Abstract
Yersinia pseudotuberculosis produces YPM (Y. pseudotuberculosis-derived mitogen), a superantigenic toxin that exacerbates the virulence of the bacterium in vivo. To date, three alleles of the superantigen gene (ypmA, ypmB, and ypmC) have been described. These genes are not found in all Y. pseudotuberculosis strains and have a low GC content, suggesting their location on mobile genetic elements. To elucidate this question, the genetic environment of the superantigen-encoding genes was characterized and 11 open reading frames (ORFs) were defined. Sequence analysis revealed that the ypm genes were not associated with plasmids, phages, transposons, or pathogenicity islands and that the superantigen genes were always located in the chromosome between ORF3 and ORF4. Nonsuperantigenic strains exhibited the same genetic organization of the locus but lacked the ypm gene between ORF3 and ORF4. A new insertion sequence, designated IS1398, which displays features of the Tn3 family, was characterized downstream of the ypmA and ypmC genes. A 13.3-kb region containing the ypm genes was not found in the genome of Y. pestis (CO92 and KIM 5 strains). We experimentally induced deletion of the ypm gene from a superantigen-expressing Y. pseudotuberculosis: using the association of aph(3′)-IIIa and sacB genes, we demonstrated that when these reporter genes were present in the ypm locus, deletion of these genes was about 250 times more frequent than when they were located in another region of the Y. pseudotuberculosis chromosome. These results indicate that unlike other superantigenic toxin genes, the Yersinia ypm genes are not associated with mobile genetic elements but are inserted in an unstable locus of the genome.
Yersinia pseudotuberculosis, a microorganism causing gastrointestinal diseases and immunopathological complications such as reactive arthritis and erythema nodosum (10, 47, 59, 60), is at present the only gram-negative bacteria known to produce a superantigenic toxin. This molecule (designated YPM for Y. pseudotuberculosis-derived mitogen) is a 14.5-kDa protein able to induce proliferation of human T lymphocytes bearing Vβ3, Vβ9, Vβ13.1, and Vβ13.2 T-cell receptor variable regions (1, 61). In vivo, YPM induces lethal shock in mice (44) and exacerbates the virulence of Y. pseudotuberculosis in a murine experimental model of systemic infection (13). To date, three YPM variants (YPMa, YPMb, and YPMc) have been described. YPMa displays 83% identity with YPMb (11, 50) and differs from YPMc only by a single substitution at position 51 of the mature protein (12). A phylogenetic analysis based on the amino acid sequences of various bacterial superantigenic toxins indicated that the YPM variants belong to a new type of bacterial superantigen family (12, 46). The ypm genes encoding the YPM toxins have not been found in the genome of Yersinia pestis, a genetically related species (49). Yersinia enterocolitica, another pathogenic Yersinia species, has been described as a mitogen-producing microorganism (57, 58); however, the T-cell specificity of the bacterial mitogen and the absence of DNA hybridization with a ypm-specific probe clearly demonstrated that the mitogen activity of Y. enterocolitica cannot be attributed to a YPM variant (12, 63).
At present, little information on the genetic location of ypm genes is available. It is now established that ypmA is not located on the virulence plasmid pYV, since the gene is present in pYV-cured strains of Y. pseudotuberculosis (11, 43). Hence, as they are not present on pYV, ypm genes are likely to be chromosomal, although no experimental evidence confirming this hypothesis has been found (43). Nevertheless, some arguments suggest that ypm genes might be harbored by a mobile genetic element. First, ypm genes are not present in all Y. pseudotuberculosis strains (22, 63), raising the question of the transmission of the superantigen genes among the Y. pseudotuberculosis population. Secondly, analysis of the nucleotide sequences of ypm genes revealed a guanine and cytosine (GC) content of between 34.6 and 35.3%, whereas the genome of Y. pseudotuberculosis has a higher GC content (46.5%) (5), thus suggesting that Y. pseudotuberculosis obtained ypm genes from a microorganism with a low GC percentage.
In this study, we addressed the question of the genetic mobility of the superantigen-encoding genes. With this goal in mind, we characterized the genetic environment of the Y. pseudotuberculosis ypm genes and compared it with the genetic organization displayed by nonsuperantigenic strains. Sequence analysis ruled out the association of the superantigen genes with mobile genetic elements but indicated that the superantigen-producing strains represent a clonal population of Y. pseudotuberculosis that has evolved concomitantly with nonsuperantigenic Y. pseudotuberculosis clones. We also demonstrated that the locus containing the ypm gene was unstable and that DNA deletion in this region can occur with high frequency without any characteristic or sequence-specific excisions.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
Forty-two Y. pseudotuberculosis strains were used in this study (Table 1). They were kindly provided by S. Aleksić (Hygiene Institut Hamburg, Hamburg, Germany), E. Carniel (Centre National de Référence des Yersinia, Institut Pasteur, Paris, France), H. Müller-Alouf (Institut Pasteur de Lille, Lille, France), J. Sundar (Statens Institutt for Folkehelse, Oslo, Norway), N. Takeda (Department of Pediatrics, Kurashiki Central Hospital, Okayama, Japan), R. Van Noyen (Imeldaziekenhuis, Bonheiden, Belgium), and G. Wauters (Université Catholique de Louvain, Louvain, Belgium). A spontaneous nalidixic acid-resistant mutant of Y. pseudotuberculosis AH (designated AH Nalr) was obtained by plating a bacterial culture onto Luria-Bertani (LB) agar containing a nalidixic acid concentration gradient. The superantigen-deficient strains were not epidemiologically related, as indicated by a NotI genomic restriction profile analysis using pulsed-field gel electrophoresis (data not shown) (30, 48).
TABLE 1.
Y. pseudotuberculosis strains used in this study
| Y. pseudotuberculosis genotype | Strain (serotype)a | Source |
|---|---|---|
| ypmA+ | AH (4b), KM (4b), MA (2b), NT (4b), ST (4b) | N. Takeda |
| 1402 (4), 1404 (6) | H. Müller-Alouf | |
| 1553 (6), 1554 (6), 1833 (4) | E. Carniel | |
| 1191/90 (4a) | S. Aleksić | |
| ypmB+ | 487/90 (6), 1093 (11), 1096 (6), 1119 (5b), 1155 (5a) | S. Aleksić |
| ypmC+ | YPT1 (3), YPT5 (3) | R. Van Noyen |
| 2887 (3), 32945 (3), 32975 (3), | E. Carniel | |
| 32977 (3), 32992 (3) | ||
| 200/90 (3), 298/89 (3), 304/89 (3), 1134/90 (3) | S. Aleksić | |
| 1216/93 (3), 776/88 (1) | J. Sundar | |
| WE31/93 (3) | G. Wauters | |
| Nonsuperantigenic | 9314/74 (1) | J. Sundar |
| 1830 (4), 2821 (5), 2843 (5), 2926 (2) | E. Carniel | |
| 199/90 (5a), 300/89 (2b), 367/89 (1a), 1432/94 (2c) | S. Aleksić | |
| 1401 (3), 1403 (5) | H. Müller-Alouf | |
| YPT12 (2a) | R. Van Noyen |
Strains in bold were chosen as representative of each subgroup.
The plasmids used in this study are listed in Table 2. Escherichia coli DH5α (28) was the host for pUC recombinant plasmids, whereas E. coli SY327λpir (41) and E. coli SM10λpir (55) were hosts for the pMM7043 suicide plasmid. Bacteria were grown at 28°C (Yersinia) or 37°C (E. coli) in LB broth or agar. Kanamycin (50 μg/ml), chloramphenicol (50 μg/ml), ampicillin (100 μg/ml), and nalidixic acid (10 μg/ml) were added to media for bacterial selection when necessary.
TABLE 2.
Plasmids used in this study
| Plasmid | Descriptiona | Reference or source |
|---|---|---|
| pUC18 and 19 | Apr cloning vector | 62 |
| pACYC184 | Source of the chloramphenicol resistance gene (cat) | 14 |
| pCVD442 | Source of the sacB gene | 19 |
| pUC1318-KmII | Source of the kanamycin resistance gene aph(3′)-IIIa | Gift from P. Trieu-Cuot |
| pCCY10 | pUC18Ω, 6.5-kb HindIII fragment of Y. pseudotuberculosis AH containing the ypmA gene | 13 |
| pFM10 | pUC18Ω, 5-kb KpnI fragment of Y. pseudotuberculosis AH containing ORF3 gene | This study |
| pSF11 | pUC19Ω, 8-kb HindIII fragment of Y. pseudotuberculosis 487/90 containing the ypmB gene | This study |
| pFM30 | pUC18Ω, 2.5-kb KpnI fragment of Y. pseudotuberculosis 487/90 containing the ypmB gene | This study |
| pSC10 | pUC18Ω, 12.7-kb PstI fragment of Y. pseudotuberculosis YPT1 containing the ypmC gene | This study |
| pSC20 | pUC18Ω, 9.7-kb PstI fragment of Y. pseudotuberculosis 9314/74 hybridizing with an ORF5- specific probe | This study |
| pMM70413 | Cmr suicide vector; pGP704 (42) derivative in which the 675-bp PstI fragment containing the bla gene was replaced by two copies in tandem of the PstI PCR-generated cat gene | This study |
| pCCY41.2 | pMM70413Ω, sacB and aph(3′)-IIIa genes inserted within ypmA | This study |
| pIL31.3 | pMM70413Ω, sacB and aph(3′)-IIIa genes inserted upstream of the urease operon promoter | This study |
Apr and Cmr, resistance to ampicillin and chloramphenicol, respectively; Ω, in vitro insertion.
DNA techniques.
Genomic DNA was extracted from bacterial cells as previously described (39). Plasmid DNA and endonuclease-restricted fragments were purified on Qiagen columns according to the manufacturer's recommendation (Qiagen S. A., Courtaboeuf, France). Southern hybridizations were carried out as previously described (13). Standard PCR amplification was performed as described elsewhere (54), whereas conditions for recombinant PCR were as presented below. DNA fragments were ligated to endonuclease-restricted vectors using T4 DNA ligase, according to standard techniques (Life Technologies, Cergy Pontoise, France) (53). Recombinant plasmids were introduced into E. coli by transformation (53).
Oligonucleotide primers and probes.
Intergenic regions were amplified with the following primers located within open reading frames (ORFs) (Fig. 1): no. 114 (5′GTGTTCCGTTTGATGAGGAGG3′), no. 93 (5′CCTGCCAATAAGCTAAGGCAG3′), no. 63 (5′GCTGTTCAGTGTTATGCCGCTG3′), no. 29 (5′GACCGCCAGCATCTACCTG3′), no. 16 (5′GCGGCAAGCTTTGAAGGGTTGTCACAATTGCACCT3′), no. 1 (5′ACACTTTTCTCTGGAGTAGCG3′), no. 2 (5′ACAGGACATTTCGTCA3′), no. 4 (5′TGTAGGAGGCAATGGATGGGG3′), no. 30 (5′GCTGCACCGTCTCTGTTATCAC3′), no. 13 (5′CCGATGCGATTAATACTGCC3′), no. 9 (5′CATGCTGGCACCTGCCTCGAA3′), no. 22 (5′GCGGATACATGCATCCGCAG3′), no. 44 (5′GGCAACTATGACACCTGCGC3′), no. 46 (5′GCCCGCAGGCACCCGGCAAAA3′), no. 56 (5′GAGTCGTTGATTGCACGCCGTAG3′), no. 101 (5′GGTCTGTGCTACGCATCGGGATC3′), no. 65 (5′GCAGTTGTGCACCCAGTTCGGC3′), no. 85 (5′CCGCGTCAATATTAACTCATTGG3′), no. 131 (5′CGACGCAGCATCGCACGGTAG3′), no. 139 (5′GTCGGTGCCGCACTCGGCATG3′), and no. 136 (5′GAAGGCTGCGGTGGGTGGAGGG3′). In nonsuperantigenic strains, the ORF3-ORF4 intergenic region was amplified with primers no. 16 and no. 30 and then sequenced with primers no. 30 and no. 58 (5′GCACCAAGGTGACGATAGGCG3′).
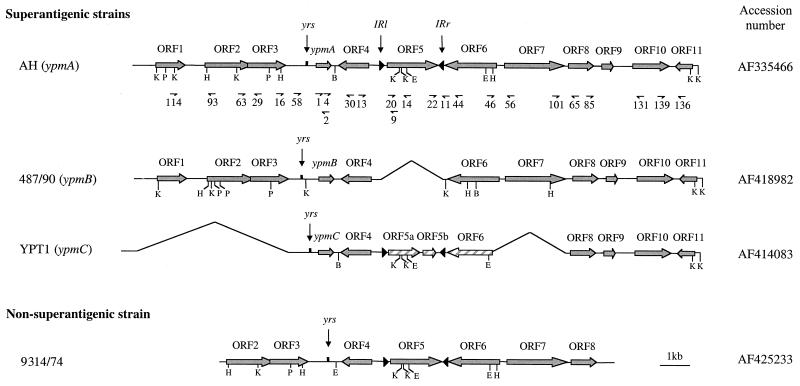
FIG. 1.
Genetic organization of the ypm gene loci from superantigenic Y. pseudotuberculosis strains AH, 487/90, and YPT1 and the corresponding locus from the nonsuperantigenic strain 9314/74. Plain grey and hatched arrows represent complete and truncated ORFs, respectively. yrs, Yersinia recombination site; IRl and IRr, left and right inverted repeats, respectively. Relevant oligonucleotides were located on the genetic map of the ypmA locus of Y. pseudotuberculosis AH. B, BamHI; E, EcoRI; H, HindIII; K, KpnI; P, PstI.
The probe specific for the three ypm alleles was generated by PCR with primers no. 1 and no. 2 (13). The IS1398-specific probe was PCR amplified with primers no. 14 (5′GTACCACCTGACCAAAGCTAG3′) and no. 20 (5′CCGGAAGCAACATCCAAGAG3′). Probes were generated by amplification in the presence of digoxigenin-11-dUTP (Roche Diagnostics, Meylan, France).
All primers used in this study were synthesized by Sigma-Genosys Ltd (Pampisford, United Kingdom) or Genset SA (Paris, France).
DNA sequencing and sequence analysis.
The recombinant plasmids used to sequence the ypm loci are described in Table 2. Plasmid pCCY10 was sequenced by a walking strategy. Since high degrees of homology between the loci of the three superantigenic alleles exist, primers generated from the pCCY10 sequence were used for the sequencing of pSF11 (locus ypmB), pSC10 (locus ypmC), and pSC20 (locus deficient in ypmA). PCR amplification products were directly sequenced as necessary. Sequencing was performed on both DNA strands. ORFs over 300 bp long were identified with ORF Finder software on the National Center for Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov). Sequences were searched against nucleotide and protein databases by using BLAST on the NCBI server. Sequences were also compared with the Y. pestis CO92 sequences presented by the Y. pestis Sequencing Group at the Sanger Center (http://www.sanger.ac.uk/Projects/Y_pestis and accession number AJ414151) (49) and with the Y. pestis KIM5 (strain P12) genomic sequence available on the University of Wisconsin Genome Project website (http://www.genome.wisc.edu). Sequence comparisons were performed with Clustal W at http://www.infobiogen.fr.
Detection of ypmA spontaneous mutants.
In order to evaluate the stability of the ypmA superantigen gene, Y. pseudotuberculosis AH was subcultured alternately at 28°C for 16 h and at 42°C for 8 h. Dilutions of bacterial cultures were plated, with isolated colonies then being tested for the presence of the ypmA gene by colony hybridization (53) using the ypm-specific probe labeled with digoxigenin.
Introduction of the aph(3′)-IIIa and sacB genes into the Y. pseudotuberculosis AH genome.
To determine the deletion frequency within the superantigen gene locus, we inserted the kanamycin resistance gene aph(3′)-IIIa and the sacB gene into ypmA of Y. pseudotuberculosis AH. The sacB gene product is toxic for gram-negative bacteria grown in the presence of sucrose (8). Only the strains in which deletion (or inactivation) of the sacB gene has occurred will grow on sucrose. To insert the aph(3′)-IIIa and sacB genes into the ypmA gene, a SpeI restriction site was generated within ypmA (250 bp from the start codon) and two XbaI sites were also created (one 550 bp upstream of the start codon and the other 386 bp downstream of the ypmA stop codon) by overlap extension using PCR (20, 27, 29). PCR amplification with no. 98Xba (5′GCTCTAGACCTTGGGCTCCGATATTGATCCATTCC3′) and no. 97Spe (5′ATTGCGCCACTAGTCCTAGTAAAATTAACGTCATATCTGCATTTAC3′) primers yielded an 823-bp fragment encompassing the upstream and 5′ regions of ypmA, whereas no. 96Spe (5′TTACTAGGACTAGTGGCGCAATGTAGGAGGCAATGGATGGGGAG3′) and no. 99Xba (5′GCTCTAGAATGCAGTAAAGAATCAGGGTGGTGTTAC3′) primers produced a 611-bp fragment covering the 3′ half of ypmA and its downstream sequence. Internal primers no. 96Spe and no. 97Spe were generated to give a 22-bp overlapping region containing an SpeI restriction site. Amplimers produced with no. 98Xba plus no. 97Spe and no. 96Spe plus no. 99Xba were purified, combined, and annealed by their 22-bp overlapping sequence and were then 3′ extended following the complementary strand. Finally, the resulting PCR product was amplified with external primers no. 98Xba and no. 99Xba to give a 1.4-kb fragment. PCR conditions have been previously described (20). The amplimer was digested with XbaI and cloned into pUC19. The sacB gene was PCR amplified with sac1 (5′GGACTAGTGGAGATCTGGCCCGTAGTCTGCAAATCCTTTT3′) and sac2 (5′GGACTAGTCCGCTCGAGGGTTAGGAATACGGTTAGCCATTTGCC3′) primers by using pCVD442 as a template. Primer sac1 contains an XhoI restriction site downstream of a SpeI site, whereas primer sac2 was synthesized with a SpeI site. The 1,877-bp fragment generated with sac1 and sac2 was purified, digested with SpeI, and cloned into the SpeI site generated within ypmA. Next, the kanamycin resistance gene aph(3′)-IIIa was PCR amplified from plasmid pUC1318-KmII by using primers kan1 (5′GCCGCTCGAGGGATTTCAGGGGGCAAGGCATAG3′) and kan2 (5′GCCGCTCGAGCAGAGTATGGACAGTTGCGGATG3′), which both contain an XhoI site. The aph(3′)-IIIa gene was cloned into the XhoI site located downstream of the sacB gene. The orientation of the two reporter genes within ypmA was established by PCR amplification. Finally, the XbaI fragment containing the aph(3′)-IIIa and sacB genes inserted into ypmA was cloned into the suicide vector pMM70413, which contains the chloramphenicol resistance gene cat. The plasmid was designated pCCY41.2. Allelic exchange was carried out between Y. pseudotuberculosis AH Nalr and E. coli SM10λpir (pCCY41.2). The first recombination event was selected for on LB agar with nalidixic acid and kanamycin. To select for the second recombination event and eliminate the suicide plasmid, we used the chloramphenicol bacteriostatic properties. Bacteria were grown in LB containing chloramphenicol until the optical density at 600 nm reached a value of between 0.2 and 0.3. Next, cycloserine was added to a final concentration of 1.8 mg/ml. This antibiotic kills bacteria in the growth phase but has no effect on bacteria in the static phase. Hence, Y. pseudotuberculosis clones that still contained the suicide plasmid grew in the presence of chloramphenicol and were killed by cycloserine. After this treatment, the chloramphenicol-sensitive, kanamycin-resistant Y. pseudotuberculosis organisms were selected for on LB agar in the presence of kanamycin. Genetic analysis by PCR of the kanamycin-resistant clones confirmed the integration of the aph(3′)-IIIa and sacB genes into the ypmA gene.
As a control, aph(3′)-IIIa and sacB genes were inserted into another Y. pseudotuberculosis AH chromosomal region, upstream of the promoter controlling the expression of the urease operon (54). First, an SpeI restriction site was introduced into a noncoding region upstream of the urease operon promoter by overlap extension, as described above. External primers UreXbaF (5′GCTCTAGAGCGGTGGTAGCCCATGGTTGCAGTCAC3′) and UreXbaR (5′GCTCTAGAGCTAAAATCAAGACAAATTATCCACCACCC3′) contain an XbaI site, whereas internal primers UreSpeF (5′GCGATTGGACTAGTGGAAATTAAATATCACAGCTAGAATAATAACTAAT3′) and UreSpeR (5′TAATTTCCACTAGTCCAATCGCATTCCACGGTTCTTTTTAGTATTAACC3′) contain an SpeI site and share a 22-bp overlapping region. The resulting fragment was digested with XbaI and cloned into pUC19 and then into the pMM70413 suicide vector. The SpeI fragment of pCCY41.2 containing the aph(3′-)IIIa and sacB genes was introduced into the newly generated SpeI site, and the suicide plasmid was designated pIL31.3. Allelic exchange for introduction of the aph(3′)-IIIa and sacB genes upstream from the urease operon of Y. pseudotuberculosis AH Nalr was carried out as above.
Measurement of the DNA deletion frequency.
Dilutions of overnight cultures of Y. pseudotuberculosis AH containing the aph(3′)-IIIa and sacB genes were plated concomitantly on LB agar lacking sodium chloride in the presence of 10% sucrose on one hand and on LB agar alone on the other. The selection frequency for sucrose-resistant mutants was calculated as the ratio of the number of CFU growing on 10% sucrose agar to the number of CFU counted on LB agar without sucrose. To confirm that sucrose resistance was due to a deletion process rather than to a point mutation in sacB, deletions of the kanamycin resistance gene were sought. For this purpose, colonies growing on 10% sucrose were streaked onto LB agar in the presence of kanamycin, and the percentage of kanamycin-sensitive bacteria among the sucrose-resistant clones was established. Finally, the deletion frequency of the aph(3′)-IIIa and sacB genes was calculated as the frequency of appearance of the sucrose-resistant mutant multiplied by the percentage of kanamycin-sensitive bacteria among the sucrose-resistant clones. Frequency values were obtained from three separate experiments.
Nucleotide sequence accession numbers.
The nucleotide sequences of the ypm loci have been submitted to GenBank and were given accession numbers AF335466 (Y. pseudotuberculosis strain AH), AF414083 (Y. pseudotuberculosis strain YPT1), AF418982 (Y. pseudotuberculosis strain 487/90), and AF425233 (Y. pseudotuberculosis strain 9314/74).
RESULTS
The ypmA gene is not associated with a mobile genetic element but is inserted in the chromosome in the vicinity of a DNA recombination site.
In order to define the genetic environment of the superantigen gene of Y. pseudotuberculosis strain AH, a 17.1-kb DNA segment encompassing the ypmA gene was sequenced and 11 ORFs were identified (Fig. 1). The same genetic organization was found in 10 other ypmA+ strains (KM, MA, NT, ST, 1191/90, 1402, 1404, 1553, 1554, and 1833) as shown by PCR amplification of the intergenic regions with primers located in ORFs 1 to 11 (Fig. 1). The ypmA locus of strain 1833 differs from strain AH only by the absence of ORF5. These ORFs encode proteins with homologies to regulatory proteins (ORF1 and ORF7), aminotransferases and synthetases (ORF3 and ORF11, respectively), dehydrogenases (ORF6 and ORF8), and membrane proteins (ORF2, ORF4, and ORF10) (Table 3). No function could be attributed to ORF5 or ORF9. It is noteworthy that the ORF4 product is highly homologous (77% identity) to PagO, a putative transmembrane protein regulated by the PhoP-PhoQ two-component system found in Salmonella enterica serovar Typhimurium (25) and, to a lesser extent (59% identity), to the Y. enterocolitica protein YomA, encoded by a gene adjacent to yadA on the virulence plasmid (56). Like ypmA, ORF4 has a low GC content (36.5%), contrasting with the higher GC content of the surrounding genes, which ranges from 43.3% for ORF11 to 55.6% for ORF5 (Table 3).
TABLE 3.
ORFs identified upstream and downstream of the ypmA gene of Y. pseudotuberculosis AH
| CDSa | Gene
|
Product
|
|||||
|---|---|---|---|---|---|---|---|
| Size (nt)b | G+C (%) | Size (aa)c | Protein similarity
|
||||
| Relevant homologous protein | % Amino acid identity (ratio) | Putative function | Accession no. | ||||
| ORF1 | 1,008 | 49.1 | 335 | GalR of E. coli | 41 (136/328) | Transcriptional regulator | AAA83860 |
| ORF2 | 1,455 | 48.5 | 484 | NhaC of Haemophilus influenzae | 47 (219/458) | Na+/H+ antiporter | Q57007 |
| ORF3 | 1,179 | 45.2 | 392 | MalY of E. coli | 36 (140/388) | Aminotransferase | P23256 |
| ypmA | 456 | 35.3 | 151 | Y. pseudotuberculosis-derived mitogen | 100 | Superantigenic toxin | D38638 |
| D38523 | |||||||
| ORF4 | 924 | 36.5 | 307 | PagO of S. enterica serovar Typhimurium | 77 (224/290) | Membrane protein | AAB82452 |
| YomA of Y. enterocolitica | 59 (173/289) | AAD16869 | |||||
| ORF5 | 1,599 | 55.6 | 532 | No homology found | |||
| ORF6 | 1,602 | 50.9 | 533 | ExaC of P. aeruginosa | 73 (372/506) | Aldehyde dehydrogenase | AAC79659 |
| ORF7 | 1,845 | 51.1 | 614 | AdhR of Pseudomonas stutzeri | 35 (226/632) | Transcriptional regulator | AAG0924 |
| ORF8 | 750 | 47.7 | 249 | YdfG of E. coli | 65 (163/247) | Oxidoreductase/dehydrogenase | F64908 |
| ORF9 | 375 | 44.3 | 124 | Hypothetical protein b1583 of E. coli | 45 (43/95) | Unknown | A64914 |
| ORF10 | 1,281 | 52.2 | 426 | b1592 of E. coli | 42 (179/424) | Chloride channel | AAC74664 |
| ORF11 | 669 | 43.3 | 222 | DTBS of E. coli | 63 (141/223) | Dethiobiotin synthetase | BAA15317 |
CDS, coding sequence.
nt, nucleotides.
aa, amino acids.
Another noticeable feature of the 17.1-kb locus is the presence (245 bp upstream of ypmA) of a 26-bp sequence that is homologous to a recombination sequence (dif) that promotes RecA-independent, site-specific recombination (33) and that might be responsible for hyperrecombination events in the E. coli chromosome terminus region (37) (Fig. 2). Another homologous sequence is present on the E. coli multidrug-resistant plasmid R1 and, like dif, is responsible for site-specific recombination (15, 16). Strong homology was also found with a phage integration locus found in Xanthomonas campestris (18). Thus, in light of the function attributed to these various sequences, the 26-bp sequence upstream of ypmA was designated yrs for Yersinia recombination site. Comparison of the nucleotide sequences revealed a 19-bp consensus sequence with two 6-bp inverted repeats (Fig. 2). Interestingly, this 19-bp common core sequence is widely distributed among various bacterial species, since it was found in the genomes of Neisseria meningitidis, S. enterica serovar Typhimurium, Pseudomonas aeruginosa, and Pasteurella multocida (Fig. 2).
FIG. 2.
Nucleotide sequence of the Yersinia recombination site (yrs) located upstream of ypmA aligned with the corresponding sequence found between ORFs YPO2283 and YPO2281 in Y. pestis CO92 (49), with the E. coli dif locus (33), with the recombination site found on plasmid R1 from E. coli (16), with the phage integration site in X. campestris (18), and with sequences from the genome of N. meningitidis strain MC58 (accession number AE002470), S. enterica serovar Typhimurium strain LT2 (accession number AE008767), P. aeruginosa strain PAO1 (accession number AE004648), and P. multocida strain PM70 (accession number AE006097). The underlined sequence corresponds to the yrs sequence. Asterisks indicate identical nucleotides, with arrows indicating the IR sequences.
A putative novel insertion sequence (IS), which we designated IS1398, was identified between ORF4 and ORF6 (Fig. 1). This 1.8-kb genetic element is composed of a single 1,599-kb ORF (ORF5) flanked by two inverted repeats (IRs) sharing 30 of 35 bp (Fig. 3). IS1398 displays several features found in the Tn1000 and Tn3 transposon families. First, the two 35-bp IRs are homologous to the Tn1000 and Tn3 IRs (77.1 and 71.4% identity, respectively) (52) and to the terminal IRs of the Pseudomonas sp. strain BW13 mercury resistance Tn3-type transposon (42). Secondly, ORF5-specific Southern hybridization revealed that IS1398, like Tn3 (35), was always present in a single copy in the Y. pseudotuberculosis genome (data not shown). Finally, a duplicate of a pentanucleotide (TTCAA) was present at the IS1398 insertion site (52). However, apart from these features held in common with Tn3-type transposons, the 532-amino-acid ORF5 product located between the two IRs did not exhibit homology with any of the transposase genes known so far, thus suggesting a new type of IS or a Tn3 transposon remnant that lost the transposase and resolvase genes. PCR amplification of ORF4-ORF5 and ORF5-ORF6 intergenic regions in 13 IS1398-positive Y. pseudotuberculosis strains (strains AH, KM, NT, ST, YPT1, YPT12, 199/90, 300/89, 367/89, 1191/90, 1402, 1432/94, and 9314/74) demonstrated that IS1398 was always present at the same location in the genome, suggesting either an absence of mobility of this genetic element or the presence of a unique IS1398 target site between ORF4 and ORF6.
FIG. 3.
Nucleotide sequence of IS1398 and its immediate environment from Y. pseudotuberculosis AH. Black boxes represent the 35-bp IRs, and grey boxes indicate the 5-bp pair repeats at the target site. The stop codon is marked with an asterisk.
A 918-bp fragment containing ypm is absent in most nonsuperantigenic Y. pseudotuberculosis strains.
In the nonsuperantigenic strain 9314/74, sequence analysis of 12.3 kb of the ypmA corresponding locus showed the same genetic organization as in strain AH, except for the absence of the superantigen gene (Fig. 1). Comparative analysis of the ORF3-ORF4 intergenic region from strains 9314/74 and AH allowed us to define a 918-bp fragment containing ypmA in strain AH and missing in strain 9314/74. Interestingly, the yrs site was still present within the ORF3-ORF4 intergenic space. Particular sequences, such as direct or inverted repeats, were not found at the ends of this 918-bp sequence in strain AH. Sequencing of the PCR product given by primers no. 16 and no. 30 revealed the same ORF3-ORF4 intergenic space as 9314/74 in 10 nonepidemiologically related ypm mutant strains (strains YPT12, 199/90, 300/89, 367/89, 1401, 1403, 1432/94, 2821, 2843, and 2926). No amplification was produced from Y. pseudotuberculosis 1830: further investigation revealed that this strain lacked ORF4 to ORF6 and part of ORF7 and that a 1.6-kb fragment was inserted downstream of yrs (Fig. 4A). These results indicate that the absence of ypm in some nonsuperantigenic strains may be the consequence of a deletion process.
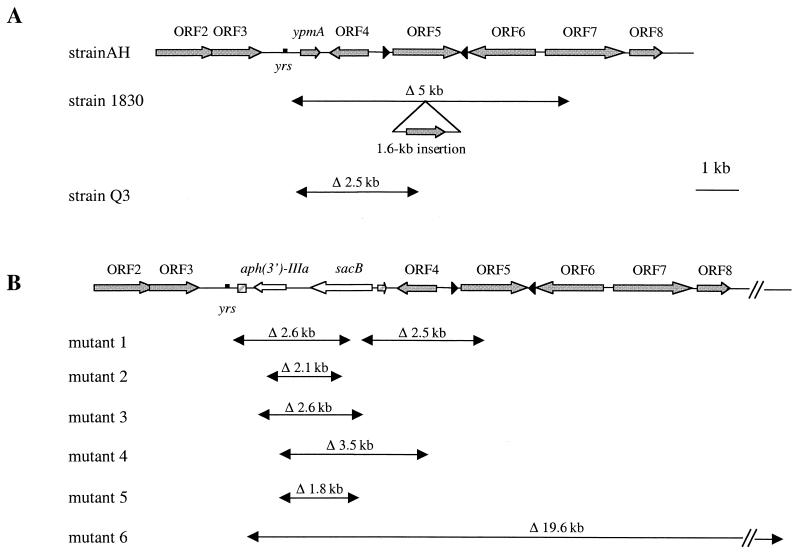
FIG. 4.
Location of deletions (Δ) observed in the ypm locus of Y. pseudotuberculosis. (A) Positions of the deletions in strain Q3, a Y. pseudotuberculosis AH derivative spontaneously cured of ypmA, and in nonsuperantigenic clinical isolate 1830. (B) Deletions in mutants 1 to 6 generated after insertion of aph(3′)-IIIa and sacB reporter genes into ypmA of Y. pseudotuberculosis strain AH with selection on sucrose LB agar and then on LB agar in the presence of kanamycin. The hatched arrow represents an incomplete ypmA gene. yrs, Yersinia recombination site.
The genetic environment of the ypmB and ypmC alleles differs from that of the ypmA locus.
To study the genetic environment of ypmB and ypmC, the two other alleles of the superantigenic gene, we cloned and sequenced the 15-kb ypmB locus of strain 487/90 and the 12.1-kb ypmC locus of Y. pseudotuberculosis YPT1. The main difference between the ypmA and ypmB loci lies in the absence of the IS1398 genetic element in strain 487/90 (Fig. 1). Genes from the ypmB locus are between 97.5% (ORF4) and 88.7% (ypm) identical to the corresponding genes at the ypmA locus. Interestingly, ypmB has the lowest identity level (88.7%) among the 12 genes studied, suggesting a greater genetic drift for the superantigen gene. PCR analysis of the intergenic regions using primers located within the ORFs of other ypmB+ strains from our collection (1093, 1096, 1155, and 1119) indicated a 1.1-kb insertion between ORF10 and ORF11 in strains 1096, 1119, and 1155.
When the superantigen gene loci of AH and YPT1 (ypmC locus) were compared, we found a 4,380-bp deletion (encompassing ORF1 to ORF3) upstream of ypmC, together with a 2,100-bp deletion downstream of ORF5 (including part of ORF6 and ORF7) (Fig. 1). Analysis of the sequence at the boundaries of the second deletion site showed that the excision had occurred at two heptanucleotide sequences (CCAATAC), suggesting a site-specific deletion. This heptanucleotide was not found flanking the first deletion. Furthermore, a frameshift due to deletion of a dinucleotide was observed within ORF5, giving rise to two smaller hypothetical proteins, ORF5a and ORF5b (Fig. 1). Besides these deletions, the genes of the ypmC locus of Y. pseudotuberculosis are over 99% identical to the corresponding genes of the ypmA locus. The genetic organization of the ypmC locus is not specific to the strain YPT1, since all other ypmC+ strains tested (WE31/93, YPT5, 200/90, 298/89, 304/89, 776/88, 1134/90, 1216/93, 2887, 32945, 32975, 32977, and 32992) have the same genetic organization, as judged by PCR analysis of the intergenic regions with primers located within the ORFs. However, we found that the deletion upstream of ypmC was about 400 bp larger in strains 2887, 32945, 32977, and 32992.
The Y. pseudotuberculosis ypmA-containing locus is not present in the Y. pestis genome.
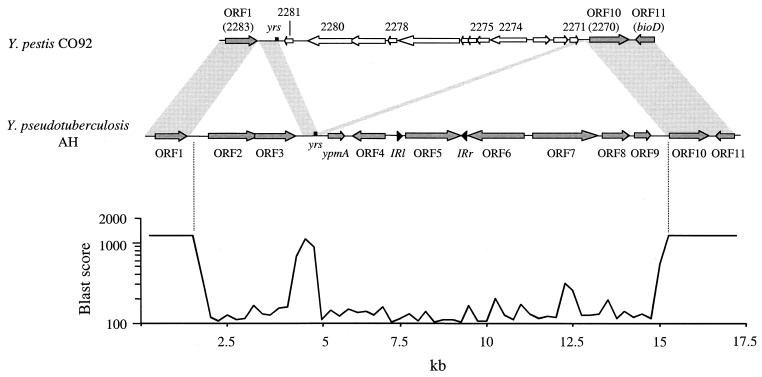
Since Y. pseudotuberculosis and Y. pestis are genetically related species (2, 45), we compared the genetic organizations of the ypmA loci of these two microorganisms (Fig. 5). Surprisingly, the 13.3-kb region of Y. pseudotuberculosis including ORF2 to ORF9 was not found in the genomes of Y. pestis CO92 and Y. pestis KIM5 when the Y. pseudotuberculosis AH sequence was compared with those presented by the Y. pestis sequencing group at the Sanger Center (49) and by the University of Wisconsin Genome project, respectively. Twelve ORFs located in a 9.3-kb locus were present between ORF1 (YPO2283) and ORF10 (YPO2270) in Y. pestis, according to the recent genome annotation (49) (Fig. 5). The products of the YPO2274 and YPO2275 genes were similar to bacteriophage I2-2 proteins, whereas YPO2278 and YPO2280 gene products were similar to Vibrio cholerae filamentous bacteriophage proteins. Interestingly, a 581-bp region of Y. pseudotuberculosis upstream of ypmA and containing the yrs site was also found in Y. pestis. However, in Y. pestis this region was not found intact but was divided into two unequal parts flanking Y. pestis ORFs: a first 411-bp part containing the intact yrs site was located between ORF1 and YPO2281, whereas a second part (187 bp) containing half of the yrs site (TACATTATGCGCA) was found upstream of ORF10 (Fig. 5). This genetic organization demonstrates that Y. pestis phage genes were inserted within this 581-bp region and that this insertion generated a duplication of half of the yrs site. This strongly suggests that yrs represents a target site for phage insertion.
FIG. 5.
Genetic organization of the Y. pseudotuberculosis AH locus containing the ypmA gene compared to the corresponding locus in Y. pestis CO92 biotype Orientalis (positions 285894 to 298332 of the genome sequence available under accession number AJ414151 [49]). Two hundred and fifty-base fragments were compared by using WU-Blast 2.0 software, and results were expressed as Blast scores. A score of 1250 represents 100% identity on 250 nucleotides. White arrows indicate Y. pestis ORFs that differ from Y. pseudotuberculosis ORFs. Shaded areas represent sequences common for Y. pestis and Y. pseudotuberculosis. Identical results were obtained when the Y. pseudotuberculosis AH sequence was compared with the genomic sequence of Y. pestis KIM5 strain P12 (http://www.genome.wisc.edu). Numbers on the Y. pestis sequence correspond to the annotation of the Y. pestis genome (49). yrs, Yersinia recombination site; bioD, dethiobiotine synthetase gene; IRl and IRr, left and right inverted repeats, respectively.
Deletion of the ypmA locus occurs with a high frequency.
Several arguments from the above nucleotide sequence analysis suggested that the ypm locus is unstable: first, partial deletions within the ypm locus were detected in strain 1830 (Fig. 4A) and in ypmC+ strains (Fig. 1), and second, the region is not present in Y. pestis, a species considered to be an emerging clone of Y. pseudotuberculosis (2) (Fig. 5). In order to address the question of the ypm region instability, we evaluated the frequency of appearance of spontaneous ypmA mutants. Hence, Y. pseudotuberculosis strain AH was subcultured alternately at 28 and 42°C in LB broth. Bacteria were tested for the presence of the superantigen gene by colony blot hybridization with a ypmA-specific probe. Among 12,000 Y. pseudotuberculosis clones examined, 1 clone (designated Q3) lost not only the ypmA gene but also ORF4 and part of IS1398, according to PCR amplification (Fig. 4A). Sequencing of the PCR products obtained with primers no. 16 and no. 11 revealed a 2,520-bp deletion in Q3. The size of the excision in Q3 contrasts with the 918-bp fragment missing in nonsuperantigenic strains such as 9314/74. Excepting the fact that the DNA fragment deletion occurred in an AT-rich region, Q3 and strain 1830 shared no obvious common genetic features at the deletion site.
In order to characterize the deletion process (frequency and site specificity) responsible for the ypmA loss observed in strain Q3, we inserted a selection gene [kanamycin resistance gene aph(3′)-IIIa] and a counterselectable marker (the sacB levane sucrase-encoding gene) into the ypmA gene of Y. pseudotuberculosis AH. Since the product of the sacB gene is toxic for gram-negative bacteria in the presence of sucrose, only clones from which sacB is deleted will grow on agar containing this sugar. Sucrose-resistant Yersinia appeared with high frequency (2.8 × 10−5 ± 7 × 10−7), and among these sucrose-resistant clones, 21.2% ± 4.2% were sensitive to kanamycin (Table 4). Overall, deletion of the sacB and aph(3′)-IIIa genes occurred with a frequency of 5.9 × 10−6 (Table 4). Six of these sucrose-resistant, kanamycin-sensitive clones were further characterized (Fig. 4B). DNA deletions ranged from 1.8 to 19.6 kb, none of which were found twice. Comparison of 100 bp at the upstream and downstream deletion sites did not reveal any features common to the mutants, thus confirming that the deletion mechanism is not site specific. The yrs site was preserved in all mutants, suggesting that these 26-bp motifs might be important for the deletion process.
TABLE 4.
Deletion frequencies of the aph(3′)IIIa and sacB genes as a function of their location in the Y. pseudotuberculosis AH genome
Mean value of three separate experiments ± standard deviation.
Sucrose-resistant clones were plated on LB agar in the presence of kanamycin. The percentage represents the ratio of kanamycin-sensitive bacteria to the sucrose-resistant bacterial population (mean value of three separate experiments ± standard deviation).
Frequency of sucrose-resistant bacteria multiplied by the proportion of kanamycin-sensitive bacteria to the sucrose-resistant bacterial population.
As a control, we placed the two reporter genes [aph(3′)-IIIa and sacB] upstream of the Y. pseudotuberculosis AH urease operon. This chromosomal region was chosen because as far as we know, no Y. pseudotuberculosis strain has been found to be deficient in urease activity due to deletion of its locus, indicating that the region is stable. Selection on 10% sucrose and then on kanamycin medium revealed that the occurrence of the sucrose-resistant, kanamycin-sensitive clones was much less frequent when the reporter genes were inserted upstream of the urease locus than when they were located within ypmA (2.4 × 10−8 versus 5.9 × 10−6, respectively) (Table 4). This means that deletions in the ypmA locus occur 245 times more frequently than those in the urease locus, demonstrating an instability of the chromosomal locus containing the superantigen gene ypmA.
DISCUSSION
Bacterial superantigenic toxin genes have been frequently associated with mobile genetic elements (21, 34, 40). Staphylococcal enterotoxin SEA and SEE genes and streptococcal exotoxin genes (speA, speC, speH, and speI) are found on phage DNA (7, 17, 21, 23, 32), whereas the enterotoxin SED gene and to a lesser extent the SEB gene are plasmid borne (3, 4, 31). Furthermore, the toxic shock syndrome toxin 1 gene and the recently described enterotoxin genes (sei, seg, sek, sel, sem, seo, and sep) appear to be genetically linked to four different staphylococcal pathogenicity islands (34, 36, 40). In this study, we demonstrate that ypm genes are chromosomally encoded and that although there is a putative phage integration site (yrs) 245 bp upstream of the ypm genes, bacteriophage proteins were not found in their vicinity. This rules out the association of a specific phage with the superantigen genes. Sequence analysis also showed that the ypm genes were associated neither with a transposon nor with a pathogenicity island as defined by Hacker and Kaper (26). For the 30 superantigen-producing strains that we tested, the ypm genes were always found at the same location in the Y. pseudotuberculosis genome, between ORF3 and ORF4. This feature is a strong argument that prompts us to question whether the ypm genes are mobile. Indeed, a number of different chromosomal locations of the ypm genes, as is the case for SEA (6), would have suggested mobility of the superantigen-encoding genes. Taken together, sequence analyses of the ypm locus demonstrated that the genetic organization of the superantigen gene of Y. pseudotuberculosis is different from that found for other superantigenic toxins.
Once the absence of an association of the ypm genes with mobile genetic elements was established, the following question remained: have nonsuperantigenic Y. pseudotuberculosis strains lost the superantigen gene or was ypm never taken up by these genomes? Nucleotide sequence analysis showed that the ORF3-ORF4 intergenic region was identical in 10 of 11 epidemiologically unrelated, nonsuperantigenic strains. Since ypm genes are not flanked by characteristic DNA sequences such as IRs, it is unlikely that a deletion occurred at the exact same location in these nonsuperantigenic strains. This suggests that the absence of the 918-bp, ypm-containing fragment between ORF3 and ORF4 is due to the nonincorporation of ypm rather than to a deletion of the gene. To test this hypothesis, we looked for spontaneous mutants and isolated mutant Q3, which did not display the ORF3-ORF4 intergenic region found in nonsuperantigenic strains but presented a larger deletion of 2.5 kb. Furthermore, when we selected deletion mutants with aph(3′)-IIIa and sacB genes, none of the mutants resembled the wild-type nonsuperantigenic strains (Fig. 4). Hence, sequence analyses and experimental data all indicate that nonsuperantigenic Y. pseudotuberculosis probably never integrated a superantigen gene into their genome.
Although Y. pestis and Y. pseudotuberculosis are closely related genetically, the 13.3-kb region between ORF1 and ORF10 containing ypmA was not present in the Y. pestis genome (strains CO92 and KIM5). Instead, Y. pestis displays a 9.3-kb locus containing 13 ORFs, corresponding to phage remnants (Fig. 5) (49). Interestingly, the only common nucleotide sequence between Y. pestis and Y. pseudotuberculosis in the ORF1-ORF10 intergenic space was a 581-bp region containing the yrs site. Sequence analysis of the boundary regions in Y. pestis revealed that the phage genes were inserted within this 581-bp region (Fig. 5) and that a part of the yrs site was duplicated. This demonstrates that, like in X. campestris (18), the yrs site can function as a phage integration site. Genetic analysis indicates how Y. pestis acquired the phage genes but cannot explain the absence of ORF2 to ORF9. Since Y. pestis is considered to be a clone of Y. pseudotuberculosis that emerged 1,500 to 20,000 years ago (2), one can speculate that the 13.3-kb locus containing ypmA (except the yrs region) was deleted from the Y. pestis genome and that this event was followed by the insertion of the bacteriophage genes. However, we can also hypothesize that the Y. pestis genome never harbored the ORF2 to ORF9 genes, suggesting that the ancestral Y. pseudotuberculosis clone (from which Y. pestis derived) did not contain these genes either. Comparison of the genetic organization in the two Yersinia species also suggested that the ORF2-ORF9 region of Y. pseudotuberculosis might represent a pathogenicity islet as described in Salmonella (24). The absence of significant inverted or direct repeats flanking this element rules out the designation of the Y. pseudotuberculosis 13.3-kb sequence as a pathogenicity islet.
Other elements indicated the occurrence of genetic events in this genomic region of Yersinia. First, we found a wild-type strain of Y. pseudotuberculosis (strain 1830) with a 5-kb deletion within the ypm locus followed by a 1.6-kb insertion of an unknown ORF (Fig. 4). Second, the strains expressing the YPMc variant—which clearly represent a clonal population (12)—display two deletions in the ypm locus (Fig. 1). Third, we were able to demonstrate experimentally the occurrence in the locus of high-frequency deletion without precise excision (strain Q3 and mutants 1 to 6) (Fig. 4; Table 4). Taken as a whole, these arguments strongly indicate the genetic instability of the region containing the ypm genes.
In this work, we also described IS1398, a novel IS with no homology to any known mobile genetic element. IS1398 displays some features of the Tn3 family, that is to say, (i) a single copy per genome (35), (ii) 35-bp IRs ending the IS and homologous to the γδ sequences of Tn1000 (52), and (iii) a pentanucleotide duplication at the genomic integration site (52) (Fig. 3). However, the functionality of this genetic element remains unproved. The small size of IS1398 (1.8kb for IS1398 versus 4.9 and 5.7 kb for Tn3 and Tn1000 [38], respectively) and the absence of homology of ORF5 with transposase (tnpA) and resolvase (tnpR) genes might suggest that IS1398 represents a Tn3-like transposon remnant. Because of the proximity of ypmA and IS1398, it was initially tempting to associate the presence of IS1398 with the heterogeneous distribution of the ypm genes among the Y. pseudotuberculosis strains. We clearly demonstrated that there was no close genetic relationship between IS1398 and ypm. Indeed, some strains harboring IS1398 do not contain a ypm gene and conversely some superantigen-expressing strains were IS1398-free. Furthermore, IS1398 is not associated with the ancestral gene ypmB, ruling out a role of IS1398 in the initial integration of ypmB into the Y. pseudotuberculosis genome.
Nucleotide sequence analysis of the ypm genes and their flanking regions sheds some light on the ypm gene evolution. The GC contents of the ypm genes (34.6% for ypmB and 35.3% for ypmA and ypmC) with regard to that of the Y. pseudotuberculosis genome as a whole (46.5%) (5) suggest a horizontal gene transfer from a microorganism with low GC content. Superantigen-producing species such as Streptococcus pyogenes, Staphylococcus aureus, and Mycoplasma arthritidis could be good candidates, since their GC contents are low (38.5, 32.8, and 30 to 32.6%, respectively) (21, 34, 51). However, the codon usage of ypmB, which is supposed to be the ancestral ypm gene, according to a recent epidemiological study (12), is different from that found in group A streptococci, S. aureus, or M. arthritidis (data not shown). This indicates either another origin for the ypm genes or a drift of the ypm sequence towards the Yersinia GC content. In the near future, the various sequencing projects of bacterial genomes may well generate important information on the possible origin of the ypm genes. How the ypmB gene originally integrated into the genome of Y. pseudotuberculosis without the presence of flanking IRs or specific sites is still speculative. The most plausible hypothesis is that ypmB integration into the Y. pseudotuberculosis genome was the consequence of a recombination event, as was the case for the BRO β-lactamase gene in Moraxella catarrhalis (9). Nevertheless, since yersiniae are non-naturally competent, the bro gene entry mechanism in M. catarrhalis cannot be extended to Y. pseudotuberculosis. Hence, the uptake of ypmB by Y. pseudotuberculosis probably required the presence of a mobilizing structure, such as a phage or a plasmid. Considering the presence of a sequence with homology to a phage integration site (the yrs site) in the ypm locus and the association of this site with phage proteins in Y. pestis, we can speculate on the involvement of a phage in the uptake of the ypm genes. After the initial integration of ypmB into the Y. pseudotuberculosis genome, the superantigen gene evolved to give ypmA and ypmC. Interestingly, a recent epidemiological study bringing together more than 2,200 strains indicated that all ypmB+ strains were found in nonhuman hosts and in the environment, whereas the ypmA and ypmC alleles were present in human pathogenic strains (22). This suggests that the genetic drift of the Y. pseudotuberculosis superantigen gene from ypmB towards the ypmA and ypmC alleles might be correlated with the increased virulence of Y. pseudotuberculosis.
Acknowledgments
C. Carnoy was supported by a grant from the Centre Hospitalier Régional et Universitaire de Lille and by the Fondation pour la Recherche Médicale. This work was supported by the Region Nord-Pas de Calais and the European Regional Development Fund.
We thank J.-P. Bohin for critical reading of the manuscript and Ingrid Loison for technical assistance.
REFERENCES
- 1.Abe, J., T. Takeda, Y. Watanabe, H. Nakao, N. Kobayashi, D. Y. M. Leung, and T. Kohsaka. 1993. Evidence for superantigen production by Yersinia pseudotuberculosis. J. Immunol. 151:4183-4188. [PubMed] [Google Scholar]
- 2.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 96:14043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altboum, Z., I. Hertman, and S. Sarid. 1985. Penicillinase plasmid-linked genetic determinants for enterotoxins B and C1 production in Staphylococcus aureus. Infect. Immun. 47:514-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayles, K. W., and J. J. Iandolo. 1989. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J. Bacteriol. 171:4799-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bercovier, H., and H. H. Mollaret. 1984. Genus Yersinia, p. 498-506. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, Md.
- 6.Betley, M. J., S. Lofdahl, B. N. Kreiswirth, M. S. Bergdoll, and R. P. Novick. 1984. Staphylococcal enterotoxin A gene is associated with a variable genetic element. Proc. Natl. Acad. Sci. USA 81:5179-5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betley, M. J., and J. J. Mekalanos. 1985. Staphylococcal enterotoxin A is encoded by a phage. Science 229:185-187. [DOI] [PubMed] [Google Scholar]
- 8.Blomfield, I. C., V. Vaughn, R. F. Rest, and B. I. Eisenstein. 1991. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol. 5:1447-1457. [DOI] [PubMed] [Google Scholar]
- 9.Bootsma, H. J., H. van Dijk, P. Vauterin, J. Verhoef, and F. R. Mooi. 2000. Genesis of BRO β-lactamase-producing Moraxella catarrhalis: evidence for transformation-mediated horizontal transfer. Mol. Microbiol. 36:93-104. [DOI] [PubMed] [Google Scholar]
- 10.Butler, T. 1983. Plague and other Yersinia infection. Plenum Press, New York, N.Y.
- 11.Carnoy, C., H. Müller-Alouf, S. Haentjens, and M. Simonet. 1998. Polymorphism of ypm, Yersinia pseudotuberculosis superantigen-encoding gene. Zentbl. Bakteriol. 29(Suppl.):397-398. [Google Scholar]
- 12.Carnoy, C., and M. Simonet. 1999. Yersinia pseudotuberculosis superantigenic toxins, p. 611-622. In J. E. Alouf and J. H. Freer (ed.), Bacterial protein toxins: a comprehensive sourcebook, 2nd ed. Academic Press, London, United Kingdom.
- 13.Carnoy, C., C. Mullet, H. Müller-Alouf, E. Leteurtre, and M. Simonet. 2000. Superantigen YPMa exacerbates the virulence of Yersinia pseudotuberculosis in mice. Infect. Immun. 68:2553-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerget, M. 1984. A 140 base-pair DNA segment from the kanamycin resistance region of plasmid R1 acts as an origin of replication and promotes site-specific recombination. J. Mol. Biol. 178:35-46. [DOI] [PubMed] [Google Scholar]
- 16.Clerget, M. 1991. Site-specific recombination promoted by a short DNA segment of plasmid R1 and by a homologous segment in the terminus region of the Escherichia coli chromosome. New Biol. 3:780-788. [PubMed] [Google Scholar]
- 17.Couch, J. L., M. T. Soltis, and M. J. Betley. 1988. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J. Bacteriol. 170:2954-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai, H., T. Y. Chow, H. J. Liao, Z. Y. Chen, and K. S. Chiang. 1988. Nucleotide sequences involved in the neolysogenic insertion of filamentous phage Cf16-v1 into the Xanthomonas campestris pv. citri chromosome. Virology 167:613-620. [PubMed] [Google Scholar]
- 19.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnenberg, M. S., J. Yu, and J. B. Kaper. 1993. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J. Bacteriol. 175:4670-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukushima, H., Y. Matsuda, R. Seki, M. Tsubokura, N. Takeda, F. N. Shubin, I. K. Paik, and X. B. Zheng. 2001. Geographical heterogeneity between Far Eastern and Western countries in prevalence of the virulence plasmid, the superantigen Yersinia pseudotuberculosis-derived mitogen, and the high-pathogenicity island among Yersinia pseudotuberculosis strains. J. Clin. Microbiol. 39:3541-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goshorn, S. C., and P. M. Schlievert. 1989. Bacteriophage association of streptococcal pyrogenic exotoxin type C. J. Bacteriol. 171:3068-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groisman, E. A., and H. Ochman. 1997. How Salmonella became a pathogen. Trends Microbiol. 5:343-349. [DOI] [PubMed] [Google Scholar]
- 25.Gunn, J. S., W. J. Belden, and S. I. Miller. 1998. Identification of PhoP-PhoQ activated genes within a duplicated region of the Salmonella typhimurium chromosome. Microb. Pathog. 25:77-90. [DOI] [PubMed] [Google Scholar]
- 26.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 27.Haentjens-Herwegh, S., C. Carnoy, F. Wallet, and R. J. Courcol. 2000. Development and use of internal positive controls for PCR detection of microorganisms, p. 547-556. In S. G. Pandalai (ed.), Recent research developments in microbiology, 4th ed., vol. 4. Transworld Research Network, Trivandrum, India.
- 28.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 29.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 30.Iteman, I., H. Najdenski, and E. Carniel. 1995. High genomic polymorphism in Yersinia pseudotuberculosis. Contrib. Microbiol. Immunol. 13:106-111. [PubMed] [Google Scholar]
- 31.Johns, M. B., Jr., and S. A. Khan. 1988. Staphylococcal enterotoxin B gene is associated with a discrete genetic element. J. Bacteriol. 170:4033-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, L. P., M. A. Tomai, and P. M. Schlievert. 1986. Bacteriophage involvement in group A streptococcal pyrogenic exotoxin A production. J. Bacteriol. 166:623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuempel, P. L., J. M. Henson, L. Dircks, M. Tecklenburg, and D. F. Lim. 1991. dif, a recA-independent recombination site in the terminus region of the chromosome of Escherichia coli. New Biol. 3:799-811. [PubMed] [Google Scholar]
- 34.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 35.Lee, C. H., A. Bhagwat, and F. Heffron. 1983. Identification of a transposon Tn3 sequence required for transposition immunity. Proc. Natl. Acad. Sci. USA 80:6765-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsay, J. A., A. Ruzin, H. F. Ross, N. Kurepina, and R. P. Novick. 1998. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol. Microbiol. 29:527-543. [DOI] [PubMed] [Google Scholar]
- 37.Louarn, J. M., J. Louarn, V. Francois, and J. Patte. 1991. Analysis and possible role of hyperrecombination in the termination region of the Escherichia coli chromosome. J. Bacteriol. 173:5097-5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maloy, S. R., J. E. Cronan, Jr., and D. Freifelder. 1994. Microbial genetics, 2nd ed., p. 239-262. Jones and Bartlett Publishers, Inc., Sudbury, Mass.
- 39.Marmur, J. 1961. A procedure for the isolation of desoxyribonucleic acid from microorganisms. J. Mol. Biol. 3:208-218. [Google Scholar]
- 40.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 41.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mindlin, S. Z., G. Y. Kholodii, Z. M. Gorlenko, S. V. Minakhina, L. S. Minakhin, E. S. Kalyaeva, A. V. Kopteva, M. A. Petrova, O. V. Yurieva, and V. G. Nikiforov. 2001. Mercury resistance transposons of Gram-negative environmental bacteria and their classification. Res. Microbiol. 152:811-822. [DOI] [PubMed] [Google Scholar]
- 43.Miyoshi-Akiyama, T., A. Abe, H. Kato, K. Kawahara, H. Narimatsu, and T. Uchiyama. 1995. DNA sequencing of the gene encoding a bacterial superantigen, Yersinia pseudotuberculosis-derived mitogen (YPM), and characterization of the gene product, cloned YPM. J. Immunol. 154:5228-5234. [PubMed] [Google Scholar]
- 44.Miyoshi-Akiyama, T., W. Fujimaki, X. J. Yan, J. Yagi, K. Imanishi, H. Kato, K. Tomonari, and T. Uchiyama. 1997. Identification of murine T cells reactive with the bacterial superantigen Yersinia pseudotuberculosis-derived mitogen (YPM) and factors involved in YPM-induced toxicity in mice. Microbiol. Immunol. 41:345-352. [DOI] [PubMed] [Google Scholar]
- 45.Moore, R. L., and R. R. Brubaker. 1975. Hybridization of deoxyribonucleotide sequences of Yersinia enterocolitica and other selected members of Enterobacteriaceae. Int. J. Syst. Bacteriol. 25:336-339. [Google Scholar]
- 46.Müller-Alouf, H., C. Carnoy, M. Simonet, and J. E. Alouf. 2001. Superantigen bacterial toxins: state of the art. Toxicon 39:1691-1701. [DOI] [PubMed] [Google Scholar]
- 47.Nakano, T., H. Kawaguchi, K. Nakao, T. Maruyama, H. Kamiya, and M. Sakurai. 1989. Two outbreaks of Yersinia pseudotuberculosis 5a infection in Japan. Scand. J. Infect. Dis. 21:175-179. [DOI] [PubMed] [Google Scholar]
- 48.Odaert, M., P. Berche, and M. Simonet. 1996. Molecular typing of Yersinia pseudotuberculosis by using an IS200-like element. J. Clin. Microbiol. 34:2231-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 50.Ramamurthy, T., K.-I. Yoshino, J. Abe, N. Ikeda, and T. Takeda. 1997. Purification, characterization and cloning of a novel variant of the superantigen Yersinia pseudotuberculosis-derived mitogen. FEBS Lett. 413:174-176. [DOI] [PubMed] [Google Scholar]
- 51.Razin, S., and E. A. Freundt. 1984. The Mycoplasmas, p. 740-793. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, Md.
- 52.Reed, R. R., R. A. Young, J. A. Steitz, N. D. F. Grindley, and M. S. Guyer. 1979. Transposition of the Escherichia coli insertion element γ generates a five-base-pair repeat. Proc. Natl. Acad. Sci. 76:4882-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 54.Sebbane, F., A. Devalckenaere, J. Foulon, E. Carniel, and M. Simonet. 2001. Silencing and reactivation of urease in Yersinia pestis is determined by one G residue at a specific position in the ureD gene. Infect. Immun. 69:170-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 56.Snellings, N. J., M. Popek, and L. E. Lindler. 2001. Complete DNA sequence of Yersinia enterocolitica serotype 0:8 low-calcium-response plasmid reveals a new virulence plasmid-associated replicon. Infect. Immun. 69:4627-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stuart, P. M., and J. G. Woodward. 1992. Yersinia enterocolitica produces superantigenic activity. J. Immunol. 148:225-233. [PubMed] [Google Scholar]
- 58.Stuart, P. M., R. K. Munn, E. Demoll, and J. G. Woodward. 1995. Characterization of human T-cell responses to Yersinia enterocolitica superantigen. Hum. Immunol. 43:269-275. [DOI] [PubMed] [Google Scholar]
- 59.Tertti, R., K. Granfors, O.-P. Lehtonen, J. Mertsola, A.-L. Mäkelä, I. Välimäki, P. Hänninen, and A. Toivanen. 1984. An outbreak of Yersinia pseudotuberculosis infection. J. Infect. Dis. 149:245-250. [DOI] [PubMed] [Google Scholar]
- 60.Tertti, R., R. Vuento, P. Mikkola, K. Granfors, A.-L. Mäkelä, and A. Toivanen. 1989. Clinical manifestations of Yersinia pseudotuberculosis infection in children. Eur. J. Clin. Microbiol. Infect. Dis. 8:587-591. [DOI] [PubMed] [Google Scholar]
- 61.Uchiyama, T., T. Miyoshi-Akiyama, H. Kato, W. Fujimaki, K. Imanishi, and X.-J. Yan. 1993. Superantigenic properties of a novel mitogenic substance produced by Yersinia pseudotuberculosis isolated from patients manifesting acute and systemic symptoms. J. Immunol. 151:4407-4413. [PubMed] [Google Scholar]
- 62.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 63.Yoshino, K.-I., T. Ramamurthy, G. B. Nair, H. Fukushima, Y. Ohtomo, N. Takeda, S. Kaneko, and T. Takeda. 1995. Geographical heterogeneity between Far East and Europe in prevalence of ypm gene encoding the novel superantigen among Yersinia pseudotuberculosis strains. J. Clin. Microbiol. 33:3356-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]