Abstract
The growth of some strains of Rhizobium leguminosarum bv. viciae is inhibited by N-(3-hydroxy-7-cis tetradecenoyl)-l-homoserine lactone (3OH-C14:1-HSL), which was previously known as the small bacteriocin before its characterization as an N-acyl homoserine lactone (AHL). Tn5-induced mutants of R. leguminosarum bv. viciae resistant to 3OH-C14:1-HSL were isolated, and mutations in two genes were identified. These genes, bisR and triR, which both encode LuxR-type regulators required for plasmid transfer, were found downstream of an operon containing trb genes involved in the transfer of the symbiotic plasmid pRL1JI. The first gene in this operon is traI, which encodes an AHL synthase, and the trbBCDEJKLFGHI genes were found between traI and bisR. Mutations in bisR, triR, traI, or trbL blocked plasmid transfer. Using gene fusions, it was demonstrated that bisR regulates triR in response to the presence of 3OH-C14:1-HSL. In turn, triR is then required for the induction of the traI-trb operon required for plasmid transfer. bisR also represses expression of cinI, which is chromosomally located and determines the level of production of 3OH-C14:1-HSL. The cloned bisR and triR genes conferred 3OH-C14:1-HSL sensitivity to strains of R. leguminosarum bv. viciae normally resistant to this AHL. Furthermore, bisR and triR made Agrobacterium tumefaciens sensitive to R. leguminosarum bv. viciae strains producing 3OH-C14:1-HSL. Analysis of patterns of growth inhibition using mutant strains and synthetic AHLs revealed that maximal growth inhibition required, in addition to 3OH-C14:1-HSL, the presence of other AHLs such as N-octanoyl-l-homoserine lactone and/or N-(3-oxo-octanoyl)-l-homoserine lactone. In an attempt to identify the causes of growth inhibition, a strain of R. leguminosarum bv. viciae carrying cloned bisR and triR was treated with an AHL extract containing 3OH-C14:1-HSL. N-terminal sequencing of induced proteins revealed one with significant similarity to the protein translation factor Ef-Ts.
Bacterial populations can coordinately regulate gene expression as a result of the production of diffusible signaling molecules. One class of such signals is the N-acyl-homoserine lactones (AHLs), which can influence diverse physiological processes in a wide range of gram-negative bacteria (16, 36). These signals can act to induce gene expression in response to bacterial cell density in a process known as quorum sensing (18).
AHL-mediated quorum sensing plays an important role in many bacteria that promote plant growth or interact with plants as pathogens or symbionts (9, 36). Rhizobium leguminosarum bv. viciae, the symbiont of pea and vetch, produces an unusually diverse range of AHLs, and the cinRI locus is at the top of a regulatory network of quorum-sensing loci in this bacterium (23). CinI produces the AHL N-(3-hydroxy-7-cis tetradecenoyl)-l-homoserine lactone (3OH-C14:1-HSL) and CinR is a LuxR-type regulator that positively regulates cinI expression in response to 3OH-C14:1-HSL. The cinR and cinI genes were so called (23) because they are involved in the production of an AHL, which was previously thought to be a bacteriocin that was referred to as small (20, 42). The purified small bacteriocin molecule (32) turned out to be an AHL identical in structure to the 3OH-C14:1-HSL, which had been isolated (19) on the basis that it induced gene expression in R. leguminosarum bv. viciae by quorum-sensing regulators of the LuxR type. Mutation of cinI or cinR greatly reduced the expression of the rhiABC operon (23). These genes are expressed in the legume rhizosphere and influence the formation of symbiotic nitrogen-fixing nodules on vetch (10). The effect of cinIR on rhiABC expression was shown to be indirect and to be mediated via rhiI and rhiR. RhiI produces N-hexanoyl-, N-heptanoyl-, and N-octanoyl-l-homoserine lactones (C6-HSL, C7-HSL, and C8-HSL, respectively), which stimulate RhiR to induce the rhiABC and rhiI operons (23, 30). Mutation of cinI or cinR also greatly reduced the production of several other AHLs not made by CinI or RhiI, indicating that there are other AHL production loci in R. leguminosarum bv. viciae strain A34 (23).
The strong growth-inhibitory effect of the presence of 3OH-C14:1-HSL on some strains of R. leguminosarum is a very unusual effect for an AHL. The growth-inhibitory effect was found to be bacteriostatic rather than due to cell death, and it was concluded that 3OH-C14:1-HSL could switch the bacteria into the stationary-growth phase, even though the cell density was low (19). Subsequently, 3OH-C14:1-HSL was shown to confer salt tolerance and long-term survival characteristics on exponential-phase cells of R. leguminosarum strains (38).
The sensitivity of R. leguminosarum bv. viciae to growth inhibition by 3OH-C14:1-HSL is conferred by a locus on the symbiotic plasmid pRL1JI (20, 42). In addition, pRL1JI represses the production of 3OH-C14:1-HSL, and this effect appears to be due, at least in part, to decreased transcription of the chromosomally located cinI gene (23). To try to understand the mechanism of growth inhibition by 3OH-C14:1-HSL, we have analyzed mutants of R. leguminosarum bv. viciae that are resistant to growth inhibition by this AHL. This has led to the identification of two adjacent luxR-type regulatory genes that control plasmid transfer, growth inhibition by 3OH-C14:1-HSL, and repression of 3OH-C14:1-HSL synthesis.
MATERIALS AND METHODS
Microbiological techniques.
Rhizobium and Agrobacterium strains were grown at 28°C on either TY complex medium (4) or Y minimal medium (34). Escherichia coli strains were grown at 37°C on L medium (31). Antibiotics were added to maintain selection for plasmids as appropriate. Culture optical densities at 600 nm (OD600) were measured using an MSE Spectro-plus spectrophotometer. β-Galactosidase activity was measured as previously described (25), using a Titertek Multiscan Plus spectrophotometer. Nodulation tests were performed on Frisson variety peas (Pisum sativum) as described previously (6), using at least 15 plants per test.
Bacteriocin-type growth inhibition test.
The strain to be tested for growth sensitivity was suspended in 10 ml of TY broth to give an OD600 of ∼0.4. This was added to 200 ml of cooled TY agar, some of which was immediately poured as a thin layer (3 to 5 mm thick) into a petri dish. The agar was allowed to set before being overlaid with a thin layer of TY agar. Growth inhibition was assessed by inoculating bacteria onto the surface and measuring halos of growth inhibition following 2 days of growth at 28°C.
A modified test was used to analyze the effects of synthetic AHLs upon growth. Synthetic AHLs (1 μl of a 1 μg/ml solution in methanol) were pipetted onto a TY plate. The plate was dried for 30 min so that the solvent could evaporate. The plate was overlaid with a thin (3 to 5 mm) layer of TY agar seeded with the appropriate sensitive strain. Growth inhibition was assessed after 2 days of growth.
Bacterial strains and plasmids.
Bacterial strains and plasmids used in the present study are listed in Table 1. Transductions were done using phage RL38 (8). Plasmids were transferred from E. coli strains into Rhizobium or Agrobacterium strains by triparental mating using a helper plasmid. Transposon mutagenesis was done by mobilizing the Tn5-carrying suicide plasmid pJB4JI (5) into either 8401 or A34 and then selecting on Y medium with streptomycin and kanamycin. 3OH-C14:1-HSL-resistant mutants of A34 were isolated by replica plating colonies of Tn5-induced mutants onto Y medium containing an extract of AHLs containing 3OH-C14:1-HSL from the culture supernatant of strain 8401. Colonies which grew well on this medium were selected and checked for resistance on the same medium.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| R. leguminosarum strains | ||
| 8401 | Strain lacking pSym | 22 |
| A34 | Derivative of 8401 carrying pRL1JI | 12 |
| A73 | Derivative of 8401, Rifr | |
| A160 | Derivative of 8401 carrying rhiR::Tn5 | 11 |
| A549 | Derivative of A34 carrrying bisR::Tn5 | This work |
| A552 | Derivative of 8401 carrying cinR1::Tn5 | 23 |
| A621 | Derivative of A34 carrying cinR1::Tn5 | 23 |
| A627 | Derivative of A34 carrying triR::Tn5 | This work |
| A664 | Derivative of A34 carrying cinI3::spc | 23 |
| A682 | Derivative of A34 carrying trbE::Tn5-lacZ | This work |
| Rhizobium sp. strain ANU265 | Plasmid-cured derivative of broad-host-range Rhizobium sp. strain NGR234 | 26 |
| A. tumefaciens C58.00 | Strain lacking the AT and Ti plasmids | 39 |
| C. violaceum CV026 | AHL biosensor strain | 24 |
| Plasmids | ||
| pIJ1891 | pLAFR3-based cloning vector | 14 |
| pIJ7500 | EcoRI fragment carrying bisR::Tn5 from A549 | This work |
| pIJ7542 | pLAFR1 cosmid carrying bisR and triR | This work |
| pIJ7546 | 1.3-kb HindIII fragment from pIJ7545, carrying bisR, in pIJ1891 | This work |
| pIJ7750 | 2.35-kb EcoRV fragment, carrying cinR and cinI, in the HincII site of pUC19 | 23 |
| pIJ7752 | 0.3-bp fragment carrying the bisR promoter cloned into pMP220 as a PstI-KpnI fragment; bisR-lacZ | This work |
| pIJ7630 | 10.8-kb EcoRI fragment from pIJ7542 carrying bisR and triR cloned in pUC18. | This work |
| pIJ7777 | Deleted derivative of pIJ7750 carrying cinI but not cinR | This work |
| pIJ7786 | EcoRI fragment carrying triR::Tn5 from A627 | This work |
| pIJ7867 | 2.2-kb SacI-EcoRI fragment carrying bisR and triR in pKT230 | This work |
| pIJ7873 | bisR and triR cloned on a PstI fragment in pBBR1-MCS5 | This work |
| pIJ7878 | triR-lacZ fusion in pMP220 | This work |
| pIJ7910 | cinI-lacZ in pMP220 | 23 |
| pIJ7986 | 1.3-kb HindIII fragment carrying bisR in the HindIII site of pIJ7750; bisR promoter is next to the pLac | This work |
| pIJ7987 | As pIJ7986, except the 1.3-kb fragment is cloned in the opposite orientation | This work |
| pIJ9036 | 6.5-kb EcoRI fragment carrying traI in pUC18 | This work |
| pIJ9071 | 6.5-kb EcoRI fragment carrying traI in pMP220 | This work |
| pBBR1-MCS5 | Broad-host-range cloning vector | 21 |
| pKT230 | IncQ broad-host-range vector | 3 |
| pMP220 | lacZ promoter vector | 35 |
Plasmid pIJ7867 was made by cloning a 2.2-kb PstI fragment carrying bisR and triR into pBluescript KS and then recloning the fragment as an EcoRI-SacI fragment into the EcoRI and SacI sites of pKT230 or as a PstI fragment into pBBR1-MCS-5 to make pIJ7873. To create pIJ7878, the intergenic region between bisR and triR was cloned from one of the ExoIII deletion constructs used for DNA sequencing into pBluescript SK as a 300-bp EcoRV-HindIII fragment, which was then recloned into pMP220 as a KpnI-EcoRI fragment. Plasmid pIJ7752 was made as follows: a 1.3-kb HindIII fragment, which carries bisR as well as the intergenic region between trbI and bisR, was cloned into pUC18. This fragment was shortened by ExoIII digestion to make a plasmid which contains only the intergenic region between trbI and bisR, including the translation start of bisR. This fragment was cloned into pBluescript KS by using the HindIII and EcoRI sites and was then recloned into pMP220 as a PstI-KpnI fragment to make pIJ7752. pIJ7777 is an ExoIII deletion derivative of pIJ7750 that was generated during the sequencing of the cinRI gene region (23).
Assay of AHLs.
Cultures were grown in TY medium for 24 or 48 h to an OD600 of approximately 0.4 or 0.8, respectively. Cells were removed by centrifugation, and AHLs were extracted as previously described (43). Aluminum-backed RP18F254S reverse-phase plates (Merck) were used for thin-layer chromatography (TLC) analysis (33). Extract (5 to 20 μl) was spotted onto the plate and dried in air. The AHLs were separated (using methanol and water [60:40] as the mobile phase) until the solvent front reached the top of the plate. The plate was removed from the chromatography tank and dried before the separation process was repeated. Chromobacterium violaceum CV026 was used as an AHL indicator organism (24), as this strain responds to exogenous short-acyl-chain AHLs by producing the purple pigment violacein.
Molecular biology techniques.
All standard DNA manipulations were carried out as described previously (31). DNA was labeled with [α-32P]dCTP by using either the Ready-To-Go kit (Pharmacia) or the Rediprime DNA labeling system (Amersham Life Science) according to the manufacturers' instructions. ExoIII deletions were made using the Erase-a-Base System (Promega). DNA was sequenced using either the ABI Prism Dye Primer cycle sequencing kit (Perkin-Elmer) or the ThermoSequenase Dye Terminator kit (Amersham) according to the manufacturers' instructions. Reactions were run on ABI 377 DNA sequencers. For PCR amplification of traI from Rhizobium sp. strain NGR234, the following primers homologous to the DNA flanking the translated region of the gene were used: p87 (5′ GAT TTG TGC TGA TTT CCC CC 3′) and p88 (5′ AGA GCG AAG CTG TTC CAC TG) (both made by Gibco BRL). Taq DNA polymerase (Pharmacia) was used throughout according to the manufacturer's instructions. PCR products were cloned using the Topoisomerase TA cloning kit (Stratagene) according to the manufacturer's instructions.
DNA sequence was analyzed using the GCG software package (version 8; Genetics Computer Group, Madison, Wis.). Homologous DNA sequences were identified from the GENEMBL library using FASTA and BLAST software, and homologous proteins were identified from the EMBL and SWISSPROT databases.
Analysis of proteins induced by 3OH-C14:1-HSL.
Strain A34 or strain A34/pIJ7867 was precultured for 2 days in 10 ml of TY medium. A small inoculum was used to inoculate 600 ml of TY medium, and this culture was grown overnight until the early exponential phase (OD600, ∼0.1). The culture was divided into three parts, and each 200-ml fraction was incubated for 2.5 h with either 2 ml of methanol, 2 ml of AHL extract from strain 8401, or 2 ml of extract from strain A552 (cinI3::spc). The amount of AHL extract used corresponded to the amount that would be present in 200 ml of the original cultures of strain 8401 or A552 grown to the early stationary phase. Following the 2.5-h incubation, the bacteria were collected by centrifugation (20 min at 4,000 × g at 4°C) and washed in 0.1 M Tris-HCl (pH 8.0). The washed cells were resuspended in 1 ml of 0.1 M Tris-HCl (pH 8.0) and then lysed by sonication (30-s pulse with a MSE Soniprep at full power, repeated six times). Cell debris was removed by centrifugation at 12,000 rpm in an Eppendorf Microcentrifuge and cooled to 4°C, and the protein content of the supernatant was estimated using Bradford reagent (Bio-Rad). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (7) and stained with Coomassie blue R250. Size markers were supplied by Sigma.
For protein sequencing, the gel (Protean II; Bio-Rad) was cast, left overnight at 4°C, and then prerun (at 80 V for 40 min) with 0.1 mM of sodium thioglycolate in the upper tank to avoid N-terminal blocking. After separation of 40 μg of proteins from a cell extract, the proteins were transferred onto polyvinylidene fluoride (Immobilon P [0.45 μm]; Millipore) using a semidry transfer apparatus (Sartorius) in CAPS buffer [10 mM 3-(cyclohexamino)-1-propanesulfonic acid (pH 11) containing 10% (vol/vol) methanol]. The membrane was stained with Coomassie blue R250 (in methanol and water [1:1]), destained in methanol and water (1:1), and dried. Bands of interest were cut out, and protein sequencing was carried out using an Applied Biosystems Procise Sequencer.
For immunoblotting, proteins (20 μg) were separated, transferred to nitrocellulose, and probed with RhiA antiserum as previously described (11). For EF-Tu detection, a monoclonal antibody to E. coli EFTu, MAb 900 (40), was used, and binding was revealed using goat anti-mouse conjugates coupled to alkaline phosphatase.
RESULTS
Isolation of 3OH-C14:1-HSL-resistant mutants.
A population of Tn5-induced mutants of R. leguminosarum bv. viciae strain A34 was replica plated onto TY plates containing 3OH-C14:1-HSL to select for mutations that caused resistance. Sensitive colonies showed poor growth after 2 days of incubation, while resistant mutants grew quickly. Resistant colonies were isolated and retested for resistance to 3OH-C14:1-HSL. This screen puts a selective pressure on the bacterial population for any spontaneous mutation that leads to resistance. Since pRL1JI confers sensitivity but is not essential for growth, deletion or loss of pRL1JI results in resistance. In light of this, we used only those mutations that cotransduced with kanamycin resistance, showing that the Tn5 insertion was linked to the resistance phenotype.
Such resistant mutants fell into two classes: those which did and those which did not produce 3OH-C14:1-HSL (see below). One mutant from each class was picked for further study, namely, A549, which produces 3OH-C14:1-HSL, and A627, which does not. The Tn5 insertions were cloned from these mutants as EcoRI fragments into pUC18, yielding pIJ7500 (from A549) and pIJ7786 (from A627). Sequencing of the DNA adjacent to the Tn5 insertions showed that a different gene was affected in each mutant, and based on the sequences, both genes were predicted to encode LuxR homologues. In A549, the affected gene was designated bisR (i.e., bacteriostasis induction sensor), and in A627, the affected gene was called triR (i.e., transfer and inhibition). DNA hybridization (using pIJ7500 and pIJ7786 as probes) showed that both contained DNA from pRL1JI, because each probe hybridized to a 10.8-kb EcoRI band with genomic DNA from A34 but not 8401 (data not shown). DNA hybridization experiments using pIJ7500 to probe pIJ7786 also revealed that both genes were located on the same 10.8-kb EcoRI fragment. Thus, two sensitivity genes on pRL1JI have been identified.
To clone the wild-type genes, we made use of the observation that one of the mutants (A549) had lost the ability to repress 3OH-C14:1-HSL production. Clones from a cosmid library were transferred to A549 and screened for repression of 3OH-C14:1-HSL, using a bacteriocin-type assay. DNA hybridization using pIJ7500 as a probe (data not shown) confirmed that a complementing cosmid (pIJ7542) contained the 10.8-kb EcoRI fragment that was mutagenized in A549.
Characterization of the gene region associated with sensitivity to 3OH-C14:1-HSL.
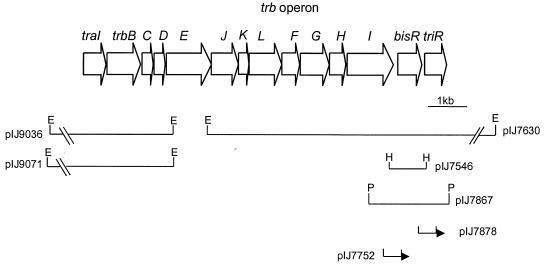
The 10.8-kb EcoRI fragment from pIJ7542 was subcloned (pIJ7630) and sequenced. The sequencing revealed that the two mutations conferring resistance to 3OH-C14:1-HSL were in genes downstream of an operon containing trb genes, which are predicted to be involved in plasmid transfer (Fig. 1). Such trb genes are highly conserved (2), and the strongest database similarity was with the trb operon from the symbiotic plasmid of Rhizobium sp. strain NGR234 and the Ti plasmid from Agrobacterium tumefaciens. In these bacteria, the trb operon is preceded by a traI gene encoding LuxI-type AHL synthases (13, 15, 17, 28). It was evident from the comparison that only part of the trb operon from pRL1JI had been cloned. Part of traI from NGR234 was amplified by PCR and used as a probe to identify a clone carrying traI from pRL1JI. DNA hybridization with A34 revealed that traI-hybridizing DNA was on a 7-kb EcoRI fragment and that this fragment was not on the cosmid (pIJ7542) carrying the trb-triR-bisR gene region. The 7-kb EcoRI hybridizing fragment was subcloned in pUC18 from a minilibrary of DNA from A34, using the traI probe amplified from NGR234 to identify a hybridizing plasmid (pIJ9036). The DNA on this fragment was sequenced, revealing that a traI-like gene is located immediately upstream of the trb operon (Fig. 1). The gene arrangement shown in Fig. 1 is the same as that for the equivalent region in NGR234 (15), except that there is one additional gene (bisR) present near the 3′ end of the trb operon. The trb gene region was confirmed to be required for transfer by isolation of a transposon insertion in the trbE gene and recombination of the mutation onto pRL1JI in A34 to generate strain A682. Normally pRL1JI is transferred at a frequency of about 10−2 per recipient (23), but strain A682 had lost the ability to transfer pRL1JI (the transfer frequency was less than 10−9 per recipient).
FIG. 1.
Map of the traI-trb operon, bisR triR gene region. Open reading frames are depicted by block arrows. Plasmids carrying different parts of the region are denoted by lines, and the positions of lacZ fusions are indicated by arrowheads. Restriction sites are marked as follows: H, HindIII; P, PstI; E, EcoRI.
Characterization of BisR and TriR.
Both BisR and TriR share significant homology to LuxR-type regulators. The predicted 246-amino acid BisR protein is most similar to CinR, the LuxR-type regulator previously isolated from this strain of R. leguminosarum bv. viciae (23), with which it shares 59% identity. Neither BisR nor CinR shares any more than 30% identity with any of the other LuxR-type regulators in this strain of Rhizobium or in other bacteria. This suggests that bisR may have arisen relatively recently from a duplication of cinR, with subsequent sequence divergence. CinR is required for expression of cinI, which encodes the protein required for production of 3OH-C14:1-HSL (23). BisR shares next highest homology with Rhodobacter sphaeroides CerR (29% identity), which is thought to regulate the cerI gene; CerI (29) directs the synthesis of N-tetradecenoyl-l-homoserine lactone (C14:1-HSL), which is clearly similar to the 3OH-C14:1-HSL made by CinI.
The triR gene product is 64% identical to TraR from the symbiotic plasmid of Rhizobium sp. strain NGR234 (15) and 32% identical to TraR from A. tumefaciens (accession no. AF057718). It is less similar to RhiR and BisR (30% and 22% identity, respectively). The predicted translation start codon of triR is a TTG codon. There is a later ATG codon (30 bp downstream of the TTG), although starting translation from this codon would result in an atypically short LuxR homologue. The amino-acid sequence encoded between the first putative start codon (TTG) and the second putative start codon (ATG) is homologous to the sequence at the beginning of other LuxR homologues, especially TraR from Rhizobium sp. strain NGR234.
There is a 183-bp intergenic region between the predicted start codon of bisR and the stop codon of the previous gene, trbI. No regions of dyad symmetry similar to lux box or tra box regulatory elements could be found. The distance between the stop codon of bisR and the predicted start of triR is 106 bp, and part of this region showed strong similarity (28 matches over 41 nucleotides) to part of the cinR-cinI intergenic region, which includes regions of dyad symmetry. The significance of this with regard to transcription initiation or attenuation remains to be determined.
Phenotypes of bisR and triR mutants.
It seemed likely that the bisR and triR genes regulate plasmid transfer. The transfer of pRL1JI was less than 10−9 plasmids per recipient using either A549 (bisR::Tn5) or A627 (triR::Tn5) as donors and strain A73 (which lacks a symbiotic plasmid) as a recipient, demonstrating that both mutations abolished plasmid transfer. Normal frequencies of plasmid transfer were restored by presence of the complementing cosmid pIJ7542 or subcloned bisR and triR on pIJ7873. This suggests that BisR and TriR are required for the normal induction of transfer of pRL1JI.
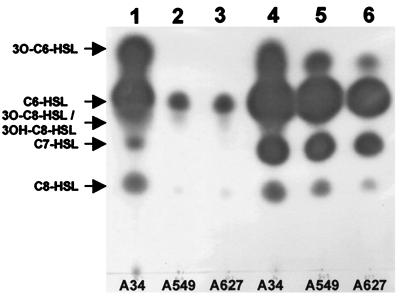
The organization of the traI-trb operon on pRL1JI (Fig. 1) is similar to that in A. tumefaciens, in which traI is regulated by TraR. Plasmid pRL1JI determines the production of several AHLs in addition to those determined by strain 8401 (23). The production of the additional AHLs is due, in part, to the presence of rhiI on pRL1JI (30) and presumably in part to that of AHLs determined by traI. The effect on AHL production of mutations in bisR (A549) and triR (A627) was assayed by TLC using C. violaceum CV026 as a detection system. Mutation of either gene significantly reduced AHL production, and this was particularly clear after 24 h of growth (Fig. 2, lanes 1 to 3). After 48 h (Fig. 2, lanes 4 to 6), the difference between the bisR or triR mutant and the wild type was less obvious as other AHLs accumulated. The role of bisR and triR in the regulation of traI will be described elsewhere.
FIG. 2.
AHL production by bisR and triR mutants. Extracts of AHLs were separated by TLC, and the AHLs produced were visualized using C. violaceum CV026 as described previously (24). Lanes 1 to 3 depict extracts of AHLs which were isolated after 24 h of growth of strains A34, A549 (bisR::Tn5), and A627 (triR::Tn5), respectively. Lanes 4 to 6 depict extracts from the same strains after 48 h growth. The migration positions of various chemically synthesized standards are indicated on the left of the TLC.
The bisR and triR mutants A549 and A627 were tested for effects of the mutations on growth in minimal and complex media and on nodulation of pea. In these tests, no significant differences were observed in comparison with the wild-type control A34 (data not shown).
BisR mediates repression of 3OH-C14:1-HSL synthesis.
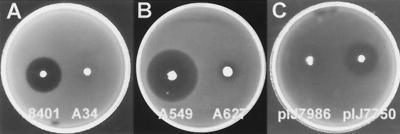
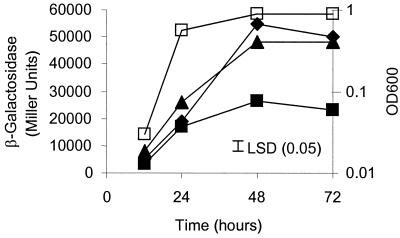
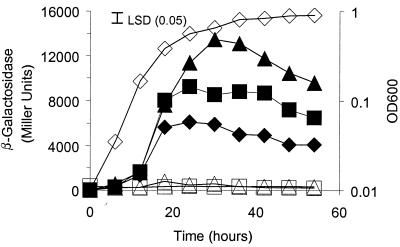
It was previously shown that expression of cinI is induced by CinR in response to CinI-made 3OH-C14:1-HSL and that pRL1JI represses cinI expression (23). A549 (bisR::Tn5) and A627 (triR::Tn5) were tested for 3OH-C14:1-HSL production in a bacteriocin-type assay; A549 (but not A627) was found to have lost repression of 3OH-C14:1-HSL production, because A549 inhibited the growth of the small bacteriocin-sensitive strain A34 (Fig. 3B) in a manner similar to that of 8401 (Fig. 3A). This indicates that bisR plays a role in repression of 3OH-C14:1-HSL synthesis. The activity of a cinI::lacZ fusion (pIJ7910) was measured in the bisR mutant and the wild type. In the bisR mutant, the level of cinI expression was equivalent to that seen in strain 8401, which lacks pRL1JI (Fig. 4).
FIG. 3.
Growth inhibition tests indicate bisR represses cinI expression. Colonies of different AHL-producing bacteria were inoculated onto a lawn of the indicator strain A34 in a small bacteriocin-type test. The results of inoculation onto the lawn with strains 8401 and A34 (A), strains A549 and A627 (B), and the E. coli DH5α strain carrying pIJ7986 (cinR, cinI, and bisR) or pIJ7750 (cinR and cinI) (C) are shown.
FIG. 4.
Assay of cinI-lacZ expression in a bisR mutant. Expression of cinI throughout growth in TY medium was assayed using pIJ7910 (cinI-lacZ), which had been introduced into the wild-type A34 strain (filled squares) and the bisR mutant A549 (triangles). For comparison, the expression of cinI-lacZ in strain 8401/pIJ7910 (derivative of A34 lacking pRL1JI) is also shown (diamonds). The growth of strain A34 is shown (open squares), and the growth rates of the other strains were similar. LSD, least significant difference.
In order to define whether bisR was the only gene from the R. leguminosarum genome required to repress 3OH-C14:1-HSL synthesis, the ability of the cin genes to synthesize 3OH-C14:1-HSL in E. coli DH5α was exploited. Plasmid pIJ7750 contains cinR and cinI cloned in pUC19, and cinR and cinI are the only two complete genes present. In a bacteriocin-type assay, E. coli DH5α/pIJ7750 inhibits growth of A34, demonstrating that cinR and cinI are sufficient to direct the synthesis of 3OH-C14:1-HSL (Fig. 3C). Deletion of part of cinR (pIJ7777) abolished 3OH-C14:1-HSL formation and the growth inhibition (data not shown), demonstrating that CinR is required for cinI expression in E. coli. A 1.3-kb HindIII fragment which carries bisR was cloned into pIJ7750 downstream of cinR and cinI, making pIJ7986 and pIJ7987 (which are both orientations of the 1.3-kb fragment). In E. coli DH5α, neither pIJ7986 (Fig. 3C) nor pIJ7987 (data not shown) could confer the ability to synthesize 3OH-C14:1-HSL, based on a bacteriocin-type bioassay using R. leguminosarum bv. viciae A34 as a sensitive strain. This demonstrates that bisR can repress 3OH-C14:1-HSL synthesis in E. coli. In R. leguminosarum bv. viciae, cloned bisR (on pIJ7546) repressed 3OH-C14:1-HSL formation by strain 8401, as tested by means of a bacteriocin-type assay (data not shown). These results suggest that BisR inhibits 3OH-C14:1-HSL synthesis by repressing transcription of cinI. This repression could occur if BisR competes with CinR for binding to the cinI promoter or if BisR forms inactive heterodimers with CinR.
BisR and 3OH-C14:1-HSL induce expression of triR.
A triR-lacZ transcriptional fusion plasmid (pIJ7878) was constructed. Growth phase-dependent induction of triR-lacZ expression was seen in the wild-type A34 (Fig. 5). Mutations in bisR, cinI (Fig. 5), and cinR (data not shown) all had the effect of abolishing triR expression. This raises the question of whether CinR regulates expression of triR directly or because of the requirement of CinR for induction of cinI, the product of which makes 3OH-C14:1-HSL. To resolve this question, 3OH-C14:1-HSL was added to cultures of A34 (wt), A549 (bisR::Tn5), A621 (cinR::Tn5), and A664 (cinI::Spc), all carrying pIJ7878 (triR-lacZ). Addition of 3OH-C14:1-HSL to the wild-type A34 strain doubled triR-lacZ expression (Table 2). With A621 (cinR) and A664 (cinI) carrying pIJ7878 (triR-lacZ), addition of 3OH-C14:1-HSL induced high levels of triR-lacZ expression from a low-level background (Table 2). However, with in the presence of A549/pIJ7878, 3OH-C14:1-HSL did not induce triR-lacZ expression (Table 2), demonstrating that the loss of triR expression in the cinI and cinR mutants is due to the absence of 3OH-C14:1-HSL. Therefore, both bisR and 3OH-C14:1-HSL are essential for expression of triR. Plasmid transfer was previously shown to be very severely reduced if both the donor and recipient bacteria were mutated in cinI and were therefore defective for 3OH-C14:1-HSL production (23). In filter matings between cinI mutants similar to those described previously (23), we found that the addition of 0.1 μM 3OH-C14:1-HSL to the mating medium could restore normal conjugation ([1.8 ± 0.3] × 10−2 transconjugants per donor) from the low rates (less than 10−7) observed when 3OH-C14:1-HSL was not added. The addition of 3OH-C14:1-HSL did not stimulate plasmid transfer by bisR or triR mutants (data not shown).
FIG. 5.
Assays of triR-lacZ expression in various mutants. Expression of triR was assayed using pIJ7878 (triR-lacZ) introduced into the wild-type strain A34 (filled diamonds), the rhiR mutant A160 (filled squares), the bisR mutant A549 (open squares), the triR mutant A627 (filled triangles), and the cinI mutant A664 (open triangles). OD600s measured using strain A34 are represented by open diamonds, and the growth rates of all the strains were similar.
TABLE 2.
Effect of 3OH-C14:1-HSL on triR-lacZ expression in mutants
| Strain carrying pIJ7878 (triR-lacZ) | β-Galactosidase activity (Miller units)
|
|
|---|---|---|
| No added AHL | +3OH-C14:1-HSL | |
| A34 (wt)a | 5,400 (±170) | 22,700 (±500) |
| A549 (bisR) | 290 (±33) | 320 (±40) |
| A621 (cinR) | 400 (±39) | 21,800 (±450) |
| A664 (cinI) | 620 (±50) | 22,100 (±440) |
wt, wild type.
The effect of mutating other genes involved in quorum sensing in R. leguminosarum bv. viciae was also analyzed using pIJ7878 (triR-lacZ). Mutations in rhiR and triR both increased expression of triR-lacZ. This effect was particularly strong in the triR mutant (Fig. 5).
A transcriptional fusion to the upstream region of bisR was also made (pIJ7752). The bisR-lacZ fusion was expressed at a low level in the early exponential phase (1420 ± 90 units), increasing to 4,180 ± 190 units in early stationary phase. Mutations in bisR, rhiR, or triR had no observed effect upon expression of bisR, but mutation of cinI or cinR reduced bisR expression by about 30% (data not shown).
Sensitivity to 3OH-C14:1-HSL is influenced by other AHLs.
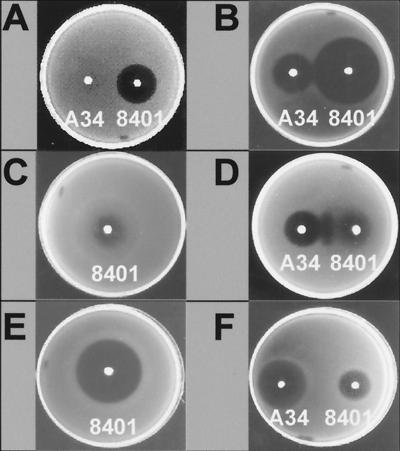
Mutations in bisR and triR indicate both genes are necessary for sensitivity to 3OH-C14:1-HSL. Both bisR and triR were cloned in pIJ7867. Strain A34 carrying pIJ7867 had enhanced growth sensitivity, based on the size of the halo produced around a colony of strain 8401 bacteria (compare Fig. 6A and B). Furthermore, growth of A34/pIJ7867 was now inhibited by strain A34 (Fig. 6B). This is consistent with the finding that strain A34 produces some 3OH-C14:1-HSL, although at a much lower level than strain 8401 (23).
FIG. 6.
Growth inhibition tests indicate that growth sensitivity to 3OH-C14:1-HSL requires the presence of other AHLs. (A) The indicator strain was A34. (B) The indicator strain was A34/pIJ7867 (bisR and triR). (A and B) Strains 8401 and A34 were inoculated on top. (C and D) The indicator strain was 8401/pIJ7867 (bisR and triR). (C) Strain 8401 was inoculated on top. (D) Both 8401 and A34 were inoculated on top. (E) The indicator strain was 8401/pIJ7867/pIJ9071 (bisR triR traI) and strain 8401 was inoculated on top. (F) The indicator strain was A. tumefaciens C58.00/pIJ7867, and 8401 and A34 were inoculated on top. A similar result to that seen in panel F was obtained if the indicator strain was ANU265 carrying pIJ7867 (data not shown).
To determine whether the bisR and triR genes can confer sensitivity to 3OH-C14:1-HSL in the absence of other pRL1JI genes, pIJ7867 was introduced into strain 8401. When 8401/pIJ7867 was grown as an underlay, growth inhibition could be seen around the colony of 8401, indicating that pIJ7867 conferred some sensitivity to 3OH-C14:1-HSL (Fig. 6C). Although there was a zone of inhibition of growth, it was not as clear as that seen with strain A34 as an underlay (compare Fig. 6A and C), and the edge of the zone was diffuse. When bisR and triR were cloned separately and introduced into R. leguminosarum strain 8401, neither gene had any discernible effect on sensitivity to 3OH-C14:1-HSL (data not shown). Therefore, bisR and triR are the pRL1JI genes which primarily determine sensitivity to 3OH-C14:1-HSL, although it would appear that other genes on pRL1JI increase sensitivity.
Surprisingly, strain A34 induced stronger inhibition of growth of 8401/pIJ7867 than did 8401 (Fig. 6D). This was the opposite of what was expected and indicates that some process which was determined by pRL1JI (in A34) contributed to the inhibition of the growth of strain 8401 carrying bisR and triR on pIJ7867. A clear additional zone of growth inhibition was seen between colonies of 8401 and A34 on a lawn of 8401/pIJ7867 (Fig. 6D). This could be explained if AHLs made by pRL1JI enhance the sensitivity of the lawn (of 8401/pIJ7867) to the 3OH-C14:1-HSL produced by 8401. One of the pRL1JI loci determining AHL production is traI, and so plasmid pIJ9071 (carrying traI) was introduced into 8401/pIJ7867 (carrying bisR and triR). The presence of traI on pIJ9071 greatly increased the sensitivity of a lawn of 8401/pIJ7867 to inhibition by 8401 (compare Fig. 6E and C). This indicates that TraI-made AHLs contribute to the growth sensitivity that is determined by bisR and triR. In other work, traI was shown to produce primarily 3O-C8-HSL and C8-HSL (V. Danino, unpublished data), and so it should be possible to test the effects of these AHLs by adding them to a lawn of 8401/pIJ7867 and measuring their effect on growth inhibition by 3OH-C14:1-HSL.
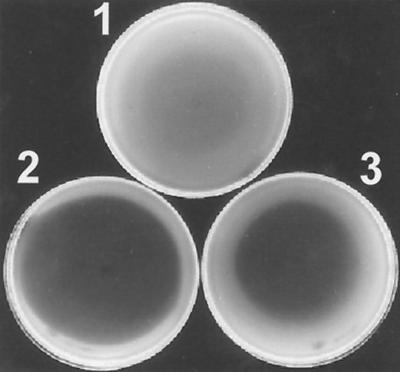
Several chemically synthesized AHLs were tested for their ability to increase the growth inhibition caused by 3OH-C14:1-HSL. The AHLs (C4-HSL, 3O-C4-HSL, C6-HSL, 3O-C6-HSL, C8-HSL, and 3O-C8-HSL) were tested separately and in combination with 3OH-C14:1-HSL for their effect on the growth of 8401/pIJ7867. Although 3OH-C14:1-HSL strongly inhibits the growth of A34, it inhibited the growth of 8401/pIJ7867 only slightly (Fig. 7). C4-HSL, 3O-C4-HSL, C6-HSL, 3O-C6-HSL, C8-HSL, and 3O-C8-HSL individually had no significant inhibitory effect (data not shown). However, 3O-C8-HSL or C8-HSL added together with 3OH-C14:1-HSL dramatically inhibited growth of 8410/pIJ7867 (Fig. 7). None of the other AHLs tested had any effect on the sensitivity of 8401/pIJ7867 to 3OH-C14:1-HSL (data not shown).
FIG. 7.
Growth inhibition assays using purified AHLs. The assays were similar to those described for Fig. 3, except AHLs were added to the plate instead of AHL-producing colonies. In the three plates, the indicator strain was 8401/pIJ7867 (bisR triR). (Plate 1) 3OH-C14:1-HSL was added to the middle of the agar. (Plate 2) Both 3OH-C14:1-HSL and 3O-C8-HSL were added to the middle of the agar. (Plate 3) Both 3OH-C14:1-HSL and C8-HSL were added to the middle of the agar.
bisR and triR confer 3OH-C14:1-HSL sensitivity to Agrobacterium and Rhizobium sp. strain NGR234.
Plasmid pIJ7867 (carrying bisR and triR) was transferred to A. tumefaciens strain C58.00. The growth of C58.00 was not inhibited by the presence of strain 8401 or A34. However, growth of C58.00/pIJ7867 was strongly inhibited by both 8401 and A34 (Fig. 6F) but not by the cinI mutant derivatives of these strains (data not shown). This demonstrates that bisR and triR can confer sensitivity to 3OH-C14:1-HSL in A. tumefaciens C58.00. The growth inhibition of C58.00/pIJ7867 by A34 was greater than that seen with 8401 (Fig. 6F), again indicating that some product made by pRL1JI enhances the growth inhibition. Similar results were observed when pIJ7867 was transferred to ANU259, a derivative of Rhizobium sp. strain NGR234 lacking its symbiotic plasmid (data not shown).
Proteins induced during growth inhibition.
It is evident that bisR and triR influence the growth sensitivity to AHLs and it is most likely that growth inhibition is caused by induction or repression of some chromosomal gene by BisR and/or TriR. We were unsuccessful in isolating Tn5-induced mutants (other than bisR and triR) that conferred resistance. Such mutants might have given an insight about the regulated gene. In the absence of such a mutant, we decided to determine whether there were major changes to the protein profile during growth inhibition by AHLs. 3OH-C14:1-HSL inhibits the growth of early exponentially growing strains containing pRLlJI about 6 h after the addition of 3OH-C14:1-HSL (19). Using the supersensitive strain A34/pIJ7867 (containing pRL1JI and cloned using bisR-triR), we investigated the effect of growth-inhibiting AHLs on protein production. After treatment with 3OH-C14:1-AHL (from a culture of strain 8401), the protein profile was analyzed by SDS-PAGE. Several proteins were induced by this treatment, including three proteins with estimated masses of 24 kDa, 29 kDa, and 37 kDa (Fig. 8, lane 2). When the same strain was treated with an extract lacking 3OH-C14:1-HSL (using an extract made from a culture of the cinI mutant A552), the induction of these proteins was not seen and the protein profile was the same as that of the control (to which no extract was added) (Fig. 8, lane 1). When a similar experiment was done using A34 rather than A34/pIJ7867 as the sensitive strain, similar proteins were induced, although at a reduced level (data not shown).
FIG. 8.
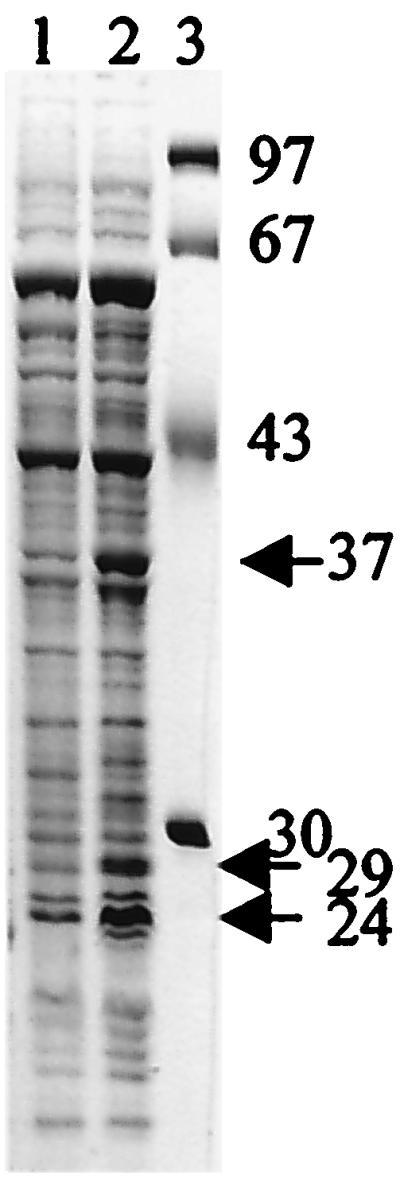
Analysis of proteins induced by growth-inhibitory AHL extract. Following growth of strain A34/pIJ7867 to an OD600 of 0.1 in TY medium, AHL extracts from 8401 (containing 3OH-C14:1-HSL) (lane 2) and the cinI mutant A552 (lacking 3OH-C14:1-HSL) (lane 1) were added. After 2.5 h of incubation, the cells were disrupted and the proteins were separated by SDS-PAGE and stained with Coomassie blue. Lane 3 shows size markers (in kDa). The proteins indicated by arrows were induced by the extract from strain 8401.
The most strongly induced protein (24 kDa) was identified by immunoblotting as RhiA (data not shown), which fits with the finding that 3OH-C14:1-HSL induces expression of the rhiABC operon (19). To identify the two other proteins, the N-terminal sequences were determined. The sequence of the 29-kDa protein was MQENQYSTQYLK(Q/A)S(S/Q)AVKNA, which matches the predicted BisR sequence. The protein which migrated to a position corresponding to an apparent mass of 37 kDa gave the sequence MEITAAMVKELREKTGAGMM, which closely matches the predicted N-terminal sequences MTVTAAMVKELREKTGAGMM and MSISAAQVKELRDLTGAGMM of the predicted EF-Ts proteins from Sinorhizobium meliloti and Mesorhizobium loti, respectively. These EF-Ts proteins are predicted to be about 32 kDa in size, which is a typical predicted mass for EF-Ts proteins. The E. coli EF-Ts protein migrates to a position corresponding to an apparent mass about 3 kDa larger than its predicted size (entry P02997 in the Swiss two-dimensional PAGE database [www.expasy.ch/ch2d]), and so the apparent size (37 kDa) of the induced protein is reasonably consistent with its being EF-Ts. There is the theoretical possibility that the specifics of the determined sequence were due to background levels of EF-Ts if the induced protein was N-terminally blocked. However, following two-dimensional electrophoresis, we observed that the induced protein migrates to a position corresponding to a different size from but at the same pI as BisR, which has a predicted pI of 5.38. Therefore, this finding gives an accurate estimate of the pI for the 37-kDa protein, which fits well with the predicted pI of 5.3 for the M. loti EF-Ts protein. Thus, we conclude that it is most probably EF-Ts that is induced. Three elongation factors are required for protein synthesis. EF-Tu associated with GTP forms a stable complex with an amino-acyl-tRNA; the binding of this ternary complex to the A site of the ribosome is associated with the hydrolysis of GTP. After release of EF-Tu-GDP, peptide bond formation takes place. EF-G is then required for the translocation of the elongating peptide from the A site to the P site of the ribosome. EF-Ts, a nucleotide exchange protein, is needed to recycle EF-Tu to its active state by exchanging GDP and GTP (41). In view of the close relation between EF-Ts and EF-Tu, we tested whether the amount of EF-Tu was increased. Using a monoclonal antibody to the E. coli EF-Tu, a protein of 43 kDa was detected (expected size for EF-Tu: 40), but the amount of this reactive protein was unchanged by the addition of growth-inhibiting AHLs (data not shown).
DISCUSSION
R. leguminosarum bv. viciae strain A34 has four AHL synthases (10, 23, 30, 44) and multiple regulators that respond to the AHLs. Two of the AHL synthases are located on the symbiotic plasmid pRL1JI, and one of these is clearly analogous to the well-described traI gene on the tumor-inducing (Ti) plasmid of A. tumefaciens. The order and location of the traI-trbBCDEJKLFGHI genes is conserved between the pRL1JI and Ti plasmids. However, in A. tumefaciens the regulator of the traI-trbBCDEJKLFGHI operon is encoded by traR, which is located at some distance from traI. In two different strains of A. tumefaciens with somewhat different gene arrangements, the traR genes are located in operons, which are induced in response to metabolites released from plants that have been transformed with genes from the Ti plasmid. The resulting spread of the Ti plasmid, which contains genes for the degradation of the plant-made metabolites (such as opines), enhances the selection and growth of specific plasmid-bearing strains around wound sites. The A. tumefaciens traR genes can be induced under the control of different types of regulation. For example, in one strain, expression of the operon in which traR is located is repressed by AccR and this repression is relieved by plant-made metabolites. In another strain, the operon containing traR is induced by the LysR-type regulator OccR in response to plant-made metabolites.
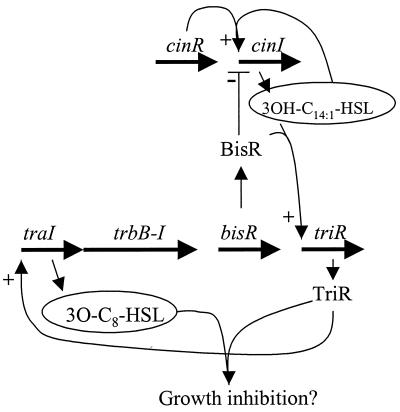
It is evident that the regulation of the equivalent gene (triR) on pRL1JI is somewhat different from the regulation of traR in A. tumefaciens, because triR is induced by BisR, another LuxR-type regulator. This induction requires the presence of CinI-made 3OH-C14:1-HSL. Therefore, control of plasmid transfer is influenced by three LuxR-type regulators, CinR (which is required for cinI expression), BisR, and TriR (Fig. 9). The observation that BisR induces triR in response to 3OH-C14:1-HSL explains the previous observation that mutation of cinI or cinR can greatly reduce plasmid transfer (23) but only if both strains in the mating are defective for 3OH-C14:1-HSL production. In addition to inducing triR, BisR plays a role in repressing cinI expression (Fig. 9), and this explains why pRL1JI reduces the amount of 3OH-C14:1-HSL formation. We could not determine whether repression of cinI expression by BisR occurs in the absence of 3OH-C14:1-HSL, because this AHL must be present to induce cinI expression by CinR. The phenotypes of the bisR and triR mutants described here show similarities to the rps (repression of the small bacteriocin) and sbs (sensitivity to the small bacteriocin) mutants described previously (42). We tried to obtain these mutants for complementation experiments but the original mutants had been lost.
FIG. 9.
Model for regulatory interactions between the cinI cinR quorum-sensing genes and the bisR, triR, and traI genes. Bold arrows designate genes, and the contiguous arrows between traI and the trb genes indicate that these probably constitute an operon.
It is not immediately obvious why the control of pRL1JI should be under the ultimate regulation of the chromosomal cinRI genes. One reason could be that induction of plasmid transfer occurs as the bacteria begin to leave the exponential phase. Alternatively, pRL1JI transfer might be strongly induced by a population of potential recipients of pRL1JI, which tend to make much higher levels of 3OH-C14:1-HSL than strains containing pRL1JI (23). Perhaps this is related to the very high plasmid transfer frequencies that are seen with pRL1JI. The regulation of plasmid transfer is more complex than that which is described here. This can be seen in part from the observation that mutations in triR and rhiR enhance expression from the triR promoter. Furthermore, in other work we have observed that mutation of traI enhances expression from the traI promoter and that the addition of TraI-made AHLs to strain A34 carrying Tn5-lacZ in trbE decreases expression of the trb operon (unpublished data).
The AHL-dependent inhibition of growth of R. leguminosarum is a curious and unusual effect of AHLs. It had previously been thought that growth inhibition required the presence of 3OH-C14:1-HSL alone, but the work described here demonstrates that the growth inhibition requires other AHLs such as 3O-C8-HSL and C8-HSL. The sensitivity is mediated by the two LuxR-type regulators encoded by bisR and triR, and a plasmid carrying both of these genes can confer growth sensitivity to different rhizobial strains and to A. tumefaciens. This suggests that BisR and/or TriR is inducing (or repressing) the expression of some gene(s) whose altered expression arrests growth. Identification of the targeted gene(s) will give an insight into the mechanism of growth inhibition. The observation that a protein showing strong similarity to the translation factor EF-Ts is present at increased levels during growth inhibition points to a possible effect on translation. Interestingly, another translation elongation factor, EF-G, was found to be present at increased levels in starved cells of R. leguminosarum bv. phaseoli (37). In E. coli, transcription of these genes decreases during starvation (1), and so further work would be required to demonstrate whether the effect observed here on levels of EF-Ts was an effect on transcription or protein stability. However, at this stage we do not know whether the observed increase in EF-Ts is an effect of growth inhibition or some direct or indirect effect of AHLs. It is noteworthy that the global regulator RsmA in P. aeruginosa negatively regulates both transcription and translation of the quorum sensing-regulated hcnA gene required for cyanide biosynthesis in stationary phase (27).
It is difficult to explain what advantage this pRL1JI-mediated AHL sensitivity system endows upon the bacteria or the plasmid; in theory, it places those cells which are carrying the pRL1JI plasmid under a selective disadvantage compared to those which are not. One possibility is that the extreme growth inhibition caused by the AHLs is an artifact of bacterial growth in the laboratory. The concentrations of AHLs to which the bacteria are exposed in high-cell-density cultures when they are growing as colonies on solid medium may be higher than those encountered in a normal environment.
In R. leguminosarum, both bisR and triR are required to confer sensitivity to 3OH-C14:1-HSL, but growth inhibition requires other AHLs (such as 3O-C8-HSL or C8-HSL) in addition to 3OH-C14:1-HSL. One model is that BisR, when activated by 3OH-C14:1-HSL (presumably upon binding), increases expression of triR and the resulting high level of expression of triR causes growth problems in the presence of 3O-C8-HSL or C8-HSL (Fig. 9). TriR, in conjunction with one of these AHLs, may regulate other genes in the bacterium, leading to a reduced rate of growth caused by some mechanism that remains to be defined. Possibly we were unable to isolate small bacteriocin-resistant mutants other than those affected in bisR or triR because other important genes are essential for growth or because there are functionally redundant copies of target genes. There are indications that translation may be affected, but at this stage we cannot determine whether the increased level of EF-Ts is a cause or an effect of the growth inhibition.
Acknowledgments
We thank Paul Williams and Siri Ram Chhabra for gifts of AHLs for use as standards, Mike Naldrett for determining N-terminal amino acid sequences, Nick Brewin for antiserum to RhiA, J. Khöl (Hannover) for antiserum to EF-Tu, and A. Davies for help with maintenance of bacterial strains and plasmids. We thank E. Watson and M. Dow for constructive comments on the manuscript.
This work was supported by a grant-in-aid, studentships (to A.W., V.D., and J.K.L.) and a grant (208/PRS12210) from the Biotechnology and Biological Sciences Research Council, and in part by contracts (B104-CT96-0181 and QLK3-CT-2000-31795) from the European Union and a gift from CERES.
REFERENCES
- 1.Albertson, N. H., and T. Nystrom. 1994. Effects of starvation for exogenous carbon on functional mRNA stability and rate of peptide chain elongation in Escherichia coli. FEMS Microbiol. Lett. 117:181-187. [DOI] [PubMed] [Google Scholar]
- 2.Alt-Morbe, J., J. L. Stryker, C. Fuqua, P. L. Li, S. K. Farrand, and S. C. Winans. 1996. The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J. Bacteriol. 178:4248-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagdasarian, M., R. Lurz, B. Ruckert, F. C. H. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number RSF1010-derived vectors and a host-vector system for cloning in Pseudomonas. Gene 16:237-247. [DOI] [PubMed] [Google Scholar]
- 4.Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 5.Beringer, J. E., J. L. Beynon, A. V. Buchanan-Wollaston, and A. W. B. Johnston. 1978. Transfer of the drug resistance transposon Tn5 to Rhizobium. Nature 276:633-634. [Google Scholar]
- 6.Beynon, J. L., J. E. Beringer, and A. W. B. Johnston. 1980. Plasmids and host range in Rhizobium leguminosarum and Rhizobium phaseoli. J. Gen. Microbiol. 120:421-429. [Google Scholar]
- 7.Bradley, D. J., E. A. Wood, A. P. Larkins, G. Galfrè, G. W. Butcher, and N. J. Brewin. 1988. Isolation of monoclonal antibodies reacting with peribacteroid membranes and other components of pea root nodules containing Rhizobium leguminosarum. Planta 173:149-160. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan-Wollaston, A. V. 1979. Generalized transduction in Rhizobium leguminosarum. J. Gen. Microbiol. 112:135-142. [Google Scholar]
- 9.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 10.Cubo, M. T., A. Economou, G. Murphy, A. W. B. Johnston, and J. A. Downie. 1992. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J. Bacteriol. 174:4026-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dibb, N. J., J. A. Downie, and N. J. Brewin. 1984. Identification of a rhizosphere protein encoded by the symbiotic plasmid of Rhizobium leguminosarum. J. Bacteriol. 158:621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downie, J. A., G. Hombrecher, Q.-S. Ma, C. D. Knight, B. Wells, and A. W. B. Johnston. 1983. Cloned nodulation genes of Rhizobium leguminosarum determine host-range specificity. Mol. Gen. Genet. 190:359-365. [Google Scholar]
- 13.Farrand, S. K. 1998. Conjugal plasmids and their transfer, p. 199-233. In H. P. Spaink, A. Kondorosi, and P. J. J. Hooykaas (ed.), The Rhizobiaceae. Kluwer Academic, Dordrecht, The Netherlands.
- 14.Finnie, C., N. M. Hartley, K. C. Findlay, and J. A. Downie. 1997. The Rhizobium leguminosarum prsDE genes are required for secretion of several proteins, some of which influence nodulation, symbiotic nitrogen fixation and exopolysaccharide modification. Mol. Microbiol. 25:135-146. [DOI] [PubMed] [Google Scholar]
- 15.Freiberg, C., R. Fellay, A. Bairoch, W. J. Broughton, A. Rosenthal, and X. Perret. 1997. Molecular basis of symbiosis between Rhizobium and legumes. Nature 387:394-401. [DOI] [PubMed] [Google Scholar]
- 16.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Ann. Rev. Microbiol. 50:727-751. [DOI] [PubMed] [Google Scholar]
- 17.Fuqua, W. C., and S. C. Winans. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176:2796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray, K. M., J. P. Pearson, J. A. Downie, B. E. A. Boboye, and E. P. Greenberg. 1996. Cell-to-cell signaling in the symbiotic nitrogen-fixing bacterium Rhizobium leguminosarum: autoinduction of a stationary phase and rhizosphere-expressed genes. J. Bacteriol. 178:372-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch, P. R. 1979. Plasmid-determined bacteriocin production by Rhizobium leguminosarum. J. Gen. Microbiol. 113:219-228. [Google Scholar]
- 21.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 22.Lamb, J. W., J. A. Downie, and A. W. B. Johnston. 1985. Cloning of the nodulation (nod) genes of Rhizobium phaseoli and their homology to R. leguminosarum nod DNA. Gene 34:235-241. [DOI] [PubMed] [Google Scholar]
- 23.Lithgow, J. K., A. Wilkinson, A. Hardman, B. Rodelas, F. Wisniewski-Dye, P. Williams, and J. A. Downie. 2000. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol. 37:81-97. [DOI] [PubMed] [Google Scholar]
- 24.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 25.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, New York, N.Y.
- 26.Morrison, N. A., C. Y. Hau, M. J. Trinick, J. Shine, and B. G. Rolfe. 1983. Heat curing of a sym plasmid in a fast-growing Rhizobium sp. that is able to nodulate legumes and the nonlegume Parasponia sp. J. Bacteriol. 153:527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. Holden, M. Camara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piper, K. R., S. Beck von Bodman, and S. K. Farrand. 1993. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362:448-450. [DOI] [PubMed] [Google Scholar]
- 29.Puskas, A., E. P. Greenberg, S. Kaplan, and A. L. Schaefer. 1997. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J. Bacteriol. 179:7530-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodelas, B., J. K. Lithgow, F. Wisniewski-Dye, A. Hardman, A. Wilkinson, A. Economou, P. Williams, and J. A. Downie. 1999. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv. viciae. J. Bacteriol. 181:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Schripsema, J., K. E. E. de Rudder, T. B. van Vliet, P. P. Lankhorst, E. de Vroom, J. W. Kijne, and A. A. N. Van Brussel. 1996. Bacteriocin small of Rhizobium leguminosarum belongs to the class of N-acyl-l-homoserine lactone molecules, known as autoinducers and as quorum sensing co-transcription factors. J. Bacteriol. 178:366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherwood, M. T. 1970. Improved synthetic medium for the growth of Rhizobium. J. Appl. Bacteriol. 33:708-713. [DOI] [PubMed] [Google Scholar]
- 35.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRLIJI. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 36.Swift, S., J. A. Downie, N. A. Whitehead, A. M. Barnard, G. P. Salmond, and P. Williams. 2001. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microb. Physiol. 45:199-270. [DOI] [PubMed] [Google Scholar]
- 37.Thorne, S. H. 1997. Stationary phase survival of Rhizobium leguminosarum. Ph.D. thesis. University of London, London, United Kingdom.
- 38.Thorne, S. H., and H. D. Williams. 1999. Cell density-dependent starvation survival of Rhizobium leguminosarum bv. phaseoli: identification of the role of an N-acyl homoserine lactone in adaptation to stationary-phase survival. J. Bacteriol. 181:981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaudequin-Dransart, V., A. Petit, C. Poncet, C. Ponsonnet, X. Nesme, J. B. Jones, H. Bouzar, W. Scott-Chilton, and Y. Dessaux. 1995. Novel Ti plasmids in Agrobacterium strains isolated from fig tree and chrysanthemum tumors and their opinelike molecules. Phytopathology 8:311-321. [DOI] [PubMed] [Google Scholar]
- 40.Weber, S., F. Lottspeich, and J. Kohl. 1995. An epitope of elongation factor Tu is widely distributed within the bacterial and archaeal domains. J. Bacteriol. 177:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weijland, A., and A. Parmeggiani. 1994. Why do two EF-Tu molecules act in the elongation cycle of protein biosynthesis? Trends Biochem. Sci. 19:188-193. [DOI] [PubMed] [Google Scholar]
- 42.Wijffelman, C. A., E. Pees, A. A. N. Van Brussel, and P. J. J. Hooykaas. 1983. Repression of small bacteriocin excretion in Rhizobium leguminosarum and Rhizobium trifolii by transmissible plasmids. Mol. Gen. Genet. 192:171-176. [Google Scholar]
- 43.Winson, M. K., M. Camara, A. Latifi, M. Foglino, S. R. Chhabra, M. Daykin, M. Bally, V. Chapon, G. P. Salmond, B. W. Bycroft, et al. 1995. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:9427-9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wisniewski-Dýe, F., J. Jones, S. R. Chhabra, and J. A. Downie. 2002. raiIR genes are part of a quorum-sensing network controlled by cinI and cinR in Rhizobium leguminosarum. J. Bacteriol. 184:1597-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]