Abstract
The visA gene of Streptomyces virginiae has been thought to be a part of the virginiamycin S (VS) biosynthetic gene cluster based on its location in the middle of genes that encode enzymes highly similar to those participating in the biosynthesis of streptogramin-type antibiotics. Heterologous expression of the visA gene was achieved in Escherichia coli by an N-terminal fusion with thioredoxin (TrxA), and the intact recombinant VisA protein (rVisA) was purified after cleavage with enterokinase to remove the TrxA moiety. The purified rVisA showed clear l-lysine 2-aminotransferase activity with an optimum pH of around 8.0 and an optimum temperature at 35°C, with 2-oxohexanoate as the best amino acceptor, indicating that VisA converts l-lysine into Δ1-piperidine 2-carboxylic acid. A visA deletion mutant of S. virginiae was created by homologous recombination, and the in vivo function of the visA gene was studied by phenotypic comparison between the wild type and the visA deletion mutant. No differences in growth in liquid media or in morphological behavior on solid media were observed, indicating that visA is not involved in primary metabolism or morphological differentiation. However, the visA mutant failed to produce VS while maintaining the production of virginiamycin M1 at a level comparable to that of the parental wild-type strain, demonstrating that visA is essential to VS biosynthesis. These results, together with the observed recovery of the defect in VS production by the external addition of 3-hydroxypicolinic acid (3-HPA), a starter molecule in VS biosynthesis, suggest that VisA is the first enzyme of the VS biosynthetic pathway and that it supplies 3-HPA from l-lysine.
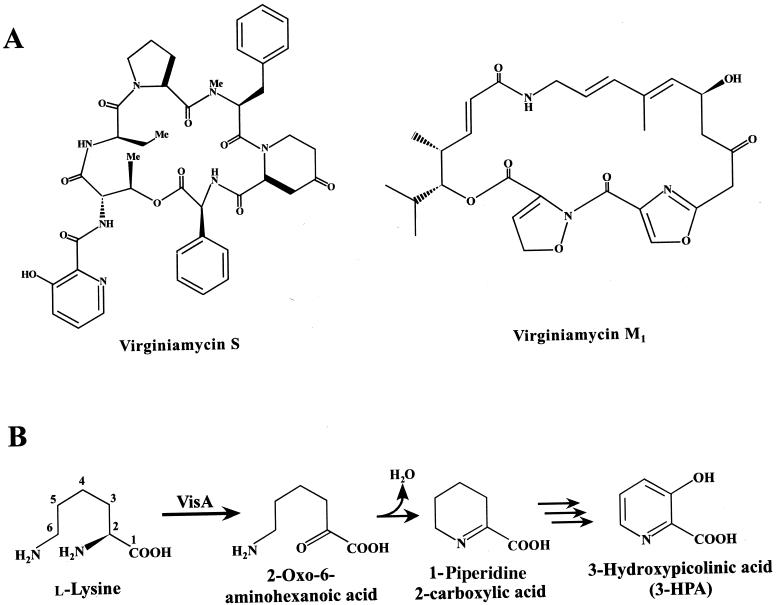
Virginiamycin S (VS) is a cyclohexadepsipeptide antibiotic of Streptomyces virginiae coproduced with a macrolide antibiotic virginiamycin M1 (VM1) as a synergistic mixture that is bactericidal against a wide range of gram-positive bacteria. VS is composed of seven amino acid moieties connected by a peptide linkage in the following order: 3-hydroxypicolinic acid (3-HPA), l-threonine, d-α-aminobutyric acid, l-proline, N-methyl-l-phenylalanine, l-4-oxopipecolic acid, and l-phenylglycine (Fig. 1A). It has been postulated that the condensation of these amino acids, as well as the final cyclization, is carried out by a nonribosomal peptide synthetase (15, 23). Based on feeding experiments, l-lysine has been confirmed to be the precursor of the 3-hydroxypicolynyl moiety as well as the 4-oxopipecolyl moiety (16). Therefore, two distinct l-lysine-converting pathways should be present in the process of VS biosynthesis to supply the necessary precursors. The one participating in 3-HPA formation is particularly important for enhancing VS productivity and/or creating novel VS derivatives having different starter molecules, as 3-HPA is considered to be the starter molecule in nonribosomal peptide synthesis.
FIG. 1.
(A) Structures of VS and VM1 produced by S. virginiae. (B) Presumed enzymatic reaction carried out by the VisA protein followed by the spontaneous nonenzymatic cyclization to form 1-piperidine 2-carboxylic acid.
The production of both VS and VM1 is known to be induced by a low-molecular-weight compound, virginiae butanolide (VB), which belongs to the γ-butyrolactone autoregulators (24). To date, its specific receptor protein, BarA, has been identified (14) and it was clarified that the binding of VB to BarA triggers the production of virginiamycins (8). Several genes relating to the regulation of virginiamycin biosynthesis have been found around the barA gene (7, 12), but the structural genes for VS and VM1 have not been identified in S. virginiae as yet.
Four plausible structural genes (visAB visCD) were recently discovered (13) downstream from the VS resistance operon (varS varR). It is possible that these genes are part of the VS biosynthetic gene cluster based on homologies of the gene products to those involved in the biosynthesis of streptogramin-type antibiotics and on their transcription pattern coinciding with VS production. It was hypothesized that the leftmost of the four genes, visA, encodes l-lysine 2-aminotransferase and may participate in one of the two l-lysine-converting pathways, especially that for 3-HPA formation (Fig. 1B), because another gene (visB) of the bicistronic visAB operon seems to encode the 3-HPA-activating enzyme.
In this study, the biochemical nature of VisA was determined by using purified recombinant VisA protein, and all of the data confirmed that VisA is an l-lysine 2-aminotransferase preferentially converting l-lysine into 2-keto-6-aminocaproate, which nonenzymatically cyclizes to form Δ1-piperidine 2-carboxylic acid. Furthermore, a visA deletion mutant was constructed to evaluate the in vivo function, and the phenotype of the visA mutant demonstrated that VisA participates in the VS biosynthetic pathway, producing the 3-HPA moiety in S. virginiae.
MATERIALS AND METHODS
Strains, growth conditions, and plasmids.
S. virginiae (strain MAFF 10-06014; National Food Research Institute, Ministry of Agriculture, Forestry, and Fisheries, Tsukuba, Japan) was grown at 28°C as described previously (22). For the genetic manipulation in Escherichia coli, E. coli strain DH5α (3) and Streptomyces lividans strain TK21 (4) were used. Streptomyces strains were grown at 28°C in yeast extract-malt extract liquid medium for the preparation of protoplasts (4), in tryptone soy broth (Oxoid, Basingstoke, Hampshire, United Kingdom) for the preparation of total DNA, and on solid intracellular serine protease medium 2 (Difco, Detroit, Mich.) for spore formation. S. lividans TK21 was obtained from the John Innes Centre, Norwich, United Kingdom. DNA manipulations in E. coli were performed as described by Sambrook et al. (17). pUC18 and pUC19 were used for genetic manipulation in E. coli, and pUC19 was used for DNA sequencing. pUWL-KS (21), an E. coli-Streptomyces shuttle vector, was used for gene replacement. pET32a(+) (Novagen, Madison, Wis.) and E. coli BL21(DE3) CodonPlus competent cells (Stratagene, La Jolla, Calif.) were used for overexpression of visA. Flavobacterium sp. strain ATCC 25311 (IFO 3048), a producer of l-lysine 6-aminotransferase, was purchased from the Institute for Fermentation, Osaka, Japan.
Chemicals.
All chemicals were of reagent or high-performance liquid chromatography (HPLC) grade and were purchased from either Nacalai Tesque (Osaka, Japan), Takara Shuzo (Shiga, Japan), Wako Pure Chemical Industries, Ltd. (Osaka, Japan), or Sigma Aldrich Japan (Tokyo, Japan). [α-32P]dCTP was purchased from ICN Biomedicals, Inc. (Costa Mesa, Calif.). VS and VM1 were purified by a previously described method (10).
Overexpression of visA and purification of recombinant VisA protein (rVisA).
To introduce new restriction sites, visA was amplified by PCR with oligonucleotides 5′-TTCCATGGTGACGGCGGCGCAGCGCTAC-3′ and 5′-TATGGATCCTCATCGGACCGCCAGCGG-3′, which contain artificial NcoI and BamHI recognition sites (underlined), respectively. The amplified product was digested with NcoI and BamHI and inserted into NcoI-BamHI-digested pET32a(+), generating pVS104, by which the visA coding region is fused with sequences encoding 160 surplus amino acids of E. coli thioredoxin (TrxA), six-histidine residues, and an enterokinase cleavage site at the 5′ end of visA. The plasmid pVS104 was transformed into E. coli BL21(DE3) CodonPlus competent cells, and the transformants were cultured at 37°C in Luria broth containing ampicillin (25 μg/ml), chloramphenicol (25 μg/ml), and betaine monohydrate (25 mM). When the optical density at 600 nm reached 0.6, isopropyl 1-thio-β-d-galactoside (IPTG) (final concentration of 0.1 mM) was added to the culture, followed by a further 16-h cultivation at 20°C. Cells were harvested by centrifugation at 7,000 × g for 15 min at 4°C, washed, and resuspended in buffer A (0.05 M potassium phosphate buffer [pH 7] containing 0.2 M KCl and 10% [vol/vol] glycerol). The cells were disrupted by sonication for 3 min (50% duty cycle and output level 3, Branson Sonifier 250) in an ice bath followed by centrifugation at 10,000 × g for 15 min at 4°C to obtain a crude cell extract.
The crude extract was applied to an Ni-nitrilotriacetic acid (NTA) column (bed volume of 1 ml; Qiagen, Tokyo, Japan) at room temperature, and unbound proteins were washed out with buffer A containing 40 mM imidazole. The TrxA-VisA fusion protein was eluted with buffer A containing 500 mM imidazole. After dialysis against a 500-fold volume of buffer A at 4°C for 6 h to remove imidazole and concentration by ultrafiltration (Mr cutoff of ≥10,000) (Advantec, Tokyo, Japan), the TrxA-VisA protein was cleaved by recombinant enterokinase (Novagen) for 12 h at 20°C. The solution containing intact VisA and His6-tagged TrxA was loaded onto the Ni-NTA column again, and the recombinant VisA was purified in the pass-through fractions, whereas the His6-tagged TrxA and the uncleaved TrxA-VisA remained bound to the column.
Preparation of crude cell extract from wild-type S. virginiae.
S. virginiae cultivation was initiated by inoculating 2.1 ml of preculture into 70 ml of f-medium (7.5 g of Bacto Casitone/liter, 7.5 g of yeast extract/liter, 15 g of glycerol/liter, and 2.5 g of NaCl/liter) in a 500-ml baffled flask. After cultivation at 28°C for 14 h on a reciprocating shaker (120 strokes per min), cells were harvested by centrifugation (3,000 × g, 10 min, 4°C). The cells were suspended in buffer B (buffer A containing 1 mM phenylmethylsulfonyl fluoride and 1 mM dithiothreitol) and disrupted by sonication as described above. After centrifugation (10,000 × g, 15 min, 4°C), the supernatant was obtained as a crude cell extract and used as the source of native VisA protein.
Flavobacterium sp. strain IFO 3048 was cultured in a medium (10 g of polypeptone/liter, 2 g of yeast extract/liter, and 1 g of MgSO4·7H2O/liter) at 30°C on a reciprocating shaker (120 strokes per min) until the absorbance at 600 nm reached 0.6. The cells were harvested by centrifugation, suspended in buffer B, and disrupted by sonication, as described above, to obtain the cell extract.
Enzyme assay and protein determination.
The lysine 2-aminotransferase activity was assayed as described by Soda et al. (19). The standard incubation mixture contained protein (up to 150 μl), 5 mM l-lysine, 100 mM 2-oxobutyrate, 70 μM pyridoxal 5′-phosphate (PLP), and 100 μM potassium phosphate (pH 8.0) in a final volume of 250 μl. The reaction was initiated by the addition of the protein and the mixture was incubated for 20 min at 30°C followed by the addition of a 50% trichloroacetic acid-absolute ethanol (1:10) mixture (125 μl) to terminate the reaction. The precipitated protein was removed by centrifugation. The piperidine carboxylate formed from l-lysine by transamination and nonenzymatic cyclization was derivatized with o-aminobenzaldehyde by mixing 250 μl of the deproteinized supernatant with 250 μl of 40 mM o-aminobenzaldehyde in 200 mM potassium phosphate (pH 8.0). A yellow-orange-colored dihydroquinazolinium complex developed during incubation at 37°C overnight, and the absorbance at 450 nm was measured. The amount of the dihydroquinazolinium complex was calculated by using the absorption coefficient of 1.25 × 106 cm2 mol−1, as determined by Jordan (5). One unit of enzyme activity was defined as the amount of enzyme catalyzing the formation of 1.0 μmol of 1-piperidine 2-carboxylic acid per min (18).
Aminotransferase activities toward 5-hydroxylysine and ornithine were measured by using protocols similar to that used for lysine 2-aminotransferase activity. With the ornithine aminotransferase assay, the amount of the dihydroquinazolinium complex was determined at 440 nm by using the absorption coefficient of 1.9 × 106 cm2 mol−1 reported by Voellmy and Leisinger (20).
Lysine 6-aminotransferase activity was assayed by the same method as that used for the lysine 2-aminotransferase assay, except that the reaction mixture contained 2-oxoglutarate instead of 2-oxobutyrate.
Protein concentrations were determined by the method of Bradford (1), with bovine serum albumin used as a standard.
Construction of a visA deletion mutant.
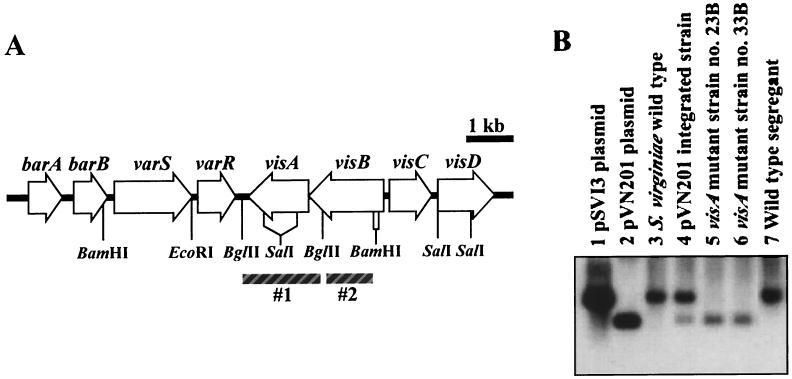
A 3.8-kb EcoRI-BamHI fragment extending from 1.1 kb downstream of visA to 1.5 kb upstream of visA (see Fig. 4) was subcloned into pUC19 to generate pSVI3. To disrupt the visA gene, the 849-bp SalI-SalI fragment encoding the internal 283 amino acids of VisA (Pro111 to Asp393) was deleted from pSVI3 by SalI-digestion and self-ligation, and the resulting 3.0-kb EcoRI-BamHI fragment was subcloned into EcoRI-BamHI-digested pUWL-KS to generate pVS201. After being propagated once in S. lividans TK21, the plasmid pVS201 was transformed into S. virginiae as described previously (6). To confirm the integration of pVS201, transformants were checked by Southern blot hybridization with a BglII-BglII fragment (Fig. 4) as a probe. Thiostrepton-sensitive strains resulting from the second crossover were selected on solid intracellular serine protease medium 2 from the thiostrepton resistance transformants after three rounds of growth in liquid tryptone soy broth medium in the absence of thiostrepton. The visA deletion mutant was identified by Southern blot hybridization by using the same probe as above.
FIG. 4.
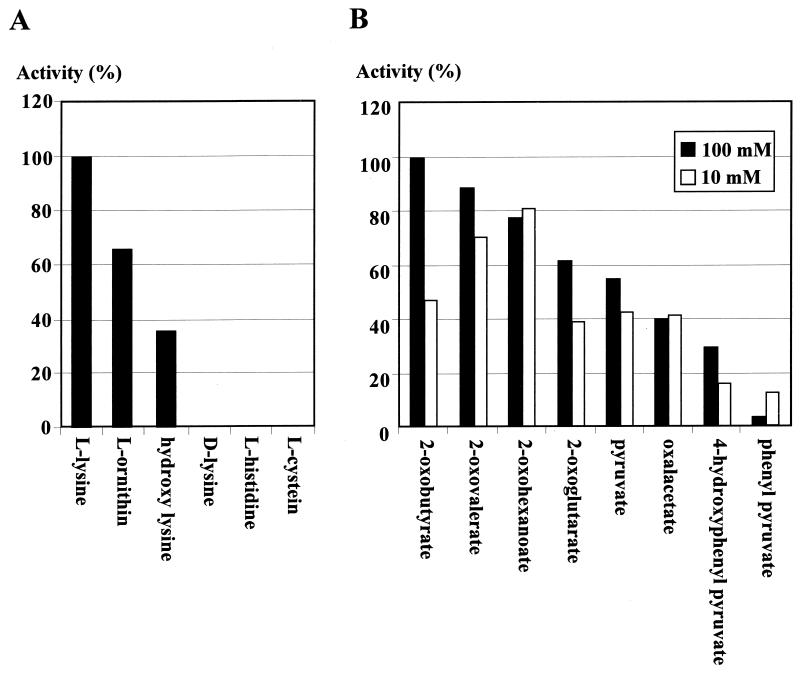
Amino donor (A) and amino acceptor (B) specificities of purified rVisA. Activities toward several amino acids were measured at a final concentration of 5 mM with 100 mM 2-oxobutylate as the amino acceptor. Activities toward several keto acids were assayed at a final concentration of either 10 or 100 mM with 5 mM l-lysine as the amino donor.
Determination of virginiamycins.
The amounts of VS and VM1 were determined by reverse-phase HPLC on a Cosmosil 5C18-AR column (10 mm by 250 mm, Waters type) (Nacalai) under the following conditions: 50% CH3CN as a mobile phase, flow rate of 2 ml/min, and detection at 305 nm. Peaks and retention times of the virginiamycins were determined by using authentic samples.
RNA preparation and Northern blot analysis.
Total RNA was isolated by the method of Kirby et al. (9), with modifications described by Hopwood et al. (4), and was quantified by absorbance at 260 nm. RNA (10 μg) was loaded on each lane, electrophoresed on a 1.2% agarose gel, and transferred to Hybond N+ (Amersham Pharmacia Biotech) according to the manufacturer's recommendations. Hybridization was carried out at 65°C for 1 h in Rapid-hyb buffer (Amersham Pharmacia Biotech) followed by three washings of the blot at 50°C for 10 min with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate [pH 7.7]) containing 0.1% sodium dodecyl sulfate. The BglII-BglII fragment was used as a specific probe for visB (see Fig. 4). The DNA fragments were labeled with [α-32 P]dCTP with the random primer DNA labeling kit (version 2) (Takara Shuzo Co.).
RESULTS AND DISCUSSION
Overproduction and purification of rVisA.
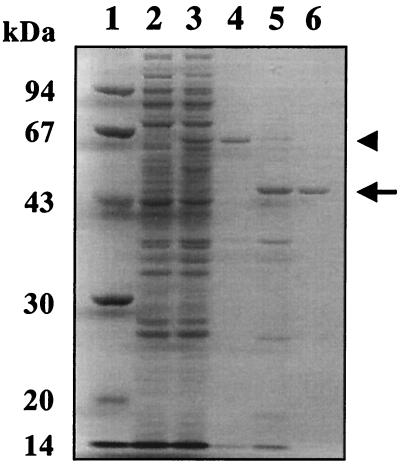
To determine the in vitro function of VisA, the visA gene was overexpressed in E. coli under the control of the T7 promoter. A protein of 62 kDa was observed in the extracts of IPTG-induced cells carrying the visA gene in pET32a(+) (pVS104) (see Materials and Methods for construction) (Fig. 2, lane 3), whereas the corresponding band was missing in the extracts of cells carrying vector pET32a(+) alone (Fig. 2, lane 2). The molecular mass of 62 kDa agreed well with that estimated from the nucleotide sequences of visA plus trxA. After enterokinase treatment, which cleaves between the TrxA and VisA portions, the 62-kDa protein shifted to a 45-kDa protein which corresponded well with the molecular mass of intact VisA calculated from the nucleotide sequence (Mr = 45,546). The 45-kDa protein was purified as rVisA, and the identity was confirmed by N-terminal amino acid sequencing (LMTAAQRYLVPFQ). The fusion with TrxA was essential for sufficient production of soluble VisA in E. coli: the overexpression of visA alone or His6-tagged visA resulted in the formation of an inclusion body in the crude extract and hindered further purification.
FIG. 2.
Overexpression and purification of rVisA. A sample from each purification step was separated on a sodium dodecyl sulfate-10% polyacrylamide gel and stained with Coomassie brilliant blue R-250 (17). Lane 1, molecular mass (in kilodaltons) standard; lane 2, crude extract (10 μg) of E. coli BL21(DE3) CodonPlus containing pET32a(+) as a negative control; lane 3, crude extract (10 μg) of E. coli BL21(DE3) CodonPlus containing pVN104; lane 4, eluate (7 μg) with buffer A containing 500 mM imidazole from Ni-NTA affinity chromatography; lane 5, the product cleaved by enterokinase; lane 6, purified VisA. The arrow indicates the position of VisA, and the arrowhead indicates the position of the TrxA-VisA fusion protein.
Because VisA is highly homologous to l-lysine 2-aminotransferases (13), the activity was at first assayed by using the crude cell extract containing the TrxA-VisA fusion protein and by monitoring the colored complex formation between the enzymatic product and o-aminobenzaldehyde according to the method of Soda et al. (19). The crude extract showed clear activity of 0.07 U/mg of protein, whereas no activity (<0.0001 U/mg) was detected with control extracts prepared from E. coli cells harboring pET32a(+) alone. These findings indicate that VisA is a kind of l-lysine aminotransferase, although it is not clear at this stage which of the two amino groups of l-lysine is transaminated because transamination of either the 2-amino or 6-amino group resulted in a compound that can develop a similar color complex with o-aminobenzaldehyde.
To clarify this point, rVisA was purified from the crude extract containing TrxA-fused rVisA. After adsorption and elution from an Ni-NTA column, the TrxA-VisA fusion protein showed activity of 0.35 U/mg of protein. After cleavage with enterokinase and passage through an Ni-NTA column, the rVisA was purified to homogeneity and showed activity of 0.07 U/mg of protein. The activity loss occurred primarily during the enterokinase treatment and the second Ni-NTA column step, due probably to the tendency of the intact rVisA protein to aggregate without the TrxA moiety. However, the enzyme activity was proportional to the amount of purified rVisA, and the reaction proceeded linearly for up to 10 h with 6 μg of purified rVisA under the standard assay conditions, confirming that it was not TrxA but rVisA that catalyzed the transamination of l-lysine. No requirement for the external PLP was observed, suggesting that rVisA had a high binding affinity toward PLP and contained a sufficient amount of the coenzyme (data not shown).
Characterization of rVisA.
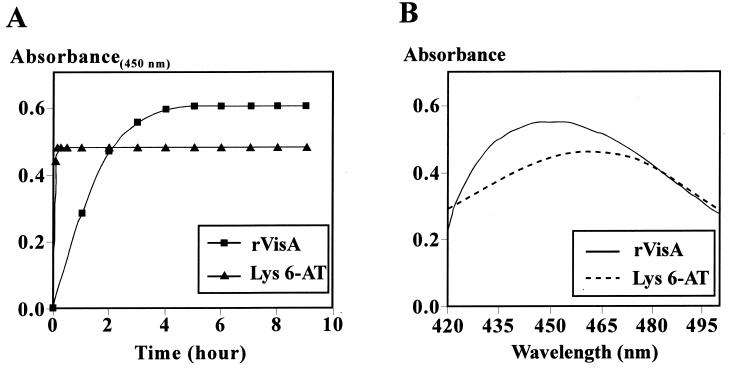
The enzymatic characteristics of VisA were examined with the purified rVisA. Because either the 2- or the 6-amino group of lysine could be transaminated, yielding piperidine 2-carboxylate or piperidine 6-carboxylate, respectively, the reaction product of rVisA was determined based on the method reported by Soda et al. (19); specifically, o-aminobenzaldehyde reacts slowly with piperidine 2-carboxylate but very quickly with piperidine 6-carboxylate, forming dihydroquinazolinium complexes with absorption maximums at 450 nm and at 465 nm, respectively. As shown in Fig. 3, it took 5 h for the rVisA-catalyzed transamination product to complete the formation of the dihydroquinazolinium complex (Fig. 3A), and the complex showed an absorption maximum at 450 nm (Fig. 3B). In contrast, when the product formed by l-lysine 6-aminotransferase from Flavobacterium sp. reacted with o-aminobenzaldehyde, the reaction was complete within 10 min and the maximum absorption of the complex was observed at 465 nm. As such, VisA appears to exhibit l-lysine 2-aminotransferase activity.
FIG. 3.
(A) Reaction time course of o-aminobenzaldehyde toward the transamination product of rVisA or lysine 6-aminotransferase (Lys 6-AT). The reaction was monitored by the absorbance reading at 450 nm. (B) Absorption spectra of the dihydroquinazolinium complex derived from the transamination product of rVisA or lysine 6-aminotransferase (Lys 6-AT).
The substrate specificity of rVisA was determined with several amino acids as amino donors and with several keto acids as amino acceptors (Fig. 4). At a fixed concentration of 5 mM, l-lysine served as the best substrate, while l-ornithine and 5-hydroxy-l-lysine gave moderate activity and d-lysine did not, confirming that VisA is an l-lysine-2-aminotransferase. The Km for l-lysine was determined to be 0.92 mM under the standard assay conditions. Because VisA showed relatively broad substrate specificity, such as that with 5-hydroxylysine, it may be possible to create novel VS structures by feeding l-lysine derivatives, if the subsequent biosynthetic enzymes accept the modified precursors.
Using l-lysine as the amino donor, 2-oxobutyrate is the best amino acceptor at a concentration of 100 mM while 2-oxohexanoate is best at a concentration of 10 mM (Fig. 4B). This phenomenon seems to reflect the inhibitory nature of some keto acids at higher concentrations (100 mM), as it was observed that imidazole pyruvate shows the highest aminotransferase activity at 10 mM for NikC l-lysine 2-aminotransferase, whereas inhibition occurs at concentrations greater than 25 mM (2). VisA was found to have an optimum temperature of 35°C (139% activity compared to that at 30°C), and a broad pH optimum between pH 7.5 and 8.5.
Construction and characterization of a visA deletion mutant.
To investigate the in vivo function of VisA in S. virginiae, the visA gene in the chromosome was disrupted by replacing it with the mutated visA on pVS201 by homologous recombination. In the mutated visA gene on pVS201 (for details, see Materials and Methods), the 849-bp SalI-SalI fragment was deleted, which resulted in the in-frame deletion of 283 amino acids from VisA. Thiostrepton-resistant colonies of S. virginiae were selected as the candidates of the transformants, and after single-colony isolation in the presence of thiostrepton (50 μg/ml), Southern blot hybridization was performed to confirm the integration of pVS201 (Fig. 5B, lane 4). The plasmid-integrated strain was put through three rounds of cultivation in liquid media without thiostrepton to facilitate the second crossover. As expected, two types of thiostrepton-sensitive colonies were obtained: namely, ΔvisA strains (in which the wild-type visA gene was replaced with the visA deletion mutant) (Fig. 5B, lane 5 and 6) and regenerated wild-type strains (Fig. 5B, lane 7). One of these ΔvisA mutants (strain 23B) was used for further investigation.
FIG. 5.
(A) Gene organization around the visA gene in S. virginiae. Probes are indicated by shaded boxes. The BglII-BglII fragment (#1) was used for the Southern hybridization analysis of visA. The BglII-BamHI fragment (#2) was used for Northern hybridization analysis of a visB-visA transcript (see Fig. 6) (7, 12, 14). barA, VB receptor gene (8); barB, a kind of regulatory gene (unpublished data); varS, VS resistance gene (11); varR, varS repressor gene (12). (B) Southern blot analysis of wild-type S. virginiae, pVN201 integrated strain, ΔvisA strains, and the wild-type segregant strain. The BglII-BglII fragment containing visA was used as a probe against BglII-digested genomic DNA of various strains and control plasmids. pSVI3 and pVN201 are the control plasmids containing visA and ΔvisA, respectively.
Transcriptional analysis of the VS biosynthetic genes upstream of ΔvisA.
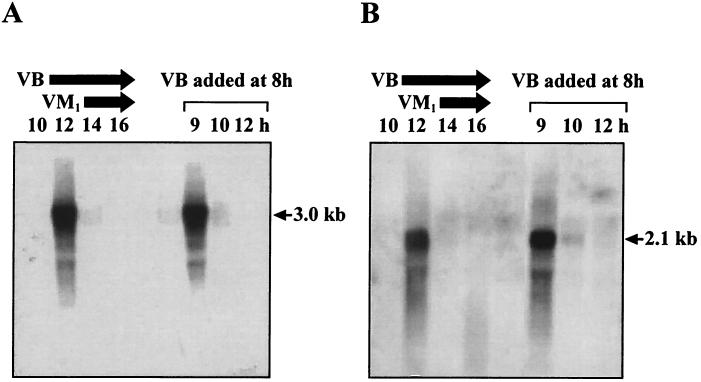
Because the visA gene forms a bicistronic operon with the upstream visB (13), the effects of the visA disruption on transcription of the visB-visA operon were investigated by Northern blot hybridization with RNA samples of the ΔvisA mutant after a 7- to 14-h cultivation with the visB-specific probe (Fig. 6B) (Fig. 5A shows the position of the probe). Similar to the case of wild-type S. virginiae (Fig. 6A), no transcription was observed until 10 h of cultivation. At 12 h of cultivation, i.e., 1 h after VB production (at 11 h) or 2 h before VM1 production (14 h) (6), intense transcription of the expected 2.1-kb transcript, rather than that of the wild-type strain (3.0 kb), was detected. The highly temporal transcription, as well as the induction by VB, was completely indistinguishable from that in the wild-type strain, indicating that ΔvisA mutation did not affect either the operon structure or the regulation of visB-visA transcription. Similarly, transcription of the visC-visD operon further upstream was not affected by the ΔvisA mutation (data not shown).
FIG. 6.
Northern blot hybridization analysis of the visB-visA transcript in the wild-type strain (A) and in the ΔvisA strain (B) during cultivation. The visB probe was used. Total RNA was extracted from cells cultivated at 28°C for the indicated period. Cultivation without VB addition is shown on the left side of each panel. Under the experimental conditions, the production of VB and VM1 started at 11 and 14 h of cultivation, respectively, as shown by the arrows above the lanes. Cultivation with VB added at 8 h is shown on the right side of each panel. To determine the effects of VB on the transcription of these genes, VB was added to the culture at 8 h to a final concentration of 64 ng/ml.
Phenotype of the visA mutant strain.
The growth characteristics of the ΔvisA strain on several kinds of solid media as well as in liquid media were almost the same as those of the wild-type strain, indicating that VisA does not participate in the primary metabolism essential for growth or in the control of morphological differentiation in S. virginiae.
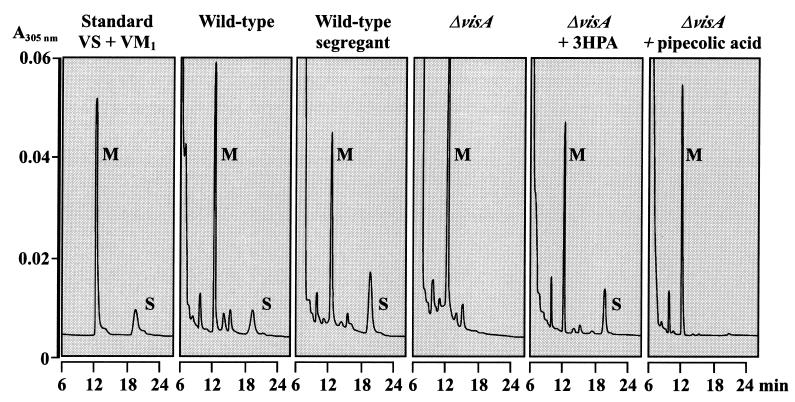
Since two l-lysine molecules are incorporated into VS and because VisA may participate in one of the two pathways converting l-lysine into the necessary precursors, VS and VM1 production in the ΔvisA strain were examined by HPLC (see Materials and Methods). Although a wild-type parental strain as well as the wild-type segregant began to produce both VS and VM1 after 12 h of cultivation, the ΔvisA strain produced only VM1 after 12 h of cultivation but failed to produce VS even after 48 h of cultivation (Fig. 7). This result indicates that VisA is an essential component in VS biosynthesis.
FIG. 7.
HPLC analysis of the culture filtrate from (left to right) 3.5 nmol (each) of authentic VM1 and VS, the S. virginiae wild-type strain, the wild-type segregant strain, the ΔvisA strain, the ΔvisA strain grown in the medium containing 1 mM 3-HPA, and the ΔvisA strain grown in the medium containing 2 mM dl-pipecolic acid. The strains were grown for 24 h in f-medium. Peaks of VM1 (M) and VS (S) are indicated.
As for the more-detailed function of VisA in VS biosynthesis, based on the enzymatic activity of l-lysine 2-aminotransferase of VisA, VisA seems to participate in supplying either 3-HPA or pipecolic acid from l-lysine. VisA has previously been identified as being involved in the 3-HPA biosynthetic pathway (13), as visA forms an operon with upstream visB, the product of which appears to activate 3-HPA to an adenylated intermediate. In the present study, the involvement of VisA in the 3-HPA production pathway was confirmed by our observation that the defect in the VS production can be recovered by the external addition of 1 mM 3-HPA (Fluka and Sigma Aldrich Japan) to the culture of the ΔvisA strain, whereas no recovery of VS production occurs with the addition of pipecolic acid (Fig. 7).
The deletion of the visA gene clearly showed that VisA is important for supplying 3-HPA from l-lysine via 1-piperidine 2-carboxylate. However, there is another gene (visC) that can be postulated to encode lysine cyclodeaminase, which also produces 1-piperidine 2-carboxylate from l-lysine. Actually, the formation of 1-piperidine 2-carboxylate in the crude lysate of the ΔvisA mutant strain was only slightly reduced in relation to that of the wild-type strain (data not shown). Although it is not completely clear at present why VS biosynthesis was terminated even with sufficient enzyme activity to produce 1-piperidine 2-carboxylate, it seems that the downstream activities converting 1-piperidine 2-carboxylate into the final precursor, 3-HPA, may be tightly coupled with the activity of VisA. Actually, the addition of picolinic acid instead of 3-HPA to the culture of the ΔvisA mutant strain did not result in a recovery of VS production (data not shown), suggesting that even the hydroxylation of picolinic acid is severely impaired by the absence of the VisA protein.
REFERENCES
- 1.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 2.Burntner, C., and C. Bormann. 1998. The Streptomyces tendae Tu901 l-lysine 2-aminotransferase catalyzes the initial reaction in nikkomycin D biosynthesis. Eur. J. Biochem. 254:347-355. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 4.Hopwood, D. A., M. J. Bibb, K. F. Chater, C. J. Bruton, H. M. Kieser, D. J. Lydiate, C. P. Smith, J. M. Ward, and H. Schrempf. 1985. Genetic manipulation of Streptomyces: a laboratory manual. The John Innes Foundation, Norwich, United Kingdom.
- 5.Jordan, B. 1985. Untersuchungen zu Aminossurestoffwechsel und Nikkomycinbildung in Streptomyces tendae. Ph.D. thesis. Universität Münster, Münster, Germany.
- 6.Kawachi, R., T. Nihira, and Y. Yamada. 1997. Development of a transformation system in Streptomyces virginiae. Actinomycetologica 11:46-53. [Google Scholar]
- 7.Kawachi, R., U. Wangchaisoonthorn, T. Nihira, and Y. Yamada. 2000. Identification by gene deletion analysis of a regulator, VmsR, that controls virginiamycin biosynthesis in Streptomyces virginiae. J. Bacteriol. 182:6259-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinoshita, H., H. Ipposhi, S. Okamoto, H. Nakano, T. Nihira, and Y. Yamada. 1997. Butyrolactone autoregulator receptor protein (BarA) as a transcriptional regulator in Streptomyces virginiae. J. Bacteriol. 179:6986-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby, T., E. Fox-Carter, and M. Guest. 1967. Isolation of deoxyribonucleic acid and ribosomal ribonucleic acid from bacteria. Biochem. J. 104:258-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, C.-K., M. Minami, S. Sakuda, T. Nihira, and Y. Yamada. 1996. Stereospecific reduction of virginiamycin M1 as the virginiamycin resistance pathway in Streptomyces virginiae. Antimicrob. Agents Chemother. 40:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, C.-K., Y. Kamitani, T. Nihira, and Y. Yamada. 1998. Identification and in vivo functional analysis of a virginiamycin S resistance gene (varS) from Streptomyces virginiae. J. Bacteriol. 181:3293-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namwat, W., C.-K. Lee, H. Kinoshita, Y. Yamada, and T. Nihira. 2001. Identification of the varR gene as a transcriptional regulator of virginiamycin S resistance in Streptomyces virginiae. J. Bacteriol. 183:2025-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namwat, W., Y. Kamioka, H. Kinoshita, and T. Nihira. 2002. Characterization of virginiamycin S biosynthetic genes from Streptomyces virginiae. Gene 286:283-290. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto, S., K. Nakamura, T. Nihira, and Y. Yamada. 1995. Virginiae butanolide binding protein from Streptomyces virginiae. J. Biol. Chem. 270:12319-12326. [DOI] [PubMed] [Google Scholar]
- 15.Paris, J. M., J. C. Barriere, C. Smith, and P. E. Bost. 1990. The chemistry of pristinamycins, p. 183-248. In G. Lukacs and M. Ohno (ed.), Recent progress in the chemical synthesis of antibiotics. Springer-Verlag, Berlin, Germany.
- 16.Reed, J. W., M. B. Purvis, D. G. I. Kingston, A. Biot, and F. Gossele. 1989. Biosynthesis of antibiotics of the virginiamycin family. 7. Stereo- and regiochemical studies on the formation of the 3-hydroxypicolinic acid and pipecolic acid units. J. Org. Chem. 54:1161-1165. [Google Scholar]
- 17.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Soda, K., and H. Misuno. 1971. l-lysine-α-ketoglutarate aminotransferase (Achrobacter liquidum). Methods Enzymol. 17B:222-228. [Google Scholar]
- 19.Soda, K., H. Misono, and T. Yamamoto. 1968. l-lysine-α-ketoglutarate aminotransferase. I. Identification of a product, Δ1-piperideine-6-carboxylic acid. Biochemistry 7:4102-4109. [DOI] [PubMed] [Google Scholar]
- 20.Voellmy, R., and T. Leisinger. 1975. Dual role for N2-acetylornithine 5-aminotransferase from Pseudomonas aeruginosa in arginine biosynthesis and arginine catabolism. J. Bacteriol. 122:799-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wehmeier, U. F. 1995. New multifunctional Escherichia coli-Streptomyces shuttle vectors allowing blue-white screening on X-Gal plates. Gene 165:149-150. [DOI] [PubMed] [Google Scholar]
- 22.Yamada, Y., K. Sugamura, K. Kondo, M. Yanagimoto, and H. Okada. 1987. The structure of inducing factors for virginiamycin production in Streptomyces virginiae. J. Antibiot. 40:496-504. [DOI] [PubMed] [Google Scholar]
- 23.Yamada, Y., T. Nihira, and S. Sakuda. 1997. Butyrolactone autoregulators, inducers of virginiamycin in Streptomyces virginiae: their structures, biosynthesis, receptor proteins, and induction of virginiamycin biosynthesis, p. 63-79. In W. R. Strolhl (ed.), Biotechnology of antibiotics. Marcel Dekker, Inc., New York, N.Y.
- 24.Yamada, Y., and T. Nihira. 1999. Microbial hormones and microbial chemical ecology. Compr. Nat. Prod. Chem. Pergamon 8:377-413. [Google Scholar]