Abstract
Sequencing a 8,519-bp segment of the Sulfolobus acidocaldarius genome revealed the existence of a tightly packed bipolar pyrimidine gene cluster encoding the enzymes of de novo UMP synthesis. The G+C content of 35.3% is comparable to that of the entire genome, but intergenic regions exhibit a considerably lower percentage of strong base pairs. Coding regions harbor the classical excess of purines on the coding strand, whereas intergenic regions do not show this bias. Reverse transcription-PCR and primer extension experiments demonstrated the existence of two polycistronic messengers, pyrEF-orf8 and pyrBI-orf1-pyrCD-orf2-orf3-orf4, initiated from a pair of divergent and partially overlapping promoters. The gene order and the grouping in two wings of a bipolar operon constitute a novel organization of pyr genes that also occurs in the recently determined genome sequences of Sulfolobus solfataricus P2 and Sulfolobus tokodaii strain 7; the configuration appears therefore characteristic of Sulfolobus. The quasi-leaderless pyrE and pyrB genes do not bear a Shine-Dalgarno sequence, whereas the initiation codon of promoter-distal genes is preceded at an appropriate distance by a sequence complementary to the 3′ end of 16S rRNA. The polycistronic nature of the pyr messengers and the existence of numerous overlaps between contiguous open reading frames suggests the existence of translational coupling. pyrB transcription was shown to be approximately twofold repressed in the presence of uracil. The mechanism underlying this modulation is as yet unknown, but it appears to be of a type different from the various attenuation-like mechanisms that regulate pyrB transcription in bacteria. In contrast, the pyrE-pyrB promoter/control region harbors direct repeats and imperfect palindromes reminiscent of target sites for the binding of a hypothetical regulatory protein(s).
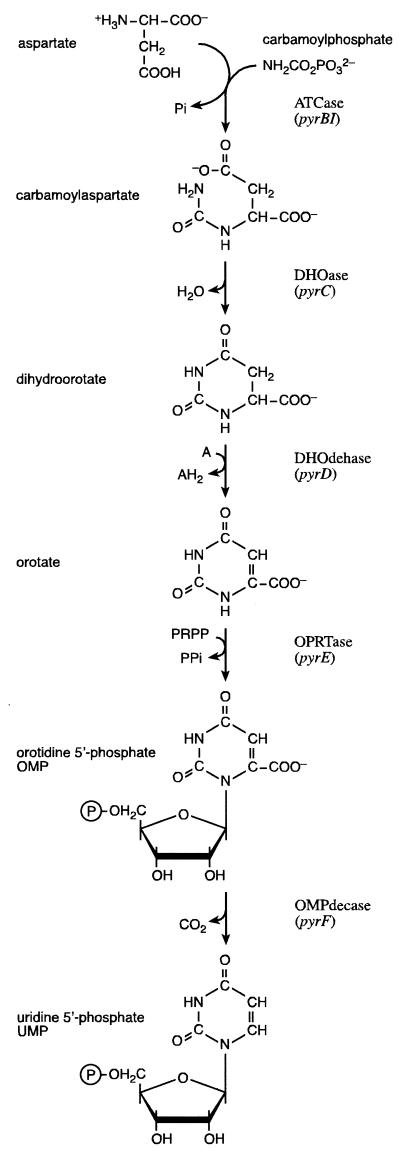
De novo synthesis of UMP is universally performed in six steps catalyzed by conserved enzymes in all three domains of life (Fig. 1). In lower eukaryotes, such as the yeast Saccharomyces cerevisiae, the carbamoylphosphate synthetase (CPSase) (EC 6.3.5.5) and aspartate carbamoyltransferase (ATCase) (EC 2.1.3.2) activities are fused in a single polypeptide chain, also containing an inactive version of dihydroorotase (DHOase) (EC 3.5.2.3). In animals the pyrimidine biosynthetic activities are highly organized; thus, the trifunctional CAD protein shows CPSase, ATCase, and DHOase activities assembled in a single polypeptide chain, and similarly, orotate phosphoribosyltransferase (OPRTase) (EC 2.4.2.10) and orotidine-5′-monophosphate decarboxylase (OMPdecase) (EC 4.1.1.23) are fused in a bifunctional protein. In bacteria and archaea the different reactions are performed by monofunctional enzymes, but some of them do interact, at least transiently. Thus, in Pseudomonas putida and Pseudomonas aeruginosa, pyrB overlaps a pyrC′ gene encoding a nonfunctional DHOase, which is required for the assembly of the functional dodecameric ATCase (49), and feedback inhibition and thermostability of ATCase from the extreme thermophilic bacterium Thermus ZO5 are conferred by coexpression of pyrB and the adjacent bbc (for “between b and c”) and pyrC genes, respectively (58, 59). In hyperthermophilic bacteria and archaea, CPSase or carbamate kinase-like CPSase appears to interact physically with ATCase and its paralogue, ornithine carbamoyltransferase, from the arginine biosynthetic pathway, thereby allowing substrate channeling and protection of the thermolabile and potentially toxic carbamoylphosphate from the hot aqueous environment (35, 57, 43, 44, 40). Indeed, since the pathway includes several thermolabile and energy-rich substrates and precursors, some of which decompose into toxic degradation products, the very possibility of pyrimidine biosynthesis is not obvious in hot environments. Yet all investigated hyperthermophilic bacteria and archaea use the same, classical route; evidently, this situation necessitates adapted strategies.
FIG. 1.
De novo synthesis of UMP. Enzymes and their corresponding gene symbols are indicated.
The pyr genes are organized in a vast number of different ways; most microorganisms exhibit an intermediate scattering, with monocistronic and polycistronic messengers. In Escherichia coli and Salmonella enterica serovar Typhimurium, the pyr genes are all scattered throughout the chromosome; only carA and carB, encoding the glutamine amidotransferase and catalytic subunits of CPSase, and pyrB and pyrI, encoding the catalytic and regulatory subunits of ATCase, are grouped into two small operons (for a review, see reference 42). The other extreme occurs in Bacillus subtilis, where 10 cistrons, comprising the pyrimidine biosynthetic genes, the gene for the regulator (pyrR), and the gene for a uracil permease (pyrP), are grouped in a compact, unipolar operon (45). A similar situation is found with the extreme thermophilic gram-positive bacterium Bacillus caldolyticus (17, 18). In the completely sequenced genomes of several euryarchaeota, including methanogens, halophiles, and hyperthermophilic Thermococcales, the pyrimidine genes are highly dispersed, with only pyrB and pyrI showing more systematic clustering. An intermediate situation prevails with Aeropyrum pernix (three small clusters, pyrBI, pyrDC, and pyrFE) and Pyrobaculum aerophilum (pyrFB and pyrDEI clusters), the only crenarchaeotes not belonging to the Sulfolobales genus for which the entire genome sequences have been established (31, 15).
In Bacteria, pyrimidine-specific control of gene expression is exerted by a multitude of distinct mechanisms. Remarkably, even within a single organism like E. coli, the scattered genes and operons are noncoordinately regulated by multiple mechanisms, including UTP-sensitive transcription attenuation (pyrBI and pyrE), UTP-sensitive reiterative transcription (pyrBI and carAB), nucleotide-pool-dependent translational control (pyrC and pyrD), mRNA stability (pyrF) or still, as demonstrated for the carAB operon, complex transcription initiation control imposed by the concerted action of several multifunctional proteins, some of which combine catalytic and regulatory activities (5, 6, 20, 26, 33, 42). The pyrimidine operon of B. subtilis is submitted to transcriptional attenuation exerted by PyrR, an RNA binding protein that also has residual uracil phosphoribosyltransferase activity (54, 56). UTP-dependent binding of PyrR to three untranslated regions of the polycistronic mRNA disrupts the formation of the antiterminator stem-loop structures (3). An analogous mechanism appears to be operative in the gram-positive bacteria B. caldolyticus (17, 18), Lactobacillus plantarum (12), Lactococcus lactis (38, 39), and Enterococcus faecalis (36). An alternative PyrR-dependent attenuation mechanism was proposed in the extreme thermophilic gram-negative bacterium Thermus strain Z05 (58). In this organism, transcriptional attenuation would result from the coupling of transcription and translation of a short open reading frame (ORF), whose ribosome binding site is occluded by pyrimidine-dependent binding of PyrR to the cognate mRNA. PyrR binding would prevent translation of the leader peptide, thus promoting the formation of the terminator structure that leads to reduced expression of the downstream genes. Data on modulation of pyrimidine biosynthetic enzyme specific activities in archaea are extremely scarce and to the best of our knowledge have been reported only for Sulfolobus acidocaldarius (23; also this work); no information is yet available on the mechanism(s) that imposes pyrimidine-specific control of gene transcription in archaea.
In this study we present the isolation, organization, and characterization of the pyrimidine gene cluster of S. acidocaldarius, an aerobic thermoacidophilic sulfur-oxidizing crenarchaeote that grows optimally around 80°C and pH 3.0 (4). In several aspects, S. acidocaldarius is the best-studied member of the Sulfolobales; its genome sequence has not yet been established, whereas those of the relatives Sulfolobus solfataricus P2 and Sulfolobus tokodaii strain 7 have recently been determined (52, 32). We demonstrate that the pyrimidine gene cluster of S. acidocaldarius constitutes a bipolar operon transcribed from two major promoters, an unprecedented organization for pyrimidine genes that turns out to be characteristic of Sulfolobus. We determined transcription initiation sites and polycistronic messenger compositions and demonstrate pyrimidine-specific modulation of transcription initiation. We performed amino acid sequence comparisons and analyzed in silico the nucleotide sequence in terms of transcription and translation initiation and stop signals.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Genotypes and descriptions of strains and plasmids are given in Table 1. S. acidocaldarius (type strain DSM 639) was grown aerobically at 75°C on a rotary shaker platform in either complex medium or minimal medium as described previously (11, 13). Growth conditions for E. coli were described previously (19). Ampicillin was used at a concentration of 25 μg/ml, kanamycin at 30 μg/ml, tetracycline at 15 μg/ml, and uracil at 50 μg/ml.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| S. acidocaldarius DSM639 | Wild-type | Deutsche Sammlung von Microorganismen |
| E. coli | ||
| XL1-Blue | F′::Tn10 proA+B+ lacIq Δ(lacZ)M15/recA1 endA1 gyrA96 (Nalr) thi hsdR17(r− m+) supE44 relA1 lac | Stratagene |
| JM101 | F′ traD36 lacIq Δ(lacZ) M15 proA+B+/supE thi Δ(lac-proAB) strA slcB15 endA hspR4 | 41 |
| HMS174 (DE3)/pLysS | F−recA1 hsdR (r− m+) Rifr (DE3)/pLysS (Cmr) | Novagen |
| Plasmids | ||
| pUC18 | Apr, lacZ cloning vector | 60 |
| pGEM-7 Zf(+) | Apr, cloning vector | Promega |
| pKK223-3 | Apr, IPTG-inducible expression vector | Pharmacia |
| pET3a | Apr, IPTG-inducible and T7 RNA-polymerase dependent expression vector | Novagen |
| pSPYR3 | 9.6-bp PstI genomic fragment of S. acidocaldarius in pKK223-3 | 11 |
| pSPYRE | 1,493-bp SacI genomic fragment of S. acidocaldarius cloned as BamHI PCR product in pGEM7 Zf(+) | This study |
| pSPYRF | 1,316-bp EcoRI genomic fragment of S. acidocaldarius cloned as BamHI PCR product in pGEM7 Zf(+) | This study |
| pET3PYRE | pyrE coding region of S. acidocaldarius in pET3a | This study |
DNA preparations and manipulations, sequencing strategy, and amino acid sequence comparisons.
Plasmid DNA extraction was based on the alkaline sodium dodecyl sulfate lysis method and performed with the commercial Qiaprep Spin Miniprep kit (Qiagen). Oligonucleotides were purchased from Gibco BRL (Table 2). Nuclease digestion, ligation, dephosphorylation, and phosphorylation of DNA fragments and oligonucleotides were performed with commercial enzymes and buffers according to the manufacturer's instructions (Roche). Competent cells were prepared by the CaCl2 method (10). DNA sequencing was performed by the enzymatic dideoxy chain-terminating method with double-stranded plasmid DNA as the template (47). The nucleotide sequence of the 6,845-bp PstI fragment of plasmid pSPYR3 was determined for both strands by subcloning of the PstI-XbaI borders (540 and 1,000 bp) and internal XbaI subfragments (290, 400, 2,200, 2,400 bp) and the generation of nested series of deletions by partial ExoIII nuclease digestion. The correct order of the XbaI subfragments in the PstI clone was determined by sequencing the boundaries using as a template pSPYR3 plasmid DNA and as primers oligonucleotides designed on the basis of the established sequences of the subclones. Amino acid sequences of S. acidocaldarius enzymes were used as queries to retrieve similar sequences from databases using the BLASTP program. Multiple and pairwise alignments of amino acid sequences were generated using the Clustal W program (55).
TABLE 2.
Oligonucleotide used in this work
| Oligonucleotide | Sequence | Use |
|---|---|---|
| E3Sac | 5′-CGGGATCCCTGCTTCGTATACTAACAAGG-3′ | Inverse PCR, pyrE |
| E5Bam | 5′-CGGGATCCGCTATGGATATTGTCTTACCAC-3′ | Inverse PCR, pyrE |
| PYRF1 | 5′-CGGGATCCCATTCTCAGCGACGAGATTTGAC-3′ | Inverse PCR, pyrF |
| PYRF2 | 5′-CGGGATCCGCTCATATCTGATTATATGGTG-3′ | Inverse PCR, pyrF |
| PYRENde | 5′-GGAATTCCATATGGATTTCGTGAAAGCTCTACTTG-3′ | Amplification coding region, pyrE |
| PYREBam | 5′-CGGGATCCCTAGCTTTTTCCAATATTTTTCACC-3′ | Amplification coding region, pyrE |
| PYRB | 5′-CTGTTAGGGCAAATATGTCCTC-3′ | Primer extention |
| PYRC | 5′-GAGATCAACTGATGCAGGCAG-3′ | Primer extention |
| PYRD | 5′-GGTTCCAATGGTGAATAAGTTAGG-3′ | Primer extention |
| PYRE | 5′-GGCTAACTTTACCTGATGTTAAC-3′ | Primer extention |
| ORF1 | 5′-GCCCAAGAGCTTTTTCCCCT-3′ | Primer extention |
| ORF4 | 5′-CACAGAAAAAGGATCTATAATTGC-3′ | Primer extention |
| 1a | 5′-GTGGACAAGACTACTAAGGC-3′ | RT-PCR |
| 1b | 5′-CCTAAGACATTAAGTACTGCG-3′ | RT-PCR |
| 2a | 5′-GAGATGTGAGTATTGTGAGAC-3′ | RT-PCR |
| 2b | 5′-CGTAATCTGCAACTGTCAGG-3′ | RT-PCR |
| 3a | 5′-GTTATAGCCTGTGTTGAGGGAC-3′ | RT-PCR |
| 3b | 5′-GAGATCAACTGATGCAGGCAG-3′ | RT-PCR |
| 4a | 5′-GATAGTTGAAGGTAAATTGGC-3′ | RT-PCR |
| 4b | 5′-GGTTCCAATGGTGAATAAGTTAAC-3′ | RT-PCR |
| 5a | 5′-CCAAGACCTCCTTCTAGCAATG-3′ | RT-PCR |
| 5b | 5′-GTCAGTATATAAGGTAAGCG-3′ | RT-PCR |
| 10b | 5′-CACAGAAAAAGGATCTATAATTGC-3′ | RT-PCR |
Reverse transcriptase primer extension.
Total RNA was prepared with the Life Technologies procedure using the Trizol reagent (8) from 200-ml cultures of S. acidocaldarius cells grown in complex medium, minimal medium, and minimal medium supplemented with uracil (50 μg/ml) and arrested in the exponential phase. Total RNA (100 μg) was mixed with about 40,000 cpm of 5′-32P-end-labeled oligonucleotide primer, and after overnight hybridization at 42°C this was elongated with 10 U of avian myeloblastosis virus reverse transcriptase (Roche) at 40°C for 1 h as described previously (7). Chain-terminating DNA sequencing reactions of the noncoding strand obtained with pSPYR3 plasmid DNA as the template and the same 5′-end-labeled nucleotides used as the primer were applied as reference ladders.
Reverse transcription-PCR (RT-PCR).
Total RNA was extracted with the RNeasy Midi kit (Qiagen) according to the manufacturer's instructions from S. acidocaldarius cells grown in minimal medium and harvested in the exponential phase. To eliminate all possible traces of contaminant DNA, a supplementary DNase I treatment was performed by incubating 1 U of enzyme (RNase free; Roche) per μg of RNA at 37°C for 30 min. DNase I was inactivated by incubation at 75°C for 5 min. cDNA synthesis was performed in a total volume of 20 μl, with 1.0 μg of RNA, 30 pmol of oligonucleotide, 1.0 mM concentration (each) of the four deoxynucleoside triphosphates, 10 mM dithiothreitol, and 50 U of Expand Reverse Transcriptase (Roche) in the commercial buffer and in the presence of 20 U RNase inhibitor (Roche). RNA and primer were heated at 65°C for 10 min and then mixed with the other components and incubated at 42°C for 90 min. The reaction was stopped by cooling on ice. Controls were performed in the absence of Expand reverse transcriptase. Three-microliter cDNA aliquots were used as a template in the PCR amplification step with different combinations of oligonucleotide pairs (0.6 μM each) in a total volume of 50 μl, with a 0.2 mM concentration (each) of the four deoxynucleoside triphosphates and 1.5 U of Pfu DNA polymerase (Promega) and in the commercial buffer. Initial denaturation was for 5 min at 94°C, followed by 30 cycles of synthesis comprising 1 min of denaturation at 94°C, 30 s of annealing at 50°C, and elongation at 72°C for 2 min per kilobase. The amplification was ended with a 10-min elongation at 72°C. Seventeen-microliter aliquots of the various PCRs were analyzed by agarose gel electrophoresis.
Enzyme assays.
ATCase activity was measured using the colorimetric method of Prescott and Jones on dialyzed cell extracts as described previously (11). For OPRTase assays, bacterial pellets from 100-ml cultures were resuspended in 3 ml of 100 mM Tris-HCl, pH 8.8, and sonicated for 6 min. Cell debris was removed by centrifugation. The assay mixture contained 300 μmol of Tris-HCl (pH 8.8), 0.25 μmol of sodium orotate, 25 μmol of MgCl2, and 0.4 μmol of 5′-phospho-α-d-ribosyl-pyrophosphate (PRPP) and bacterial extract in a final volume of 3 ml. The reaction was monitored by measuring the decrease in absorbance measured at 295 nm. Protein was measured by the method of Lowry et al. (37).
Nucleotide sequence accession number.
The 8,519-bp-long sequence has been deposited in the EMBL data bank under accession number AJ459777.
RESULTS
Cloning and nucleotide sequence determination of the pyrimidine gene cluster of S. acidocaldarius.
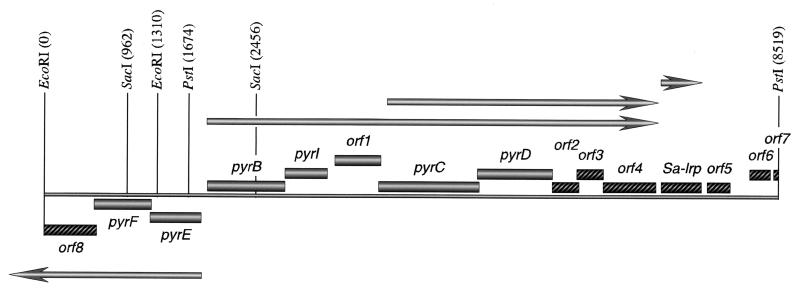
We previously reported the isolation of the S. acidocaldarius pyrB and pyrI genes on a 6.9-kb genomic PstI fragment (Fig. 2) by complementation of an E. coli mutant deficient for ATCase (11). A Southern blot analysis performed under stringent conditions indicated that this fragment indeed originates from S. acidocaldarius (data not shown). The complete 6,845-nucleotide (nt)-long sequence was established, and subsequently extended with another 1,674 bp (Fig. 2, left-hand border EcoRI-PstI segment) by sequencing two clones obtained by inverse PCR with SacI- and EcoRI-digested genomic DNA, respectively. Sequence analysis of the 8,519-bp-long contig and comparisons with databases revealed the presence of 13 complete ORFs and two truncated ones, some of which are homologous to pyrimidine genes of other archaea, bacteria, and eukaryotes (see below). Besides the previously identified contiguous pyrBI genes (11), the fragment also contains the pyrimidine genes pyrC, pyrD, pyrE, and pyrF encoding DHOase, dihydroorotate dehydrogenase (DHOdehase), OPRTase, and OMPdecase, respectively (Fig. 2). pyrI and pyrC are separated by the 177-amino-acid (aa)-long orf1, involved in the electron transport associated with the formation of orotate (see below). pyrD is followed by six relatively short ORFs (see below), one of which corresponds to Sa-lrp, encoding an Lrp-like archaeal transcription regulator (7, 13). Thus, the entire 8,519-bp-long EcoRI-PstI segment of the S. acidocaldarius genome contains the information for the pyrE, pyrF, and truncated orf8 gene products on one strand, and for the pyrB, pyrI, orf1, pyrC, pyrD, orf2, orf3, orf4, Sa-lrp, orf5, orf6, and truncated orf7 gene products on the complementary strand (Fig. 2). The G+C content of the cluster is 35.3%, comparable to that of the entire genome (36 to 38%) (61, 24).
FIG. 2.
Schematic drawing of the pyrimidine gene cluster of S. acidocaldarius and surrounding genes. Grey boxes represent pyrimidine biosynthetic genes. Cross-hatched boxes indicate potential ORFs with unknown function (orf2 to orf8) and the transcriptional regulator Sa-Lrp. Arrows represent mRNA molecules and indicate the start, approximate stop, and orientation of transcription. Positions of the recognition sites for the restriction enzymes EcoRI, SacI, and PstI are indicated (in nucleotides).
This divergent pyr gene cluster encodes the functional enzymes responsible for de novo UTP synthesis, as demonstrated by sequence analysis of mutant alleles and enzyme assays. The pyrB allele from mutant DG64, affected in ATCase activity (23), proved to bear a substitution of phenylalanine (TTT) for the strictly conserved proline (CCT, position 130), right in a highly conserved stretch (HPTQ) that is part of the active site. Similarly, we have shown that the pyrE alleles of mutants DG96 and MR450, affected in the OPRTase activity (23), bear the same single A-T base pair deletion in an A7 stretch that appears to be a hot spot for pyrE mutations (22). The mutation results in a frame shift and the introduction of a premature stop codon (TGA) at position 191. Enzyme assays demonstrated that the S. acidocaldarius pyrE gene encodes a functional and thermophilic OPRTase. Indeed, cell extracts of E. coli strain HMS174(DE3)pLysS transformed with plasmid pET3PYRE, induced with isopropyl-β-d-thiogalactopyranoside (IPTG), and heated for 5 min at 55, 60, 70, or 80°C exhibited a threefold-higher OPRTase activity when assayed at 66°C than at 37°C, whereas no activity was measurable in extracts of noninduced controls (not shown). Moreover, the cloned S. acidocaldarius pyrE gene can complement a pyrE mutant of Pyrococcus abyssi for growth at 90°C (S. Lucas, L. Toffin, Y. Zivanovic, D. Charlier, H. Moussard, P. Forterre, D. Prieur, and G. Erauso, submitted for publication).
Amino acid sequence comparisons.
The sequence analysis, enzyme purification, and characterization, as well as the phylogeny, of S. acidocaldarius ATCase have been published previously (11, 34). Briefly, S. acidocaldarius ATCase is allosterically regulated, belongs to the class B enzymes composed of catalytic (pyrB) and regulatory (pyrI) chains, and is clearly homologous to other ATCases of archaeal, bacterial, or eukaryotic origin. Now that new sequences are available, the best fits are with S. tokodaii (70 and 66% amino acid sequence identity for the catalytic and regulatory chains, respectively) and S. solfataricus (69 and 59%) followed by P. aerophilum (53 and 51%), A. pernix (50 and 35%), and Pyrococcus horikoshii (50 and 47%).
Sequence comparisons of active DHOases and silent DHOase domains of multifunctional enzymes indicate that DHOases fall into two main phylogenetically distinct groups (14). Type I includes the silent DHOase-like sequences of several fluorescent Pseudomonas species, the monofunctional active enzyme of mesophilic and thermophilic gram-positive bacteria, the active monofunctional Ura2 gene product of S. cerevisiae, active multifunctional hamster CAD, and all the archaeal DHOases (monofunctional) identified till now. Type II enzymes are predominantly found in bacteria, gram-negatives and cyanobacteria (active enzymes), but also the inactive DHOase-like sequence embedded in the multifunctional Ura4 gene product from S. cerevisiae belongs to the Type II. The best scores for S. acidocaldarius DHOase are with the crenarchaeotes: S. tokodaii (69% identity), S. solfataricus (57%), A. pernix (34%), and P. aerophilum (31%). These enzymes are clearly Type I enzymes (14), and S. acidocaldarius DHOase also shows the conserved stretch (consensus: PGLV) of 4 aa (PASV, positions 45 to 48), typical for Type I enzymes, that immediately precedes the aspartate residue of the first DHOase signature, DLHVHVRGA (positions 49 to 57 in S. acidocaldarius DHOase). Sequence analyses strongly suggest that S. acidocaldarius pyrC translation initiates at a TTG codon and overlaps the C terminus of orf1 by 10 aa (Fig. 3), rather than at the in-phase ATG codon 99 (nt) downstream. Several arguments support this proposal: the TTG codon is preceded by a good Shine-Dalgarno (SD) sequence (Table 3), whereas the ATG codon is not; downstream initiation would result in a truncated DHOase missing 34 aa residues that show good sequence conservation with other DHOase sequences; moreover, this 102-nt-long stretch separating the TTG and ATG codons shows all the characteristics of a coding region (Pu/Py ratio, 2.90; G+C content, 34.3%) rather than of an intergenic part (see also below, gene organization in the divergent gene cluster) and finally, a similar overlap of PyrC with the C terminus of Orf1 appears to exist in S. solfataricus, though not in S. tokodaii.
FIG. 3.
Nucleotide sequences of the border regions of adjacent genes in the divergent pyrimidine gene cluster. Potential start codons are indicated in bold capitals and are overlined; stop codons are indicated in bold capitals and underlined. Potential SD sequences are indicated in bold capitals.
TABLE 3.
Function and characteristics of ORFs in the 8,519-bp genomic segment of S. acidocaldarius
| Gene | Function | Length of ORF (in aa) | Potential initiation codon | Stop codon | Potential SD sequence | Pu:Py ratio | G+C (mol %) | 5′-overlap (in nt) | 3′-overlap (in nt) |
|---|---|---|---|---|---|---|---|---|---|
| orf8 | Hypothetical protein | 202 (truncated) | ATG | ?a | GAaGGTaA | 1.164 | 36.2 | 26 | ? |
| pyrF | OMPdecase | 219 | TTG | TGA TAA | GGTGA | 1.266 | 32.6 | 14 | 26 |
| pyrE | OPRTase | 197 | ATG | TAG | None | 1.309 | 36.2 | 14 | |
| pyrB | ATCase catalytic | 299 | TTG | TGA | None | 1.342 | 35.0 | ||
| pyrI | ATCase regulatory | 164 | ATG | TGA | GGTGA | 1.800 | 34.8 | ||
| orfI | DHOdehase electron transfer | 177 | TTG | TGA | TGATCC | 1.414 | 36.4 | 29 | |
| pyrC | DHOase | 389 | TTG | TAA | AGGTGA | 1.432 | 37.0 | 29 | 23 |
| pyrD | DHOdehase catalytic | 291 | TTG | TGA | GAGGTG | 1.473 | 37.6 | 23 | 2 |
| orf2 | Hypothetical protein | 103 | ATG | TAA | GAG-TGA | 1.836 | 38.2 | 2 | 29 |
| orf3 | Hypothetical protein | 102 | ATG | TGA | GAGG | 1.597 | 34.0 | 29 | 2 |
| orf4 | Conserved hypothetical | 203 | ATG | TGA | None | 1.061 | 37.6 | 2 | |
| Sa-lrp | Transcriptional regulator | 145 | ATG | TAA | None | 1.450 | 31.8 | ||
| orf5 | Hypothetical Zn finger | 99 | ATG | TAA | GGTGAT | 2.000 | 40.0 | ||
| orf6 | Hypothetical protein | 80 | ATG | TAA | None | 2.076 | 28.8 | ||
| orf7 | Hypothetical protein | 18 (truncated) | ATG | ? | GGTG | 1.040 | 50.9 | ? |
?, unknown.
The formation of orotate constitutes the sole redox reaction in de novo UMP biosynthesis. On the basis of phylogenetic studies and enzyme characterizations, DHOdehase sequences have been subdivided into two major groups (29, 53). Class I enzymes (predominantly present in gram-positive bacteria and archaea) are cytosolic homodimers (pyrD encoded) that use fumarate as the electron acceptor (type 1A) or heterotetramers (pyrD-pyrK, pyrD-pyrDII) (1, 30) that use NAD+ as the electron acceptor (type 1B), whereas class II enzymes (gram-negative bacteria, higher eukaryotes) are monomeric enzymes attached to the membrane that use different kinds of quinones. Class I and class II enzymes use a different amino acid as the catalytic base, cysteine and serine, respectively. Sequence alignments and experimental data indicate that S. solfataricus (53) and also S. acidocaldarius (by sequence similarity only) DHOdehase belongs to the class I enzymes, but the S. solfataricus enzyme is unable to use any of the natural electron acceptors used in known DHOdehase types and uses serine as catalytic base, unique for a cytosolic DHOdehase (53). This serine residue (position 120 of S. solfataricus DHOdehase) is also conserved in the S. acidocaldarius (position 121) and S. tokodaii enzymes. The exact nature of the natural electron acceptor of the Sulfolobus enzymes is still unknown.
In gel filtration experiments, the catalytic subunit (pyrD) of S. solfataricus DHOdehase was found to comigrate with another protein that turned out to be the product of orf1 (53). orf1 shows only very weak amino acid sequence similarity to the pyrK (pyrDII)-encoded electron transfer subunits and is much shorter, but it bears an iron-sulfur cluster. Since orf1 from S. acidocaldarius (177 aa) and that from S. solfataricus (208 aa) show 38.9% sequence identity (41% to S. tokodaii 7 [197 aa]), Sulfolobus DHOdehases appear to be heteromeric enzymes constituted by a catalytic subunit and a new type of electron transfer subunit (53).
The best fits of S. acidocaldarius OPRTase are with the crenarchaeotes S. tokodaii (57% identity), S. solfataricus (55%), A. pernix (39%), and P. aerophilum (36%). The PRPP binding and active site is well conserved (VIVVDDVATTGGS, residues 108 to 120 in the S. acidocaldarius enzyme).
The best fits of S. acidocaldarius OMPdecase are with archaeal homologues, the best being with S. tokodaii (60% identity), S. solfataricus (48%), A. pernix (43%), M. jannashii (32%), and P. abyssi (31%).
orf2 (encoding 103 aa), orf3 (encoding 102 aa), and orf6 (encoding 80 aa) encode very small, hypothetical proteins of unknown function. Since they are conserved and similarly organized in S. solfataricus and S. tokodaii, they are most likely expressed. The fact that orf2, orf3, and orf4 are cotranscribed with the pyr genes (see below) and show the classical excess of purines on the coding strand and the typical G+C value of coding parts (Table 3) further supports this proposal. Sa-Lrp has been purified from the original host (13). Orf4 (204 aa) is well conserved among very divergent archaea and bacteria, but its function is unknown. Orf5 (99 aa) shows a Zn finger motif and is homologous to the hypothetical DNA-directed RNA polymerase subunit M of S. solfataricus and S. tokodaii. The 18-aa-long truncated Orf7 is homologous to the N termini of 506- and 489-aa-long conserved hypothetical proteins with unknown function from S. solfataricus and S. tokodaii, respectively. This ORF is embedded in the same genetic environment in S. acidocaldarius and S. tokodaii, but not in S. solfataricus. Similarly, the 203-aa-long truncated Orf8 is homologous to a hypothetical protein of unknown function from S. solfataricus (NP342138) and S. tokodaii (NP377445), but these are not contiguous to pyrF.
Gene organization in the divergent pyrimidine cluster.
The genetic information comprised within the 8,519-bp stretch containing the divergent pyr cluster is extremely tightly packed. ORFs cover more than 94% of the sequence, and the gene density of 1.643 per kb largely exceeds those determined for the complete S. solfataricus P2 and S. tokodaii 7 genomes (1.013 and 1.049, respectively). Considering only those genes clearly involved in pyrimidine biosynthesis, the density becomes 1.333 ORFs per kb, which is still high with respect to gene densities calculated for archaeal genomes. In seven instances, adjacent ORFs overlap over a short distance, varying between 2 and 29 nt (Table 3; Fig. 3). Of these, five show an overlap at both the N and C termini, resulting in 1.5% of sequence information being translated into two different proteins. In three other instances, the stop and start codons of contiguous ORFs are separated by a short nucleotide stretch of variable length comprised of between 1 and 133 nt (Table 4).
TABLE 4.
Characteristics of intergenic regions
| Location | Length (nt) | Pu/Py ratio | G+C (mol %) |
|---|---|---|---|
| pyrE-pyrB | 71 | 1.03 | 26.8 |
| pyrB-pyrI | 1 | ||
| pyrI-orf1 | 85 | 1.073 | 34.1 |
| orf4-Sa-lrp | 60 | 1.14 | 18.3 |
| Sa-lrp-orf5 | 66 | 0.784 | 24.2 |
| orf5-orf6 | 133 | 0.847 | 27.8 |
| orf6-orf7 | 96 | 1.133 | 31.4 |
Of the 15 ORFs, 10 (66.7%) likely start with an ATG codon and five (33.3%) start with TTG, an observation in good agreement with the statistics on the utilization of ATG (65%), TTG (28%), and GTG (14%) as initiation codons in a 156-kb sequence of the related S. solfataricus (51). The most frequent stop codon utilized in the divergent gene cluster is TGA (53.8%), followed by TAA (38.5%) and TAG (7.7%) (Table 3); pyrF shows a pair of adjacent TGA and TAA stop codons. This occurrence of stop codons is unlike the situation reported for the 156-kb sequence of S. solfataricus (51) where TAA is predominant (50%). The coding parts show a purine bias (Pu/Py ratios varying between 1.164 and 2.076 for the individual genes; mean value of 1.416 for the 15 ORFs) (Table 3) close to the typical 55% A+G (ratio of 1.222) found in all sequenced genomes, including archaea, bacteria, and eukaryotes, except for orf4 (Pu/Py ratio of 1.061). In contrast, the intergenic regions with Pu/Py ratios of between 0.78 and 1.133 do not show this bias (Table 4).
Experimental identification of the transcription starts indicate that the 5′ mRNA leader sequences of the two polycistronic pyr messengers (pyrE-F-orf8 and pyrB-I-orf1-pyrC-D-orf2-orf3-orf4; see below) and of monocistronic Sa-lrp (13) are very short and do not harbor a ribosome binding site upstream of the initiation codon. A similar situation might prevail for orf6. Within the polycistronic mRNAs (see below), however, the situation is different and the initiation codon is nearly always preceded at an appropriate distance by a sequence that shows extensive complementarity to the 3′ end of the 16S rRNA (Table 3; Fig. 3). This is not the case for orf4, but as the ATG initiation codon overlaps the stop codon of orf3, translation might possibly proceed without the need for reassociation of ribosomes on the polycistronic messenger.
Polycistronic messenger analyses and potential transcription stop signals.
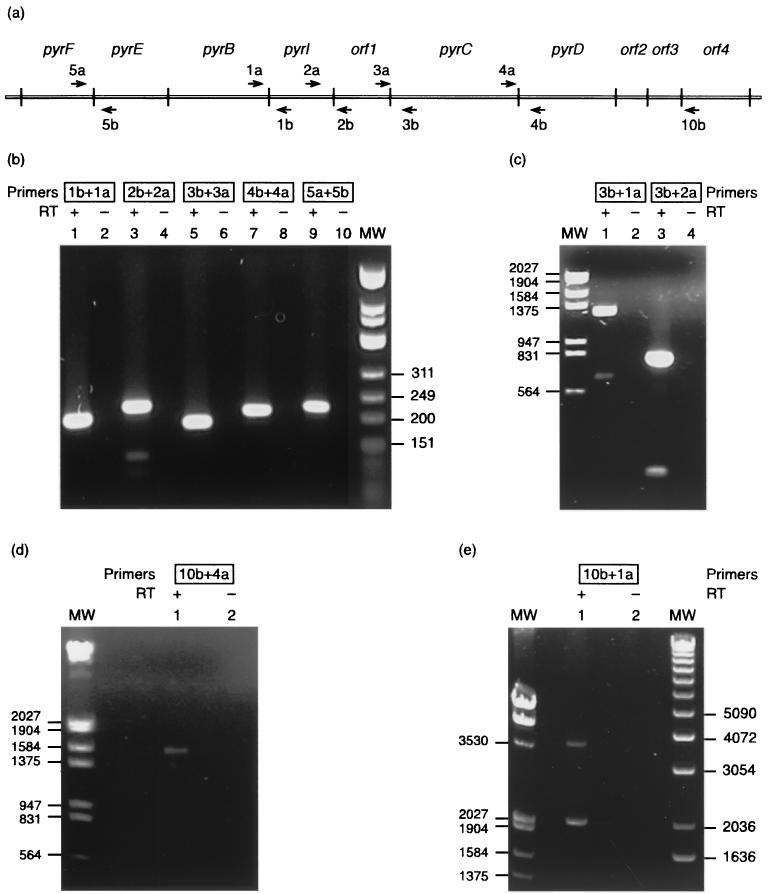
The existence of polycistronic pyr messengers was demonstrated by RT-PCR experiments (Fig. 4). To demonstrate that two or more genes are transcribed as a single mRNA molecule, we used total RNA as template, reverse transcriptase, and pairs of oligonucleotides hybridizing to complementary strands of different genes, in PCR amplification reactions. In the first step, the oligonucleotide complementary to the mRNA of the downstream gene was used to synthesize cDNA. In the second amplification reaction, the oligonucleotide complementary to the cDNA and located in a different gene was added (for details, see Materials and Methods). In the sole presence of RNA as a template, the amplification reaction will be possible only if the mRNA molecule indeed spans the different genes. Thus, we have demonstrated that pyrF and pyrE are part of the same mRNA molecule, initiated at the pyrE promoter (see below for the initiation site). Indeed, using the pair of oligonucleotides 5a (hybridizing to the N-terminal part of the pyrF messenger, for cDNA synthesis) and 5b (hybridizing to the C terminus of pyrE, for further amplification), we detected the expected 226-bp fragment (Fig. 4a and b, lane 9), whereas no amplification at all was detected in the absence of reverse transcriptase in the assay (lane 10). Similarly, we have demonstrated cotranscription of pyrI and pyrB (oligonucleotides 1b and 1a; 204-bp fragment, lanes 1 and 2), of orf1 and pyrI (oligonucleotides 2b and 2a; 231-bp fragment, lanes 3 and 4), of pyrC and orf1 (oligonucleotides 3b and 3a; 193-bp fragment, lanes 5 and 6), and of pyrD and pyrC (oligonucleotides 4b and 4a; 217-bp fragment, lanes 7 and 8). In further combinations we demonstrated cotranscription of pyrI, orf1, and pyrC (oligonucleotides 3b and 2a, 816-bp fragment; Fig. 4c, lanes 3 and 4), of pyrB, pyrI, orf1, and pyrC (oligonucleotides 3b and 1a; 1,329-bp fragment, Fig. 4c, lanes 1 and 2) and of pyrC, pyrD, orf2, orf3, and orf4 (oligonucleotides 10b and 4a; 1,600-bp fragment, Fig. 4d, lanes 1 and 2). These various combinations already strongly suggested that transcription initiated at the pyrB promoter (see below) might proceed till the end of orf4. This was further proven by an RT-PCR experiment using the pair of oligonucleotides 10b and 1a, which produced the expected 3,861-bp fragment (Fig. 4e). Previously, members of our group had shown that the Sa-lrp gene is transcribed as a monocistronic messenger that most likely stops at the type I transcriptional stop (5′-TTTTTATT) located 1 nt downstream of the TAA stop codon and that there is only very little readthrough from orf4 into Sa-lrp (13). Thus, the bipolar pyrimidine operon from S. acidocaldarius is transcribed as two polycistronic messengers initiated from the divergent pair of pyrE and pyrB promoters (see below). Transcription initiated at the pyrB promoter does not proceed into Sa-lrp and most likely stops at the type I transcriptional stop signal 5′-TTTTTT, located 22 nt downstream of the TGA stop codon of orf4 (13). Similar thymine stretches (5′-TTTTCTT and 5′-TTTTATTTT) are found 70 and 68 nt downstream of the TAA stop codons of orf5 and orf6, respectively. Such a run af T residues is not present in the region 3′ of the pyrF, pyrE, pyrB, pyrI, orf1, pyrC, pyrD, orf2, and orf3 genes and occurs in four instances only in the 7,636-nt-long coding part of the cluster (one in pyrF, two in pyrB, one in orf4; they are far more numerous on the noncoding strand). The presence of these thymine runs at the 3′ end of mono- and polycistronic messengers appears therefore statistically significant. No potential transcriptional stop signal (type I or type II) could be identified at the 3′ end of pyrF. This observation, and the physical overlap of the coding parts of pyrF and truncated orf8, suggest that transcription initiated at pyrE proceeds into orf8. In contrast, orf5 and orf6 might produce monocistronic messengers.
FIG. 4.
(a) Schematic presentation of the bipolar pyrimidine operon. The approximate position of oligonucleotides used as primers in the RT-PCRs and their polarity are indicated by small arrows. (b, c, d, and e) Analysis by agarose gel electrophoresis of double-stranded DNA fragments generated in the RT-PCRs performed with S. acidocaldarius total RNA, Expand reverse transcriptase (when indicated), Pfu DNA polymerase, and different combinations of primers, as indicated. MW, molecular size markers.
Transcription initiation and promoter sequences in the bipolar pyrimidine operon.
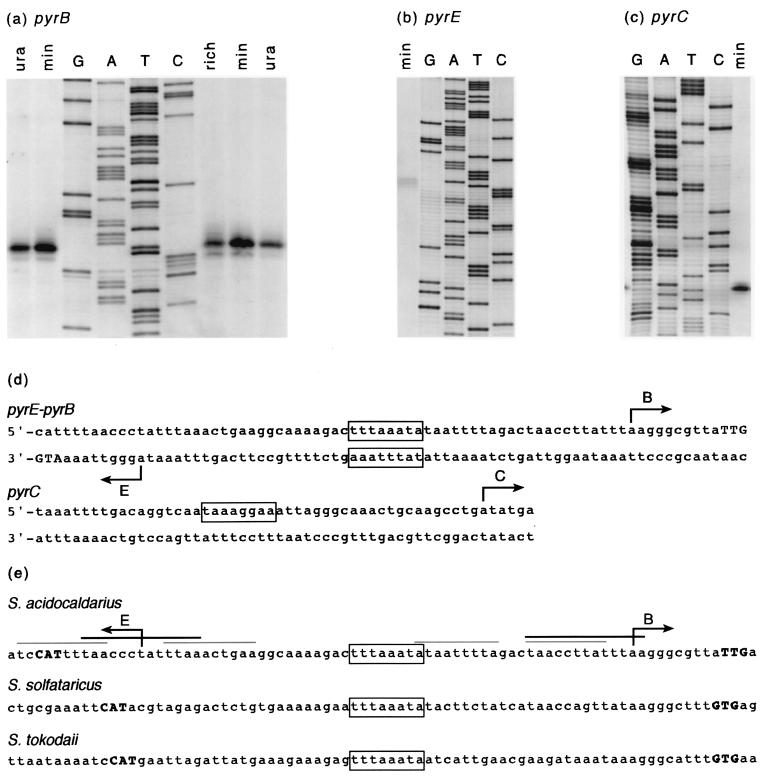
Start points of transcription were determined by primer extension using total RNA extracted from S. acidocaldarius cells grown in minimal medium and 5′-32P-end-labeled oligonucleotides complementary to the pyrE, pyrB, pyrC, pyrD, orf1, and orf4 messengers (Table 2). The autoradiograph (Fig. 5a) indicates that pyrB transcription is initiated with an A residue, 10 nt upstream of the TTG initiation codon (Fig. 5d). Similarly, pyrE transcription, proceeding in the opposite direction, was found to initiate with an A residue 9 nt upstream of the ATG initiation codon (Fig. 5b). Consequently, the start sites for divergent transcription are separated by a 52-nt-long stretch only (Fig. 5d). Its G+C content of 21.2% (26.8% for the extended 71-nt-long noncoding region) is considerably lower than that of the complete cluster (35.3%) but comparable to the mean value of 25% for intergenic regions in S. solfataricus (50). No transcription start could be identified in front of orf1, pyrD, and orf4 (not shown), but a cDNA product corresponding to a potential initiation at an A residue within the pyrC coding part was detected (Fig. 5c and d). The start site of the monocistronic Sa-lrp gene was determined previously (7, 13); transcription initiation of orf5, orf6, and of the truncated orf7 and orf8 has not been investigated. Three of the identified transcripts (pyrE, pyrB, and Sa-lrp) initiate with a purine residue that is preceded by a thymine, thus creating the pyrimidine-purine dinucleotide required for correct start site selection of archaeal promoters (25). A good match to the consensus TATA box of archaeal promoters is the 5′-TTTAAATA sequence, ideally centered around −26.5 nt upstream of the pyrB start site (Fig. 5d). This sequence is preceded (positions −36 to −31) by a sequence stretch, 5′-AAAGAC, that shows good sequence conservation with the transcription factor B responsive element sequence of archaeal promoters, a purine-rich stretch with a consensus RNWAAW (R = purine; W = A or T) (2). The potential TATA box element of the pyrE promoter (5′-TATTTAAA), comprising between −23 and −30 upstream of the pyrE transcription start, largely overlaps the TATA box element of the pyrB promoter (Fig. 5d). Therefore, this region is apparently shared by the divergent pair of pyrE and pyrB promoters. The hypothetical TATA box element (5′-TAAAGGAA) comprising between −23 and −30 nt upstream of the potential pyrC transcription start showed only weak sequence identity with the consensus (Fig. 5d). Therefore, the detected cDNA product might correspond to a discrete degradation product of the longer mRNA molecule initiated at pyrB, or it could result from a premature arrest of the reverse transcriptase. The lack of a stable hairpin structure in the region makes the latter unlikely, however.
FIG. 5.
(a) Quantitative determination of pyrB transcripts and mapping of the potential transcription initiation site by primer extension. Lanes 1 and 2, pyrB primer extension reactions with 100 μg of total RNA extracted from S. acidocaldarius cells grown in minimal medium supplemented with uracil (50 μg/ml) and minimal medium, respectively, and arrested in the exponential phase. Lanes 7 to 9, primer extension reactions with 100 μg of total RNA from independent cultures grown on complex medium, minimal medium, and minimal medium supplemented with uracil, as indicated. G, A, T, and C, chain terminating DNA sequencing reactions of the noncoding strand obtained with the same 32P-labeled oligonucleotide used to perform the extension reactions. (b) Mapping of the potential transcription initiation site of pyrE by primer extension. The extension reaction was performed with 100 μg of total RNA extracted from cells grown in minimal medium. (c) Mapping of the potential transcription initiation site located within the pyrC gene. The extension reaction was performed with 100 μg of total RNA extracted from cells grown in minimal medium. (d) Nucleotide sequences of the pyrE-pyrB intergenic region and of the potential internal promoter located in the pyrC coding region. Arrows indicate the start site and orientation of transcription. Potential TATA promoter elements are boxed. (d) Comparison of the pyrE-pyrB intergenic regions of S. acidocaldarius, S. solfataricus, and S. tokodaii. Sequences have been aligned with respect to the strictly conserved TATA box elements. The most important direct repeats (black bars) and imperfect palindromic sequences (grey bars) present in the S. acidocaldarius sequence are indicated. Translation initiation codons are in bold capitals (complementary strand for pyrE), and arrows indicate the start sites and orientations of transcription.
Regulation of pyrB transcription initiation.
To determine the effect of nutrient composition on the abundance of pyrB transcription, we performed primer extension experiments with total RNA extracted from S. acidocaldarius cells grown on complex medium or on minimal medium either devoid of or supplemented with uracil. The densitometric analysis of the autoradiograph (Fig. 5a) indicated that the pyrB transcript is about 2.0-fold more abundant in the absence than in the presence of uracil and about 2.5-fold more abundant on minimal medium than on complex medium (mean for two assays performed with independent RNA preparations). Similarly, an approximately twofold difference was also observed in the ATCase specific activities measured in cell extracts of S. acidocaldarius cells grown in the presence or absence of uracil. Therefore, expression of the S. acidocaldarius pyrimidine biosynthetic genes is regulated, at least in part, at the transcriptional level.
DISCUSSION
In this study we present the sequence determination and analysis of an 8,519-bp segment of the S. acidocaldarius genome carrying an extremely tightly packed divergent pyrimidine gene cluster that exhibits a novel organization. Primer extension and RT-PCR experiments indicate that the pyrimidine genes are transcribed as two polycistronic messengers, pyrBI-orf1-pyrCD-orf2-orf3-orf4 and pyrEF. The latter most likely also includes orf8, as suggested by the physical overlap of the pyrF and hypothetical orf8 cistrons and the lack of an identifiable transcription stop signal at the 3′ end of pyrF. A similar clustering of pyr genes occurs in the entirely sequenced genomes of S. solfataricus P2 (52) and S. tokodaii strain 7 (32) and appears therefore characteristic of Sulfolobus. Beyond the orf5 and pyrF genes, however, the similarity in genome organization vanishes. Also novel is the gene order orf1-pyrC-pyrD, in which pyrC is intercalated between the electron transfer and catalytic subunit of DHOdehase; in bacteria, pyrK (pyrDII) and pyrD are generally contiguous.
The seven cistrons involved in de novo synthesis of UMP are tightly packed, and only pyrB and pyrI, encoding the catalytic and regulatory subunits of ATCase, respectively, show no overlap at either the 5′ or 3′ end: pyrB and pyrI are separated by a single nucleotide, and pyrI and orf1 are separated by 85 nt. The start points of the divergent transcripts initiated at the pyrE and pyrB promoters are separated by 52 nt only, and the corresponding initiation codons are separated by 71 nt. This short intergenic region appears to contain a pair of overlapping divergent promoters showing a good match to the consensus TATA and transcription factor B responsive element promoter elements. This peculiar situation constitutes an interesting model system for the study of basal and regulated transcription in hyperthermophilic archaea, still a poorly documented area of investigation. Indeed, the intimate intertwining of divergent promoters creates an interesting test case for the analysis of sequence determinants imposing transcription polarity, for the study of mutual influences of nearby promoters on binding of transcription factors (TBP, TFB, and TFE) and polymerase recruitment, and for the analysis of variations in template topology generated by the moving polymerases engaged in divergent transcription (positive supercoiling in front, negative ahead) on transcription initiation frequencies. This last aspect is particularly interesting, since DNA in hyperthermophiles is known to be relaxed to slightly positively supercoiled, in contrast to the negative supercoiling of mesophilic genomes.
We have shown that pyr gene expression in S. acidocaldarius is regulated at least in part at the transcriptional level. pyrB mRNA levels were found to differ by a factor of 2 in minimal medium devoid of or supplemented with uracil. On the other hand, ATCase enzyme activities measured in cultures of various pyrimidine auxotrophs grown on limiting amounts of uracil showed a 2- to 10-fold derepression (23). Taken together, these data suggest the existence of a several-fold regulation of transcription initiation that would provide S. acidocaldarius with the flexibility required to attune pyrimidine biosynthesis to the cellular needs and fluctuations in available nutrients. The mode of pyrimidine-specific repression of pyrB transcription initiation in S. acidocaldarius is not known, but the mechanism appears to be different from the various types of attenuation-like mechanisms operative in mesophilic and thermophilic bacteria. The pyrE and pyrB leader sequences are too short to contain attenuator-antiattenuator structures. In contrast, the short pyrE-pyrB intergenic region exhibits features (direct repeats and imperfect palindromic sequences) characteristic of potential binding sites for a hypothetical transcriptional regulator(s), which are at least in part conserved in S. solfataricus and S. tokodaii (Fig. 5e).
The quasi-leaderless pyrB and pyrE messengers bear no SD site, whereas in contrast, the ORFs embedded in the polycistronic messengers show a rather extensive complementarity to the 3′ end of 16S rRNA (Table 3). Interestingly, it was previously shown that the disruption of the SD sequence abolished translation, but most importantly, the effect of this disruption could be suppressed by deleting the 5′-untranslated region, thus creating a leaderless messenger (9). In all organisms, bacteria, archaea, and eucarya, leaderless messengers exist, even for abundant proteins (28). Translation initiation of the archaeal leaderless mRNAs might require the formation of a complex equivalent to the bacterial 30S-fMet-tRNAfMet-IF2 intermediate implicated in translation of such mRNAs in E. coli, as is suggested by the faithful in vitro translation of leaderless λ cI mRNA with an archaeal translation system (21).
The bipolar pyr operon of S. acidocaldarius does not harbor the genes encoding carbamoylphosphate synthetase. Studies with mutants indicate that S. acidocaldarius has only one carbamoylphosphate synthetase (23), and on the S. solfataricus P2 genome the adjacent carA and carB genes encoding the sole CPSase of this organism are embedded in an arginine gene cluster (52). This peculiar situation raises interesting questions regarding the regulation of CPSase activity in relation to the cellular needs for arginine and pyrimidine biosynthesis. A comparable situation exists in L. lactis, where the carA and carB genes encoding the sole CPSase are dispersed; carA is part of a pyr gene cluster, whereas pyrB is monocistronic, but both are submitted to pyrimidine regulation (38, 39).
Thermophilic OPRTases and DHOdecases deserve special attention. Mutants deficient in either one of these activities are resistant to 5-fluoroorotic acid and auxotrophic for uracil. Therefore, both the wild-type and mutant pyrE and pyrF alleles can be positively selected. These unique traits and the universal and ancient character of pyrimidine biosynthesis make the utilization of the pyrE and pyrF genes as genetic markers particularly attractive. S. acidocaldarius pyrE has proven to be particularly suited for studies on intragenic recombination, chromosomal marker exchange by archaeal conjugation, and genetic fidelity at high temperature (16, 22, 27, 46, 48). Furthermore, the gene has also been used for the construction of cloning vectors for the hyperthermophilic euryarchaeote Pyrococcus abyssi, where the S. acidocaldarius gene was chosen to avoid homologous recombination (S. Lucas et al., submitted).
Acknowledgments
We are grateful to D. Grogan for the gift of strains and to J.-P. Ten Have for the artwork.
This work was supported by the Fund for Joint Basic Research-Flanders (FWO-Vlaanderen, contract no. G.0069.00) and the Flanders Interuniversity Institute for Biotechnology (VIB).
REFERENCES
- 1.Andersen, P. S., J. Martinussen, and K. Hammer. 1996. Sequence analysis and identification of the pyrKDbF operon from Lactococcus lactis including a novel gene, pyrK, involved in pyrimidine biosynthesis. J. Bacteriol. 178:5005-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell, S. D., P. L. Kosa, P. S. Sigler, and S. P. Jackson. 1999. Orientation of the transcription preinitiation complex in Archaea. Proc. Natl. Acad. Sci. USA 96:13662-13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonner, E. R., J. N. D'Elia, B. K. Billips, and R. L. Switzer. 2001. Molecular recognition of pyr mRNA by the Bacillus subtilis attenuation regulatory protein PyrR. Nucleic Acids Res. 29:4851-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brock, T. D., K. M. Brock, R. T. Belly, and R. L. Weiss. 1972. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Microbiol. 84:54-68. [DOI] [PubMed] [Google Scholar]
- 5.Charlier, D., A. Kholti, N. Huysveld, D. Gigot, D. Maes, T.-L. Thia-Toong, and N. Glansdorff. 2000. Mutational analysis of Escherichia coli PepA, a multifunctional DNA-binding aminopeptidase. J. Mol. Biol. 302:411-426. [DOI] [PubMed] [Google Scholar]
- 6.Charlier, D., M. Roovers, D. Gigot, N. Huysveld, A. Piérard, and N. Glansdorff. 1993. Integration host factor (IHF) modulates the expression of the pyrimidine-specific promoter of the carAB operons of Escherichia coli K12 and Salmonella typhimurium LT2. Mol. Gen. Genet. 237:273-286. [DOI] [PubMed] [Google Scholar]
- 7.Charlier, D., M. Roovers, T.-L. Thia-Toong, V. Durbecq, and N. Glansdorff. 1997. Cloning and identification of the Sulfolobus solfataricus lrp gene encoding an archaeal homologue of the eubacterial leucine-responsive global transcriptional regulator Lrp. Gene 201:63-68. [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski, P. 1993. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cells and tissue samples. BioTechniques 15:532-534. [PubMed] [Google Scholar]
- 9.Condò, I., A. Ciammaruconi, D. Bellini, D. Ruggero, and P. Londei. 1999. Cis-acting signals controlling translation initiation in the thermophilic archaeon Sulfolobus solfataricus. Mol. Microbiol. 34:377-384. [DOI] [PubMed] [Google Scholar]
- 10.Dagert, M., and S. D. Ehrlich. 1979. Prolonged incubation in calcium chloride improves the competence of E. coli cells. Gene 6:23-28. [DOI] [PubMed] [Google Scholar]
- 11.Durbecq, V., T.-L. Thia-Toong, D. Charlier, V. Villeret, M. Roovers, R. Wattiez, C. Legrain, and N. Glansdorff. 1999. Aspartate carbamoyltransferase from the thermoacidophilic archaeon Sulfolobus acidocaldarius. Eur. J. Biochem. 264:233-241. [DOI] [PubMed] [Google Scholar]
- 12.Elagöz, A., A. Abdi, J.-C. Hubert, and B. Kammerer. 1996. Structure and organisation of the pyrimidine biosynthesis pathway genes in Lactobacillus plantarum: a PCR strategy for sequencing without cloning. Gene 182:37-43. [DOI] [PubMed] [Google Scholar]
- 13.Enoru-Eta, J., D. Gigot, T.-L. Thia-Toong, N. Glansdorff, and D. Charlier. 2000. Purification and characterization of Sa-Lrp, a DNA-binding protein from the extreme thermoacidophilic archaeon Sulfolobus acidocaldarius homologous to the bacterial global transcription regulator Lrp. J. Bacteriol. 182:3661-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields, C., D. Brichta, M. Shepherdson, M. Farinha, and G. O'Donovan. 1999. Phylogenetic analysis and classification of dihydroorotases: a complex history for a complex enzyme. Paths Pyrimidines 7:49-63. [Google Scholar]
- 15.Fitz-Gibbon, S. T., H. Ladner, U.-J. Kim, K. O. Stetter, M. I. Simons, and J. H. Miller. 2002. Genome sequence of the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. Proc. Natl. Acad. Sci. USA 99:984-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghané, F., and D. W. Grogan. 1998. Chromosomal marker exchange in the thermophilic archaeon Sulfolobus acidocaldarius: physiological and cellular aspects. Microbiology 144:1649-1657. [DOI] [PubMed] [Google Scholar]
- 17.Ghim, S.-Y., and J. Neuhard. 1994. The pyrimidine biosynthesis operon of the thermophile Bacillus caldolyticus includes genes for uracil phosphoribosyltransferase and uracil permease. J. Bacteriol. 176:3698-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghim, S.-Y., P. Nielsen, and J. Neuhard. 1994. Molecular characterization of pyrimidine biosynthesis genes from the hyperthermophile Bacillus caldolyticus. Microbiology 140:479-491. [DOI] [PubMed] [Google Scholar]
- 19.Glansdorff, N. 1965. Topography of cotransducible arginine mutations in Escherichia coli K12. Genetics 51:167-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glansdorff, N., D. Charlier, P. Chen, A. Kholti, and Y. Xu. 1998. Genetic studies on the pyrimidine paradigm in prokaryotes: new insights in regulatory mechanisms. Paths Pyrimidines 6:53-63. [Google Scholar]
- 21.Grill, S., C. O. Gualerzi, P. Londei, and U. Bläsi. 2000. Selective stimulation of translation of leaderless mRNA by initiation factor 2: evolutionary implications for translation. EMBO J. 19:4101-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grogan, D. W., G. T. Carver, and J. W. Drake. 2001. Genetic fidelity under harsh conditions: analysis of spontaneous mutation in the thermoacidophilic archaeon Sulfolobus acidocaldarius. Proc. Natl. Acad. Sci. USA 98:7928-7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grogan, D. W., and R. P. Gunsalus. 1993. Sulfolobus acidocaldarius synthesizes UMP via a standard de novo pathway: results of a biochemical-genetic study. J. Bacteriol. 175:1500-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grogan, D. W., P. Palm, and W. Zillig. 1990. Isolate B12, which harbours a virus-like element, represents a new species of the archaebacterial genus Sulfolobus. Sulfolobus shibatae, sp. nov. Arch. Microbiol. 154:594-599. [DOI] [PubMed] [Google Scholar]
- 25.Hain, J., W.-D. Reiter, U. Hüdepohl, and W. Zillig. 1992. Elements of an archaeal promoter defined by mutational analysis. Nucleic Acids Res. 20:5423-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han, X., and C. L. Turnbough, Jr. 1998. Regulation of carAB expression in Escherichia coli occurs in part through UTP-sensitive reiterative transcription. J. Bacteriol. 180:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs, K. L., and D. W. Grogan. 1998. Spontaneous mutation in a thermoacidophilic archaeon: evaluation of genetic and physiological factors. Arch. Microbiol. 169:81-83. [DOI] [PubMed] [Google Scholar]
- 28.Janssen, G. R. 1993. Eubacterial, archaebacterial and eukaryotic genes that encode leaderless mRNA, p. 59-67. In R. H. Baltz, G. D. Hegeman, and P. L. Skatrud (ed.), Industrial microorganisms: basic and applied molecular genetics. American Society for Microbiology, Washington, D.C.
- 29.Jensen, K. F., and O. Bjornberg. 1998. Evolutionary and functional families of dihydroorotate dehydrogenases. Paths Pyrimidines 6:20-28. [Google Scholar]
- 30.Kahler, A. E., and R. L. Switzer. 1996. Identification of a novel gene of pyrimidine nucleotide biosynthesis, pyrDII, that is required for dihydroorotate dehydrogenase activity in Bacillus subtilis. J. Bacteriol. 178:5013-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawarabayasi, Y., Y. Hino, H. Horikawa, S. Yamazaki, Y. Haikawa, K. Jin-No, M. Takahashi, M. Sekine, S. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishijima, H. Nakazawa, M. Takamiya, S. Masuda, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Ogushi, K. Aoki, K. Kubota, Y. Nakamura, N. NOmura, Y. Sako, and H. Kikuchi. 1999. Complete genome sequence of an aerobic hyperthermophilic crenarchaeon, Aeropyrum pernix K1. DNA Res. 6:83-101. [DOI] [PubMed] [Google Scholar]
- 32.Kawarabayasi, Y., Y. Hino, H. Horikawa, K. Jin-No, M. Takahashi, M. Sekine, S.-I. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishiijima, R. Otsuka, H. Nakasawa, M. Takamiya, Y. Kato, T. Yoshizawa, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K.-I. Aoki, S. Masuda, M. Yanagii, M. Nishimura, A. Yamagishi, T. Oshimla, and H. Kikuchi. 2001. Complete genome sequence of an aerobic thermoacidophilic crenarchaeon, Sulfolobus tokodaii strain 7. DNA Res. 8:123-140. [DOI] [PubMed] [Google Scholar]
- 33.Kholti, A., D. Charlier, D. Gigot, N. Huysveld, and N. Glansdorff. 1998. pyrH-encoded UMP-kinase directly participates in pyrimidine-specific modulation of promoter activity in Escherichia coli. J. Mol. Biol. 280:571-582. [DOI] [PubMed] [Google Scholar]
- 34.Labedan, B., A. Boyen, M. Baetens, D. Charlier, P. Chen, R. Cunin, V. Durbecq, N. Glansdorff, G. Hervé, C. Legrain, Z. Liang, C. Purcarea, M. Roovers, R. Sanchez, T.-L. Thia-Toong, M. Van de Casteele, F. Van Vliet, Y. Xu, and Y.-F. Zhang. 1999. The evolutionary history of carbamoyltransferases: a complex set of paralogous genes was already present in the last universal common ancestor. J. Mol. Evol. 49:461-473. [DOI] [PubMed] [Google Scholar]
- 35.Legrain, C., M. Demarez, N. Glansdorff, and A. Piérard. 1995. Ammonia-dependent synthesis and metabolic channeling of carbamoyl phosphate in the hyperthermophilic archaeon Pyrococcus furiosus. Microbiology 141:1093-1099. [DOI] [PubMed] [Google Scholar]
- 36.Li, X., G. M. Weinstock, and B. E. Murray. 1995. Generation of auxotrophic mutants of Enterococcus faecalis. J. Bacteriol. 177:6866-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowry, O. H., N. J. Rosenbrough, A. L. Farr, and R. J. Randall. 1951. Protein measurements with folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 38.Martinussen, J., and K. Hammer. 1998. The carB gene encoding the large subunit of carbamoylphosphate synthetase from Lactococcus lactis is transcribed monocistronically. J. Bacteriol. 180:4380-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinussen, J., J. Schallert, B. Andersen, and K. Hammer. 2001. The pyrimidine operon pyrRPB-carA from Lactococcus lactis. J. Bacteriol. 183:2758-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massant, J., P. Verstreken, V. Durbecq, A. Kholti, C. Legrain, S. Beeckmans, P. Cornelis, and N. Glansdorff. 2002. Metabolic channeling of carbamoyl phosphate, a thermolabile intermediate: evidence for physical interaction between carbamate kinase-like carbamoyl phosphate synthetase and ornithine carbamoyltransferase from the hyperthermophile Pyrococcus furiosus. J. Biol. Chem. 277:18517-18522. [DOI] [PubMed]
- 41.Messing, J. 1983. New M13 vectors for cloning. Methods Enzymol. 101:499-560. [DOI] [PubMed] [Google Scholar]
- 42.Neuhard, J., and R. A. Kelln. 1996. Biosynthesis and conversion of pyrimidines, p. 580-599. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 43.Purcarea, C., D. R. Evans, and G. Hervé. 1999. Channeling of carbamoyl phosphate to the pyrimidine and arginine biosynthetic pathways in the deep sea hyperthermophilic archaeon Pyrococcus abyssi. J. Biol. Chem. 274:6122-6129. [DOI] [PubMed] [Google Scholar]
- 44.Purcarea, C., G. Hervé, R. Cunin, and D. R. Evans. 2001. Cloning, expression, and structure analysis of carbamate kinase-like carbamoyl phosphate synthetase from Pyrococcus abyssi. Extremophiles 5:229-239. [DOI] [PubMed] [Google Scholar]
- 45.Quinn, C. L., B. T. Stephenson, and R. L. Switzer. 1991. Functional organization and nucleotide sequence of the Bacillus subtilis pyrimidine biosynthetic operon. J. Biol. Chem. 266:9113-9127. [PubMed] [Google Scholar]
- 46.Reilly, M. S., and D. W. Grogan. 2001. Characterization of intragenic recombination in a hyperthermophilic archaeon via conjugational DNA exchange. J. Bacteriol. 183:2943-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt, K. J., K. E. Beck, and D. W. Grogan. 1999. UV stimulation of chromosomal marker exchange in Sulfolobus acidocaldarius: implications for DNA repair, conjugation and homologous recombination at extremely high temperatures. Genetics 152:1407-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schurr, M. J., J. F. Vickrey, A. P. Kumar, A. L. Campbell, R. Cunin, R. C. Benjamin, M. S. Shanley, and G. A. O'Donovan. 1995. Aspartate transcarbamoylase genes of Pseudomonas putida: requirement for an inactive dihydroorotase for assembly into the dodecameric holoenzyme. J. Bacteriol. 177:1751-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sensen, C. W., R. L. Charlebois, C. Chow, Ib G. Clausen, B. Curtis, W. F. Doolittle, M. Duguet, G. Erauso, T. Gaasterland, R. A. Garrett, P. Gordon, I. Heikamp de Jong, A. C. Jeffries, C. Kozera, N. Medina, A. De Moors, J. van der Oost, H. Phan, M. A. Ragan, M. E. Schenk, Q. She, R. K. Singh, and N. Tolstrup. 1998. Completing the sequence of the Sulfolobus solfataricus P2 genome. Extremophiles 2:305-312. [DOI] [PubMed] [Google Scholar]
- 51.Sensen, C. W., H.-P. Klenk, R. K. Singh, G. Allard, C. C.-Y. Chan, Q. Y. Liu, S. L. Penny, F. Young, M. E. Schenk, T. Gaasterland, W. F. Doolittle, M. A. Ragan, and R. L. Charlebois. 1996. Organizational characteristics and information content of an archaeal genome: 156 kb of sequence from Sulfolobus solfataricus P2. Mol. Microbiol. 22:175-191. [DOI] [PubMed] [Google Scholar]
- 52.She, Q., R. K. Singh, F. Confalonieri, Y. Zivanovic, G. Allard, M. J. Awayez, C. C.-Y. Chan-Weiher, I. G. Clausen, B. A. Curtis, A. De Moors, G. Erauso, C. Fletcher, P. M. K. Gordon, I. Heinkamp-de Jong, A. C. Jeffries, C. J. Kozeka, N. Medina, X. Peng, H. P. Thi-Ngoc, P. Redder, M. E. Schenk, C. Theriault, N. Tolstrup, R. L. Charlebois, W. F. Doolittle, M. Duguet, T. Gaasterland, R. A. Garrett, M. A. Ragan, C. W. Sensen, and J. Van der Oost. 2001. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. USA 98:7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sørensen, P. G. 2001. A new type of dihydroorotate dehydrogenase-type 1S-from the thermoacidophilic archaeon Sulfolobus solfataricus. Ph.D. thesis. University of Copenhagen, Copenhagen, Denmark.
- 54.Switzer, R. L., R. J. Turner, and Y. Lu. 1999. Regulation of the Bacillus subtilis pyrimidine biosynthetic operon by transcriptional attenuation: control of gene expression by a mRNA-binding protein. Prog. Nucleic Acid Res. Mol. Biol. 62:329-367. [DOI] [PubMed] [Google Scholar]
- 55.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner, R. J., Y. Lu, and R. L. Switzer. 1994. Regulation of the Bacillus subtilis pyrimidine biosynthetic (pyr) gene cluster by an autogenous transcriptional attenuation mechanism. J. Bacteriol. 176:3708-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van de Casteele, M., C. Legrain, L. Desmarez, P. G. Chen, A. Piérard, and N. Glansdorff. 1997. Molecular physiology and carbamoylation under extreme conditions: what can we learn from extreme thermophilic microorganisms? Comp. Biochem. Physiol. 118A:463-473. [DOI] [PubMed] [Google Scholar]
- 58.Van de Casteele, M., P. Chen, M. Roovers, C. Legrain, and N. Glansdorff. 1997. Structure and expression of a pyrimidine gene cluster from the extreme thermophile Thermus strain ZO5. J. Bacteriol. 179:3470-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van de Casteele, M., L. Desmarez, C. Legrain, P. G. Chen, K. Van Lierde, A. Piérard, and N. Glansdorff. 1994. Genes encoding aspartate carbamoyltransferase of Thermus aquaticus ZO5 and Thermotoga maritima MSB8: modes of expression in E. coli and properties of their products. Biocatalysis 112:135-179. [Google Scholar]
- 60.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertional mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 61.Zillig, W., K. O. Stetter, S. Wunderl, W. Schulz, H. Priess, and I. Scholz. 1980. The Sulfolobus-Caldariella group: taxonomy on the basis of the structure of DNA-dependent RNA polymerases. Arch. Microbiol. 125:259-269. [Google Scholar]