Abstract
Using a lysine-specific cleavable cross-linking reagent ethylene glycolbis(sulfosuccimidylsuccinate) (Sulfo-EGS), we studied conformational motion in the surface loops of Escherichia coli FepA during its transport of the siderophore ferric enterobactin. Site-directed mutagenesis determined that Sulfo-EGS reacted with two lysines, K332 and K483, and at least two other unidentified Lys residues in the surface loops of the outer membrane protein. The reagent cross-linked K483 in FepA L7 to either K332 in L5, forming a product that we designated band 1, or to the major outer membrane proteins OmpF, OmpC, and OmpA, forming band 2. Ferric enterobactin binding to FepA did not prevent modification of K483 by Sulfo-EGS but blocked its cross-linking to OmpF/C and OmpA and reduced its coupling to K332. These data show that the loops of FepA undergo conformational changes in vivo, with an approximate magnitude of 15 Å, from a ligand-free open state to a ligand-bound closed state. The coupling of FepA L7 to OmpF, OmpC, or OmpA was TonB independent and was unaffected by the uncouplers CCCP (carbonyl cyanide m-chlorophenylhydrazone) and DNP (2,4-dinitrophenol) but completely inhibited by cyanide.
In spite of evidence for conformational change within the surface loops of Escherichia coli ligand-gated porins (LGP) during binding and transport of ferric siderophores (3, 6, 16, 17, 22, 27, 32), few differences appeared in the X-ray determinations of loop conformations of ligand-free and ligand-bound FhuA (11, 23). This discrepancy raised two possibilities: either prior data implying loop motion in vivo were misinterpreted, or the X-ray analysis captured only one conformation of LGP loops that was perhaps predisposed by either the in vitro crystallographic environment or by the nature of the crystals themselves. In most porin crystals, for example, including those of FepA (5) and FhuA (11, 23), packing interactions involved the loops (7, 37). Purified LGP, furthermore, suffer about a 100-fold decrease in affinity for their respective siderophores, supporting the notion that the X-ray data on FepA and FhuA did not fully describe the loop conformations that occur in vivo. Crystallographic experiments with FecA, an LGP that transports ferric citrate, now affirm this concept: the loop conformations of ligand-free and ligand-bound FecA are distinctly different (10). FecA is a homolog of FepA and FhuA: its relationship to FepA is so close that FecA also transports ferric enterobactin (FeEnt), albeit with lower affinity (46-48). Here we report experiments with FepA that preceded (38) the FecA crystal structure, but recapitulate, in vivo, the conformational motion that was observed in FecA: FepA L7 changes from an open to a closed state when FeEnt binds.
The crystal structure of FepA revealed an important feature of its surface loops: they are flexible and, in some cases (L4, L5, and L7), too flexible to permit their crystallographic description. The X-ray analysis did not reveal many details of the ligand-binding site within FepA because the crystals showed only weak occupancy by FeEnt (in the outermost loop regions). In FhuA, however, ferrichrome bound deep within the vestibule formed by the surface loops. In the crystallized forms of both FepA and FhuA, their 11 surface loops consolidated and closed above the membrane surface, whether or not FeEnt or ferrichrome were present.
Treatment of E. coli with the homobifunctional cross-linking reagent ethylene glycolbis(sulfosuccimidylsuccinate) (Sulfo-EGS) produced two prominent products containing FepA, with molecular masses of 100 kDa (band 1) and 120 kDa (band 2) (39). Band 2 contained FepA coupled to OmpF and/or OmpC (which were indistinguishable because of their identical N termini) and OmpA. The cross-linking reactions were independent of TonB (39), but preincubation of FepA with FeEnt or deletion of its N-terminal 150 residues inhibited them, presumably because ligand binding or removal of the globular domain closes the receptor (39). The identification in the present study of FepA residues that participate in the cross-linking reactions further delineates the nature and extent of LGP conformational dynamics in vivo, which are unaffected by TonB but inhibited by cyanide.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Cross-linking reactions were performed in the fepA backgrounds of KDF541 (F− thi entA pro trp rpsL recA azi fepA fhuA cir) (36), KDF571 (isogenic with KDF541, but tonB) (36), and KNO16, which we obtained from AN718 (F− uncA401 argH pyrE entA [13]) by spontaneous colicin B resistance (36). fepA clones were identified by Western blots with α-FepA monoclonal antibody (MAb) 45 (28) and verified by the ability of pITS23 (fepA+; full promoter; on the low-copy-number vector pHSG575) (39) to restore growth stimulation by FeEnt and ColB susceptibility (28, 29).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblots.
Proteins were separated on 10% acrylamide slabs with either 0.26% (2) or 0.07% (15) bisacrylamide and were either stained with Coomassie blue or electrophoretically transferred to nitrocellulose paper. Western immunoblots were reacted with α-FepA MAbs 41 or 45, developed with 125I-labeled protein A (6), visualized by exposure to X-ray films, and quantitated by image analysis with a Storm Scanner (Molecular Dynamics).
Sulfo-EGS cross-linking.
Bacteria grown in morpholinepropanesulfonic acid (MOPS) minimal medium or sucrose gradient-purified outer membrane fractions were suspended in phosphate-buffered saline (PBS) at 109 cells/ml or 10 mg/ml, respectively. Sulfo-EGS (Pierce) was added to 4 mM, and the samples were incubated for 2 h at 0°C. Reactions were quenched by incubation with 50 mM Tris-Cl (pH 7.4) for 15 min, and cells or outer membrane proteins were pelleted by centrifugation, solubilized in sample buffer, subjected to SDS-PAGE, and then stained with Coomassie blue or transferred to nitrocellulose and stained with α-FepA MAb 45 and 125I-labeled protein A. When indicated, 5 μM FeEnt was added to the cells prior to exposure to Sulfo-EGS. Cross-linked bands were excised, cleaved by incubation with hydroxylamine, electroeluted in an ISCO concentrator, and reelectrophoresed. Cells were identically treated when prepared for quantitative binding analysis, except that bacteria cross-linked in the presence of FeEnt were warmed to 37°C for 10 min. to allow internalization of the siderophore.
Cross-linking in the presence of energy inhibitors.
After growth for 5 h in MOPS minimal medium, energy inhibitors were added to the cultures, and they were incubated for an additional hour at 37°C. Cells were concentrated and resuspended in PBS containing the same concentration of inhibitor, chilled to 0°C, and then subjected to Sulfo-EGS in the usual manner. The concentrations of the energy inhibitors were previously shown to completely inhibit FeEnt transport (35).
Cross-linking of BSA.
Bovine serum albumin (BSA; 10 mg/ml; Gibco-BRL) was dialyzed against PBS, and aliquots (150 μg) were prepared in PBS with or without energy inhibitors and incubated on ice for 15 min. Sulfo-EGS was then added to a final concentration of 1.5 mM. The samples were incubated for 2 h on ice and quenched by the addition of 50 mM Tris (pH 7.4) for 15 min; 4-μg aliquots were analyzed by SDS-PAGE and Coomassie blue staining.
Siderophore binding.
The adsorption of 59FeEnt (6, 30, 39) was measured with KDF541 expressing FepA and its mutant or cross-linked derivatives.
RESULTS
Identity of Sulfo-EGS-cross-linked residues in FepA.
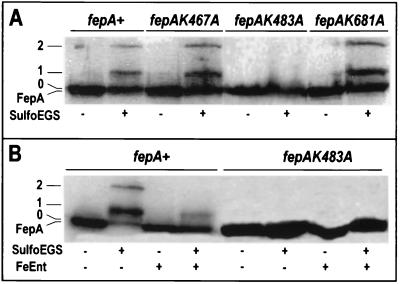
The ability of FeEnt to prevent the cross-linking of FepA to other outer membrane proteins (39) (Fig. 1) suggested that conformational changes occur in its suface loops during the siderophore binding reaction. The first step in characterizing the loop motion that gave rise to different cross-linking reactions was the identification of the reactive residue(s) in FepA. Sulfo-EGS reacts with primary amines, and its molecular mass (660 Da) restricts its reactivity to the cell surface of E. coli; we sought to identify the Lys residues in FepA that it modified. The observation of two cross-linked bands containing FepA, one of which included multiple proteins (band 2, OmpF/C and OmpA [39]), suggested that the reagent reacted with two or more Lys side chains. We initially surveyed a series of deletion mutants that individually removed each of FepA's 11 cell surface loops (30). Among the loop deletions, only five changed the cross-linking reactions (data not shown), in loops 2 (residues 98 to 123), 4 (residues 315 to 326), 7 (residues 467 to 497), 8 (residues 546 to 560), and 11 (residues 681 to 691). The altered reactivity of FepAΔL2, FepAΔL4, and FepAΔL8 must have resulted from structural aberrations in the remaining loops, because these three deletions did not remove any lysines. FepAΔL7 and FepAΔL11 removed three Lys residues: K467 and K483 in L7 and K681 in L11. We individually changed these residues to Ala to eliminate their reactive primary amine side chains. The resulting mutants were phenotypically indistinguishable from wild-type FepA, except for a 10-fold reduction in the ColB sensitivity of cells expressing FepAK483A. However, immunoblots of lysates from cross-linked cells showed that the K483A mutation prevented the formation of bands 1 and 2 (Fig. 1), whereas the K467A and K681A mutations had no visible effects. These initial data showed that K483 was one Sulfo-EGS-reactive surface residue that was responsible for the formation of bands 1 and 2 and suggested that FepA L7 undergoes conformational changes in the presence or absence of the ferric siderophore.
FIG. 1.
FepAK483 participates in the formation of complex 2. (A) KDF541containing pITS23 or its derivatives that expressed wild-type or mutant FepA proteins were incubated in the the absence (−) or presence (+) of Sulfo-EGS. Cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and visualized with α-FepA MAb 45 and 125I-labeled protein A. (B). KDF541/pITS23 and KDF541/pfepAK483A were incubated in the absence (−) or presence (+) of Sulfo-EGS, either with or without prior incubation with FeEnt (5 μM).
Composition of band 1.
Hydroxylamine cleaves the cross-links created by Sulfo-EGS (1), and we used this property to identify cell envelope proteins linked to FepA in band 2: OmpF/C and OmpA (39). We sought to determine the components of the 100-kDa band 1 by the same methods, but the only protein we observed after the cleavage and reelectrophoresis of band 1 was FepA, despite repeated efforts with sufficient protein concentrations to allow visualization of the anticipated ∼20-kDa protein.
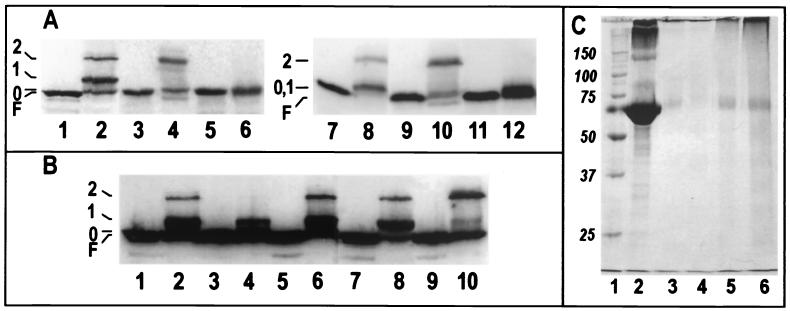
However, in another SDS-PAGE system (15) the bands of interest migrated differently. Gels of this composition revealed that in lysates from bacteria treated with Sulfo-EGS, the previously perceived “wild-type” FepA band consisted of FepA proteins that were modified by the reagent (now designated band 0; Fig. 2): the alternative SDS-PAGE system revealed that no wild-type FepA remained after cross-linking. Nonspherical or incompletely denatured proteins may display anomalous electrophoretic mobility (8, 9, 14, 25, 42), raising the possibility that Sulfo-EGS created internal cross-links within FepA that resulted in bands 0 and 1. To assess this idea, we mutagenized (to Ala) all of the Lys residues within 16 Å of K483: K167, K328, K332, K375, K406, K503, K535, K560, K634, K635, and K639. The substitution mutants retained wild-type FeEnt uptake ability and susceptibility to colicins B and D, but the cross-linking profile of K332A was altered, identifying it as the second residue involved in the formation of band 1 (Fig. 2A). In strains expressing FepAK332A, band 1 disappeared and band 2 doubled in intensity, showing that, in two competing reactions, Sulfo-EGS may conjugate K483 to other outer membrane proteins or to K332 in FepA L4. The abrogation of the internal cross-link with K332 also better revealed band 0, which contained previously unseen products involving other lysines (designated Lys X). We did not further pursue these reactions, which involved the attachment of Sulfo-EGS at sites that were not related to the cross-linking of FepA to other outer membrane proteins. In summary, bound FeEnt prevented the formation of band 2 and reduced the amount of band 1: the former complex of proteins resulted from FepAK483 conjugating to either OmpF/C or OmpA; band 1 derived from an internal cross-link between K483 and K332.
FIG. 2.
(A) Reactivity of K332 and the effects of energy poisons. KDF541 containing pITS23 (lanes 1, 2, 7, and 8), pfepAK332A (lanes 3, 4, 9, and 10), or pfepAK483A (lanes 5, 6, 11, and 12) was incubated in the the absence (odd-numbered lanes) or presence (even-numbered lanes) of Sulfo-EGS. Cell lysates were resolved on 10% acrylamide-0.26% bisacrylamide (lanes 1 to 6) or 10% acrylamide-0.067% bisacrylamide (lanes 7 to 12)slab gels, transferred to nitrocellulose, and visualized with a mixture of α-FepA MAbs 41 and 45 and 125I-labeled protein A. (B). Sulfo-EGS treatment of bacteria prepared in the presence of energy inhibitors. KDF541/pITS23 was either untreated (odd lanes) or exposed to Sulfo-EGS (even lanes) in the presence energy inhibitors at the following concentrations: no energy inhibitors (lanes 1 and 2), 20 mM cyanide (lanes 3 and 4), 0.1 mM CCCP (lanes 5 and 6), 2 mM DNP (lanes 7 and 8), and 10 mM azide (lanes 9 and 10). (C) Sulfo-EGS cross-linking of purified BSA in the presence or absence of energy inhibitors. A total of 25 μg of BSA was left untreated (lane 2) or was cross-linked in the absence (lane 3) or presence of 20 mM cyanide (lane 4), 10 mM azide (lane 5), or 100 mM azide (lane 6). The samples were solubiized in sample buffer, subjected to SDS-PAGE, and stained with Coomassie blue. Cross-linked BSA was unable to enter the gel slab (lanes 3 to 6); image analysis (not shown) revealed that only sodium azide had a small inhibitory effect on the cross-linking reaction (<1%). Molecular mass markers appear in lane 1.
Effects of energy inhibitors on the cross-linking profile.
The proton motive force (PMF)-deflating agents CCCP (carbonyl cyanide m-chlorophenylhydrazone) and DNP (2,4-dinitrophenol) did not influence the formation of bands 1 and 2 at the concentrations we used (Fig. 2). To address the possibility that the ATP synthase acted in reverse to sustain PMF, we conducted Sulfo-EGS reactions in an unc strain (KNO16). However, in KNO16, in the presence of DNP and CCCP, Sulfo-EGS produced bands 1 and 2 in the same manner (data not shown). Thus, PMF depletion did not affect the conformation of FepA in a way that was detectable by the cross-linking analysis.
On the other hand, cyanide prevented cross-links to OmpF/C and OmpA and reduced the amount of band 1, just like the effect of bound FeEnt. Cyanide inhibits energy metabolism by blocking electron flow through cytochrome oxidase, and these data suggested suggested either a need for energy to generate the open form of FepA or a direct chemical inhibition of Sulfo-EGS reactivity by the poison. However, chemical inhibition by cyanide was unlikely, because it did not prevent the formation of band 1. Azide, another inhibitor of electron transport, did not affect band 2 but also diminished the amount of band 1 (Fig. 2b). We tested the possibility that cyanide and azide directly interfere with Sulfo-EGS reactivity by studying their effects on the cross-linking of BSA (Fig. 2C). Cyanide had no effect on the BSA cross-linking reactions, and azide only slightly inhibited the reagent (<1%), which was considerably less than its effects on band 1. Finally, the effects of cyanide and azide were the same in the unc strain, KNO16 (data not shown).
Ligand binding by cross-linked FepA proteins.
We studied the effect of Sulfo-EGS treatment on the ability of cells expressing FepA and FepAK483A to bind 59FeEnt. We first cross-linked in the absence of the ligand and determined the effects on 59FeEnt adsorption. We then cross-linked cells in the presence of FeEnt and subsequently measured 59FeEnt binding. That experiment had several stages. We incubated bacteria at 0°C with saturating FeEnt and divided the sample, exposing half to Sulfo-EGS and half to buffer. After quenching of the cross-linking reaction, dilution, and centrifugation, we resuspended the aliquots of (live) bacteria in MOPS medium at 37°C for 10 min to allow transport of the bound FeEnt. Finally, we chilled the cells and determined their affinity and capacity for 59FeEnt.
The binding assays revealed several things about the modified proteins (Table 1). (i) For bacteria expressing FepA, treatment with Sulfo-EGS reduced affinity for FeEnt 80-fold and its binding capacity by 50%. (ii) Bound FeEnt partially protected the wild-type receptor from Sulfo-EGS: in this case the cross-linker only decreased affinity eightfold, but it still dropped capacity ca. 50%. (iii) Bacteria expressing FepAK483A (unmodified) showed about a 10-fold reduction in affinity for FeEnt (no change in capacity). (iv) Treatment of cells expressing FepAK483A with Sulfo-EGS reduced affinity for 59FeEnt ninefold further, without affecting capacity. (v) Again, bound FeEnt protected against Sulfo-EGS: in its presence the cross-linker did not alter the affinity of FepAK483A for 59FeEnt and only slightly decreased capacity.
TABLE 1.
Adsorption of 59FeEnt to wild-type FepA and FepAK483a
| Plasmid and treatment | Binding capacity (pmol/109 cells) | Kd (nM) | Fold decrease (affinity) |
|---|---|---|---|
| pITS23 | 50 | 0.1 | 0 |
| pITS23 + Sulfo-EGS | 25 | 8.0 | 80 |
| pITS23 + FeEnt | 53 | 0.2 | 0 |
| pITS23 + FeEnt + Sulfo-EGS | 20 | 0.8 | 8 |
| pfepAK483A | 47 | 1.3 | 12 |
| pfepAK483A + Sulfo-EGS | 45 | 11.4 | 87 |
| pfepAK483A + FeEnt | 47 | 1.4 | 10 |
| pfepAK483A + FeEnt + Sulfo-EGS | 38 | 1.5 | 10 |
Bacteria were subjected to Sulfo-EGS in the presence or absence of FeEnt, and the 59FeEnt-binding capacity (pmol/109 cells) and binding affinity were determined. The experiments were performed twice, and mean values were plotted with GRAFIT (Erithacus) to yield the binding parameters. The mean standard error of the fitted curves that produced the capacity values was 4.7% with a standard deviation of 2%; the mean standard error of the fitted curves that produced the Kd values was 19%, with a standard deviation of 7%. The fold reduction in affinity was calculated relative to that of the wild type.
Image analysis of Figs 1 and 2 indicated that Sulfo-EGS converted one-third of FepA to band 2 (K483-OmpF/C and -OmpA), one-third to band 1 (K483-K332), and one-third to band 0 (Sulfo-EGS-LysX). The reduction in binding capacity derived primarily from reaction of Sulfo-EGS with K483, because FepAK483A showed no loss of capacity after exposure to the reagent (from points i and iv above). Thus, reactions of Sulfo-EGS with K332 or LysX, which occurred in cells expressing K483A, did not decrease capacity. K483 is accessible in both ligand-free and the ligand-bound FepA because the capacity loss occurred in the absence or presence of FeEnt (points i and ii above). The latter finding indicated that FeEnt binding did not prevent the cross-linking of FepA to OmpF/C/A by steric hindrance of the Sulfo-EGS reaction with K483. Instead, these data support the alternative explanation that FeEnt inhibited cross-linking to other outer membrane proteins by inducing a conformational change from the open to the closed form.
The effects of Sulfo-EGS on affinity agreed with these interpretations. The 8-fold reduction in affinity by reaction of Sulfo-EGS with K483 and the 10-fold reduction that occurred upon mutagenesis of K483 to Ala were comparable (points ii and iii above). The further, ∼10-fold reductions in affinity that modification of K332 and LysX engendered were seen in both FepA and FepA483A (points i and iv above), and FeEnt binding prevented such decreases (points i, ii, iv, and v).
DISCUSSION
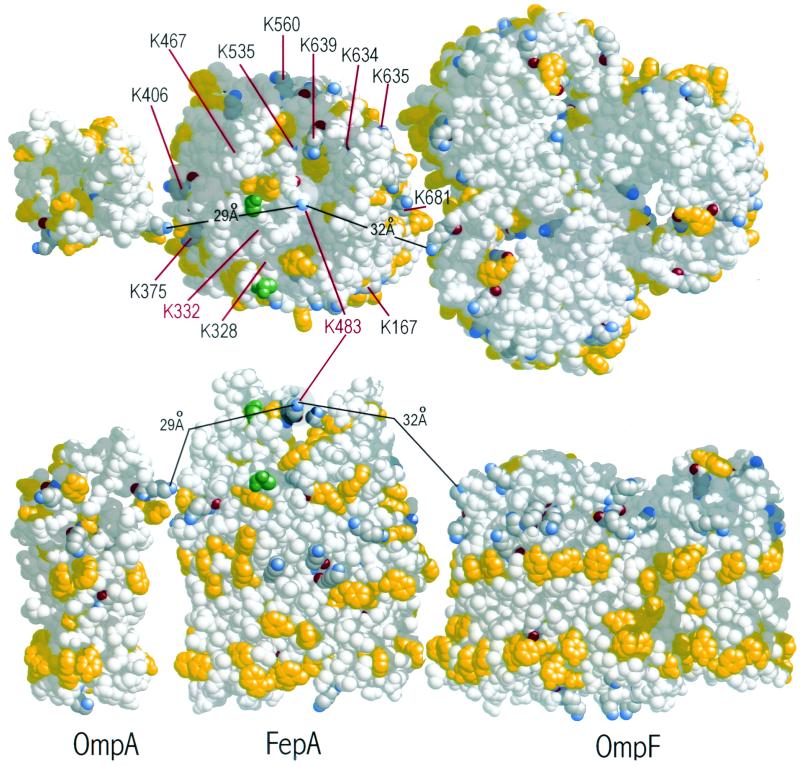
Although the crystal structures of many E. coli outer membrane proteins are known (e.g., OmpF [7], LamB [37], OmpA [31], FepA [5], FhuA [11, 23], phospholipase A2 [41], OmpT [43], and TolC [18]), the nature and extent of conformational motion within these proteins in vivo is unknown. FepAK332 resides in an unsolved region of L4 (5); K483 resides in L7, and only the α and β carbons of its side chain were crystallographically described, presumably because of the flexibility of the loop (Fig. 3). In the crystalline, closed form, K483 localizes above the center of the FepA β-barrel (5), and the cross-linking of K483 to OmpF/C and OmpA is inconsistent with this position. The distance from K483 (β carbon) to the rim of the FepA barrel varies between 20 Å and 24 Å; the distance to residues of OmpF/C or OmpA is closer to 30 Å; because they contain smaller surface loops that project less above the membrane bilayer than those of FepA. Even considering the additional 4 Å of the γ, δ, and ɛ atoms of the K483 side chain, the 16-Å length of Sulfo-EGS is insufficient (by ca. 15 Å) to reach other outer membrane proteins (Fig. 3). Steric factors are relevant, because the X-ray data portrayed K483 sunken within the FepA vestibule. To surmount such a 15-Å gap between participant lysines requires flexibility and motion in FepA L7, of the exact the magnitude that was seen on ligand binding by FecA (10). Our results demonstrate that such motion occurs not just in FecA but also in FepA in vivo, relocating the ɛ-amine of K483 at least 15 Å closer to neighboring proteins in the outer membrane membrane bilayer. These data raise the possibility that such conformational changes are a general facet of siderophore transport dynamics, even though these changes were not observed in the FhuA crystal structure.
FIG. 3.
Space-filling representations of OmpA, FepA, and OmpF showing a view of their surface Lys residues, including those in FepA that we mutagenized (enumerated) (top), and a side view in which the outer membrane proteins are aligned by their girdles of aromatic amino acids (yellow) at the internal and external interfaces of the outer membrane bilayer (bottom). Lys residues are shown in CPK colors. FepA residues 323 and 335, which define the terminal crystallographically solved portions of L4, are green. K332 exists somewhere between these two amino acids, close to residue 335. K483 is highlighted in the center of FepA.
These considerations depict large movements in the binding site that support our postulate of an open form that closes when the ferric siderophore binds or when it is extracted from its native environment of the outer membrane (6, 16, 29, 32, 39). Other experiments with both FepA and FhuA suggested that they are different in vivo and in vitro (6, 24, 32, 46, 48), although exact explanations of these discrepancies were unknown. In bacteria, for instance, the FepA-FeEnt binding reaction has a Kd of 0.1 to 0.2 nM (6, 30; the present study), whereas for purified FepA the Kd is 15 to 20 nM (12, 32, 47, 48). Similar differences exist for the FhuA-Fc binding reaction (24, 39). Although these experiments evaluated only L7, its motion suggests that other loops, which we did not observe, may undergo similar dynamics. The flexibility of L4 and L5 seen in the crystal stucture and experiments with fluorescent and paramagnetic probes attached to sites in other loops (6, 16, 32) support this idea.
LGP-mediated metal transport is TonB (33, 44) and energy dependent (45), and the energetic requirement at least in part involves proton motive force (4). The Sulfo-EGS cross-linking reactions of FepA to OmpF/C and OmpA were TonB independent (39) and unaffected by PMF deflaters (CCCP and DNP) but eliminated by the electron transport inhibitor cyanide. This effect was not chemical inhibition of the cross-linking reaction, suggesting that cyanide blocks adoption of the open form by FepA. This phenomenon may involve energy, but more work is needed to understand the effect of cyanide, especially because azide, another electron transport inhibitor, did not block the formation of band 2. Pugsley and Reeves (35) saw elimination of FeEnt uptake by exposure of E. coli to electron transport inhibitors, but their studies did not address the outer membrane transport stage. The LGP requirements for energy and TonB are considered inseparable, in that TonB was proposed to transfer (PMF) energy from the inner membrane to the outer membrane (19-21, 33, 34), but the participation of TonB in energy metabolism or transfer has never been biochemically demonstrated. In our experiments, cyanide and the tonB marker produced different effects on FepA.
Previous experiments with formaldehyde showed low-level cross-linking between FepA and TonB (34, 40). Our experiments do not clarify the proposed relationship between the two cell envelope proteins, because in spite of the fact that Sulfo-EGS reacts exclusively with the primary ɛ-amine of Lys, which formaldehyde also activates, the larger size of the former reagent restricts its targets to residues on the bacterial cell surface. Formaldehyde may enter the periplasm by diffusion through general porins, is membrane permeant, and the full spectrum of its chemical reactivity in biological systems is unknown (26). So the absence of FepA-TonB complexes in our experiments neither conflicts with prior data nor further elucidates the relationship between these two cell envelope proteins.
The evidence for a biphasic mechanism of FepA binding in vivo is substantial. In addition to biphasic adsorption kinetics (32), mutations in two different regions of the FepA vestibule affect the binding reaction (6, 29). In this context, the conditions that prevented the Sulfo-EGS-mediated conjugation of FepAL7 to OmpF/C and OmpA (deletion of the N domain, treatment with cyanide, and FeEnt binding) suggest that the formation of the ligand-free open state requires the presence of the globular domain within the β-barrel, and perhaps also, non-PMF energy. Entry of metal chelates into the vestibular “mouth”of LGP, where they interact with binding determinants contained therein, initiates loop closing. The avidity of the binding interactions (Kd ≈ 10−10 M) likely provides the energy for this structural rearrangement to the closed form, in which the metal complex sits above the N-terminal globular domain, poised for internalization.
Acknowledgments
We thank Marjorie Montague for excellent technical support and Zhenghua Cao, Raj Annamalai, Jin Bo, and Wallace Kaserer for helpful discussions.
This work was supported by NIH grant GM53836 and OCAST grant 00072.
REFERENCES
- 1.Abdella, P. M., P. K. Smith, and G. P. Royer. 1979. A new cleavable reagent for cross-linking and reversible immobilization of proteins. Biochem. Biophys. Res. Commun. 87:734-742. [DOI] [PubMed] [Google Scholar]
- 2.Ames, G. F. 1974. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs: membrane, soluble, and periplasmic fractions. J. Biol. Chem. 249:634-644. [PubMed] [Google Scholar]
- 3.Bos, C., D. Lorenzen, and V. Braun. 1998. Specific in vivo labeling of cell surface-exposed protein loops: reactive cysteines in the predicted gating loop mark a ferrichrome binding site and a ligand-induced conformational change of the Escherichia coli FhuA protein. J. Bacteriol. 180:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbeer, C. 1993. The proton motive force drives the outer membrane transport of cobalamin in Escherichia coli. J. Bacteriol. 175:3146-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 6.Cao, Z., Z. Qi, C. Sprencel, S. M. Newton, and P. E. Klebba. 2000. Aromatic components of two ferric enterobactin binding sites in Escherichia coli fepA. Mol. Microbiol. 37:1306-1317. [DOI] [PubMed] [Google Scholar]
- 7.Cowan, S. W., T. Schirmer, G. Rummel, M. Steiert, R. Ghosh, R. A. Pauptit, J. N. Jansonius, and J. P. Rosenbusch. 1992. Crystal structures explain functional properties of two E. coli porins. Nature 358:727-733. [DOI] [PubMed] [Google Scholar]
- 8.Creamer, L. K., and T. Richardson. 1984. Anomalous behavior of bovine alpha s1- and beta-caseins on gel electrophoresis in sodium dodecyl sulfate buffers. Arch. Biochem. Biophys. 234:476-486. [DOI] [PubMed] [Google Scholar]
- 9.Dunker, A. K., and A. J. Kenyon. 1976. Mobility of sodium dodecyl sulphate-protein complexes. Biochem. J. 153:191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 12.Fiss, E. H., P. Stanley-Samuelson, and J. B. Neilands. 1982. Properties and proteolysis of ferric enterobactin outer membrane receptor in Escherichia coli K12. Biochemistry 21:4517-4522. [DOI] [PubMed] [Google Scholar]
- 13.Gibson, F., G. B. Cox, J. A. Downie, and J. Radik. 1977. Partial diploids of Escherichia coli carrying normal and mutant alleles affecting oxidative phosphorylation. Biochem. J. 162:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossman, S. H., B. Le Jeune, and D. Mixon. 1983. A comparison of native and covalently crosslinked creatine kinases: denaturation and reassembly. Arch. Biochem. Biophys. 224:449-455. [DOI] [PubMed] [Google Scholar]
- 15.Hancock, R. W., and V. Braun. 1976. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and φ80 to Escherichia coli. J. Bacteriol. 125:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, X., M. A. Payne, Z. Cao, S. B. Foster, J. B. Feix, S. M. Newton, and P. E. Klebba. 1997. Ligand-specific opening of a gated-porin channel in the outer membrane of living bacteria. Science 276:1261-1264. [DOI] [PubMed] [Google Scholar]
- 17.Klug, C. S., and J. B. Feix. 1998. Guanidine hydrochloride unfolding of a transmembrane beta-strand in FepA using site-directed spin labeling. Protein Sci. 7:1469-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 19.Larsen, R. A., D. Foster-Hartnett, M. A. McIntosh, and K. Postle. 1997. Regions of Escherichia coli TonB and FepA proteins essential for in vivo physical interactions. J. Bacteriol. 179:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen, R. A., M. G. Thomas, and K. Postle. 1999. Proton motive force, ExbB and ligand-bound FepA drive conformational changes in TonB. Mol. Microbiol. 31:1809-1824. [DOI] [PubMed] [Google Scholar]
- 21.Larsen, R. A., G. E. Wood, and K. Postle. 1993. The conserved proline-rich motif is not essential for energy transduction by Escherichia coli TonB protein. Mol. Microbiol. 10:943-953. (Erratum, 12: 857, 1994.) [DOI] [PubMed] [Google Scholar]
- 22.Liu, J., J. M. Rutz, P. E. Klebba, and J. B. Feix. 1994. A site-directed spin-labeling study of ligand-induced conformational change in the ferric enterobactin receptor, FepA. Biochemistry 33:13274-13283. [DOI] [PubMed] [Google Scholar]
- 23.Locher, K. P., B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771-778. [DOI] [PubMed] [Google Scholar]
- 24.Locher, K. P., and J. P. Rosenbusch. 1997. Oligomeric states and siderophore binding of the ligand-gated FhuA protein that forms channels across Escherichia coli outer membranes. Eur. J. Biochem. 247:770-775. [DOI] [PubMed] [Google Scholar]
- 25.Matagne, A., B. Joris, and J. M. Frere. 1991. Anomalous behavior of a protein during SDS-PAGE corrected by chemical modification of carboxylic groups. Biochem. J. 280:553-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Means, G. E., and R. E. Feeney. 1971. Chemical modification of proteins. Holden-Day, Inc., San Francisco, Calif.
- 27.Moeck, G. S., P. Tawa, H. Xiang, A. A. Ismail, J. L. Turnbull, and J. W. Coulton. 1996. Ligand-induced conformational change in the ferrichrome-iron receptor of Escherichia coli K-12. Mol. Microbiol. 22:459-471. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, C. K., V. I. Kalve, and P. E. Klebba. 1990. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J. Bacteriol. 172:2736-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton, S. M., J. S. Allen, Z. Cao, Z. Qi, X. Jiang, C. Sprencel, J. D. Igo, S. B. Foster, M. A. Payne, and P. E. Klebba. 1997. Double mutagenesis of a positive charge cluster in the ligand-binding site of the ferric enterobactin receptor, FepA. Proc. Natl. Acad. Sci. USA 94:4560-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton, S. M., J. D. Igo, D. C. Scott, and P. E. Klebba. 1999. Effect of loop deletions on the binding and transport of ferric enterobactin by FepA. Mol. Microbiol. 32:1153-1165. [DOI] [PubMed] [Google Scholar]
- 31.Pautsch, A., and G. E. Schulz. 1998. Structure of the outer membrane protein A transmembrane domain. Nat. Struct. Biol. 5:1013-1017. [DOI] [PubMed] [Google Scholar]
- 32.Payne, M. A., J. D. Igo, Z. Cao, S. B. Foster, S. M. Newton, and P. E. Klebba. 1997. Biphasic binding kinetics between FepA and its ligands. J. Biol. Chem. 272:21950-21955. [DOI] [PubMed] [Google Scholar]
- 33.Postle, K. 1990. TonB and the gram-negative dilemma. Mol. Microbiol. 4:2019-2025. [DOI] [PubMed] [Google Scholar]
- 34.Postle, K. 1993. TonB protein and energy transduction between membranes. J. Bioenerg. Biomembr. 25:591-601. [DOI] [PubMed] [Google Scholar]
- 35.Pugsley, A. P., and P. Reeves. 1977. Uptake of ferrienterochelin by Escherichia coli: energy-dependent stage of uptake. J. Bacteriol. 130:26-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutz, J. M., J. Liu, J. A. Lyons, J. Goranson, S. K. Armstrong, M. A. McIntosh, J. B. Feix, and P. E. Klebba. 1992. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science 258:471-475. [DOI] [PubMed] [Google Scholar]
- 37.Schirmer, T., T. A. Keller, Y. F. Wang, and J. P. Rosenbusch. 1995. Structural basis for sugar translocation through maltoporin channels at 3.1 Å resolution. Science 267:512-514. [DOI] [PubMed] [Google Scholar]
- 38.Scott, D. C. 2001. Ph.D. dissertation. University of Oklahoma, Norman.
- 39.Scott, D. C., Z. Cao, Z. Qi, M. Bauler, J. D. Igo, S. M. Newton, and P. E. Klebba. 2001. Exchangeability of N termini in the ligand-gated porins of Escherichia coli. J. Biol. Chem. 276:13025-13033. [DOI] [PubMed] [Google Scholar]
- 40.Skare, J. T., and K. Postle. 1991. Evidence for a TonB-dependent energy transduction complex in Escherichia coli. Mol. Microbiol. 5:2883-2890. [DOI] [PubMed] [Google Scholar]
- 41.Snijder, H. J., R. L. Kingma, K. H. Kalk, N. Dekker, M. R. Egmond, and B. W. Dijkstra. 2001. Structural investigations of calcium binding and its role in activity and activation of outer membrane phospholipase A from Escherichia coli. J. Mol. Biol. 309:477-489. [DOI] [PubMed] [Google Scholar]
- 42.Sweadner, K. J. 1990. Anomalies in the electrophoretic resolution of Na+/K+-ATPase catalytic subunit isoforms reveal unusual protein: detergent interactions. Biochim. Biophys. Acta 1029:13-23. [DOI] [PubMed] [Google Scholar]
- 43.Vandeputte-Rutten, L., R. A. Kramer, J. Kroon, N. Dekker, M. R. Egmond, and P. Gros. 2001. Crystal structure of the outer membrane protease OmpT from Escherichia coli suggests a novel catalytic site. EMBO J. 20:5033-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, C. C., and A. Newton. 1971. An additional step in the transport of iron defined by the tonB locus of Escherichia coli. J. Biol. Chem. 246:2147-2151. [PubMed] [Google Scholar]
- 45.Wang, C. C., and A. Newton. 1969. Iron transport in Escherichia coli: roles of energy-dependent uptake and 2,3-dihydroxybenzoylserine. J. Bacteriol. 98:1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou, X. H., and D. van der Helm. 1993. A novel purification of ferric citrate receptor (FecA) from Escherichia coli UT5600 and further characterization of its binding activity. Biometals 6:37-44. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, X. H., D. van der Helm, and J. Adjimani. 1993. Purification of outer membrane iron transport receptors from Escherichia coli by fast protein liquid chromatography: FepA and FecA. Biometals 6:25-35. [DOI] [PubMed] [Google Scholar]
- 48.Zhou, X. H., D. van der Helm, and L. Venkatramani. 1995. Binding characterization of the iron transport receptor from the outer membrane of Escherichia coli (FepA): differentiation between FepA and FecA. Biometals 8:129-136. [DOI] [PubMed] [Google Scholar]