Abstract
The effect of increasing trehalose concentrations on the kinetics of the plasma membrane H+-ATPase from Kluyveromyces lactis was studied at different temperatures. At 20°C, increasing concentrations of trehalose (0.2 to 0.8 M) decreased Vmax and increased S0.5 (substrate concentration when initial velocity equals 0.5 Vmax), mainly at high trehalose concentrations (0.6 to 0.8 M). The quotient Vmax/S0.5 decreased from 5.76 μmol of ATP mg of protein−1 min−1 mM−1 in the absence of trehalose to 1.63 μmol of ATP mg of protein−1 min−1 mM−1 in the presence of 0.8 M trehalose. The decrease in Vmax was linearly dependent on solution viscosity (η), suggesting that inhibition was due to hindering of protein domain diffusional motion during catalysis and in accordance with Kramer's theory for reactions in solution. In this regard, two other viscosity-increasing agents, sucrose and glycerol, behaved similarly, exhibiting the same viscosity-enzyme inhibition correlation predicted. In the absence of trehalose, increasing the temperature up to 40°C resulted in an exponential increase in Vmax and a decrease in enzyme cooperativity (n), while S0.5 was not modified. As temperature increased, the effect of trehalose on Vmax decreased to become negligible at 40°C, in good correlation with the temperature-mediated decrease in viscosity. The trehalose-mediated increase in S0.5 was similar at all temperatures tested, and thus, trehalose effects on Vmax/S0.5 were always observed. Trehalose increased the activation energy for ATP hydrolysis. Trehalose-mediated inhibition of enzymes may explain why yeast rapidly hydrolyzes trehalose when exiting heat shock.
When yeast cells are subjected to stress conditions such as dehydration, high salt concentrations, or high temperatures, the trehalose-synthesizing pathway is activated and cytoplasmic trehalose rises to 0.5 M and above (16-18, 38). It has been demonstrated previously that under these stress conditions trehalose stabilizes the structure of membranes and proteins as well as the activity of enzymes (8, 16, 34, 35). As soon as the stress condition is over, trehalose is rapidly hydrolyzed (18), suggesting that the accumulation of this disaccharide may not be compatible with certain cell functions (43).
The properties of trehalose in solution might explain both the mechanism of protection observed when the cell is under stress and the need to rapidly hydrolyze the disaccharide once conditions return to normal (26, 32). It has been reported previously that trehalose promotes the preferential hydration of protein surfaces (13) and favors the most compact state of the protein, inhibiting intramolecular motions that might result in denaturation, i.e., trehalose confers thermal stability to proteins (4, 34) as evidenced by the increase in their transition temperature (24). However, proteins are dynamic molecules that often need to undergo conformational changes while performing specific functions such as enzymatic catalysis or ligand binding (12, 30), and therefore, it has been proposed elsewhere that those enzymes undergoing major conformational changes during their catalytic cycle are inhibited by viscogenic agents such as polyols (9), as protein backbone motions would be hindered in a highly viscous solution (30). In studies of carbon-monoxy-myoglobin, a trehalose-mediated inhibition of carbon monoxide release upon flash photolysis has been reported (15). The mechanism underlying inhibition has been proposed to be the trehalose-mediated hindering of protein conformational changes (6, 15). Additionally, it has been demonstrated elsewhere that trehalose reduces the motional freedom of the myoglobin loop and helix structures by forming a tight hydrogen bond network around the protein (7). A second effect of trehalose, also related to immobilization of protein structures, seems to be the inhibition of the heat shock protein-catalyzed refolding of partially denatured proteins, observed immediately after a period of stress (39). It has been proposed that a high concentration of trehalose would stabilize the partially denatured conformation of proteins, interfering with the refolding catalyzed by the heat shock proteins (39).
E1E2-ATPases undergo major conformational changes during their catalytic cycle, alternating between a nonphosphorylated E1 state and a phosphorylated E2 state (10). The structures of states E1 and E2 are different, as suggested by their sensitivity to proteases and antibodies (10, 29). The Ca2+-ATPase in the sarcoplasmic reticulum, whose three-dimensional structure has been defined elsewhere (41); the plasma membrane Na+-K+-ATPase, which is an antiporter (19); and the H+-ATPases from the plasma membrane of plants and fungi (EC 3.6.1.35) (37) are E1E2-ATPases (10). The H+-ATPase from the plasma membrane of diverse fungi and yeasts is the primary pump needed to generate the proton gradient that energizes all secondary active transport in and out of the cell (33, 37). The physiological significance of H+ transport in yeast is illustrated by the lethality of the deletion of the gene encoding the H+-ATPase (36). Furthermore, during heat shock a high H+-ATPase activity is required to maintain the ionic gradients and active transport activities across the plasma membrane, which undergoes an increase in permeability as temperature rises (5).
The inactivation effects of different stress conditions on the Kluyveromyces lactis H+-ATPase have been tested, and the inactivation kinetics have been reported elsewhere (34, 35). During freeze-drying, the H+-ATPase is denatured unless high concentrations of monosaccharides or disaccharides are present, trehalose being the most effective protective agent (35). In similar studies of the thermoinactivation of the H+-ATPase, it was observed that the temperature range in which full protection by trehalose occurs is that at which cytoplasmic trehalose accumulation enhances yeast survival during heat shock (34).
It was decided to analyze whether trehalose inhibited the isolated K. lactis H+-ATPase at different temperatures. We found that, at 20°C, trehalose inhibited the activity of the H+-ATPase mainly through a decrease in Vmax, which was linearly dependent on solvent viscosity as predicted by Kramer's theory (22). In addition, sucrose and glycerol exhibited the same viscosity-inhibitory activity correlation as trehalose. As temperature increased, the ATPase activity increased exponentially while the trehalose effect on Vmax diminished in proportion with the decrease in trehalose solution viscosity. Enzyme inhibition at low temperatures may explain why, at 30°C and below, yeast cells do not accumulate trehalose and why these cells rapidly hydrolyze the accumulated trehalose as soon as they exit a heat shock period. In contrast, at heat shock temperatures ATP hydrolysis is not inhibited.
MATERIALS AND METHODS
Materials.
Trehalose, ATP-disodium salt, pyruvate kinase type II from rabbit muscle, lactate dehydrogenase type XI from rabbit muscle, sodium azide, NADH, N-tetradecyl-N,N-dimethyl-3-ammonium-1-propanesulfonate (Zwittergent 3,15), and phosphoenolpyruvate mono-(cyclohexylammonium) salt were from Sigma Chemical Co. (St. Louis, Mo.). Zymolyase-20T was from ICN Pharmaceuticals Inc. (Costa Mesa, Calif.). All other reagents were of the best quality available commercially.
Enzyme purification.
Isolation was performed essentially as described before (14). Briefly, the yeast K. lactis strain WM27 was grown in yeast extract-peptone-dextrose medium at 30°C for 20 h. Then, cells were harvested at mid-log phase by centrifugation. Yeast cells were incubated at 30°C in 1 M sorbitol, pH 7.0, containing zymolyase-20T (20 U/g [wet weight]) for 1 to 2 h until spheroplasts were detected by the decrease in turbidity of an aliquot (50 μl) immersed in distilled water (3 ml). Spheroplasts were disrupted by sonication, and the plasma membrane was obtained by differential centrifugation (14). The H+-ATPase was purified from the plasma membrane as described by Bowman and Slayman (3) and modified by Guerra et al. (14). Briefly, the plasma membrane was diluted to 2 mg/ml in buffer A (0.6 M KCl, 75 mM Tris, 6 mM EDTA, pH 7.2)-1 mM EGTA-0.09% (wt/vol) deoxycholate. The mixture was incubated for 10 min at 4°C and then centrifuged at 4°C and 100,000 × g for 1 h. The pellet was resuspended in 10 ml of buffer B (0.3 M KCl, 25 mM Tris [pH 7.5], 45% [vol/vol] glycerol, and 2 mM EDTA), and then the protein concentration was measured and adjusted to 5 mg/ml in the same buffer B. Azolectin was added to a final concentration of 5 mg/ml, and Zwittergent 3,14 was slowly added to a final Zwittergent/protein ratio of 0.85 (wt/wt). The suspension was homogenized and centrifuged at 4°C and 100,000 × g for 1 h. The supernatant was subjected to centrifugation at 100,000 × g for 14 h on a discontinuous glycerol gradient (65, 60, 55, and 50% [vol/vol] glycerol in 10 mM Tris [pH 7.0], 1 mM EDTA, 0.1% cholate, 1 mg of azolectin/ml). A Beckman ultracentrifuge and 60 Ti fixed-angle rotor were used. The lower half of the supernatant and the pellet were recovered, diluted twofold, and centrifuged at 100,000 × g for 2 h. The ATPase was suspended from the pellet in a small volume of 1 mM EGTA-Tris, pH 7.0, and kept at −70°C until used. On sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the 100,000-Mr band corresponding to the plasma membrane H+-ATPase was about 50% of the total protein yield. The H+-ATPase specific activity in this preparation, measured at 30°C, was 18.9 ± 0.3 μmol of ATP mg of protein−1 min−1. Protein concentration was determined by the method of Lowry et al. (25) with bovine serum albumin as standard.
Measurements of H+-ATPase activity.
The ATP saturation kinetics of the plasma membrane H+-ATPase were evaluated as described elsewhere for the Ca2+-ATPase from sarcoplasmic reticulum (41), by using the enzyme-coupled assay described by Anderson and Murphy (2). The assay medium (2 ml) was 10 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid); pH 7.0], 80 mM KCl, 5 mM sodium azide, 5 mM phosphoenolpyruvate, 200 μM NADH, 12.5 IU of pyruvate kinase, 10.45 IU of lactic dehydrogenase, and 5 mM MgCl2. Increasing concentrations of ATP (0.25 to 5.0 mM) and trehalose (0 to 0.8 M) were added as indicated. The assay solution was incubated at the indicated temperature for 10 min, and the ATPase was added (4.3 μg of protein in 4 μl) to start the reaction. The absorbance decay at 340 nm was recorded continuously in an Aminco DW2000 double-wavelength spectrophotometer in split mode, equipped with a cell with a thermostat. Initial velocities of ATP hydrolyzed (expressed as micromoles of ATP milligram of protein−1 minute−1) were calculated from the slope of the linear portion of each trace, with an NADH extinction coefficient of 6,200 M cm−1. The experiment was performed at temperatures between 20 and 40°C as indicated. Additionally, it was determined that the coupled enzyme assay was not affected by temperature or by trehalose and, thus, the observed variations in rate resulted specifically from the enzyme activity response to the concentration of ATP.
Viscosity measurements.
A falling-ball-type viscometer (Gilmont Instruments, Barrington, Ill.) mounted in a constant-temperature chamber was employed. Trehalose solutions (0 to 0.8 M) were prepared in 10 mM PIPES, pH 7.0. The viscometer was filled with each trehalose solution, degassed by vacuum application, and allowed to equilibrate at each temperature for 10 min. Once the assay temperature was reached, the time of ball descent was measured and used to calculate the viscosity (26) by using equation 1:
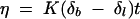 |
(1) |
where η is the viscosity in centipoise, K is the viscometer constant, δb is the density of the ball, δl is the density of the liquid, and t is the time of ball descent. Experimental data were reproducible, and standard deviations were less than 5%.
Data analysis.
Initial velocities of ATP hydrolysis were plotted against the concentration of ATP. The iterative program Microcal Origin 6.0 (Microcal Software Inc., Northampton, Mass.) was used to analyze the data by nonlinear regression. The Hill equation (equation 2) was used in the fitting of the initial velocity data:
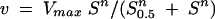 |
(2) |
where v is the initial velocity, Vmax is the maximum velocity, S is the concentration of the varied substrate, S0.5 is the substrate concentration when v = 0.5 Vmax, and n is the Hill number. From the kinetic parameters obtained with equation 2, the quotient Vmax/S0.5 was calculated. This is an estimate of the efficiency of the enzyme.
The energy of activation (Ea) for the hydrolysis of ATP was calculated by using the logarithmic form of the Arrhenius equation (equation 3) by plotting log Vmax versus 1/T and calculating the Ea from the slope value:
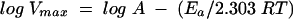 |
(3) |
where R is the gas constant (8.314 J K−1 mol−1), T is the absolute temperature, and A is a constant.
The dependence of solution viscosity on solute concentration expressed as mass fraction c (0 < c < 1) (23) was analyzed by using the treatment described by Mooney (28). Thus, the equation was as follows:
 |
(4) |
where η is the macroscopic viscosity of the solvent, A is characteristic of the solute hydrodynamic interactions, and B is proportional to a self-crowding factor.
Kramer's theory (22) was used to analyze the possible mechanism of inhibition. This theory describes the effect of solvent properties on the behavior of protein reactions involving domain diffusional motions. In a reaction evolving from the substrate S, through the activated state X, to the product P (S→X→P), the rate at which the activated state is reached and the product is obtained is inhibited by the friction of the protein with the solvent. In turn, friction is a function of viscosity η. Thus, the reaction rate constant depends linearly on η (equation 5) as described by Jacob and Schmid (21):
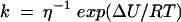 |
(5) |
where k is the rate constant for the reaction (Vmax for enzyme-catalyzed reactions), ΔU is the height of the potential energy barrier, R is the gas constant, and T is the absolute temperature. To assess whether the reaction performed by the H+-ATPase follows Kramer's relation, the relative Vmax data, defined as Vmax0/Vmax, were plotted against the relative viscosity, defined as η/η0, where Vmax0 and η0 are the Vmax and the viscosity in the absence of trehalose, respectively, and Vmax and η are the observed values at each trehalose concentration assayed (20).
RESULTS
ATP saturation kinetics of the K. lactis plasma membrane H+-ATPase: effect of trehalose.
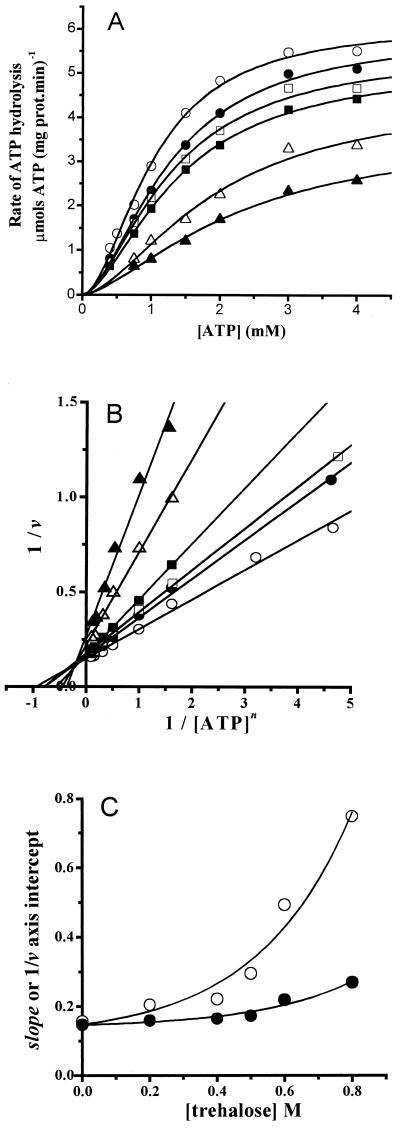
Initial velocity rates were obtained at 20°C and in the presence of an ATP concentration ranging from 0.4 to 4 mM. Experiments were performed in the absence and in the presence of different trehalose concentrations. Data were fitted to the Hill equation by nonlinear regression (Fig. 1A ). Under our experimental conditions, the rate of ATP hydrolysis displayed a sigmoid dependence upon ATP concentration (Fig. 1A). The estimated kinetic parameters for the enzyme are listed in Table 1. In the absence of trehalose, these parameters were similar to those reported elsewhere for the H+-ATPases from Neurospora crassa and Saccharomyces cerevisiae (29, 33), as expected from the high degree of identity observed in the gene (PMA1) coding for the plasma membrane H+-ATPase in these organisms (27, 31). Trehalose inhibited the H+-ATPase, mainly through a decrease in Vmax (Table 1). S0.5 values increased slowly at the lower trehalose concentrations tested (0.2 to 0.4 M). However, at the higher trehalose concentrations tested (above 0.5 M) S0.5 values varied more steeply (Table 1). The decrease in Vmax plus the increase in S0.5 resulted in a decrease in Vmax/S0.5 compared to that of the control: 2.4- and 3.6-fold lower at 0.6 and 0.8 M trehalose, respectively (Table 1). In contrast, the estimated Hill number values (n) were not modified, indicating that the mechanism of cooperativity was not sensitive to trehalose (Table 1).
FIG. 1.
Trehalose-mediated inhibition of the plasma membrane H+-ATPase. Initial rates of ATP hydrolysis were measured at 20°C as described in Materials and Methods. The reaction mixture contained 10 mM PIPES (pH 7.0), 80 mM KCl, 5 mM sodium azide, 5 mM phosphoenolpyruvate, 200 μM NADH, 12.5 IU of pyruvate kinase, 10.45 IU of lactic dehydrogenase, 5 mM MgCl2, and the indicatedconcentrations of ATP; the final volume was 2 ml. The H+-ATPase (4.3 μg of protein in 4 μl) was added to start the reaction. (A) Plot of ATP hydrolysis versus ATP concentration at the following molar concentrations of trehalose: 0 (○), 0.2 (•), 0.4 (□), 0.5 (▪), 0.6 (▵), and 0.8 (▴). The points are the means of three independent experiments. In all cases, the standard deviation was less than 5%. The lines are the result of the best fit to the Hill equation (equation 2) by nonlinear regression. (B) Lineweaver-Burk plot of the data in panel A; the lines were obtained by linear regression of the data. (C) Replots of the slope (○) and the 1/v axis intercept (•) in panel B. The lines are the best fit to the equation y = a + b·exp(c·x), where a is the ordinate, b and c are constants, and x is the trehalose concentration.
TABLE 1.
Kinetic parameters of the H+ -ATPase activity assayed at different temperatures and in the presence of different concentrations of trehalosea
| Temp (°C) | [Trehalose] (M) | Vmax (μmol of ATP mg of protein−1 min−1) | S0.5 (mM) | n | Vmax/S0.5 (μmol of ATP mg of protein−1 min−1/mM−1) |
|---|---|---|---|---|---|
| 20 | 0.0 | 6.62 | 1.15 | 1.68 | 5.76 |
| 0.2 | 5.80 | 1.21 | 1.67 | 4.79 | |
| 0.4 | 5.59 | 1.29 | 1.70 | 4.33 | |
| 0.5 | 5.22 | 1.38 | 1.66 | 3.78 | |
| 0.6 | 4.49 | 1.87 | 1.66 | 2.40 | |
| 0.8 | 3.73 | 2.29 | 1.52 | 1.63 | |
| 25 | 0.0 | 10.47 | 1.01 | 1.49 | 10.37 |
| 0.2 | 9.14 | 1.10 | 1.44 | 9.51 | |
| 0.4 | 8.93 | 1.16 | 1.50 | 8.22 | |
| 0.5 | 7.87 | 1.37 | 1.50 | 6.49 | |
| 0.6 | 7.43 | 1.57 | 1.49 | 4.73 | |
| 0.8 | 6.33 | 1.95 | 1.44 | 3.25 | |
| 30 | 0.0 | 18.58 | 1.08 | 1.46 | 17.20 |
| 0.2 | 17.49 | 1.16 | 1.46 | 15.08 | |
| 0.4 | 15.63 | 1.18 | 1.48 | 13.25 | |
| 0.5 | 14.38 | 1.27 | 1.47 | 11.32 | |
| 0.6 | 13.22 | 1.78 | 1.40 | 7.43 | |
| 0.8 | 11.49 | 2.23 | 1.47 | 5.15 | |
| 35 | 0.0 | 28.41 | 1.02 | 1.42 | 27.85 |
| 0.2 | 27.09 | 1.12 | 1.49 | 24.19 | |
| 0.4 | 26.88 | 1.16 | 1.43 | 23.17 | |
| 0.5 | 24.49 | 1.27 | 1.40 | 19.28 | |
| 0.6 | 22.81 | 1.75 | 1.45 | 13.03 | |
| 0.8 | 20.97 | 2.34 | 1.39 | 8.96 | |
| 40 | 0.0 | 40.13 | 1.01 | 1.20 | 39.73 |
| 0.2 | 40.27 | 1.25 | 1.20 | 32.22 | |
| 0.4 | 39.85 | 1.30 | 1.19 | 30.65 | |
| 0.5 | 39.30 | 1.67 | 1.18 | 23.53 | |
| 0.6 | 40.04 | 2.64 | 1.18 | 15.17 | |
| 0.8 | 38.08 | 3.63 | 1.20 | 10.49 |
The data in Fig. 1A are depicted in Fig. 1B as a plot of 1/v against 1/[ATP]n (Fig. 1B), where n is the Hill number value estimated as described above, by nonlinear regression of the data obtained at 20°C and at each trehalose concentration. The lines intersect at the left of the 1/v axis, indicating the mixed nature of the trehalose-mediated inhibition of the enzyme. In Fig. 1C, the slope (appS0.5n/appVmax) and 1/v intercept (1/appVmax) values were replotted against the concentrations of trehalose. Remarkably, the lines observed in Fig. 1C were nonlinear and, instead, bent upward as trehalose concentration increased; this might have been expected, since trehalose does not seem to bind to a specific site in the enzyme and, therefore, its effects cannot be analyzed as if this disaccharide were a classical enzyme-binding inhibitor. The possible mechanisms underlying the nonlinearity of the trehalose-mediated inhibition are discussed below.
Temperature dependence of the trehalose effect on the H+-ATPase.
Trehalose accumulation in the yeast cytoplasm during heat shock (37 to 40°C) results in enhanced survival, apparently through the protection of protein structure against denaturation (18). On the other hand, enzyme catalysis is sensitive to temperature (42), and so it was decided to determine the effect of temperature on the kinetics of the H+-ATPase in the absence and presence of trehalose (Fig. 2). Increasing the temperature of assay resulted in an exponential increase in Vmax (Fig. 2 and Table 1) and in a decrease in enzyme cooperativity, while the S0.5 was only slightly modified, suggesting that the affinity of the enzyme for ATP is not sensitive to temperature (Table 1). As a result of the increase in Vmax and the lack of modifications in S0.5, the catalytic efficiency increased with temperature (Table 1). In regard to the trehalose effect on enzyme activity, it was observed that, as the reaction temperature increased, the effect of trehalose on Vmax gradually decreased, becoming negligible at 40°C (Table 1). In contrast, the trehalose-induced increase in S0.5 was constant at all temperatures tested. Thus, the estimated catalytic efficiency Vmax/S0.5 exhibited a trehalose-dependent decrease (Table 1). The combined effects of temperature at 40°C (heat shock temperature) and trehalose (0.6 and 0.8 M) resulted in a calculated Vmax/S0.5 which was similar to that estimated in the absence of the disaccharide at 25°C and only slightly lower than that observed at 30°C (Table 1). Trehalose did not affect the cooperative behavior of the enzyme at any of the temperatures tested (Table 1). Therefore, at high temperatures, the H+-ATPase is only marginally inhibited by trehalose and its activity is comparable to that obtained when the enzyme is assayed in the absence of trehalose at the optimal temperature for yeast growth of 30°C (17).
FIG. 2.
Temperature effects on the kinetics of the plasma membrane H+-ATPase. Experimental conditions were as described for Fig. 1. The assay temperatures were 20°C (○), 25°C (•), 30°C (□), 35°C (▪), and 40°C (▵). The points are the means of three independent experiments. The standard deviations were less than 5% of the values. The lines are the result of the best fit to the Hill equation by nonlinear regression (equation 2).
Effect of trehalose on the energy of activation for ATP hydrolysis.
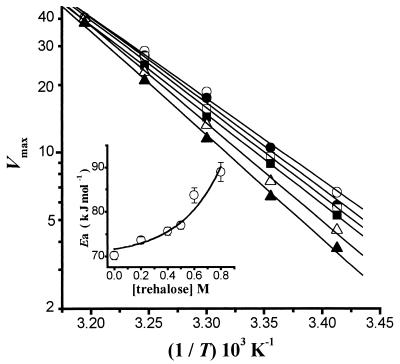
The observed trehalose-induced decrease in Vmax suggested that trehalose increases the energy of activation (Ea) for the plasma membrane H+-ATPase-catalyzed reaction. To further analyze this, Ea was determined at different trehalose concentrations by means of the Arrhenius equation (Fig. 3). In the absence of trehalose, Ea was equal to 70.2 ± 0.9 kJ mol−1. This value was similar to that reported elsewhere for the S. cerevisiae enzyme (33), suggesting that the two enzymes have similar mechanisms of catalysis. The presence of increasing concentrations of trehalose resulted in an increase in the energy barrier for ATP hydrolysis, as indicated by the rise in Ea as a function of trehalose concentration (Fig. 3, inset), e.g., in the presence of 0.8 M trehalose, Ea increased to 89.0 ± 2.2 kJ mol−1. Such an increase seemed to be exponential (Fig. 3, inset), probably as a result of the exponential increase in solvent viscosity induced by trehalose (see below).
FIG. 3.
Arrhenius plot for the plasma membrane H+-ATPase in the presence of different concentrations of trehalose. The points are the calculated Vmax at each trehalose concentration and temperature. Trehalose molar concentrations were 0 (○), 0.2 (•), 0.4 (□), 0.5 (▪), 0.6 (▵), and 0.8 (▴). The lines were obtained by linear regression. (Inset) Plot of the energy of activation (Ea) versus trehalose concentration. Ea values were calculated from the line slopes as described by the Arrhenius equation (equation 3), and the line was the result of the fitting as described for Fig. 1C.
Effect of trehalose and temperature on viscosity.
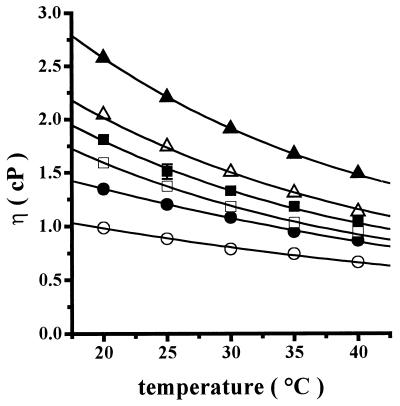
Kramer's theory for reactions in solution, which is applicable to protein folding and enzyme kinetics, states that solution viscosity has a negative effect on the substrate probability of reaching the activated state and proceeding toward the product (21, 22), i.e., increasing solvent viscosity should result in lower protein conformational motility and lower reaction rates. In this regard, it has been reported elsewhere that solvent viscosity is affected by changes in carbohydrate concentration and temperature (26, 32). Thus, we began by testing the effect of increasing trehalose concentrations on viscosity at different temperatures (Fig. 4). At each temperature tested, increasing trehalose concentrations increased exponentially the viscosity of the solution (η) (Fig. 4), e.g., at 20°C η increased from 0.98 ± 0.03 cP in the absence of trehalose to 2.58 ± 0.10 cP in the presence of 0.8 M trehalose (Fig. 4). In addition, increasing temperatures from 20 to 40°C resulted in a decrease in the trehalose-promoted viscosity, e.g., at 0.5 M trehalose, η decreased from 1.81 ± 0.04 cP at 20°C to 1.04 ± 0.03 cP at 40°C (a viscosity value similar to that of water at 25°C) (Fig. 4).
FIG. 4.
Effects of temperature on the viscosity of trehalose solutions. Each trehalose solution was prepared, poured into the viscometer, and degassed. Then the viscometer was immersed in a water jacket connected to a circulating water bath (Polyscience 9000) at the indicated temperature. Temperature in the viscometer was allowed to equilibrate 10 min before the falling times were read. The reaction mixture contained 10 mM PIPES, pH 7.0, and the following molar concentrations of trehalose: 0 (○), 0.2 (•), 0.4 (□), 0.5 (▪), 0.6 (▵), and 0.8 (▴).
Correlation between viscosity and H+-ATPase inhibition.
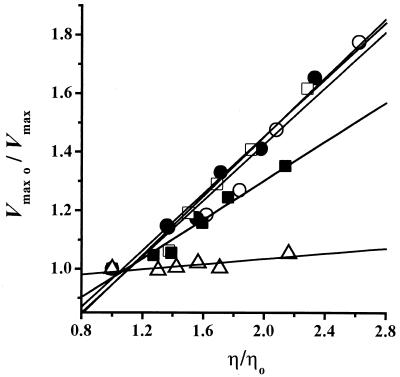
In order to address the possible mechanism underlying the nonlinear inhibition of the H+-ATPase by increasing trehalose concentrations (Fig. 1C), as well as the lack of Vmax inhibition by increasing temperature (Table 1), the data were analyzed as described by Jacob and Schmid (21) by using Kramer's theory (21, 22). If a reaction obeys Kramer's theory, a linear relationship should be observed when the rate constant of the reaction is plotted against solution viscosity (20, 21). Thus, the relative Vmax values (Vmax0/Vmax) were plotted against the relative viscosity values (η/η0) at each temperature tested (Fig. 5), and a linear dependence was observed as predicted (21, 22). Thus, the trehalose-mediated inhibition of the H+-ATPase is most likely caused by the increase in the viscosity of the solution. In addition, although trehalose still was able to increase solvent viscosity at 40°C (Fig. 4), it seems that, at this temperature, the enzyme gains sufficient energy to overcome the frictional effect and, as a result, structural movements involved in catalysis are not inhibited.
FIG. 5.
Effects of medium viscosity on the Vmax displayed by the H+-ATPase. Experiments were performed at different temperatures. The relative Vmax (Vmax0/Vmax) was plotted against the relative viscosity (η/η0). η0 is the viscosity of the solution in the absence of trehalose, and Vmax0 is the Vmax in the absence of trehalose. Temperatures were 20°C (○), 25°C (•), 30°C (□), 35°C (▪), and 40°C (▵). The lines are the result of linear regressions of the data.
H+-ATPase inhibition by other viscosity-generating solutes.
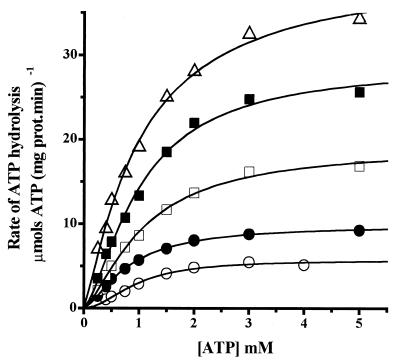
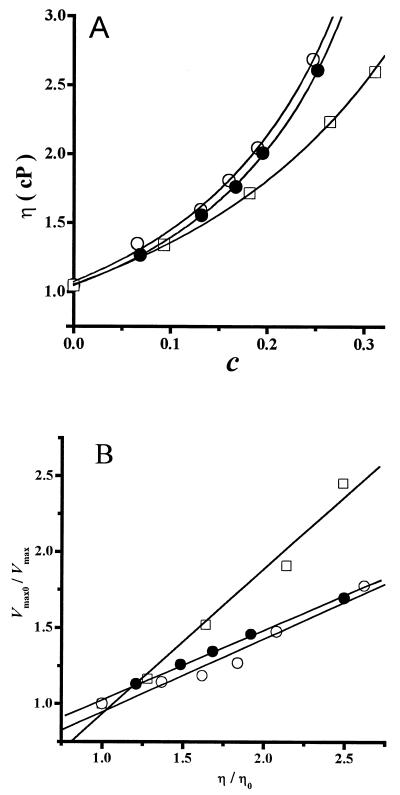
In addition to trehalose, sucrose and glycerol were used to test the generality of the correlation between increased viscosity and inhibition of the H+-ATPase. Both of these compounds are widely used to preserve molecule function and/or structure during handling or storage. At 20°C, sucrose displayed the same inhibition pattern as trehalose (data not shown). In contrast, higher glycerol concentrations were needed to inhibit the H+-ATPase (1 to 3.5 M), although the enzyme was inhibited to a higher extent than in the presence of trehalose (Fig. 6). In solutions containing increasing concentrations of sucrose or glycerol, it was observed that viscosities were similar to those obtained for trehalose when they were referred to solute mass fraction as described by the Mooney equation (equation 4) (Fig. 7A). Both sucrose and glycerol inhibited the H+-ATPase through a decrease in Vmax. Furthermore, plotting the relative Vmax0/Vmax against the relative viscosity (η/η0) resulted in the linear relation (Fig. 7B) predicted by Kramer's equation (equation 5). For comparison, the data for trehalose were also included in Fig. 7B, and it may be observed that sucrose and trehalose displayed the same relation between Vmax and η, while glycerol produced higher inhibition of Vmax as η increased (Fig. 7B). The pattern of enzyme inhibition correlated with the solution viscosity increase as predicted by Kramer's theory (21, 22) and indicating that enzyme domain immobilization was probably involved in inhibition.
FIG. 6.
Glycerol-mediated inhibition of the plasma membrane H+-ATPase. Experimental conditions were as described for Fig. 1. Glycerol molar concentrations were 0 (○), 1 (•), 2 (□), 3 (▪), and 3.5 (▵). The lines are the result of fitting the data to the Hill equation (equation 2) by nonlinear regression.
FIG. 7.
Correlation between viscosity and H+-ATPase inhibition by trehalose, sucrose, or glycerol. Viscosity measurements were performed as described for Fig. 4 at 20°C. Enzyme activity was measured as described for Fig. 1. Solutes were trehalose (○), sucrose (•), or glycerol (□). (A) Plot of viscosity (η) versus mass fraction (c). The solid lines were the result of fitting the data to the Mooney equation (equation 4) by nonlinear regression. (B) Plot of the relative Vmax (Vmax0/Vmax) against the relative viscosity (η/η0) in the presence of different viscosity-generating agents. The lines are linear regressions of the data.
DISCUSSION
The H+-ATPase from K. lactis, like other P-ATPases, possesses three cytoplasmic domains that undergo major conformational changes while alternating between two widely different states, E1 and E2 (29), as the enzyme alternatively binds substrates, hydrolyzes ATP, and pumps protons (29, 33, 41). Trehalose inhibited the H+-ATPase through a decrease in Vmax and in Vmax/S0.5 (Table 1). Since trehalose does not bind to the enzyme, the observed upward, nonlinear inhibition cannot be accounted for by the same mechanisms involved in parabolic inhibition caused by an enzyme-binding inhibitor (Fig. 1C).
Kramer's theory predicts that solvent viscosity inhibits conformational changes in proteins and that this results in inhibition of enzymatic catalysis (22). Indeed, the trehalose-, sucrose-, or glycerol-mediated inhibition of Vmax increased linearly with the viscosity of the solution (Fig. 5 and 7B). When the effect of trehalose was tested at increasing temperatures, inhibition of Vmax decreased, and this was related to the decrease in viscosity. In addition, as temperature provided the system with higher energy, frictional restriction was overcome and as a result protein conformational changes involved in catalysis were facilitated. Furthermore, our data suggest that inhibition resulted from an increase in the activation energy for ATP hydrolysis, i.e., the energy barrier separating the two conformational states (E1 and E2) increased (Fig. 3) (33). In studies of the H+-ATPase from Schizosaccharomyces pombe, Felix et al. (11) concluded that, at high temperatures, trehalose protects the structure of the enzyme by stabilizing state E2. Thus, it would be safe to assume that, in the H+-ATPase from S. pombe, the transition from a trehalose-stabilized E2 state to the E1 conformation would require higher energy. In addition, it was recently reported elsewhere that, in the cold shock protein (CspB) folding reaction, the diffusion of protein chain segments relative to the solvent is required to cross the activated state, and this folding reaction displays an inverse, linear dependence on solvent viscosity (20), again in agreement with Kramer's theory (22). Our data confirm the proposal that, in enzymes where a major conformational change is involved in catalysis, this change becomes the rate-limiting step in highly viscous solutions (30). In contrast, as temperature increases, viscosity decreases and conformation interconversion is facilitated; in this case, the chemical reaction may become the rate-limiting step (30).
The large increase in S0.5 mainly observed at high trehalose concentrations (0.6 and 0.8 M) may be due to inhibition of the diffusion of the substrate (ATP) across the trehalose mesh surrounding the protein (32). In agreement with our results, it has been reported elsewhere that high concentrations of sorbitol and dextran inhibit the diffusion of ATP away from yeast mitochondria (1).
The estimated catalytic efficiency (Vmax/S0.5) of the H+-ATPase was similar at 40°C in the presence of 0.8 M trehalose and at 25°C in the absence of trehalose (Table 1). Thus, at the high temperatures where trehalose protects the H+-ATPase from denaturation (34), enzyme activity is near normal values, i.e., at heat shock temperatures trehalose would be a compatible solute (40). In contrast, trehalose-mediated inhibition of the H+-ATPase becomes important at temperatures where no enzyme protection is needed, i.e., 20 to 30°C. In this regard, it has been reported elsewhere that, in the cytoplasm of yeast, viscosity is twice as high as water (44). Such viscosity does allow for the rotational motion of some enzymes such as hexokinase and phosphoglycerate kinase, while motional restriction of others, such as pyruvate kinase, is observed (44). Thus, viscosity should be taken into consideration when the application of enzyme kinetics studies to the in situ situation is attempted.
It may be hypothesized that, in vivo, the synthesis and accumulation of trehalose have to be finely regulated depending on environmental conditions: (i) during heat shock, yeast cells accumulate trehalose in order to protect enzymes from denaturation while allowing for the high rates of catalysis needed to survive stress (5), and (ii) as soon as the heat shock condition is over, yeast cells hydrolyze trehalose, thus allowing viscosity-sensitive enzymes to display full activity (this report) and facilitating destabilization and refolding of those proteins that were partially denatured during the stress period (39).
The plasma membrane H+-ATPase from K. lactis exhibited sigmoidal kinetics, with a mean Hill number of 1.68 at 20°C, i.e., this enzyme should be added to the growing number of E1E2-ATPases where cooperativity has been detected (10, 29). The cooperativity of diverse H+-ATPases has led to the proposal that the functional enzyme is probably not a monomer, but a dimer or even a hexamer (29).
The trehalose concentration at which H+-ATPase inhibition was observed was similar to that reported elsewhere to protect the enzyme against high temperatures (34) or against lyophilization (35). Thus, it is suggested that both the protection and the inhibition are due to the same trehalose-protein interaction mechanism. This mechanism would account for both the advantage of accumulating cytoplasmic trehalose during stress and the need to hydrolyze this disaccharide immediately after the stress period is over (18, 43). Understanding the mechanism by which trehalose protects macromolecules against heat and desiccation may lead to the design of conditions under which trehalose may be applied to enzyme-dependent industrial processes.
Acknowledgments
This work was partially funded by grants from CONACYT, 27568-N, and from DGAPA-UNAM IN207600. J.G.S. is a CONACYT Ph.D. fellow.
Rodrigo A. Díaz-Ruiz performed the viscosity measurements. Ramón Méndez and Norma Silvia Sánchez are acknowledged for their technical assistance.
REFERENCES
- 1.Aflalo, C. 1997. Localized firefly luciferase probes ATP at the surface of mitochondria. J. Bioenerg. Biomembr. 29:549-559. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. W., and A. J. Murphy. 1983. Alterations in the structure of the ribose moiety of ATP reduce its effectiveness as a substrate for the sarcoplasmic reticulum ATPase. J. Biol. Chem. 258:14276-14278. [PubMed] [Google Scholar]
- 3.Bowman, B. J., and C. W. Slayman. 1979. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J. Biol. Chem. 254:2928-2934. [PubMed] [Google Scholar]
- 4.Butler, S. L., and J. J. Falke. 1996. Effects of protein stabilizing agents on thermal backbone motions: a disulfide trapping study. Biochemistry 35:10595-10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coote, P. J., M. V. Jones, I. J. Seymour, D. L. Rowe, D. P. Ferdinando, A. J. McArthur, and M. B. Cole. 1994. Activity of the plasma membrane H+-ATPase is a key physiological determinant of thermotolerance in Saccharomyces cerevisiae. Microbiology 140:1881-1890. [DOI] [PubMed] [Google Scholar]
- 6.Cordone, L., P. Galajda, E. Vitrano, A. Gassmann, A. Ostermann, and F. Parak. 1998. A reduction of protein specific motions in co-ligated myoglobin embedded in a trehalose glass. Eur. Biophys. J. 27:173-176. [DOI] [PubMed] [Google Scholar]
- 7.Cottone, G., L. Cordone, and G. Ciccotti. 2001. Molecular dynamics simulation of carboxy-myoglobin embedded in a trehalose-water matrix. Biophys. J. 80:931-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowe, J. H., L. M. Crowe, and R. Mouradian. 1983. Stabilization of biological membranes at low water activities. Cryobiology 20:346-356. [DOI] [PubMed] [Google Scholar]
- 9.Demchenko, A. P., O. I. Ruskyn, and E. A. Saburova. 1989. Kinetics of the lactate dehydrogenase reaction in high-viscosity media. Biochim. Biophys. Acta 998:196-203. [DOI] [PubMed] [Google Scholar]
- 10.Dunbar, L. A., and M. J. Caplan. 2000. The cell biology of ion pumps: sorting and regulation. Eur. J. Cell Biol. 79:557-563. [DOI] [PubMed] [Google Scholar]
- 11.Felix, C. F., C. C. Moreira, M. S. Oliveira, M. Sola-Penna, J. R. Meyer-Fernandes, H. M. Scofano, and A. Ferreira-Pereira. 1999. Protection against thermal denaturation by trehalose on the plasma membrane H+-ATPase from yeast. Synergetic effect between trehalose and phospholipid environment. Eur. J. Biochem. 266:660-664. [DOI] [PubMed] [Google Scholar]
- 12.Frauenfelder, H., F. Parak, and R. D. Young. 1988. Conformational substrates in proteins. Annu. Rev. Biophys. Biophys. Chem. 17:451-479. [DOI] [PubMed] [Google Scholar]
- 13.Gekko, K., and S. N. Timasheff. 1981. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry 20:4667-4676. [DOI] [PubMed] [Google Scholar]
- 14.Guerra, G., S. Uribe, and J. P. Pardo. 1995. Reactivity of the H+-ATPase from Kluyveromyces lactis to sulfhydryl reagents. Arch. Biochem. Biophys. 321:101-107. [DOI] [PubMed] [Google Scholar]
- 15.Hagen, S. J., J. Hofrichter, and W. A. Eaton. 1995. Protein reaction kinetics in a room-temperature glass. Science 269:959-962. [DOI] [PubMed] [Google Scholar]
- 16.Hottiger, T., C. De Virgilio, M. N. Hall, T. Boller, and A. Wiemken. 1994. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur. J. Biochem. 219:187-193. [DOI] [PubMed] [Google Scholar]
- 17.Hottiger, T., T. Boller, and A. Wiemken. 1987. Rapid changes of heat and desiccation tolerance correlated with changes of trehalose content in Saccharomyces cerevisiae cells subjected to temperature shifts. FEBS Lett. 220:113-115. [DOI] [PubMed] [Google Scholar]
- 18.Hottiger, T., P. Schmutz, and A. Wiemken. 1987. Heat-induced accumulation and futile cycling of trehalose in Saccharomyces cerevisiae. J. Bacteriol. 169:5518-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, Y. K., and J. H. Kaplan. 2000. Site-directed chemical labeling of extracellular loops in a membrane protein. The topology of the Na,K-ATPase alpha-subunit. J. Biol. Chem. 275:19185-19191. [DOI] [PubMed] [Google Scholar]
- 20.Jacob, M., M. Geeves, G. Holterman, and F. X. Schmid. 1999. Diffusional crossing in a two-state protein folding reaction. Nat. Struct. Biol. 6:923-926. [DOI] [PubMed] [Google Scholar]
- 21.Jacob, M., and F. X. Schmid. 1999. Protein folding as a diffusional process. Biochemistry 38:13773-13779. [DOI] [PubMed] [Google Scholar]
- 22.Kramers, H. A. 1940. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 7:284-304. [Google Scholar]
- 23.Lavalette, D., C. Tetreau, M. Tourbez, and Y. Blouquit. 1999. Microscopic viscosity and rotational diffusion of proteins in a macromolecular environment. Biophys. J. 76:2744-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, T. Y., and S. N. Timasheff. 1996. On the role of surface tension in the stabilization of globular proteins. Protein Sci. 5:372-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 26.Magazù, S., G. Maisano, P. Migliardo, H. D. Middendorf, and V. Villari. 1998. Hydration and transport properties of aqueous solutions of α-α-trehalose. J. Chem. Phys. 109:1170-1174. [Google Scholar]
- 27.Miranda, M., J. Ramírez, A. Peña, and R. Coria. 1995. Molecular cloning of the plasma membrane H+-ATPase from Kluyveromyces lactis: a single nucleotide substitution in the gene confers ethidium bromide resistance and deficiency in K+ uptake. J. Bacteriol. 177:2360-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mooney, M. 1951. The viscosity of a concentrated suspension of spherical particles. J. Colloid Sci. 6:162-170. [Google Scholar]
- 29.Nakamoto, R. K., and C. W. Slayman. 1989. Molecular properties of the fungal plasma-membrane H+-ATPase. J. Bioenerg. Biomembr. 21:621-632. [DOI] [PubMed] [Google Scholar]
- 30.Nieslanik, B. S., M. J. Dabrowski, R. P. Lyon, and W. M. Atkins. 1999. Stopped-flow kinetic analysis of the ligand-induced coil-helix transition in glutathione S-transferase A1-1: evidence for a persistent denatured state. Biochemistry 38:6971-6980. [DOI] [PubMed] [Google Scholar]
- 31.Ozier-Kalogeropoulos, O., A. Malpertuy, J. Boyer, F. Tekaia, and B. Dujon. 1998. Random exploration of the Kluyveromyces lactis genome and comparison with that of Saccharomyces cerevisiae. Nucleic Acids Res. 26:5511-5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rampp, M., C. Buttersack, and H. D. Ludemann. 2000. c,T-dependence of the viscosity and the self-diffusion coefficients in some aqueous carbohydrate solutions. Carbohydr. Res. 328:561-572. [DOI] [PubMed] [Google Scholar]
- 33.Rao, R., R. K. Nakamoto, S. Varjovski-Almeida, and C. W. Slayman. 1992. Structure and function of the yeast plasma-membrane H+-ATPase. Ann. N. Y. Acad. Sci. 671:195-203. [DOI] [PubMed] [Google Scholar]
- 34.Sampedro, J. G., P. Cortés, R. A. Muñoz-Clares, A. Fernández, and S. Uribe. 2001. Thermal inactivation of the plasma membrane H+-ATPase from Kluyveromyces lactis. Protection by trehalose. Biochim. Biophys. Acta 1544:64-73. [DOI] [PubMed] [Google Scholar]
- 35.Sampedro, J. G., G. Guerra, J. P. Pardo, and S. Uribe. 1998. Trehalose-mediated protection of the plasma membrane H+-ATPase from Kluyveromyces lactis during freeze-drying and rehydration. Cryobiology 37:131-138. [DOI] [PubMed] [Google Scholar]
- 36.Serrano, R., M. C. Kielland-Brandt, and G. R. Fink. 1986. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ K+), K+- and Ca2+-ATPases. Nature 319:689-693. [DOI] [PubMed] [Google Scholar]
- 37.Serrano, R., J. M. Villalba, M. G. Palmgren, F. Portillo, A. Parets-Soler, M. Roldan, C. Ferguson, and C. Montesinos. 1992. Studies of the plasma membrane H+-ATPase of yeast and plants. Biochem. Soc. Trans. 20:562-566. [DOI] [PubMed] [Google Scholar]
- 38.Sharma, S. C. 1997. A possible role of trehalose in osmotolerance and ethanol tolerance in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 152:11-15. [DOI] [PubMed] [Google Scholar]
- 39.Singer, M. A., and S. Lindquist. 1998. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1:639-648. [DOI] [PubMed] [Google Scholar]
- 40.Singer, M. A., and S. Lindquist. 1998. Thermotolerance in Saccharomyces cerevisiae: the Yin and Yang of trehalose. Trends Biotechnol. 16:460-468. [DOI] [PubMed] [Google Scholar]
- 41.Toyoshima, C., M. Takasako, H. Nomura, and H. Ogawa. 2000. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 405:647-655. [DOI] [PubMed] [Google Scholar]
- 42.Vrbjar, N., G. A. Simatos, and K. M. Keough. 1990. Temperature dependence of kinetic parameters of (Ca2+ + Mg2+)-ATPase in rabbit and winter flounder sarcoplasmic reticulum. Biochim. Biophys. Acta 1030:94-100. [DOI] [PubMed] [Google Scholar]
- 43.Wera, S., E. De Schrijver, I. Geyskens, S. Nwaka, and J. M. Thevelein. 1999. Opposite roles of trehalase activity in heat-shock recovery and heat-shock survival in Saccharomyces cerevisiae. Biochem. J. 343:621-626. [PMC free article] [PubMed] [Google Scholar]
- 44.Williams, S., P. M. Haggie, and K. M. Brindle. 1997. 19F NMR measurements of the rotational mobility of proteins in vivo. Biophys. J. 72:490-498. [DOI] [PMC free article] [PubMed] [Google Scholar]