Abstract
A Zn2+ transport system encoded by the zntB locus of Salmonella enterica serovar Typhimurium has been identified. The protein encoded by this locus is homologous to the CorA family of Mg2+ transport proteins and is widely distributed among the eubacteria. Mutations at zntB confer an increased sensitivity to the cytotoxic effects of Zn2+ and Cd2+, a phenotype that suggests that the encoded protein mediates the efflux of both cations. A direct analysis of transport activity identified a capacity for Zn2+ efflux. These data identify ZntB as a zinc efflux pathway in the enteric bacteria and assign a new function to the CorA family of cation transporters.
Zinc is the second most abundant transition metal in biological systems (6, 11, 24). It is an essential element that is employed in a wide range of biochemical and biophysical roles. It is required to maintain the structural stability of macromolecules and to serve as a cofactor for more than 300 metabolic enzymes. It also plays a prominent role in gene expression as a structural component in a large number of Zn2+-dependent transcription factors (4, 19). While the cellular requirement for zinc is absolute, excess concentrations of the cation are highly toxic. Consequently, the ability to maintain the intracellular Zn2+ concentration within very narrow limits is a fundamental property of all living cells. Enteric bacteria are currently thought to maintain Zn2+ homeostasis through the activities of at least four Zn2+-specific transport systems. Zinc uptake is facilitated by the combined activities of the ZnuABC and ZupT transport systems (10, 13). The ZnuABC system is the primary Zn2+ influx pathway and is induced in response to zinc deprivation (16). Bacteria respond to high levels of exogenous Zn2+ by increased expression of the ZntA and ZitB efflux systems (2, 5, 9, 17). The previously uncharacterized open reading frame b1342 of Escherichia coli encodes a protein that is homologous to the CorA family of cation transporters (3, 20). In Salmonella enterica serovar Typhimurium and E. coli, CorA functions as the primary influx pathway for Mg2+ and is distinguished by its unique ability to mediate both the influx and efflux of Mg2+ (8, 22, 23). In an effort to ascertain the function of the peptide encoded by b1342, our laboratory identified the corresponding allele in S. enterica serovar Typhimurium and disrupted it by the insertion of an antibiotic resistance cassette. In this report, we show that the serovar Typhimurium homolog of b1342 encodes an additional zinc transport system. Mutations at this locus resulted in an increased sensitivity to cytotoxic levels of Zn2+ and Cd2+ and a reduced capacity for zinc efflux. Consequently, the mutated gene and its protein product were designated ZntB.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Plasmids were maintained in E. coli Top10F′ unless indicated otherwise.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| Serovar Typhimurium | ||
| RS404 | Nominal wild-type strain 14028s | ATCC |
| RS1100 | zntB1::ΩCm | This study |
| MM281 | corA45::Tn10Δ16Δ17(Tc) mgtCB::Mu dJ mgtA::Mu dJ | 21 |
| E. coli | ||
| GR480 | E. coli ΔzntA ΔzitB ΔzupD ΔznuABC ΔyiiP | C. Rensing; 9, 10 |
| RS1220 | E. coli ΔzntA ΔzitB ΔzupD ΔznuABC ΔyiiP zntB1::ΩCm | This study |
| Top10F′ | F′ {lacIq Tn10(Tc)} mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araΔ139 (ara-leu)7697 galU galK rpsL endA nupG | Invitrogen |
| Plasmids | ||
| pKD46 | oriR101 repA101(Ts) P-araB-gam-bet-exo | 7 |
| pAJW54 | Serovar Typhimurium zntB+ native promoter in pLG338/IBI30 (low copy) | This study |
| pRS310 | Serovar Typhimurium corA+ cw in pCR2.1 | 21 |
| pRS319 | M. jannaschii corA+ in pCR2.1 | 21 |
Culture media and reagents.
Bacteria were grown at 37°C with aeration in Luria-Bertani (LB) medium or on LB agar plates (14), except when otherwise stated. Minimal medium was based on the N medium of Nelson and Kennedy (15), supplemented with 0.25% glucose, 0.02% Casamino Acids, and 1 mM MgSO4. Antibiotics were added to complex and minimal culture media, respectively, at the following concentrations: sodium ampicillin salt (100 and 30 μg/ml), kanamycin sulfate (50 and 100 μg/ml), chloramphenicol (25 and 10 μg/ml). Restriction endonucleases, T4 DNA ligase, and shrimp alkaline phosphatase were obtained from Promega Corporation. Sequencing enzymes and associated biochemicals were obtained from Epicentre Technologies. 65ZnCl2 was purchased from Perkin Elmer Life Sciences, Inc. Additional chemicals were obtained from standard suppliers.
DNA manipulations and zntB mutant construction.
Construction of the serovar Typhimurium zntB mutant strain was performed as described previously by Datsenko and Wanner (7) with the following modifications: pKD46 was first moved into serovar Typhimurium 14028s by electroporation. The zntB1::ΩCm mutagenic cassette was gel purified and transformed into 14028s/pKD46. Chloramphenicol-resistant transformants were subsequently cured of pKD46 by growth at 40°C. Chromosomal integration of the mutagenic cassette was confirmed by PCR and sequenced using oligonucleotides external to the integrated cassette. Plasmid DNA was prepared from 5-ml cultures using the Qiaprep Spin Miniprep kit obtained from Qiagen. Genomic DNA was isolated using a PureGene purification kit obtained from Gentra Systems. Restriction endonuclease digestion, DNA ligation, and transformation of linear and plasmid DNA were performed as described previously (1).
Quantitation of cation sensitivity.
Susceptibility profiles were generated by disk diffusion and growth curve analyses. Bacterial lawns were spread on N-minimal agar plates, and 8-mm sterile filter disks were placed in the center. Lethal levels of ZnSO4, CoCl2, NiCl2, MnCl2, and CdCl2 (100 mM, 100 mM, 1 M, 1 M, and 100 mM, respectively) were added to the disks. Zones of inhibition were measured after 24 h of growth at 37°C. In addition, growth was monitored over time in N-minimal media containing various levels of ZnSO4. Overnight cultures were diluted 1:100 into appropriate growth assay media and readings of the optical density at 600 nm (OD600) were made every hour until stationary phase was reached.
65Zn2+ uptake.
Overnight cultures were diluted 100-fold into supplemented N-minimal medium and grown at 37°C with aeration to an OD600 of 0.4. Cells were then washed twice with ice-cold N-minimal medium without MgSO4, Casamino Acids, and glucose supplementation. The pellet was resuspended in wash buffer to a final OD600 of between 1.0 and 2.0. Uptake was measured at 37°C in N-minimal medium containing 10 mM MgSO4, 0.25% glucose, and 6 μM 65ZnCl2. The reaction was initiated by addition of prewarmed cells. At timed intervals, 100-μl samples were removed and filtered through nitrocellulose filters (0.45-μm pore size; Whatman). Filters were washed with 8 ml of cold wash buffer containing 0.5 mM EDTA. Radioactivity was analyzed by gamma counting. A background value, obtained from 1 ml of assay mixture without the addition of cells, was subtracted from all readings.
65Zn2+ efflux.
Cells were grown and washed as stated above with the following changes. Washed cells were resuspended in N-minimal medium supplemented with glucose, MgSO4, and Casamino Acids and containing 100 μM MnCl2. Cells were incubated at 37°C for 1 h to allow Mn2+ to bind intracellular sites and allow efflux. 65ZnCl2 was added to 600-μl cell suspension aliquots to a final concentration of 5 μM and incubated for 20 min. Cells were pelleted at 12,000 × g for 3 min, the supernatant was removed, and the cells were resuspended in 600 μl of assay buffer (N-minimal medium with nutrient supplements and 10 μM ZnSO4). At timed intervals, 100-μl aliquots of the cell suspension were removed and collected on nitrocellulose filters as described above. Radioactivity was analyzed by gamma counting.
DNA sequencing and PCR.
DNA sequencing was performed by the dideoxy chain termination method of Sanger et al. (18) as modified by Tabor and Richardson (25), using T7 DNA polymerase obtained from Epicentre Technologies. Initial reactions were performed using M13 −20 promoter primers obtained from LI-COR Biosciences. Subsequent reactions employed synthetic oligodeoxynucleotide primers complementary or identical to segments within a previously sequenced segment. PCRs were performed using the Opti-Prime PCR optimization kit obtained from Stratagene and oligodeoxynucleotide primers obtained from Integrated DNA Technologies.
Nucleotide sequence accession number.
The nucleotide sequence of the S. enterica serovar Typhimurium zntB locus was deposited in the nucleotide database of the National Center for Biotechnology Information with the accession number AF308568.
RESULTS
Disruption of zntB increases sensitivity to zinc.
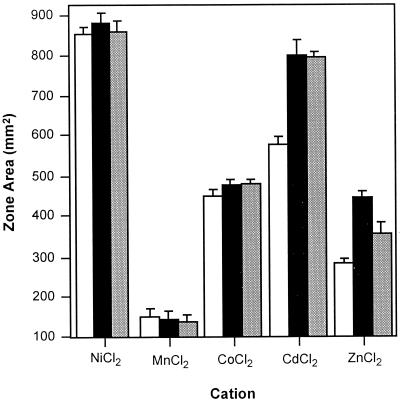
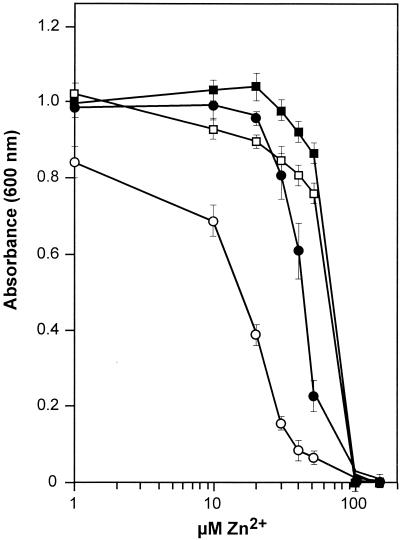
Allelic replacement techniques were used to disrupt the S. enterica serovar Typhimurium homolog of b1342 by the insertion of a chloramphenicol resistance cassette into the coding sequence, producing strain RS1100 (7). Bacteriophage P22-mediated cotransduction techniques were used to determine the position of the zntB locus on the serovar Typhimurium chromosome. The insertion mutation mapped to centisome 36 and was 100% linked to oxrA(fnr), which is in agreement with the recently completed genome sequence. The effects of the insertion mutation on metal sensitivity were determined by disk diffusion assay. Disruption of this locus did not significantly alter the sensitivities to cobalt, nickel, or manganese compared to those of the wild-type strain RS404. However, the loss of this allele had a marked effect on resistance levels to zinc and cadmium (Fig. 1). The increased zones of sensitivity displayed by the mutant suggest that the product of b1342 might participate in the extrusion of Zn2+ and possibly Cd2+. Consequently, b1342 and its gene product were designated ZntB (for Zn2+ transport). Expression of a wild-type zntB allele in the mutant background partially restored resistance to zinc but had no measurable effect on resistance to cadmium. Growth of the zntB mutant (RS1100) and wild-type (RS404) strains was measured in minimal growth media containing a range of inhibitory zinc concentrations (Fig. 2). The wild type displayed half-maximal growth in medium containing 60 μM Zn2+. By comparison, the zntB mutant displayed half-maximal growth at 20 μM Zn2+. The zinc-sensitive phenotype displayed by RS1100 could be partially rescued through the introduction of a low-copy-number plasmid encoding the wild-type zntB allele (pAJW54). This strain (RS1100/pAJW54) attained half-maximal growth at 45 μM Zn2+.
FIG. 1.
Cation sensitivity profiles of serovar Typhimurium strains RS404 (white), RS1100 (black), and RS1100/pAJW54 (gray). Cells were grown overnight in supplemented N-minimal medium, and 100 μl of the culture was spread onto N-minimal agar plates. An 8-mm filter disk was placed in the center and saturated with 17 μl of a 100 mM cation solution of ZnSO4, CdCl2, and CoCl2 and 1 M solutions of MnCl2 and NiCl2. The areas of the resulting inhibition zones were determined after 18 h of incubation at 37°C. Values presented are averages of three independent trials, with standard deviations of the means indicated.
FIG. 2.
Effects of zinc on growth of serovar Typhimurium strains RS404 (□), RS404/pAJW54 (▪), RS1100 (○), and RS1100/pAJW54 (•). Overnight cultures were prepared in supplemented N-minimal medium and diluted 1:500 into fresh N-minimal medium with the indicated concentrations of ZnCl2. Cell growth was measured as the OD600 as the cultures entered late log phase after 6 h of aerobic incubation at 37°C. The data are the averages of six independent trials, with standard deviations of the means indicated.
ZntB does not function as a Mg2+ uptake system.
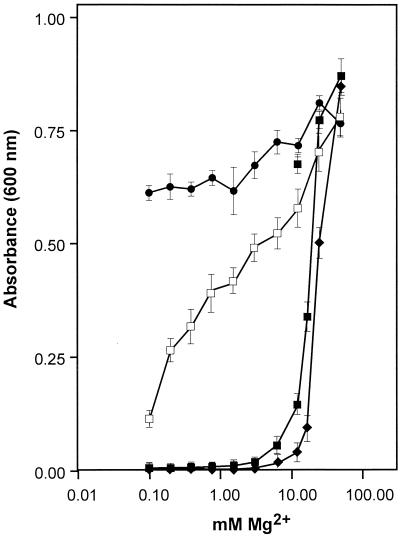
ZntB is homologous to the CorA family of cation transporters (12, 20). In serovar Typhimurium and E. coli, CorA functions as the primary influx and efflux pathway for Mg2+ (22, 23). The ability of ZntB to function as a Mg2+ transport system was tested. Strain MM281 carries insertion mutations in each of the three known Mg2+ transport loci (mgtA, mgtCB, and corA) which render the cell unable to grow unless supplemented with 100 mM Mg2+. The Mg2+-dependent growth of MM281 can be rescued by introducing a plasmid encoding a functional Mg2+ transport system (21). Strain RS1225 (MM281/pAJW54) displayed a Mg2+ growth dependence that was indistinguishable from that exhibited by MM281 (Fig. 3). In stark contrast, strains expressing CorAs from serovar Typhimurium (MM281/pRS310) and the archaeon Methanococcus jannaschii (MM281/pRS319) displayed a pronounced leftward shift in Mg2+ dependence for growth. Thus, at the level of growth, ZntB does not function as a Mg2+ uptake system.
FIG. 3.
Magnesium-dependent growth of serovar Typhimurium strains MM281 (♦), MM281/pRS310 (•), MM281/pRS319 (□), and MM281/pAJW54 (▪). Overnight cultures were prepared in LB growth medium supplemented with 100 mM Mg2+. Prior to the assay, the cell cultures were washed twice with medium containing no supplemental Mg2+ and diluted 1:500 into LB medium containing the indicated concentration of MgSO4. Cell growth was measured as the OD600 after 8 h of aerobic incubation at 37°C. The data presented are the averages of five independent trials, with standard deviations of the means indicated.
65Zn2+ transport assays.
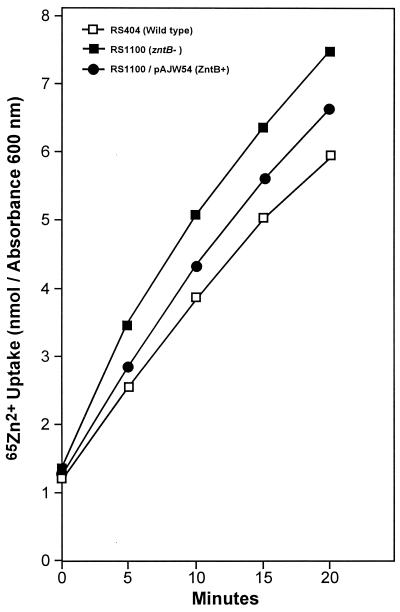
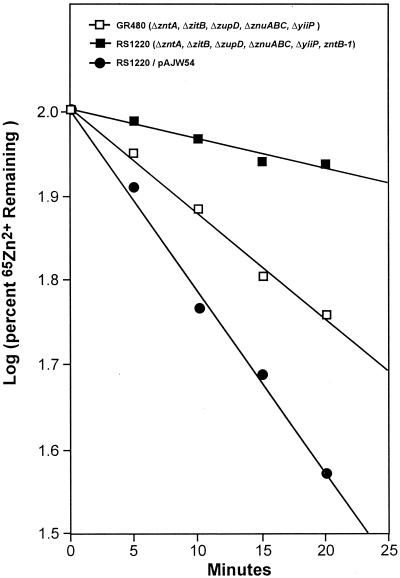
To characterize the transport activity of ZntB, uptake of 65Zn2+ was measured in RS404 (wild-type serovar Typhimurium), RS1100 (zntB mutant), and the complementing strain RS1100/pAJW54. All three strains exhibited similar rates of uptake. The total amount of 65Zn2+ accumulated by the zntB mutant was 1.2-fold greater than the wild-type control accumulation. Expression of zntB from pAJW54 reduced the level of zinc accumulation to 1.1-fold of the levels demonstrated by the wild type (Fig. 4). These data indicate that ZntB does not facilitate zinc uptake, but rather suggest a possible role in the efflux of zinc. This was directly tested by introducing pAJW54 into a Zn2+ transport-deficient strain of E. coli and measuring the rate of 65Zn2+ efflux. GR480 contains mutations in each of the known Zn2+ transport loci (zntA, zitB, zupD, znuABC, and yiiP) but retains a functional zntB allele (9, 10). This gene was disrupted by the insertion of a chloramphenicol resistance cassette producing strain RS1220 (7). The presence of the single chromosomal zntB allele carried in strain GR480 resulted in an efflux rate that was 5-fold greater than the rate for the transport-deficient strain (RS1220). Expression of ZntB from pAJW54 further increased the rate of 65Zn2+ efflux 8.8-fold (Fig. 5). Thus, ZntB is able to facilitate the efflux of zinc.
FIG. 4.
Effects of zntB expression on Zn2+ uptake. Bulk uptake of 65Zn2+ was measured in serovar Typhimurium strains RS404 (wild type □), RS1100 (zntB1 ▪), and RS1100/pAJW54 (•). Cation uptake was measured for 20 min at 37°C as described in Materials and Methods. The data shown are from a single representative experiment.
FIG. 5.
Effects of zntB expression on Zn2+ efflux. Efflux was measured at 37°C in E. coli strains GR480 (□), RS1220 (▪), and RS1220/pAJW54 (•) as described in Materials and Methods. GR480 carries chromosomal deletions in each of the known zinc transport loci (ΔzntA, ΔzitB, ΔzupD, ΔznuABC, and ΔyiiP) but retains a functional zntB allele. RS1220 was derived from GR480 by introducing an additional mutation in zntB. Plasmid pAJW54 encodes a wild-type zntB allele from serovar Typhimurium. The data shown are the averages of three independent experiments. Variability was less than ±5% at each point. Correlation coefficients derived from linear regression analysis were >0.95 for each individual experiment.
DISCUSSION
In this report, we demonstrate that the zntB locus of S. enterica serovar Typhimurium encodes a protein that is involved in the transmembrane flux of zinc. Mutations at zntB render the cell hypersensitive to the cytotoxic effects of zinc, as indicated by disk diffusion analysis and growth characterization. This phenotype suggests that ZntB plays a role in the efflux of this cation. A direct examination of transport activity revealed that zntB mutations diminish the capacity to extrude Zn2+ without significantly affecting the uptake activity. Moreover, this transport deficiency could be complemented by a plasmid encoding a wild-type zntB allele. The ZntB transporter is homologous to the CorA Mg2+ transport protein, which is the defining member of a large class of unusual proteins that appear to be widely distributed throughout the bacteria and the archaea (20, 22). In S. enterica serovar Typhimurium and E. coli, CorA functions as the primary influx pathway for magnesium and is responsible for more than 95% of the total magnesium accumulated under normal growth conditions. The ZntB transporter, however, is not able to function as a Mg2+ uptake pathway. Our results identify ZntB as a novel transport system for Zn2+ in the enteric bacteria and, as such, assign a new function to the ubiquitous CorA family of cation transporters.
Acknowledgments
This work was supported by a grant from The Welch Foundation (Y1485) to R.L.S.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. A. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Wiley-Interscience, New York, N.Y.
- 2.Beard, S. J., R. Hashim, J. Membrillo-Hernandez, M. N. Hughes, and R. K. Poole. 1997. Zinc(II) tolerance in Escherichia coli K-12: evidence that the zntA gene (o732) encodes a cation transport ATPase. Mol. Microbiol. 25:883-891. [DOI] [PubMed] [Google Scholar]
- 3.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 4.Bohm, S., D. Frishman, and H. W. Mewes. 1997. Variations of the C2H2 zinc finger motif in the yeast genome and classification of yeast zinc finger proteins. Nucleic Acids Res. 25:2464-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brocklehurst, K. R., J. L. Hobman, B. Lawley, L. Blank, S. J. Marshall, N. L. Brown, and A. P. Morby. 1999. ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol. Microbiol. 31:893-902. [DOI] [PubMed] [Google Scholar]
- 6.Coleman, J. E. 1992. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 61:897-946. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson, M. M., D. A. Bagga, C. G. Miller, and M. E. Maguire. 1991. Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol. Microbiol. 5:2753-2761. [DOI] [PubMed] [Google Scholar]
- 9.Grass, G., B. Fan, B. P. Rosen, S. Franke, D. H. Nies, and C. Rensing. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 183:4664-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grass, G., M. D. Wong, B. P. Rosen, R. L. Smith, and C. Rensing. 2002. ZupT is a Zn(II) uptake system in Escherichia coli. J. Bacteriol. 184:864-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, E. P. 1997. Metal ions and synaptic transmission: think zinc. Proc. Natl. Acad. Sci. USA 94:13386-13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehres, D., C. H. Lawyer, and M. E. Maguire. 1998. The CorA magnesium transporter gene family. Microb. Comp. Genom. 43:151-169. [DOI] [PubMed] [Google Scholar]
- 13.Lewis, D. A., J. Klesney-Tait, S. R. Lumbley, C. K. Ward, J. L. Latimer, C. A. Ison, and E. J. Hansen. 1999. Identification of the znuA-encoded periplasmic zinc transport protein of Haemophilus ducreyi. Infect. Immun. 67:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 15.Nelson, D. L., and E. P. Kennedy. 1971. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J. Biol. Chem. 246:3042-3049. [PubMed] [Google Scholar]
- 16.Patzer, S. I., and K. Hantke. 1998. The ZnuABC high-affinity uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199-1210. [DOI] [PubMed] [Google Scholar]
- 17.Rensing, C., B. Mitra, and B. P. Rosen. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 94:14326-14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schjerling, P., and S. Holmberg. 1996. Comparative amino acid sequence analysis of the C6 zinc cluster family of transcriptional regulators. Nucleic Acids Res. 24:4599-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, R. L., J. L. Banks, M. D. Snavely, and M. E. Maguire. 1993. Sequence and topology of the CorA magnesium transport systems of Salmonella typhimurium and Escherichia coli. Identification of a new class of transport protein. J. Biol. Chem. 268:14071-14080. [PubMed] [Google Scholar]
- 21.Smith, R. L., E. Gottlieb, L. M. Kucharski, and M. E. Maguire. 1998. Functional similarity between archaeal and bacterial CorA magnesium transporters. J. Bacteriol. 180:2788-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, R. L., and M. E. Maguire. 1998. Microbial magnesium transport: unusual transporters searching for identity. Mol. Microbiol. 28:217-226. [DOI] [PubMed] [Google Scholar]
- 23.Snavely, M. D., J. B. Florer, C. G. Miller, and M. E. Maguire. 1989. Magnesium transporter in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J. Bacteriol. 171:4761-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suhy, D. A., K. D. Simon, D. I. H. Linzer, and T. V. O'Halloran. 1999. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J. Biol. Chem. 247:9183-9192. [DOI] [PubMed] [Google Scholar]
- 25.Tabor, S., and C. C. Richardson. 1989. Effect of manganese ions on the incorporation of dideoxynucleotides by bacteriophage T7 DNA polymerase and Escherichia coli DNA polymerase I. Proc. Natl. Acad. Sci. USA 86:4076-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]