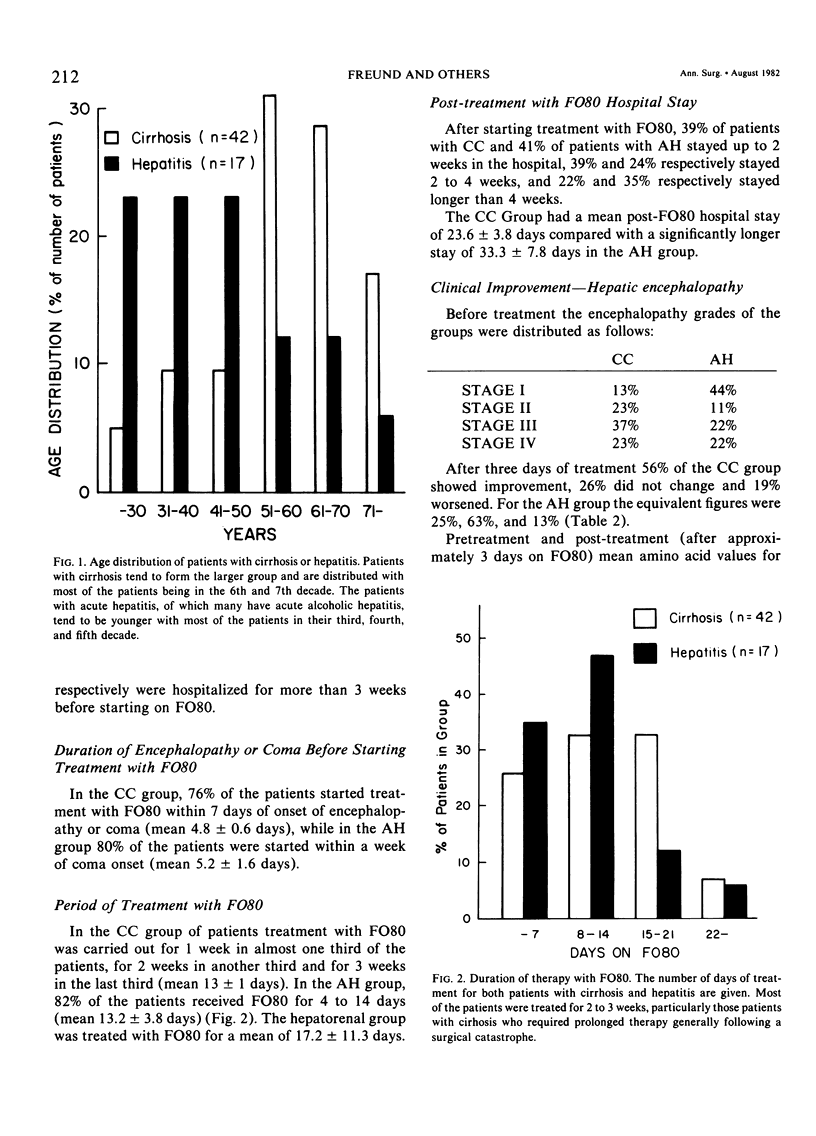
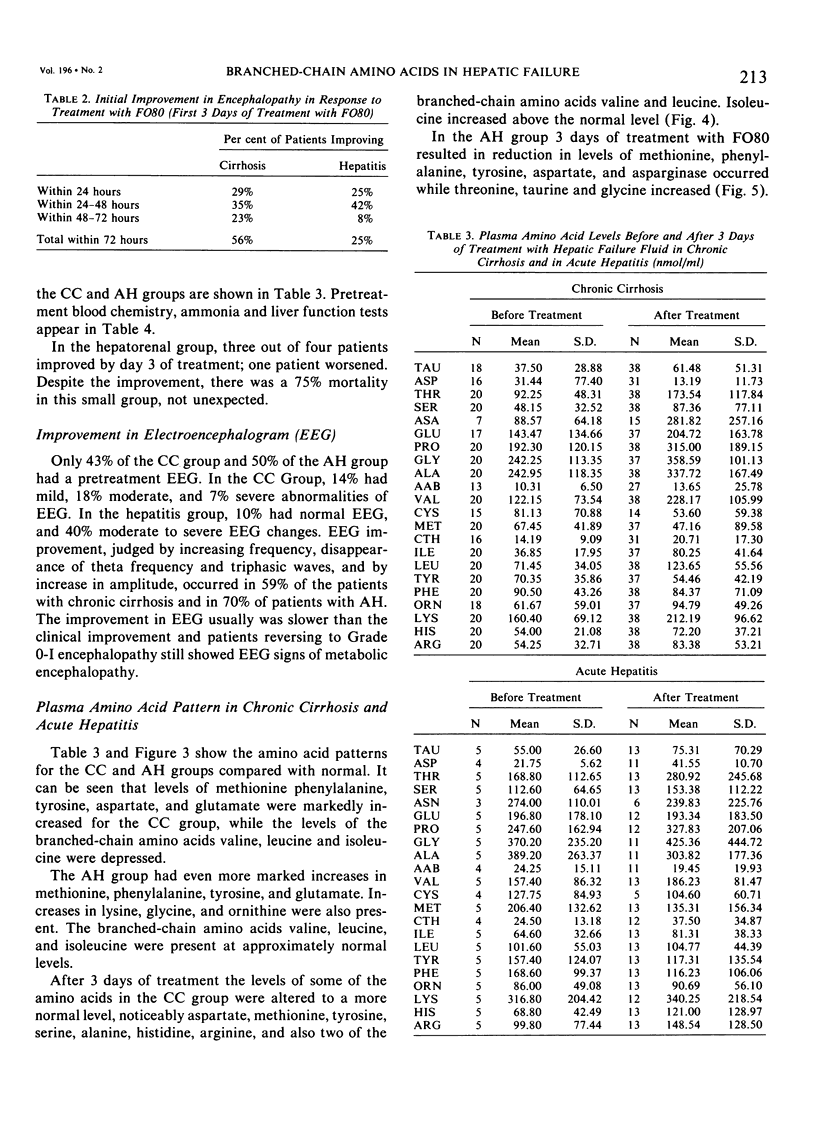
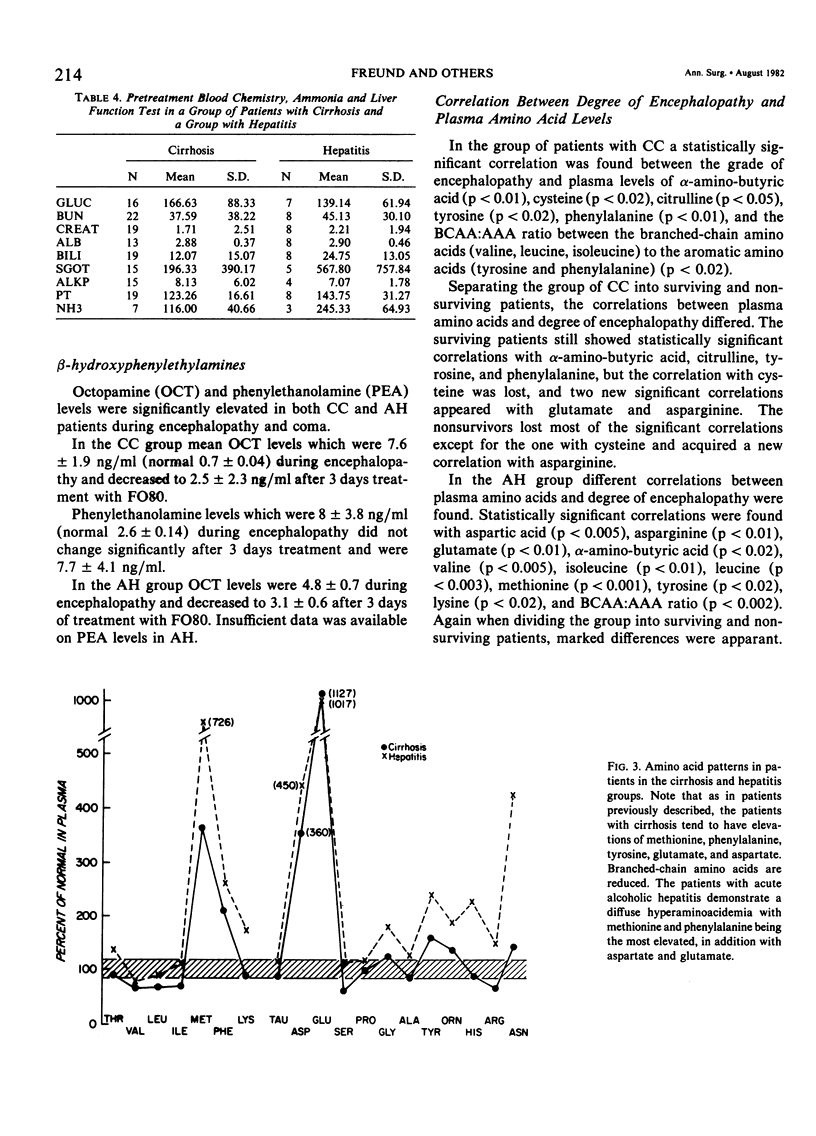
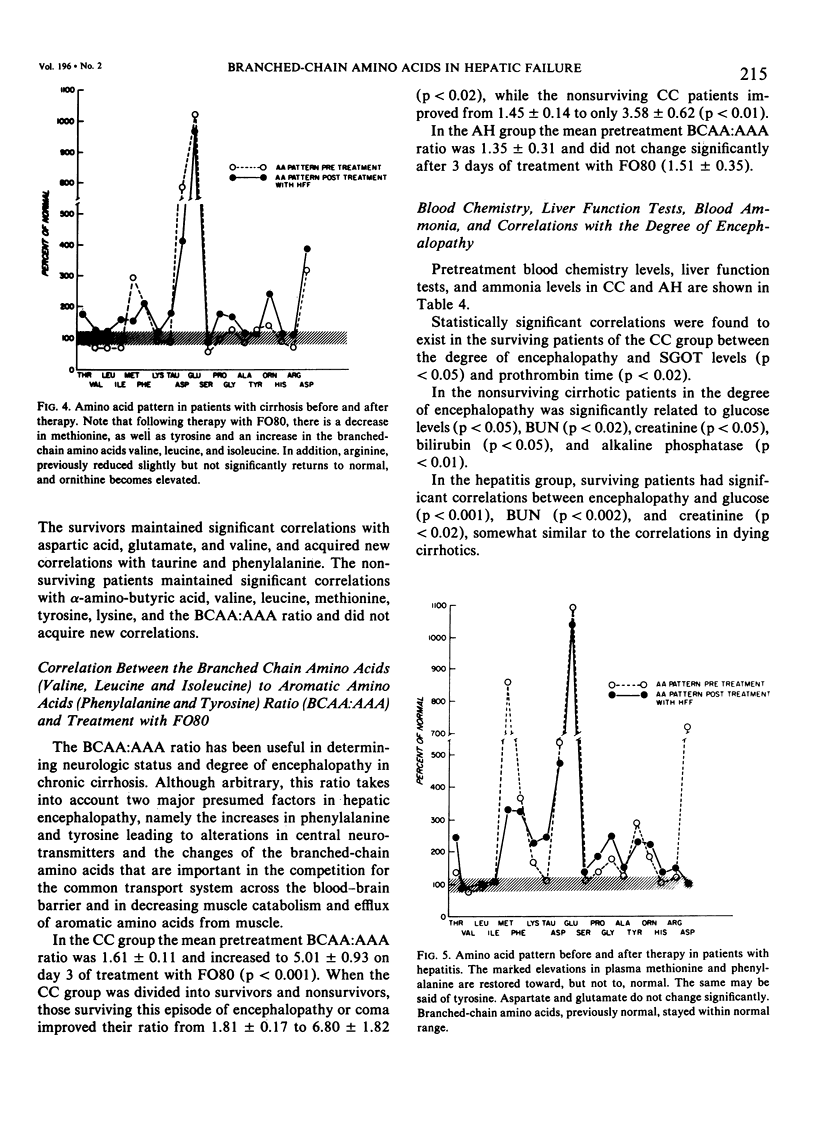
Abstract
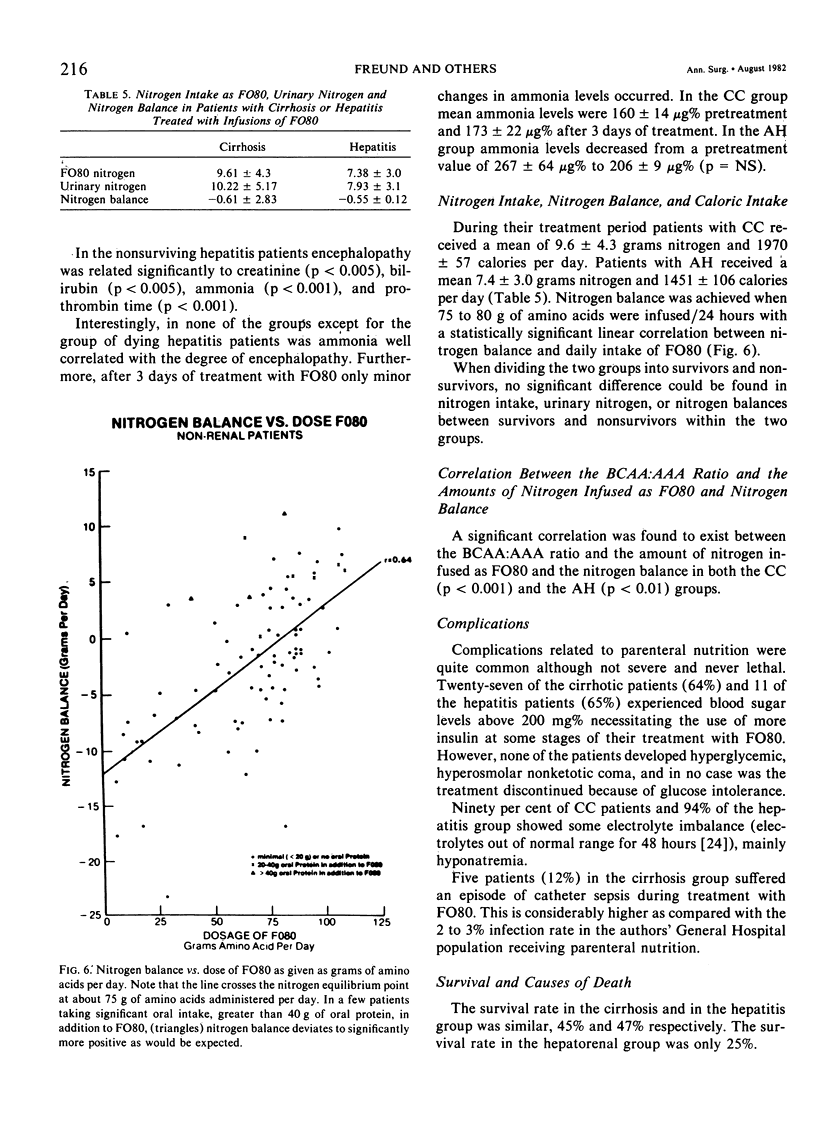
Hospitalized patients with hepatic insufficiency often suffer from severe catabolic states and are in urgent need of nutritional support during their acute illness. Protein intolerence, however, remains a significant problem with respect to the provision of adequate nutrition, either enterally or parenterally. The following report is an anecdotal series of 63 consecutive patients in a large urban hospital treated prospectively with nutritional support using a prototype high branched-chain amino acid solution (FO80) given by technique of total parenteral nutrition by the subclavian or internal jugular route with hypertonic dextrose. Sixty-three patients, of which 42 had chronic liver disease (cirrhosis) with acute decompensation and 17 with acute hepatic injury as well as four with hepatorenal syndrome, are the subject of this report. All required intravenous nutritional support and were either intolerant to commercially available parenteral nutrition solutions or were in hepatic encephalopathy at the time they were initially seen. The cirrhotic patients had been hospitalized for a mean of 14.5 ± 1.9 days before therapy, had a mean bilirubin of 13 mg/100 ml, and had been in coma for 4.8 ± 0.7 days despite standard therapy. Patients with acute hepatitis had been in the hospital for 16.2 ± 4.1 days before therapy, had a mean bilirubin of 25 mg/100 ml, and had been in coma 5.2 ± 1.6 days before therapy. Routine tests of liver function, blood chemistries, amino acids, EEGs, and complex neurological testing including Reitan trailmaking tests were used in the evaluation of these patients. Up to 120 grams of synthetic amino acid solution with hypertonic dextrose was tolerated in these patients with improvement noted in encephalopathy of at least one grade in 87% of the patients with cirrhosis and 75% of the patients with hepatitis. Nitrogen balance was achieved when 75 to 80 grams of synthetic amino acids were administered. Survival was 45% in the cirrhotic group and 47% in the acute hepatitis group. Encephalopathy appeared to correlate with individual amino acids differentially in the various groups and with the ratio between the aromatic and the branched-chain amino acids. Ammonia did not correlate with either the degree of encephalopathy or improvement therefrom. In 24 Patients therapy for hepatic encephalopathy was limited to infusion of the branched-chain enriched amino acid solution only, with wake-up in 66% of this group. The results strongly suggest that in protein intolerant patients requiring nutritional support, infusion with branchedchain enriched amino acid solutions is well tolerated with either no worsening of or improvement in hepatic encephalopathy coincident with the achievement of nitrogen equilibrium and adequate nutritional support.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS R. D., FOLEY J. M. The neurological disorder associated with liver disease. Res Publ Assoc Res Nerv Ment Dis. 1953;32:198–237. [PubMed] [Google Scholar]
- Abel R. M., Beck C. H., Jr, Abbott W. M., Ryan J. A., Jr, Barnett G. O., Fischer J. E. Improved survival from acute renal failure after treatment with intravenous essential L-amino acids and glucose. Results of a prospective, double-blind study. N Engl J Med. 1973 Apr 5;288(14):695–699. doi: 10.1056/NEJM197304052881401. [DOI] [PubMed] [Google Scholar]
- Beaubernard C., Salomon F., Grange D., Thangapregassam M. J., Bisbuth J. Experimental hepatic encephalopathy. Changes of the level of wakefulness in the rat with portacaval shunt. Biomedicine. 1977 Jun;27(4):169–171. [PubMed] [Google Scholar]
- Buse M. G., Reid S. S. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975 Nov;56(5):1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A., Ziparo V., James J. H., Fischer J. E. Loss of day-night rhythm in rats after portacaval shunt. Surg Forum. 1979;30:388–390. [PubMed] [Google Scholar]
- EDMUNDS L. H., Jr, WILLIAMS G. M., WELCH C. E. External fistulas arising from the gastro-intestinal tract. Ann Surg. 1960 Sep;152:445–471. doi: 10.1097/00000658-196009000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeid A. M., Soeters P. B., Murray P., Fischer J. E. Release of vasoactive intestinal peptide (VIP) by intraluminal osmotic stimuli. J Surg Res. 1977 Jul;23(1):25–30. doi: 10.1016/0022-4804(77)90185-8. [DOI] [PubMed] [Google Scholar]
- Elsass P., Lund Y., Ranek L. Encephalopathy in patients with cirrhosis of the liver. A neuropsychological study. Scand J Gastroenterol. 1978;13(2):241–247. doi: 10.3109/00365527809181755. [DOI] [PubMed] [Google Scholar]
- Fischer J. E., Baldessarini R. J. False neurotransmitters and hepatic failure. Lancet. 1971 Jul 10;2(7715):75–80. doi: 10.1016/s0140-6736(71)92048-4. [DOI] [PubMed] [Google Scholar]
- Fischer J. E., Baldessarini R. J. Pathogenesis and therapy of hepatic coma. Prog Liver Dis. 1976;5:363–397. [PubMed] [Google Scholar]
- Fischer J. E., Funovics J. M., Aguirre A., James J. H., Keane J. M., Wesdorp R. I., Yoshimura N., Westman T. The role of plasma amino acids in hepatic encephalopathy. Surgery. 1975 Sep;78(3):276–290. [PubMed] [Google Scholar]
- Fischer J. E., Rosen H. M., Ebeid A. M., James J. H., Keane J. M., Soeters P. B. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery. 1976 Jul;80(1):77–91. [PubMed] [Google Scholar]
- Fischer J. E., Yoshimura N., Aguirre A., James J. H., Cummings M. G., Abel R. M., Deindoerfer F. Plasma amino acids in patients with hepatic encephalopathy. Effects of amino acid infusions. Am J Surg. 1974 Jan;127(1):40–47. doi: 10.1016/0002-9610(74)90009-9. [DOI] [PubMed] [Google Scholar]
- Freund H., Hoover H. C., Jr, Atamian S., Fischer J. E. Infusion of the branched chain amino acids in postoperative patients. Anticatabolic properties. Ann Surg. 1979 Jul;190(1):18–23. doi: 10.1097/00000658-197907000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund H., Yoshimura N., Fischer J. E. The effect of branched chain amino acids and hypertonic glucose infusions on postinjury catabolism in the rat. Surgery. 1980 Apr;87(4):401–408. [PubMed] [Google Scholar]
- James J. H., Escourrou J., Fischer J. E. Blood-brain neutral amino acid transport activity is increased after portacaval anastomosis. Science. 1978 Jun 23;200(4348):1395–1397. doi: 10.1126/science.663619. [DOI] [PubMed] [Google Scholar]
- James J. H., Freund H., Fischer J. E. Amino acids in hepatic encephalopathy. Gastroenterology. 1979 Aug;77(2):421–423. [PubMed] [Google Scholar]
- James J. H., Ziparo V., Jeppsson B., Fischer J. E. Hyperammonaemia, plasma aminoacid imbalance, and blood-brain aminoacid transport: a unified theory of portal-systemic encephalopathy. Lancet. 1979 Oct 13;2(8146):772–775. doi: 10.1016/s0140-6736(79)92119-6. [DOI] [PubMed] [Google Scholar]
- Knell A. J., Davidson A. R., Williams R., Kantamaneni B. D., Curzon G. Dopamine and serotonin metabolism in hepatic encephalopathy. Br Med J. 1974 Mar 23;1(5907):549–551. doi: 10.1136/bmj.1.5907.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Faraj B. A., Caplan D. B., Ali F. M., Camp V. M., Ahmann P. A. Prolactin and the encephalopathy of Reye's syndrome. Lancet. 1979 Nov 24;2(8152):1097–1100. doi: 10.1016/s0140-6736(79)92504-2. [DOI] [PubMed] [Google Scholar]
- Odessey R., Khairallah E. A., Goldberg A. L. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem. 1974 Dec 10;249(23):7623–7629. [PubMed] [Google Scholar]
- Oldendorf W. H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971 Dec;221(6):1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Ono J., Hutson D. G., Dombro R. S., Levi J. U., Livingstone A., Zeppa R. Tryptophan and hepatic coma. Gastroenterology. 1978 Feb;74(2 Pt 1):196–200. [PubMed] [Google Scholar]
- Rosen H. M., Soeters P. B., James J. H., Hodgman J., Fisher J. E. Influences of exogenous intake and nitrogen balance on plasma and brain aromatic amino acid concentrations. Metabolism. 1978 Apr;27(4):393–404. doi: 10.1016/0026-0495(78)90095-1. [DOI] [PubMed] [Google Scholar]
- Rosen H. M., Yoshimura N., Hodgman J. M., Fischer J. E. Plasma amino acid patterns in hepatic encephalopathy of differing etiology. Gastroenterology. 1977 Mar;72(3):483–487. [PubMed] [Google Scholar]
- Salerno F., Dioguardi F. S., Abbiati R. Tryptophan and hepatic coma. Gastroenterology. 1978 Oct;75(4):769–770. [PubMed] [Google Scholar]
- Sapir D. G., Walser M. Nitrogen sparing induced early in starvation by infusion of branched-chain ketoacids. Metabolism. 1977 Mar;26(3):301–308. doi: 10.1016/0026-0495(77)90077-4. [DOI] [PubMed] [Google Scholar]
- Smith A. R., Rossi-Fanelli F., Freund H., Fischer J. E. Sulfur-containing amino acids in experimental hepatic coma in the dog and the monkey. Surgery. 1979 Jun;85(6):677–683. [PubMed] [Google Scholar]
- Soeters P. B., Fischer J. E. Insulin, glucagon, aminoacid imbalance, and hepatic encephalopathy. Lancet. 1976 Oct 23;2(7991):880–882. doi: 10.1016/s0140-6736(76)90541-9. [DOI] [PubMed] [Google Scholar]
- WARREN K. S., SCHENKER S. EFFECT OF AN INHIBITOR OF GLUTAMINE SYNTHESIS (METHIONINE SULFOXIMINE) ON AMMONIA TOXICITY AND METABOLISM. J Lab Clin Med. 1964 Sep;64:442–449. [PubMed] [Google Scholar]
- Warbritton J. D., Geyer M. A., Jeppsson B. W., Fischer J. E. Behavioral model of early hepatic encephalopathy in rats. Surg Forum. 1979;30:394–396. [PubMed] [Google Scholar]


