Abstract
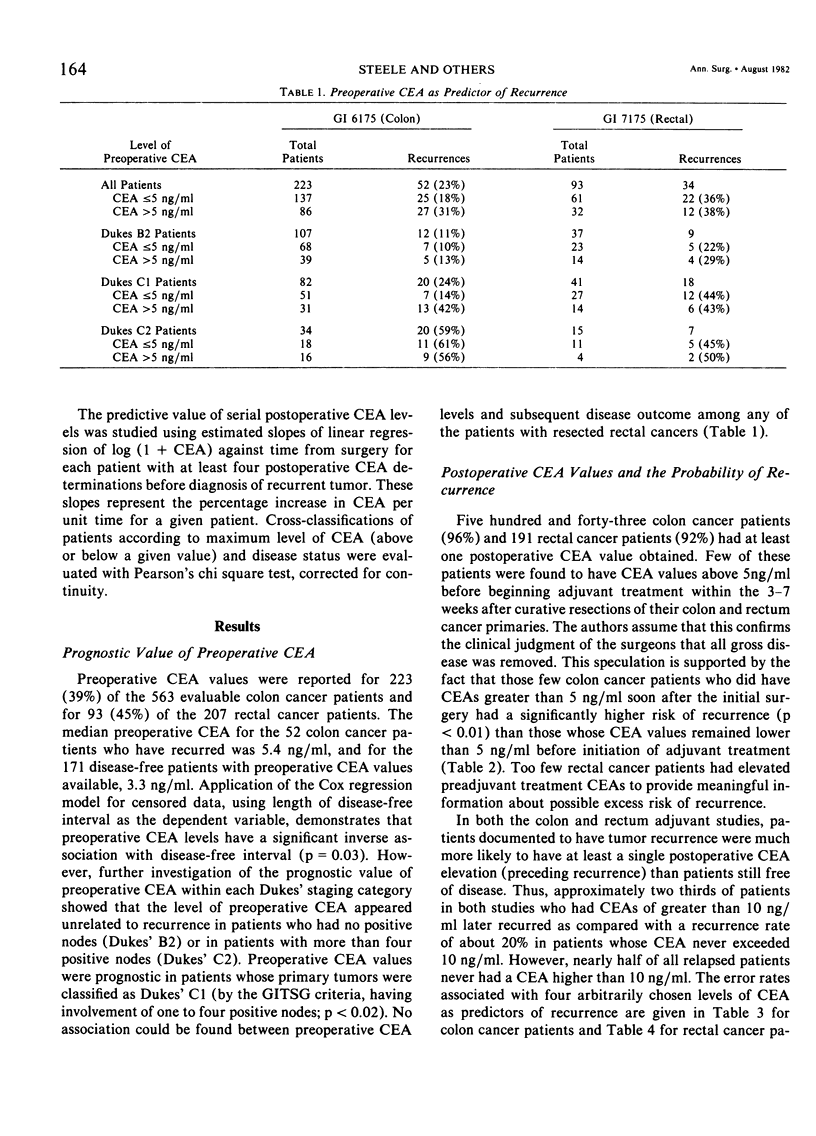
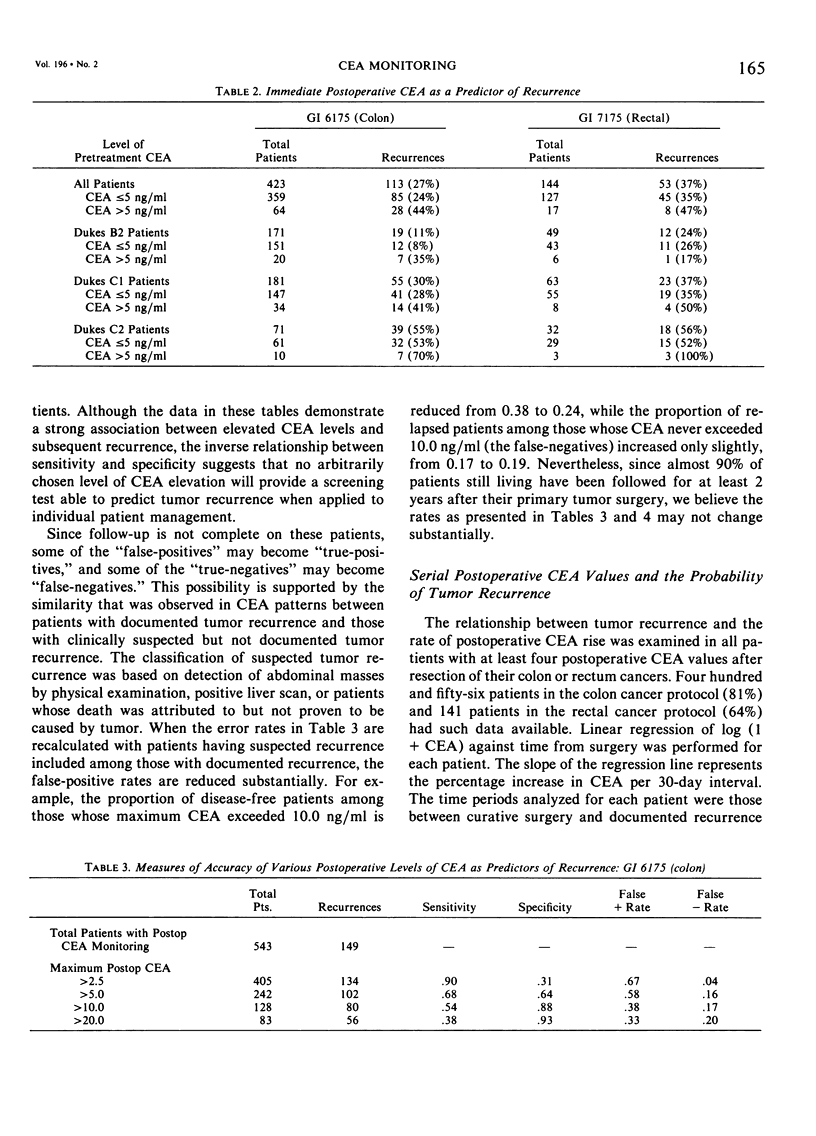
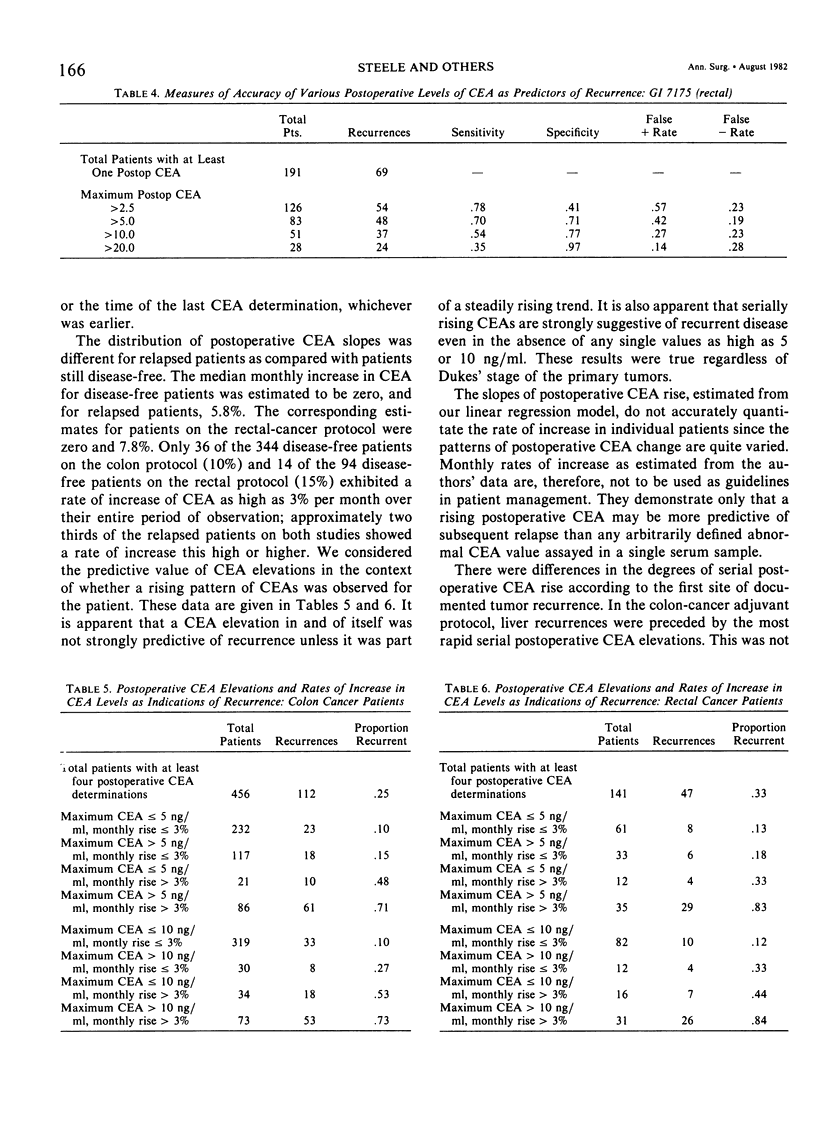
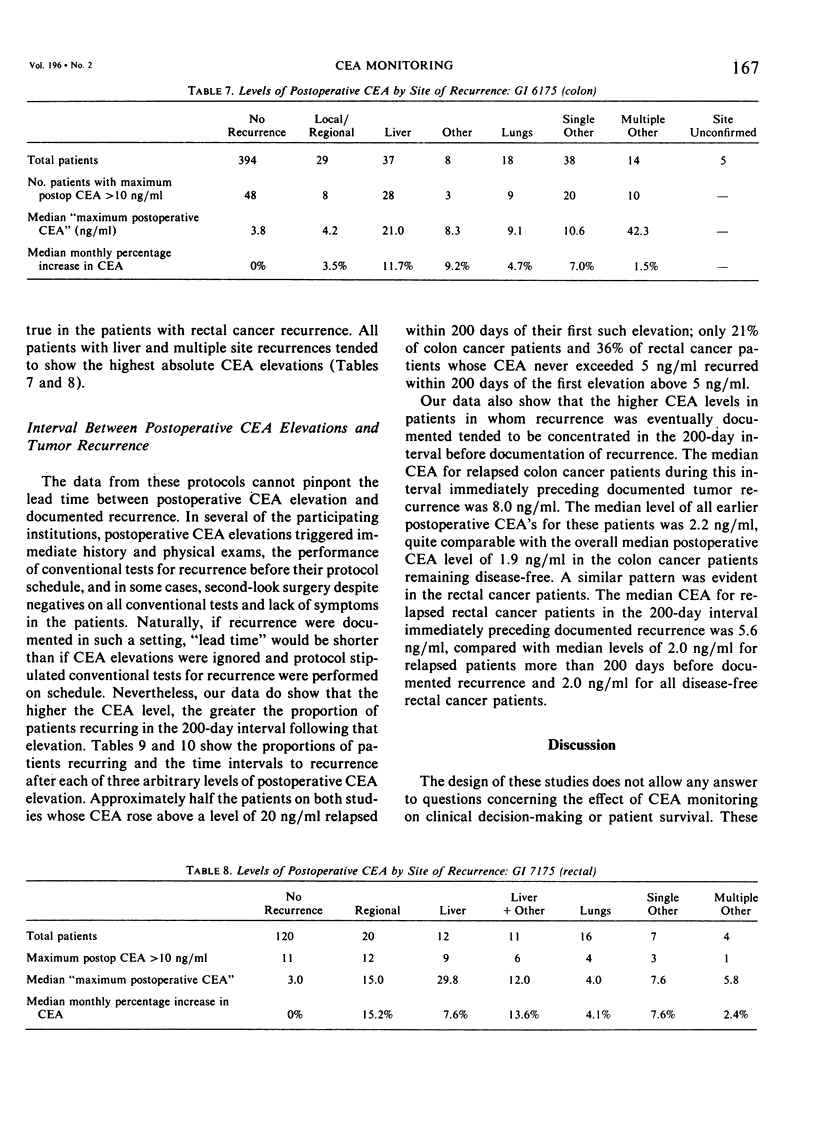
The Gastrointestinal Tumor Study Group (GITSG) has since 1975 included protocols for monitoring carcinoembryonic antigen (CEA) levels in its colorectal cancer adjuvant trials. Among the 563 patients on the colon cancer study (GI 6175) and the 207 patients on the rectal cancer study (GI 7175), one third had preoperative CEA determinations and more than 90% had some postoperative CEA monitoring. Colon cancer patients whose preoperative CEA was greater than 5 ng/ml had a greater probability of recurring than those whose values were lower (33% versus 18% recurrence with 21 months minimum follow-up; p < 0.05). The prognostic value of preoperative CEA was apparent only in patients with Dukes' C1 colon tumors. Preoperative CEA values were not of prognostic significance among the rectal adenocarcinoma patients. Although elevated levels of CEA after resection of either colon or rectum cancers were strongly associated with subsequent tumor recurrence, no single CEA value, arbitrarily defined as “elevated”, provided an adequate screening test with both high sensitivity and high specificity. Postoperative CEA elevations were more strongly predictive of recurrence when part of a steadily rising trend. In the colon cancer study, the median monthly increase in CEA for disease-free patients was estimated to be zero, and for the relapsed patients 5.8%. The corresponding estimates for patients on the rectal cancer protocol were zero and 7.8%. Only 36 of the 344 disease-free patients on the colon protocol and 14 of the 94 disease-free patients on the rectal protocol (15%) exhibited a rate of increase of CEA as high as 3% per month over the entire period of observation. Two thirds of the relapsed patients on both studies showed a rate of increase this high or higher. The patterns of CEA rise in individual patients were quite varied, however, and monthly rates of increase as established in our study are not to be used as guidelines in patient management.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTLER V. B., COLLER F. A. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954 Jun;139(6):846–852. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein B. R., Steele G. D., Jr, Ensminger W., Kaplan W. D., Lowenstein M. S., Wilson R. E., Forman J., Zamcheck N. The use and limitations of serial plasma carcinoembryonic antigen (CEA) levels as a monitor of changing metastatic liver tumor volume in patients receiving chemotherapy. Cancer. 1980 Jul 15;46(2):266–272. doi: 10.1002/1097-0142(19800715)46:2<266::aid-cncr2820460208>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Goslin R., O'Brien M. J., Steele G., Mayer R., Wilson R., Corson J. M., Zamcheck N. Correlation of Plasma CEA and CEA tissue staining in poorly differentiated colorectal cancer. Am J Med. 1981 Aug;71(2):246–253. doi: 10.1016/0002-9343(81)90125-x. [DOI] [PubMed] [Google Scholar]
- Goslin R., Steele G., Jr, Macintyre J., Mayer R., Sugarbaker P., Cleghorn K., Wilson R., Zamcheck N. The use of preoperative plasma CEA levels for the Stratification of patients after curative resection of colorectal cancers. Ann Surg. 1980 Dec;192(6):747–751. doi: 10.1097/00000658-198012000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera M. A., Chu T. M., Holyoke E. D. Carcinoembryonic antigen (CEA) as a prognostic and monitoring test in clinically complete resection of colorectal carcinoma. Ann Surg. 1976 Jan;183(1):5–9. doi: 10.1097/00000658-197601000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin P. T., Zamcheck N., Holyoke E. D. A carcinoembryonic antigen standardization experiment for the conduct of multi-institutional clinical trials. Gastrointestinal Tumor Study Group. Cancer Treat Rep. 1979 Nov-Dec;63(11-12):2031–2033. [PubMed] [Google Scholar]
- Martin E. W., Jr, James K. K., Hurtubise P. E., Catalano P., Minton J. P. The use of CEA as an early indicator for gastrointestinal tumor recurrence and second-look procedures. Cancer. 1977 Feb;39(2):440–446. doi: 10.1002/1097-0142(197702)39:2<440::aid-cncr2820390212>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Minton J. P., Martin E. W., Jr The use of serial CEA determinations to predict recurrence of colon cancer and when to do a second-look operation. Cancer. 1978 Sep;42(3 Suppl):1422–1427. doi: 10.1002/1097-0142(197809)42:3+<1422::aid-cncr2820420807>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Moertel C. G., Schutt A. J., Go V. L. Carcinoembryonic antigen test for recurrent colorectal carcinoma. Inadequacy for early detection. JAMA. 1978 Mar 13;239(11):1065–1066. [PubMed] [Google Scholar]
- Steele G., Jr, Zamcheck N., Wilson R., Mayer R., Lokich J., Rau P., Maltz J. Results of CEA-initiated second-look surgery for recurrent colorectal cancer. Am J Surg. 1980 Apr;139(4):544–548. doi: 10.1016/0002-9610(80)90335-9. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Rao B., Pinsky C. M., Hoffman R. G., Stearns M., Schwartz M. K., Oettgen H. F. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978 Aug 31;299(9):448–451. doi: 10.1056/NEJM197808312990904. [DOI] [PubMed] [Google Scholar]
- Wanebo J. H., Stearns M., Schwartz M. K. Use of CEA as an indicator of early recurrence and as a guide to a selected second-look procedure in patients with colorectal cancer. Ann Surg. 1978 Oct;188(4):481–493. doi: 10.1097/00000658-197810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]


