggest that measurements of NptA activity were possible in an environment almost devoid of host-derived background activity.
FIG. 5.
Phosphate uptake in E. coli cells transformed with pML-NPTtac. Phosphate uptake was measured in 1 mM Pi with 10 μCi of 32Pi per ml and 100 mM NaCl (or 100 mM choline chloride in the absence of Na+), pH 7.5. Means ± SEMs are shown (n = 4). (A) Pi uptake without induction of NptA. ▪, Pi uptake in the presence of sodium; ▴, Pi uptake in the absence of sodium. (B) Pi uptake after induction of NptA expression. ▪, Pi uptake in the presence of sodium; ▴, Pi uptake in the absence of sodium; ▾, Pi uptake in the presence of sodium and 10 μM monensin. (C) Sodium-dependent components of the reactions in shown in panels A and B (uptake in the presence of sodium − uptake in the absence of sodium). ▪, sodium-dependent Pi uptake in noninduced cells; ▴, sodium-dependent Pi uptake after induction of NptA expression.
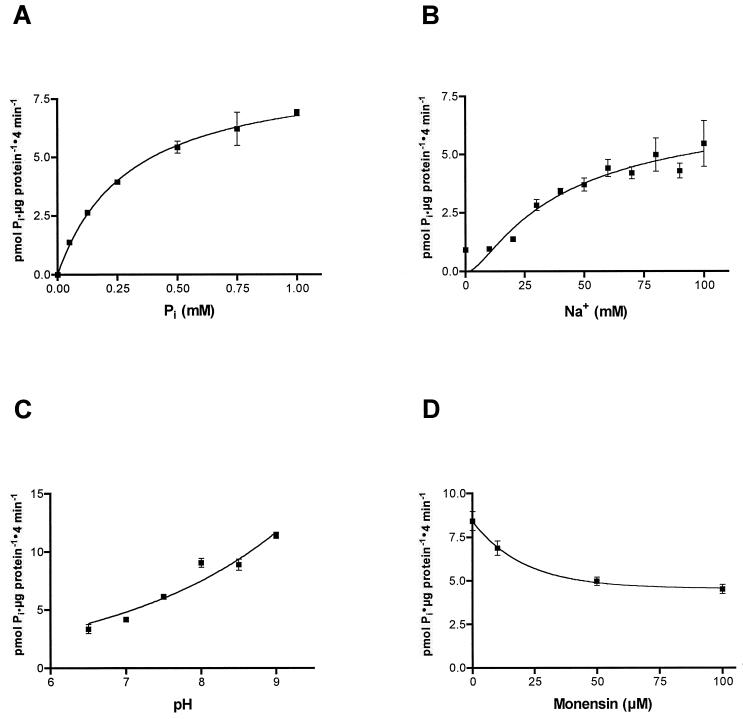
At the 4-min time point selected for kinetic characterization of NptA activity, the sodium-independent component of Pi uptake amounted to 8% or less of the total uptake measured, allowing characterization of NptA with minimal influences from E. coli phosphate uptake systems. The addition of the ionophore monensin to the reaction disrupts the maintenance of the sodium concentration gradient across the cell membrane. The results show that addition of monensin to a final concentration of 10 μM significantly inhibits sodium dependent uptake of phosphate (Fig. 5B). The inhibition was found to be concentration dependent (Fig. 6D), with a Ki of 14.5 ± 2.0 μM (mean ± standard error of the mean [SEM]; n = 4).
FIG. 6.
Effects of extracellular Pi, Na+, pH, and monensin on phosphate uptake in E. coli cells transformed with pML-NPTtac after induction. Phosphate uptake was measured in 1 mM Pi (varied in panel A) with 10 μCi of 32Pi per ml and 100 mM NaCl (in panel B NaCl was replaced with choline chloride to preserve osmolarity) at pH 7.5 (varied in panel C). Means ± SEMs are shown (n = 4). (A) Effect of Pi on sodium-dependent phosphate uptake. (B) Effect of Na+ on sodium-dependent phosphate uptake. (C) Effect of pH on sodium-dependent phosphate uptake. (D) Effect of monensin on sodium-dependent phosphate uptake. As sodium-independent uptake amounted to less than 8% of the measured uptake in noninduced cells at the selected time point (4 min), sodium-dependent uptake was taken as being equal to uptake in the presence of sodium in these experiments.
Biochemical properties of NptA.
Experiments were performed to determine the effect of Pi and Na+ concentrations on 32P-labeled Pi uptake in E. coli carrying the nptA gene under inducing conditions. Cell suspensions were incubated with various concentrations of Pi in the presence of excess NaCl (100 mM) or vice versa (Fig. 6A and B, respectively). It can be seen that the activity increases with increasing concentrations of both Pi and Na+. The Pi dependence showed a hyperbolic behavior, while the Na+ curve was sigmoidal, suggesting multiple Na+ binding. From the data it is possible to calculate the apparent Km with respect to Pi (300 ± 18 μM [mean ± SEM; n = 4]) and with respect to Na+ (74.6 ± 30.8 mM [mean ± SEM; n = 4]). The Vmax with respect to Pi was found to be 8.85 ± 0.2 pmol/min/mg of protein (mean ± SEM; n = 4).
The sensitivity of NptA to pH was investigated by altering the pH of the buffer used in the assay mixture. The results in Fig. 6C show that the enzyme activity decreases with decreasing pH and that at pH 6.5 the activity is less than half of that observed at pH 9.0.
DISCUSSION
The present work describes the cloning and characterization of the nptA gene from V. cholerae, encoding a type II sodium-dependent phosphate transport protein (NaPi-2) previously identified only in eukaryotes. In higher vertebrates these proteins function in the initial uptake of phosphate in the small intestine and reabsorption of inorganic phosphate from excreted fluid in the kidneys.
Expression of the cloned nptA gene in E. coli clearly demonstrates that the gene product is a functional Na+-Pi cotransporter catalyzing the sodium-dependent uptake of Pi. The striking similarity in structure between the eukaryotic and prokaryotic proteins reflects a similarity in function with respect to the uptake of phosphate and sodium ions and the requirement for a sodium gradient for activity.
Overexpression of the cloned nptA gene in E. coli repressed the background Pi uptake observed in noninduced cells, presumably due to the accumulation of high intracellular phosphate concentrations resulting from nptA expression during growth in phosphate-rich medium. This indicated immediately that the NptA protein was able to profoundly affect the Pi balance of cells in which it was expressed. A low background in induced cells, together with improved methodology for the rapid removal of cells from reaction mixtures, allowed the accurate and reproducible measurement of NptA-driven phosphate uptake under different conditions. The results of these experiments indicate that the apparent Km values of the NptA protein with respect to Pi and Na+ (300 μM and 75 mM, respectively) are comparable to but slightly higher than those obtained for different eukaryotic NaPi-2 proteins representing the two types (a and b) expressed in Xenopus oocytes, i.e., 30 to 250 μM for Pi and 35 to 60 mM for Na+ (7, 9, 12, 13, 16, 11, 26).
With respect to the observed pH dependence of the NptA protein, there was a reduction in activity at low pH similar to that seen in NaPi-2a proteins from higher organisms although this characteristic does not correlate with the 478REK sequence thought to be responsible for proton sensitivity in these proteins (10). Overall these results indicate that NptA is a sodium-dependent phosphate cotransporter sharing many characteristics with the animal NaPi-2a cotransporter. However, detailed kinetic and electrophysiological characterization of NptA would require patch-clamp studies in a model system such as Xenopus oocytes (8); such investigations are ongoing.
The V. cholerae amino acid sequence has an overall identity of 33% to the human sequence, with an overall homology of 50% (38 and 58%, respectively, if one excludes the large deletions). Comparison with the overall identity between rat type II and III sodium-phosphate cotransporters, which is only 15% despite the fact that they share a similar function (21), makes this level of similarity all the more striking.
Structural predictions suggest that the NptA protein and mammalian transporters share similar protein architecture, with the exception of the possible absence of the last transmembrane helix and the concomitant change in C-terminal orientation (Fig. 2B). However, computerized predictions of protein topology are putative, and the orientation of the extracellular loops in NptA must be confirmed experimentally. Nonetheless, it is noteworthy that all major deletions in the V. cholerae protein occur either at the C terminus, deleting the last transmembrane helix, or in the N-terminal part of the extracellular loop between membrane-spanning domains III and IV (21). In eukaryotes this loop is heavily glycosylated and probably has regulatory functions that are absent from the V. cholerae protein. The topological model presented in Fig. 2B is based on the model of the mammalian cotransporter as presented by Werner and Kinne (38). The hinge regions HI to HIV have some helical characteristics, and if taken as transmembrane helices, they would produce a quite different topology. In Fig. 2B they are assigned as membrane-associated regions in accordance with the model of the animal cotransporter.
BLAST searches revealed that the similarity between the higher eukaryotic Npt-2a genes and nptA is much greater than similarities between nptA and bacterial genes for which scores were recorded. It can be concluded that the Npt-2a and nptA genes are closely related and have probably not arisen as a result of convergent evolution. Phylogenetic analysis, however, suggests that there is a considerable evolutionary distance between them (Fig. 3), raising the possibility that nptA might be an ancestral gene.
Preliminary results of PCR analysis of strains from a range of other Vibrio species that exist in similar environments suggest that nptA is confined to V. cholerae and may potentially be a diagnostic feature that is independent of other virulence markers. This together with its similarity to eukaryotic Npt-2 genes and the absence of similar genes in other sequenced bacterial chromosomes raises the alternative possibility that V. cholerae has acquired the gene from a higher eukaryotic source. This has recently been noted for other bacteria with either symbiotic or pathogenic associations with plants or animals (20, 40). Arguing against this, however, are the G+C content (47.9% for nptA compared to 47.7% for chromosome I [14]) and the codon usage in the nptA gene, which are consistent with those of the V. cholerae chromosome I as a whole.
Significantly, in V. cholerae the nhaR gene, encoding the NhaA Na+/H+ antiporter-affecting protein regulator, was found to be located immediately upstream of the nptA gene, reading in the opposite direction. It appears that the two genes are linked in some way, with preliminary results suggesting that the nhaR gene may have a role in the regulation of nptA expression (M. Lebens et al., unpublished data). Furthermore, repeated sequence motifs reminiscent of the E. coli phosphate (Pho) box consensus within the intergenic region between nhaR and nptA suggest that despite the absence of an nptA-like gene from other bacteria, phoRB may be involved in controlling expression of one or both of the genes. Indeed, the promoter structures for the phoB and phoR genes (35) (accession number AF043352) and for the pstS homologue retrieved from the V. cholerae genome have sequence similarities with the Pho box structures upstream of nptA (data not shown). The exact mechanisms involved in regulation of nptA expression are currently under investigation but appear to be complex. Their elucidation will help to shed light on the physiological role of nptA in V. cholerae, which remains unclear.
The recent publication of the entire V. cholerae genome (14) reveals that Pit and Pst homologues are present. These both have a much higher affinity for Pi than does NptA, with apparent Kms of between 25 and 38 μM for the constitutive Pit system (42) and 0.2 μM for the inducible Pst system (29, 42). Furthermore, we have demonstrated that in V. cholerae, similar to in other bacteria, the uptake of phosphate is highly up-regulated when cells are grown under conditions of phosphate starvation. In contrast to E. coli, V. cholerae exhibited a weak sodium-dependent Pi uptake after growth in minimal medium. This uptake was enhanced 2.5 times by phosphate starvation. However, reporter gene studies failed to show any induction of nptA transcription under these conditions. This suggests that the enhanced sodium-dependent Pi uptake measured was due not to an activation of the nptA gene but to some other process by which Pit and Pst activities are enhanced by an increase in the potential of the sodium gradient across the cell membrane.
nptA may have a role in the pathogenicity of V. cholerae, as it colonizes the human intestine. V. cholerae is the only Vibrio species that gives rise to epidemic cholera. The changes as it passes from its water environment to the human intestine are profound and include sudden changes in pH and the availability of both energy and phosphate. It is thus possible that NptA functions as a low-affinity, high-capacity cotransporter, possibly meeting the cells' need for large amounts of Pi during rapid growth in nutrient-rich environments. nptA may thus have a role in the cascade of events that lead to the establishment of the infection that distinguishes V. cholerae from other vibrios, which, even when carrying the same virulence factors, do not give rise to such devastating epidemics. Alternatively, responses to levels of phosphate in the environment may influence the ability of strains to become infectious. In the absence of any investigation of phosphate metabolism in V. cholerae and given the obvious differences from other gram-negative organisms highlighted by the present findings, these questions remain to be answered.
Acknowledgments
The work presented in this paper was funded by a grant from the Swedish Medical Council (MFR), project no. K2001-06X-03382, and by SBL Vaccine AB, Stockholm, Sweden.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs, Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames, G. F.-L. 1986. Bacterial periplasmic transport systems: structure, mechanism and evolution. Annu. Rev. Biochem. 55:397-426. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. A., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1998. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 4.Bartilson, M., and V. Shingler. 1989. Nucleotide sequence of the catechol 2,3 dioxygenase encoding gene of phenol-catabolizing Pseudomonas CF600. Gene 85:233-238. [DOI] [PubMed] [Google Scholar]
- 5.Biber, J., M. Custer, S. Magagnin, G. Hayes, A. Werner, M. Lötscher, B. Kaissling, and H. Murer. 1996. Renal Na/Pi-cotransporters. Kidney Int. 49:981-985. [DOI] [PubMed] [Google Scholar]
- 6.Boyer, H. W., and D. Roulland-Dessoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 7.Busch, A. E., C. A. Wagner, A. Schuster, S. Waldegger, J. Biber, H. Murer, and F. Lang. 1995. Properties of electrogenic Pi transport by human renal brush border Na+/Pi transporter. Am. J. Soc. Nephrol. 6:1547-1551. [DOI] [PubMed] [Google Scholar]
- 8.Busch, A., J. Biber, H. Murer, and F. Lang. 1996. Electrophysiological insights of type I and II Na/Pi cotransporters. Kidney Int. 49:986-987. [DOI] [PubMed] [Google Scholar]
- 9.Busch, A., S. Waldegger, T. Herzer, J. Biber, D. Markovic, G. Hayes, H. Murer, and F. Lang. 1994. Electrophysiological analysis of Na+/Pi cotransport mediated by a transporter cloned from rat kidney and expressed in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 91:8205-8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De la Horra, C., N. Hernando, G. Lambert, I. Forster, J. Biber, and H. Murer. 2000. Molecular determinants of pH sensitivity of the type IIa Na/P(i) cotransporter. J. Biol. Chem. 274:6284-6287. [DOI] [PubMed] [Google Scholar]
- 11.Forster, I., C. A. Wagner, A. E. Busch, F. Lang, J. Biber, N. Hernando, H. Murer, and A. Werner. 1997. Electrophysiological characterization of the flounder type II Na+/Pi cotransporter (NaPi-5) expressed in Xenopus laevis oocytes. J. Membr. Biol. 160:9-25. [DOI] [PubMed] [Google Scholar]
- 12.Forster, I., N. Hernando, J. Biber, and H. Murer. 1998. The voltage dependence of of a cloned mammalian renal type II Na/Pi cotransporter. J. Gen. Physiol. 112:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann, C. M., C. A. Wagner, A. E. Busch, D. Markovich, J. Biber, F. Lang, and H. Murer. 1995. Transport characteristics of a murine renal Na/Pi cotransporter. Pflügers. Arch. 430:830-836. [DOI] [PubMed] [Google Scholar]
- 14.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn. R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, H. O. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:469-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins, D., J. Thompson, and T. J. Gibson. 1994. ClustalW: improving the sensitivity of progressive sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acid Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilfiker, H., O. Hattenhauer, M. Traebert, I. Forster, H. Murer, and J. Biber. 1998. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc. Natl. Acad. Sci. USA 95:14564-14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingram, C., M. Brawner P. Youngman, and J. Westpheling. 1989. xylE functions as an efficient reporter gene in Streptomyces spp.: use for the study of galP1, a catabolite-controlled promoter. J. Bacteriol. 171:6617-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarvis, S. M. 2000. Assay of membrane transport in cells and membrane vesicles, p. 1-20. In S. A. Baldwin (ed.), Membrane transport, a practical approach. Oxford University Press, Oxford, United Kingdom.
- 19.Levine, M. M., J. B. Kaper, D. Herrington, J. Ketley, G. Losonsky, C. O. Tacket, B. Tall, and S. Cryz. 1988. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet ii:467-470. [DOI] [PubMed]
- 20.Lopez-Lara, I. M., and O. J. Geiger. 2001. Novel pathway for phosphatidylcholine biosynthesis in bacteria associated with eukaryotes. J. Biotechnol. 91:211-221. [DOI] [PubMed] [Google Scholar]
- 21.Magagnin, S., A. Werner, D. Markovich, V. Sorribas, G. Stange, J. Biber, and H. Murer. 1993. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc. Natl. Acad. Sci. USA 90:5979-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makino, K., H. Shinagawa, M. Amemura, T. Kawamoto, M. Yamada, and A. Nakata. 1989. Signal transduction in the phosphate regulon in Escherichia coli involves phosphotransfer between PhoR and PhoB protein. J. Mol. Biol. 210:551-559. [DOI] [PubMed] [Google Scholar]
- 23.Makino, K., M. Amemura, S.-K. Kim, A. Nakata, and H. Shinagawa. 1994. Mechanism of transcriptional activation of the phosphate regulon in Escherichia coli, p. 5-12. In A. Torriani-Gorinin, E. Yagil, and S. Silver (ed.), Phosphate in microorganisms: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 24.Marck, C. 1988. “DNA Strider,” a “C” program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 16:1829-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murer, H., I. Forster, N. Hernando, G. Lambert, M. Traebert, and J. Biber. 1999. Posttranscriptional regulation of the proximal tubule NaPi-II transporter in response to PTH and dietary P(i). Am. J. Physiol. Renal Physiol. 277:F676-F684. [DOI] [PubMed] [Google Scholar]
- 26.Nalbant, P., C. Böhmer, L. Dehmelt, F. Wehner, and A. Werner. 1999. Functional characterization of a Na/Pi cotransporter (NaPi-II) from zebrafish and identification of related transcripts. J. Physiol. 520:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikata, T., K. Sakai, K. Shibata, A. Junichi, A. Kuroda, and H. Ohtake. 1996. Molecular analysis of the phosphate-specific (pst) operon of Pseudomonas aeruginosa. Mol. Gen. Genet. 250:692-698. [DOI] [PubMed] [Google Scholar]
- 28.Page, R. D. M. 1996. Treeview: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg, H., R. G. Gerdes, and K. Chegwidden. 1977. Two systems for the uptake of phosphate in Escherichia coli. J. Bacteriol. 131:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sala-Trepat, J. M., and W. C. Evans. 1971. The meta cleavage pathway of catechol by Azotobacter species: 4-oxalocrotonate pathway. Eur. J. Biochem. 20:400-413. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Sanchez, J., and J. Holmgren. 1989. Recombinant system for over-expression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc. Natl. Acad. Sci. USA 86:481-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takemaru, K., M. Mizuno, and Y. Kobayashi. 1996. A Bacillus subtilis gene cluster similar to the Escherichia coli phosphate-specific transport (pst) operon: evidence for a tandemly arranged pstB gene. Microbiology 142:692-698. [DOI] [PubMed] [Google Scholar]
- 34.von Heijne, G. 1992. Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487-494. [DOI] [PubMed] [Google Scholar]
- 35.von Kruger, W. M., S. Humphreys, and J. M. Ketley. 1999. A role for the PhoBR regulatory system homologue in the Vibrio cholerae phosphate-limitation response and intestinal colonization. Microbiology 145:2463-2475. [DOI] [PubMed] [Google Scholar]
- 36.Wanner, B. L. 1996. Phosphorus assimilation and control of the phosphate regulon, p. 1357-1381 In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 37.Webb, D. C., and G. B. Cox. 1994. Proposed mechanism for phosphate translocation by the phosphate-specific transport (Pst) system and the role of the Pst system in phosphate regulation, p. 37-42. In A. Torriani-Gorinin, E. Yagil, and S. Silver (ed.), Phosphate in microorganisms: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 38.Werner, A., and R. K. H. Kinne. 2001. Evolution of the Na-Pi cotransport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280:R301-R312. [DOI] [PubMed] [Google Scholar]
- 39.Werner, A., M. L. Moore, N. Mantei, J. Biber, G. Semenza, and H. Murer. 1991. Cloning and expression of a Na/Pi cotransport system of kidney cortex. Proc. Natl. Acad. Sci. USA 88:9608-9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilderman, P. J., A. I. Vasil, Z. Johnson, and M. L. Vasil. 2001. Genetic and biochemical analyses of a eukaryotic-like phospholipase D of Pseudomonas aeruginosa suggest horizontal acquisition and a role for persistence in a chronic pulmonary infection model. Mol. Microbiol. 39:291-303. [DOI] [PubMed] [Google Scholar]
- 41.Williams, S. G., O. Carmel-Harel, and P. A. Manning. 1998. A functional homolog of Escherichia coli NhaR in Vibrio cholerae. J. Bacteriol. 180:762-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willsky, G. R., and M. H. Malamy. 1980. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J. Bacteriol. 144:356-365. [DOI] [PMC free article] [PubMed] [Google Scholar]