Abstract
Integrated self-transmissible elements called conjugative transposons (CTns) are responsible for the transfer of antibiotic resistance genes in many different species of bacteria. One of the best characterized of these newly recognized elements is the Bacteroides CTn, CTnDOT. CTnDOT is thought to have a circular transfer intermediate that transfers to and integrates into the genome of the recipient cell. Previous investigations of the mechanism of CTnDOT integration have been hindered by the lack of an in vitro system for checking this model of integration and determining whether the CTnDOT integrase alone was sufficient to catalyze the integration reaction or whether host factors might be involved. We report here the development of an in vitro system in which a plasmid containing the joined ends of CTnDOT integrates into a plasmid carrying a CTnDOT target site. To develop this in vitro system, a His-tagged version of the integrase gene of CTnDOT was cloned and shown to be active in vivo. The protein produced by this construct was partially purified and then added to a reaction mixture that contained the joined ends of the circular form of CTnDOT and a plasmid carrying one of the CTnDOT target sites. Integration was demonstrated by using a fairly simple mixture of components, but integration was stimulated by a Bacteroides extract or by purified Escherichia coli integration host factor. The results of this study demonstrate both that the circular form of CTnDOT is the form that integrates into the target site and that host factors are involved in the integration process.
Bacteroides species are gram-negative anaerobes that account for about 25 to 30% of the bacterial population that normally resides in the human colon (14). Many Bacteroides strains carry large self-transmissible integrated elements that have been called conjugative transposons (CTns). In fact, a recent study has shown that over 80% of Bacteroides strains today contain at least one CTn and that the spread of resistance to tetracycline and erythromycin among Bacteroides strains has been mediated primarily by these elements (21). The best studied of the Bacteroides CTns is CTnDOT. Most of the CTns found in Bacteroides have proved to be closely related to this CTn.
Previous studies have shown that CTnDOT integrates site selectively into at least seven different target sites (attB) in the Bacteroides chromosome (3). There is a conserved 10-bp sequence (GTANNTTTGC) found at one end of CTnDOT that shares identity with a 10-bp sequence in all of the chromosomal attB sites (3). The deduced amino acid sequence of the CTnDOT integrase gene has low but significant homology (30 to 40%) to some members of the tyrosine recombinase family, of which the integrase of bacteriophage lambda is also a member. However, unlike the lambda integrase protein that makes staggered cuts 7 bp apart within the identical core sequences found in attB and attP, the CTnDOT Int protein appears to use a different mechanism. It makes staggered cuts 5 bp apart adjacent to the 10-bp conserved sequence (3). The sequences between the cleavage positions in the partner sites are not homologous. As a result, the cleavage and ligation reactions generate heteroduplex DNA regions at attL and attR. This distinct feature makes the recombination mechanism of the CTnDOT Int protein different from those of other members of the tyrosine recombinase family.
The integration of phage lambda requires integration host factor (IHF), whose role is to bend DNA in the attP site to facilitate formation of a higher-order nucleoprotein structure called the intasome. Previous studies of the integration of CTnDOT showed that the process was independent of homologous recombination (5). However, since nothing is known about possible Bacteroides host factors that might function in a manner analogous to IHF, it was not clear whether any factors other than the integrase encoded on CTnDOT were required for integration. We report here the development of an in vitro integration system that allows a plasmid carrying the CTnDOT joined ends to integrate into a second plasmid carrying one of the target sites into which CTnDOT integrates in vivo. This reaction requires the protein previously identified as the putative integrase and is stimulated by Escherichia coli IHF or by a Bacteroides extract.
MATERIALS AND METHODS
Reagents and enzymes.
Restriction endonucleases were obtained from New England Biolabs. Proteinase K, polyvinylalcohol (average molecular weight, 30,000 to 70,000), dimethyl sulfoxide (DMSO), spermidine, and spermine tetrahydrochloride were obtained from Sigma. Spermine tetrahydrochloride was dissolved in water to make a 100 mM solution, and the pH of the stock solution was adjusted to 6.8. Antibiotics were used at the following concentrations: ampicillin (Ap), 100 μg/ml; chloramphenicol (Cm), 20 μg/ml; rifampin (Rf), 10 μg/ml; kanamycin (Kn), 50 μg/ml.
Bacterial strains and plasmids and DNA manipulation.
The E. coli strain used for overexpression of the CTnDOT integrase protein was BL21(DE3)/plysS (Novagen). For initial development of the in vitro system, E. coli DH5αMCR (Gibco BRL) was used as the recipient. For mating experiments to confirm the activity of the tagged CTnDOT Int protein in vivo, the donor was E. coli pir+ strain BW19851 (11) and the recipient was E. coli pir-deficient strain EM24NR, which is the nalidixic acid- and rifampin-resistant RecA derivative of LE392 (18, 19). For overexpression of the CTnDOT Int protein, the plasmid pINT was constructed. Two primers (primer I, 5′-AATTAAGGCATATGAAGAGTACATTTTCAGTC; primer II, 5′-GTCTCGCTTCAAAGCTTCTCTTTATTAGATGG) were used to PCR amplify the int gene from chromosomal DNA of the Bacteroides thetaiotaomicron strain BT4107N3, which contains an integrated copy of CTnDOT. Primer I anneals to the 5′ end of the CTnDOT int gene and contains an NdeI site (CATATG, underlined in the sequence above). The ATG in this sequence is the likely start codon of the gene. Primer II anneals to the 3′ end of the int gene and includes a HindIII site (AAGCTT, underlined in the sequence above). It also includes the DNA sequence encoding the translation stop codon. The 1.3-kbp PCR product was digested with NdeI and HindIII and cloned into the expression vector pET28 (Novagen), which has the DNA sequence encoding the His6 tag upstream of its NdeI site. DNA sequence analysis confirmed that the pINT clone has the wild-type int gene linked to the DNA sequence encoding the His6 tag.
The plasmid pattB, which contained one of the CTnDOT integration sites in Bacteroides (attB), was constructed by cloning the attB PCR product into the PCR fragment cloning vector pGEM-T (Promega). The attB region (500 bp) was PCR amplified by using primers DLJ/U487F and DRJ/R2700R (3) and BT4001 chromosomal DNA. Another plasmid used in the in vitro integration assay, pattDOT, contained the 547-bp CTnDOT attachment site (attDOT) cloned into the ApaI and SstI sites of the pir-dependent plasmid pEPE (25) to generate pattDOT.
Two plasmids, pINT100 and pINT200, which contained the CTnDOT int gene under the control of the Ptac promoter in the pBR322-based plasmid pCKR101 (10), were constructed by cloning the CTnDOT int gene, which was amplified by using primer III (5′-TTTGAATTCGAAGGATTAAGGCATAT GAAGAGTACATTTCAG) and primer II. Primer III has an EcoRI site (GAATTC, underlined in the sequence above) at one end and also contains an E. coli ribosome-binding site (GAAGGA, boldface in the sequence above). The 1.3-kb PCR product was cloned into the EcoRI and HindIII sites of pCKR101 to form pINT100. The plasmid pINT was digested with XbaI and HindIII, and the DNA fragment containing the int gene was cloned into the XbaI and HindIII sites in pCKR101 to form pINT200. This construct placed the His6 tag at the N terminus of the CTnDOT int gene. The int genes of both pINT100 and pINT200 were sequenced to confirm that there were no mutations in the int gene. Both pINT100 and pINT200 have the same Ptac promoter and the same ribosome-binding site. The only difference between the two plasmids is that the pINT200 has the DNA sequence from pINT that encodes the His6 tag at the N terminus of the Int protein.
The two plasmids (pINT101 and pINT201) used in the in vivo integration assay in E. coli had the CTnDOT integration site in Bacteroides (attB) cloned into pINT100 and pINT200, respectively. To construct pINT101 and pINT201, the NcoI-PstI-digested attB region from the plasmid pattB was cloned into the NcoI and PstI sites of pMTL20 to provide flanking restriction sites for further subcloning (2). The resultant plasmid was then digested with HindIII and AatII, and the DNA fragment containing the attB region was cloned into the HindIII and AatII sites of pINT100 and pINT200 to form pINT101 and pINT201, respectively.
In vivo integration assay in E. coli.
The mating procedure was done as previously described (20). The E. coli donor was BW19851, which has both the RP4 transfer functions and pir gene integrated in its chromosome carrying the mobilizable attDOT vector, pattDOT. The base plasmid, pEPE (25), is pir dependent and carries a chloramphenicol resistance marker and the oriT of RP4. The pir mutant E. coli EM24NR recipient strains carried pINT101 or pINT201. The recipients were grown with and without 1 mM IPTG (isopropyl-1-thio-β-d-galactoside) for induction of Ptac. The recipients were ampicillin resistant due to a gene carried on the plasmid and rifampin resistant due to a chromosomal mutation. Ampicillin, rifampin, and chloramphenicol were used to select transconjugants, and the transconjugants were analyzed to verify the presence of cointegrated plasmids formed by the Int-mediated site-specific recombination between attB and attDOT.
Purification of the CTnDOT Int protein.
The plasmid pINT (Knr) was introduced into E. coli BL21(DE3)/plysS (Cmr) by transformation. Cultures were grown at 30°C overnight and subcultured (1:100) into 400 ml of fresh Luria-Bertani broth plus the antibiotics kanamycin and chloramphenicol. When the absorbance (optical density at 600 nm) of the culture reached 0.5, expression of the Int protein was induced by the addition of IPTG to a final concentration of 1 mM. After induction at 30°C for 5 h, cells were harvested by centrifugation. The cells were then resuspended in 40 ml of lysis buffer (50 mM Na2HPO4 · NaH2PO4 [pH 8.0], 300 mM NaCl, 10 mM imidazole) with the addition of lysozyme (1 mg/ml) and the protease inhibitor phenylmethylsulfonyl fluoride (50 μg/ml). The cells were sonicated and clarified by centrifugation (14,000 × g for 30 min at 4°C). The supernatant fraction was mixed with 4 ml of nickel-nitrilotriacetic acid resin (Qiagen), incubated at 4°C for 1 h, and then applied to a column (Bio-Rad) to collect flowthrough. The resin was then washed with 40 ml of wash buffer (lysis buffer with 20 mM imidazole) and 8 ml of E50 elution buffer (lysis buffer with 50 mM imidazole). The Int protein was eluted by successive washing with 4-ml aliquots of elution buffers E100, E150, E200, and E250, which contained imidazole concentrations of 100, 150, 200, and 250 mM, respectively. Samples were electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel to detect the Int protein. The fractions containing the highest amount of Int protein were combined and dialyzed at 4°C overnight against a buffer containing 50 mM Tris-HCl (pH 8.0), 1 mM dithiothreitol (DTT), 1 mM EDTA, 50 mM NaCl, and 40% glycerol.
In vitro integration assay. (i) Components of the reaction mixtures.
The final volume of each reaction mixture was 40 μl. The reaction buffer contained 20 mM Tris-HCl (pH 7.4), 5 mM DTT, 0.1 mg of tRNA/ml, 50 mM KCl, 5% polyvinylalcohol, 50 μg of bovine serum albumin/ml, and 1% glycerol. For a typical reaction, 3 μl of pattB (approximately 0.375 μg of DNA; 0.16 pmol) and 5 μl of pattDOT (approximately 0.085 μg of DNA; 0.02 pmol) were mixed with 1 μl of CTnDOT Int protein (approximately 0.16 μg/μl; 3.2 pmol) and 1 μl of Bacteroides crude extract or diluted E. coli host factor IHF. Two different IHF samples were used for in vitro reactions. In most cases, we used IHF prepared in our laboratory according to the procedure of Nash and Robertson (16). The sample had a concentration of approximately 0.48 μg/μl and was estimated to be 90% pure, as judged by Coomassie stain analysis of SDS-PAGE gels. Another IHF sample used was the kind gift of Steve Goodman (University of Southern California, Los Angeles). It had a concentration of approximately 1 μg/μl and was greater than 95% pure. HU, another E. coli protein used in the in vitro reactions, was a kind gift of Anca Segall. It is estimated to have a concentration of 0.85 μg/μl.
Bacteroides crude extracts were made as follows. Cells from 100 ml of an overnight BT4001 culture were harvested and resuspended in 6 ml of lysis buffer (50 mM Tris-HCl [pH 7.4], 10% sucrose). Cells were broken by sonication and clarified by centrifugation at 14,000 × g for 30 min. The supernatant was concentrated to a volume of about 2 ml by using a YM-3 centrifugal filter device (Millipore; molecular weight cutoff, 3,000). In some cases, the supernatant was heated at 95°C for 10 min and then cooled to room temperature for 30 min. The sample was then centrifuged for 30 min, and the supernatant was concentrated to about 2 ml as described above.
(ii) Reaction conditions.
The reaction mixtures were preincubated for 1 h at 4°C and then switched to 37°C incubation for up to 6 h. After the reaction, spermine precipitation (9) was used to recover the DNA. For each in vitro reaction, the total volume of the reaction mixture was brought to 500 μl by adding the appropriate amount of water, and spermine was added to a final concentration of 8 mM. After vortexing for 1 min, the solutions were left on ice for 2 h. The solutions were then centrifuged for 10 min at 12,000 rpm in an Eppendorf microcentrifuge. After the supernatant was carefully removed without disturbing the DNA pellet, 500 μl of extraction buffer (75% ethanol, 0.3 M NaAc, 0.01 M MgAc) was added to the DNA pellet. The samples were vortexed and left at 4°C overnight. After centrifugation for 10 min, the supernatant was removed and the DNA pellet was vacuum dried. The DNA was then resuspended in 40 μl of water and drop dialyzed against double-distilled water for 2 h on a nitrocellulose dialysis filter (Millipore; pore size, 0.025 μm).
Two approaches were used to detect integration events. First, DNA from the incubation mixtures was electroporated into the E. coli pir-deficient strain DH5αMCR and electroporants were selected on Luria-Bertani plates supplemented with ampicillin or ampicillin and chloramphenicol. The ratio of Apr Cmr colonies to Apr colonies was used to represent the integration frequency for the in vitro reaction. In a second approach, integration products were detected by Southern blot analysis. The dialyzed DNA mixtures were digested with EcoRI (10 U/reaction mixture) and BamHI (10 U/reaction mixture) at 37°C overnight. The DNA mixtures were then electrophoresed on a 1% agarose gel at constant voltage (35 mV) overnight. In some cases, the EcoRI-BamHI-digested DNA mixtures were extracted with phenol or incubated with proteinase K (200 μg/ml in a buffer containing 10 mM Tris-HCl [pH 7.8], 5 mM EDTA, and 0.5% SDS) at 37°C for 1 h. Southern blot analysis was done as described previously (22), and the 574-bp attDOT fragment was used as a fluorescence-labeled probe.
Mass spectrometry and DNA sequencing.
To determine the molecular mass of the purified CTnDOT Int protein, the E250 elution fraction from the above purification procedure was dialyzed against double-distilled water at 4°C overnight for mass spectrometry. Matrix-assisted laser desorption ionization (MALDI) mass spectrometry was performed at the University of Illinois Protein Sciences Facility with a Voyager DE-STR MALDI mass spectrometer.
Cointegrate plasmids from the in vivo and in vitro integration assays, as well as the attL and attR PCR products amplified by using mixtures of in vitro reactions as a template, were sequenced by using the primer DRJ/R2242F for attR and DLJ/U487F (3) for attL. DNA sequencing was performed at the University of Illinois Biotechnology Genetic Engineering facility with an Applied Biosystems model 373A version 2.0.1S dye terminator automated sequencer.
RESULTS
In vivo integration assay in E. coli.
Since E. coli and Bacteroides species are so distant from each other genetically, it was possible that the CTnDOT integrase protein that was obtained by overproduction in E. coli was not active. Moreover, even if the wild-type protein was active, the His6-tagged version of the protein might not be active. Accordingly, we first tested the integration efficiency of the pattDOT plasmid in a strain of E. coli that carried a plasmid containing the Bacteroides CTnDOT target site plus the untagged form of the CTnDOT int gene under control of the Ptac promoter (pINT101) (Fig. 1). The plasmid pattDOT is pir dependent, and it cannot replicate in the pir-deficient recipient used in this assay. Thus, in order for the recipient to become chloramphenicol resistant (Cmr), the incoming pattDOT must integrate into pINT101. The frequency of the Cmr Rfr Apr transconjugants was 10−4/recipient (Table 1). This frequency increased to 4 × 10−3 when the recipient was grown in IPTG to increase the concentration of Int (Table 1). The data indicate that the CTnDOT Int protein expressed in E. coli was still capable of mediating the integration reaction and that the reaction frequency was dependent upon the Int concentration.
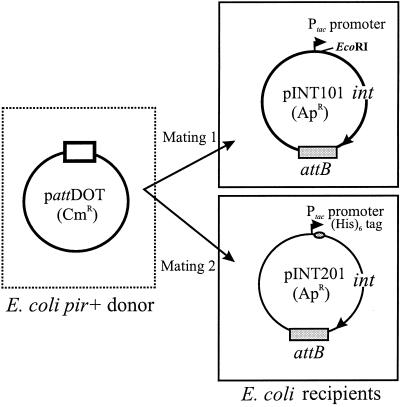
FIG. 1.
In vivo integration assay in E. coli. Two different mating experiments were done to confirm that the His6-tagged CTnDOT Int protein expressed in E. coli had all of the functions necessary to perform site-specific recombination in vivo. In the first mating, the plasmid pattDOT (Cmr, pir dependent, containing CTnDOT attachment site attDOT) was mobilized from the donor E. coli pir+ strain BW19851 into the recipient E. coli pir-deficient strain EM24NR carrying the plasmid pINT101 (Apr). The pINT101 plasmid contains the wild-type int gene (under the control of Ptac promoter) from CTnDOT and one of its chromosomal target sites (attB). The Cmr Rfr Apr transconjugants (rifampin resistance from the recipient strain EM24NR) were derived from the cointegrate plasmid due to site-specific recombination between the attDOT and attB sites. The reaction was mediated by the wild-type Int protein expressed from pINT101 in the recipient. The second mating had the same donor and a recipient that carried pINT201. pINT201 has the same attB site and expresses the His6-tagged Int protein. The Cmr Rfr Apr transconjugants were obtained at a similar frequency (10−4/recipient; see Results).
TABLE 1.
Frequencies of in vivo integration in E. colia
| Plasmid carried by: | Frequencyc | |
|---|---|---|
| Donor | Recipientb | |
| pattDOT | pINT101 | 1 × 10−4 |
| pINT101 plus 1 mM IPTG | 4 × 10−3 | |
| pattDOT | pINT201 | 5 × 10−4 |
| pINT201 plus 1 mM IPTG | 1 × 10−2 | |
| pEPE | pINT101d | <10−9 |
| pINT201d | <10−9 | |
The in vivo integration assay in E. coli is described in Materials and Methods. The donor strains are E. coli BW19851 carrying pattDOT or pEPE (25). The recipient strains are E. coli EM24NR carrying pINT101 or pINT201. pINT101 contains a wild-type CTnDOT int gene, while pINT201 contains the CTnDOT int gene behind the DNA sequences encoding a His6 tag. In addition, both plasmids have one of the Bacteroides CTnDOT target sites, attB.
The recipients were grown with and without IPTG induction of the int gene behind Ptac.
The frequency is the number of Cmr Rfr Apr transconjugants per recipient at the end of the mating. The frequency is the average of the results of three experiments.
Results were the same with or without IPTG.
We next tested plasmid pINT201, which was identical to pINT101 except that it carried the His6-tagged form of the int gene. The frequency with which Cmr Rfr Apr transconjugants were obtained was even higher than that seen with the wild-type int gene (5 × 10−4/recipient; Table 1). Again, the frequency increased about 20-fold when the recipient was grown in 1 mM IPTG to increase the concentration of the His6-tagged Int. Control experiments with the donor strain containing pEPE (parental plasmid of pattDOT) yielded no Cmr Rfr Apr transconjugants (Table 1). Cointegrate plasmids from each of the two types of mating experiments were analyzed to determine where the incoming pattDOT plasmid had integrated. In both cases, the plasmid integrated into the attB site via the joined ends of CTnDOT.
Overexpression and purification of CTnDOT Int protein.
Since the His6-tagged Int protein was clearly active in E. coli, this protein was overproduced in E. coli and purified by passage over a nickel-nitrilotriacetic acid column described in Materials and Methods for use in the in vitro assay. The majority of the Int protein was found in the fractions with imidazole concentrations of 150, 200, and 250 mM (E150, E200, and E250 fractions) (Fig. 2). The molecular mass of the protein appeared to be about 47 kDa on an SDS-PAGE gel, which is less than its real molecular mass (50 kDa) as determined by MALDI mass spectrometry (data not shown). After dialysis, all three fractions (E150, E200, and E250) showed integrase activity in the in vitro integration assay. The three fractions were combined.
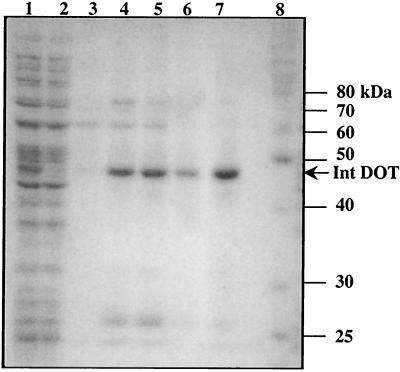
FIG. 2.
SDS-PAGE to monitor the purification of the His6-tagged CTnDOT Int protein. Lane 1 contains crude extract of the parental strain BL21(DE3)/pLysS with the plasmid pINT. Lane 2 contains crude extract of the parental strain BL21(DE3)/pLysS with the pET28 vector. Both strains were induced with IPTG at a concentration of 1 mM for 5 h. A clear strong band at about 47 kDa is seen in lane 1 but is absent from lane 2. After serial washing steps, the protein was eluted by aliquots of buffer containing 100 (E100), 150 (E150), 200 (E200), or 250 (E250) mM imidazole in the elution buffer. The E100, E150, E200, and E250 fractions are in lanes 3 to 6, respectively. Lane 7 contains E250 elution fraction after it was dialyzed (see Materials and Methods). The majority of the CTnDOT Int protein was present in the E150, E200, and E250 fractions at a position corresponding to a molecular mass of about 47 kDa. It migrated faster than its real molecular mass (50 kDa, including the His6 tag) as determined by MALDI mass spectrometry. Lane 8 contains protein molecular mass standard markers (Benchmark; Gibco).
CTnDOT Int protein catalyzes in vitro integration and requires a Bacteroides host factor(s).
The partially purified CTnDOT Int protein preparation was added to reaction mixtures containing the two plasmids pattDOT(Cmr) and pattB(Apr). An integration event that combined those two plasmids would form a cointegrate plasmid that could confer an Apr Cmr phenotype after electroporation of the reaction mixtures into an E. coli pir-deficient strain, e.g., DH5αMCR. The integration frequency was calculated as the ratio of Apr Cmr colonies to Apr colonies. When all of the reaction mixture components were combined and then electroporated into E. coli, Apr Cmr colonies were detected, but only at a relatively low frequency (10−7 to 10−6; Table 2, reaction mixture 1). The addition of Bacteroides crude extract stimulated the reaction by at least 100-fold (10−5 to 10−4; Table 2, reaction mixture 2). This indicates that site-specific recombination mediated by CTnDOT Int protein requires a Bacteroides-encoded host factor(s). After being heated at 95°C for 10 min, a Bacteroides crude extract could still stimulate the reaction as well as the original crude extract (data not shown). Negative control experiments were done with the following two-plasmid systems: pattDOT and pGEM-T (parental plasmid for pattB, used as a no attB control), and pattB and pEPE (parental plasmid for pattDOT, used as a no attDOT control). Either Bacteroides crude extract or E. coli IHF (see below) was included in the control reaction mixes. No Apr Cmr colonies were detected in either case after electroporation (Table 2, reaction mixtures 5 and 6). A third control experiment was done similarly without the addition of CTnDOT Int protein. Again, no Apr Cmr colonies were observed (Table 2, reaction mixture 4).
TABLE 2.
Frequencies of the in vitro integration assaya
| Reaction mixture | Presence (+) or absence (−) of indicated substance
|
Frequencyb | ||||
|---|---|---|---|---|---|---|
| attB | attDOT | Int | Bacteroides crude extractc | IHFc | ||
| Experiments | ||||||
| 1 | + | + | + | − | − | 10−7-10−6 |
| 2 | + | + | + | + | − | 10−5-10−4 |
| 3 | + | + | + | − | + | 10−3-10−2 |
| Controls | ||||||
| 4 | + | + | − | − | + | <10−7 |
| 5 | − | + | + | − | + | <10−7 |
| 6 | + | − | + | − | + | <10−7 |
The in vitro integration assays were performed as described in Materials and Methods. The integration frequency is expressed as the ratio of Apr Cmr colonies to Apr colonies from the electroporation method.
The frequency of integration decreases by about 10-fold after phenol extraction, as shown by the decrease from 10−3 to 10−4 after phenol extraction for reaction with IHF.
Either Bacteroides crude extract or IHF was added to the control reaction mixtures; both additives gave the same results.
To confirm that the plasmids in the Apr Cmr colonies were formed by site-specific recombination instead of homologous or illegitimate recombination, the attR and attL sites of six cointegrate plasmids were sequenced. They all had the correct attR and attL junctions. This indicates that the recombination was site specific and occurred between the attB and attDOT sites. We were also concerned that the integration reaction might be occurring after electroporation of the DNA into cells rather than in the reaction mixture, because Int protein bound to DNA could enter the cells and promote recombination inside the cells. To check this possibility, the reaction mixture was extracted with phenol to inactivate any Int protein that was bound to the DNA. After electroporation, Apr Cmr colonies were still observed, although the integration frequency was about 10-fold lower than that of samples that were not extracted with phenol. This indicates that a significant amount of cointegrate plasmids are formed in the in vitro reactions. The lower integration frequency for phenol-treated samples is possibly due to loss of the DNA that was covalently bound by Int protein, thus lowering the concentration of DNA in the aqueous fraction.
Coupling sequences for the in vitro integration assay.
When the conjugative transposon CTnDOT excises itself from the Bacteroides chromosome, the Int protein presumably makes staggered cuts 5 bp adjacent to the left and right ends. The 5-base overhang from one end is then joined to the overhang of the other end to form a circular intermediate that has 5 bp of heteroduplex DNA in the middle of the two ends. The 5-bp DNA heteroduplexes, called coupling sequences, are resolved after excision. After conjugal transfer of the CTnDOT into a recipient, the single-stranded DNA recircularizes and replicates into a double-stranded circular intermediate carrying the resolved coupling sequences from the donor chromosome. In the following integration step, staggered cuts are made again on the coupling sequences in the circular intermediate as well as the potential coupling sequences adjacent to a target site in the recipient chromosome. After integration, two DNA heteroduplex regions are formed (Fig. 3) at the left (attL) and right (attR) junctions. The DNA heteroduplexes are believed to be resolved later by either DNA replication or DNA mismatch repair systems. As a result, the original coupling sequences from the donor chromosome are located in either attL or attR in the recipient chromosome, with an equal chance that one sequence of the other is present (Fig. 3).
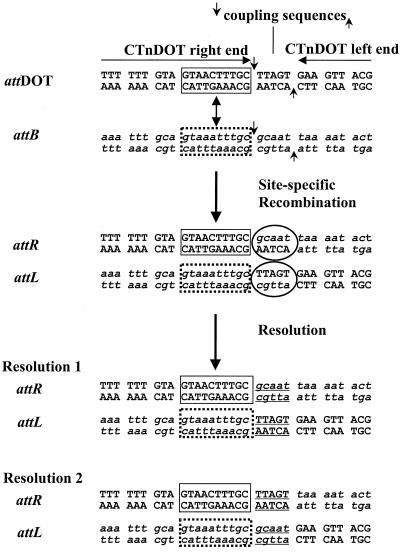
FIG. 3.
Coupling sequences for the in vitro integration assay. The donor DNA pattDOT contains the CTnDOT joined ends (left and right end joined together) with the coupling sequence TTAGT in the middle. The target DNA pattB contains one of the CTnDOT target sites in Bacteroides (attB) and the potential coupling sequence GCAAT. The 10-bp conserved sequence (GTANNTTTGC) between attDOT and attB is indicated in the boxed regions. During site-specific recombination mediated by the CTnDOT Int protein, 5-base staggered cuts were made at the borders of the coupling sequences (four DNA nick sites indicated by arrows), and DNA heteroduplex regions (indicated by circles) were formed in the attR and attL regions as a result of strand exchange and ligation. When the reaction mixtures were used as a template for PCR to amplify the attR or attL region, DNA heteroduplexes were resolved in PCRs. Sequencing data of the PCR products show that two different coupling sequences (GCAAT and TTAGT) were seen in the attR and attL regions.
If the CTnDOT Int protein forms expected recombination products during the in vitro integration assay, one would predict that the staggered cuts, strand exchange, and ligation reactions still make the heteroduplex coupling sequences in both the attL and attR regions (Fig. 3). To test this, reaction mixtures were directly used as templates in PCRs to amplify the attL and attR regions. Any DNA heteroduplex regions would be resolved by PCR amplification, but the resolved regions should have mixtures of each heteroduplex sequence, because both strands were used as the template for the PCRs. The PCR products of attL and attR were directly sequenced as described in Materials and Methods. Not only were the expected sequences of attL or attR obtained, but also a clear mixture of TTAGT (coupling sequences from donor DNA pattDOT; Fig. 3) and GCAAT (coupling sequences from target DNA pattB; Fig. 3) was seen in the sequencing chromatographs of both the attL and attR PCR products. This finding shows that staggered cuts were made and heteroduplexes were formed during the in vitro reactions. Since full-length PCR products were obtained from both attL and attR, it indicates that some recombination reactions went through the DNA cleavage, strand exchange, and religation steps.
The attL and attR PCR products were also cloned into the vector pGEM-T, and over 10 individual clones were sequenced. The coupling sequences (TTAGT) from the donor DNA pattDOT could be found in both attL and attR clones at a similar frequency. This further confirmed that the coupling sequence from donor DNA could be relocated in either the attL or attR regions after integration. All these results strongly suggest that during the in vitro integration assay, the CTnDOT Int protein makes staggered cuts to generate and relocate coupling sequences as it does in vivo.
E. coli IHF can substitute for Bacteroides host factor(s) to stimulate the integration frequency.
The E. coli host factors IHF and HU are known to be able to promote recombination in other systems, and they are also heat stable. Thus, purified IHF or HU was added into the in vitro integration assay to replace the Bacteroides crude extract. In most cases, an IHF preparation that was about 90% pure and prepared in our laboratory was used (see Materials and Methods). Another IHF sample used (over 95% pure) was the gift of Steve Goodman. Both IHF samples significantly stimulated the reaction (to 10−3 to 10−2; Table 2, reaction mixture 3). The IHF did not alter the negative results seen in the control samples in reaction mixtures 4 to 6 of Table 2. Since the two IHF preparations gave essentially identical results, these experiments suggest that IHF is the protein in the preparations that stimulates the in vitro reaction. However, we could not unequivocally rule out the possibility that a low level of host protein contaminant present in both IHF samples is required for the reaction.
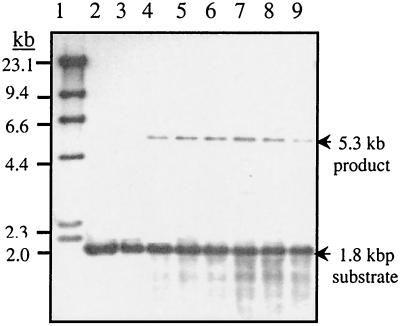
With IHF present in the reactions, we were able to detect an integration product by Southern blot analysis with fluorescence-labeled 574-bp attDOT DNA as a probe (Fig. 4, lanes 1 to 5). The reaction mixture was digested with EcoRI and BamHI and then run on an agarose gel. This enzyme combination cuts the plasmid pattDOT to produce a 1.8-kb DNA fragment containing attDOT. If site-specific recombination occurs and pattDOT is integrated into the plasmid pattB (3.5 kb) to form a cointegrate plasmid, an extra band (5.3 kb) is produced. When high concentrations of IHF (>0.25 μg/reaction mixture) were used, the integration reaction was less efficient, as shown in Fig. 4, lane 1. Excess IHF also inhibits site-specific recombination in the lambda in vitro system (23). When the IHF concentration was too low (<0.008 μg/reaction mixture), no significant integration product was observed by Southern blotting (Fig. 4, lanes 6 and 7).
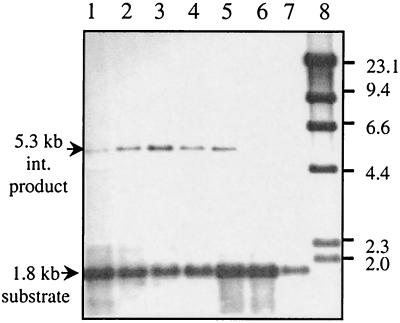
FIG. 4.
E. coli host factor IHF can stimulate the in vitro integration reactions. Different concentrations of IHF (lane 1, 0.25 μg; lane 2, 0.125 μg; lane 3, 0.063 μg; lane 4, 0.032 μg; lane 5, 0.016 μg; lane 6, 0.008 μg; lane 7, 0.004 μg) were used in each reaction mixture. The products from the reaction mixtures were digested by EcoRI and BamHI. The 1.8-kb DNA substrate is the digested DNA fragment containing attDOT from the plasmid pattDOT. The 5.3-kb integration product (lanes 1 to 5) is the digested DNA fragment containing attDOT from the final reaction product cointegrate plasmid. The integration product is not seen when the concentration of IHF is too low (<0.008 μg/reaction mixture; lanes 6 and 7). Lane 8, molecular size markers.
Another purified E. coli host factor, HU, was also tested in the reaction. Unlike IHF, very low levels of integration products were observed by Southern blot analysis with a wide range of HU concentrations (data not shown). This suggests that HU cannot substitute for the Bacteroides host factor(s) in stimulating the integration reaction. However, it is possible that the conditions used for the reaction might not be optimal for HU activity.
The frequency of the in vitro integration assay is also dependent on the quantity of Int and the molar ratio of Int to IHF.
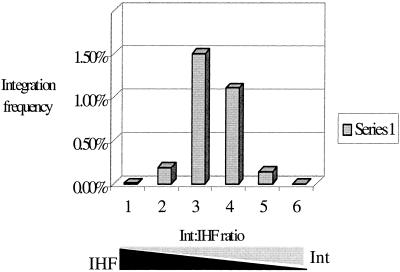
When IHF was maintained at a constant level while the CTnDOT Int concentration was varied in the in vitro reactions, the integration frequency increased as the Int concentration increased (data not shown). This suggests that integration is dependent on the quantity of Int protein added to the reaction mixture. We also determined the effect of different molar ratios of Int to IHF. The results are shown in Fig. 5. When the Int-to-IHF molar ratio was between 1:1.8 (lane 3) and 2.2:1 (lane 4), the integration frequency rose to the level of 10−2 in the electroporation assay, the highest frequency observed so far. When the Int to IHF ratio was over 35.6:1 (lane 6) or below 1:28 (lane 1), the integration frequency went down to a barely detectable level of <10−6 in both cases.
FIG. 5.
The effect of the molar ratio of Int to IHF on the in vitro integration frequency. The integration frequency was determined as the percentage of Apr Cmr colonies relative to Apr colonies. Different molar ratios of Int to IHF were used in the standard reactions. Lane 1, 0.02 μg of Int (0.4 pmol) and 0.25 μg of IHF (11.5 pmol); lane 2, 0.04 μg of Int (0.8 pmol) and 0.125 μg of IHF (5.75 pmol); lane 3, 0.08 μg of Int (1.6 pmol) and 0.063 μg of IHF (2.88 pmol); lane 4, 0.16 μg of Int (3.2 pmol) and 0.032 μg of IHF (1.44 pmol); lane 5, 0.32 μg of Int (6.4 pmol) and 0.016 μg of IHF (0.072 pmol); lane 6, 0.64 μg of Int (12.8 pmol) and 0.008 μg of IHF (0.36 pmol). The molar ratios of Int to IHF in lanes 1 to 6 were therefore 1:28, 1:7, 1:1.8, 2.2:1, 8.9:1, and 35.6:1, respectively.
Time course of the in vitro integration assay.
We also examined integration as a function of time. After incubation at 37°C for 1 h, the integration product was clearly seen (Fig. 6, lane 4), and the intensity of the product band was highest at 6 h of incubation (Fig. 6, lane 7). After that, the intensity of the band decreased. A possible explanation is that the final product is somehow unstable. However, several small DNA bands accumulated as the incubation time increased (Fig. 6, lanes 7, 8, and 9). They were not present in the control experiments after 6 h of incubation (Fig. 6, lanes 2 and 3). The bands were still present even after the reaction mixtures were treated with proteinase K or extracted with phenol (data not shown) to release proteins bound to DNA. It is not likely that the products are due to an artifact of the use of spermine in the DNA clean-up steps, because all of the samples, including the controls, were processed in the same way. These bands might be the degradation products of contaminating nuclease, recombination intermediates from multiple-step reactions, or hairpin structures formed by strand cleavage and intrastrand ligation. The origin of these products is currently under investigation.
FIG. 6.
Time course of the in vitro integration assay. Lane 1 contains λ HindIII DNA standards. Lane 2 contains a reaction mixture lacking Int after 6 h of incubation at 37°C. Lane 3 contains a reaction mixture lacking IHF after 6 h of incubation at 37°C. Lanes 4 to 9 contain reaction mixtures with incubation times at 37°C of 1, 2, 4, 6, and 8 h and overnight, respectively. The 5.3-kb integration product was seen clearly after incubation for 1 h (lane 4), and its intensity peaked after incubation for 6 h (lane 7). With the increase in incubation time, there were some other bands that accumulated at the bottom of the gel. Notice that these weak bands are not present in the two controls (lanes 2 and 3).
Optimal condition for the in vitro integration assay.
In a standard in vitro integration reaction, the reaction buffer contained 20 mM Tris-HCl (pH 7.4), 5 mM DTT, 0.1 mg of tRNA/ml, 50 mM KCl, 5% polyvinylalcohol, 50 μg of bovine serum albumin/ml, and 1% glycerol. Other components, such as pattDOT, pattB, CTnDOT Int protein, and Bacteroides crude extract or E. coli IHF, were essential for the reaction. Based on the standard reaction, we tested whether the addition or elimination of certain components, such as polyvinylalcohol, Mg2+, spermidine, ATP, or DMSO, affects the reaction. These reagents are known to affect recombination reactions in other systems. Surprisingly, these reagents did not have significant effects on the integration frequency in the CTnDOT in vitro system. The results are summarized in Table 3. Polyvinylalcohol is not essential, but the elimination of it from the reaction decreased the integration frequency by about twofold compared to that of the standard reaction. Addition of DMSO to a concentration of 10% did not stimulate the reaction. The reaction also did not require Mg2+ or an exogenous energy source such as ATP. Interestingly, the addition of spermidine (2 mM) decreased the integration frequency by about 10-fold. In contrast, some in vitro recombination systems, such as lambda (15) and Tn5 (8), are stimulated by the inclusion of spermidine in the reactions.
TABLE 3.
Conditions for the in vitro integration assay
| Conditiona | Integration frequency (10−3)b |
|---|---|
| Standard | 3.6 |
| −Polyvinylalcohol | 1.2 |
| +Mg2+ | 1.6 |
| +Spermidine | 0.4 |
| +ATP | 2.7 |
| +DMSO | 2.6 |
The standard reaction conditions are described in Materials and Methods. The E. coli host factor IHE was used in all of the reactions. Elimination of any component from the standard conditions is indicated with a “−” and addition of any component to the standard conditions is indicated with a “+”. The final concentrations for added components were as follows: Mg2+, 5 mM; spermidine, 2 mM; ATP, 2 mM; and DMSO, 10%.
The integration frequency is expressed as the ratio of Apr Cmr colonies to Apr colonies from the electroporation method (see Materials and Methods). Each value was the average of the results of four individual reactions.
DISCUSSION
We have described here the purification of the CTnDOT Int protein and its use in an in vitro integration system that simulates the integration of CTnDOT in vivo in Bacteroides hosts. This is the first Int protein from a Bacteroides conjugative transposon to be purified in E. coli and confirmed to be active by an in vitro enzymatic assay. It is perhaps surprising that although Bacteroides spp. are not closely related to E. coli, the activity of E. coli-expressed CTnDOT Int protein was nonetheless functional and did not require a specific modification from the Bacteroides host. This finding may indicate a feature of the CTns that gives them such a broad host range, namely, their ability to integrate in a diverse set of bacterial hosts.
Previously, we tried to understand why the wild-type Bacteroides conjugative transposon CTnDOT tagged with an antibiotic gene that is expressed in E. coli did not integrate into the E. coli chromosome (B. J. Paszkiet, unpublished data). We constructed several mini-elements that contained the attDOT site and the CTnDOT int gene. All of them integrated into the E. coli chromosome at a low frequency (10−7 to 10−6 transconjugants per recipient), indicating that the expression of CTnDOT protein in E. coli from its native promoter is poor.
In this work, we constructed an in vivo integration assay in E. coli by using plasmids with the CTnDOT int gene behind the E. coli Ptac promoter. Without inducing Int expression, the integration frequency was still 10−5 to 10−4 transconjugants per recipient. With the induction of Int expression by 1 mM IPTG, however, the integration frequency increased to up to 10−3 to 10−2 transconjugants per recipient. This result suggests that expression of Int protein is one of the important limitations that determine the integration frequency of the mini-element (or even the wild-type element) in E. coli.
Another possibility for the low integration frequency of the mini-elements or the whole CTnDOT element in E. coli is that the host factors from E. coli cannot work as well as Bacteroides host factor(s) to stimulate integration events. From the in vivo and in vitro integration assays described here, it is clear that the E. coli host factor IHF can at least partially complement the function of the Bacteroides host factor(s). Preliminary results from gel shift assays (A. Gupta, unpublished data) also show that purified CTnDOT Int protein can shift radiolabeled attDOT DNA only if E. coli IHF protein is included. This suggests that E. coli IHF is able to facilitate the binding of CTnDOT Int to its putative binding sites in attDOT to form a stable protein-DNA complex that might be important for integration. Inspection of the attDOT sequence did not reveal any obvious candidates for an IHF binding site. Genomic sequences for the B. thetaiotaomicron 5482A (ATCC 29148) strain used in this study and for Bacteroides fragilis 638, which has been used in studies of Bacteroides CTns and other integrated mobilizable elements, are becoming available. Currently there appear to be several Bacteroides sequences related to ihfA and ihfB, although there is significant amino acid sequence divergence. This observation might indicate that IHF proteins made by diverse host bacteria can function interchangeably in site-specific recombination.
Another E. coli host factor, HU, is a nonspecific DNA-binding protein that has been shown to be able to replace IHF in the assembly of a functional attL intasome for lambda recombination (7). HU was also tested in this study in the in vitro system, and only low levels of integration products were detected. Thus, HU may not be as evolutionarily universal as IHF in its ability to function. Based on the preliminary genome sequences of B. thetaiotaomicron 5482, we have already identified two putative IHF subunit homologues and two HU subunit homologues. We are in the process of isolating these genes and purifying these proteins to use in the in vitro integration system.
Components such as DMSO, spermidine, and Mg2+ were able to stimulate the frequency of many in vitro recombination systems. DMSO is believed to be able to decrease the requirements for the formation of the stable protein-DNA complexes for Mu (6, 13) and IS911 (24) in vitro systems. Spermidine was able to stimulate lambda (15), Tn5 (8), and Tn10 (1) in vitro reactions. In the presence of Mg2+, MuA protein binds to Mu end sequences and the reaction efficiently undergoes the subsequent DNA cleavage and strand exchange steps (12). However, the CTnDOT in vitro reaction does not require DMSO, spermidine, or Mg2+.
CTnDOT Int protein belongs to the tyrosine recombinase family and has three out of the four conserved Arg-His-Arg-Tyr residues of this family (3). Lambda integrase is also a member of this family, and a variety of experiments have shown that DNA cleavage, strand exchange, and religation reactions during lambda integration are coordinated. After lambda Int binds to donor and target DNA, the conserved residue Tyr-342 serves as a nucleophile to attack the phosphodiester bond at the junction of the core-type site and the overlap region. Int forms a covalent phosphotyrosyl bond with the 3′ end of the DNA at the cleavage site, and this is believed to conserve the energy required for the strand exchange step (17). A similar nucleophilic attack occurs and forms another phosphotyrosyl bond in the partner site. The reaction proceeds when the free 5′-OH groups at the cleavage sites in the top strands attack the phosphotyrosyl linkage in the partner site to ligate the DNA and form a Holliday junction. Another round of strand exchange and religation occurs for the bottom strands to complete the lambda integration reaction. The detailed mechanism of the CTnDOT integration reaction is not known. However, the fact that the CTnDOT Int has a conserved Tyr residue together with the finding that no external energy source, such as ATP, is required for the in vitro reactions suggests that CTnDOT Int has a similar mechanism for DNA cleavage and the subsequent strand exchange step. In other words, it is possible that the CTnDOT Int might be able to use the conserved Tyr to form the phosphotyrosyl bond and conserve the energy required for the strand exchange step. The correct attL and attR junctions could be amplified by PCR after the reaction mixtures from in vitro reactions were used as templates. This suggests that all three steps, DNA cleavage, strand exchange, and ligation, occur in the in vitro reactions. In the future, it will be informative to identify different intermediates from the above three steps, because the study of these intermediates will provide insights into understanding the molecular basis of CTnDOT recombination.
Coupling sequences are a unique feature of CTnDOT recombination. This is true of the gram-positive CTn, Tn916, as well as CTnDOT. From the in vitro work described here, it is clear that the formation of coupling sequences does not require any proteins from CTnDOT other than Int. The Int protein presumably recognizes a 10-bp conserved sequence (GTANNTTTGC) in both attDOT and attB and makes 5-bp staggered cuts adjacent to the conserved sequences. This is reminiscent of some restriction endonucleases that can recognize certain DNA sequences but cut DNA outside of the recognition sites. However, unlike restriction endonucleases, the CTnDOT Int belongs to a recombinase family that can perform DNA cleavage, strand exchange, and religation by a single protein. The presence of coupling sequences makes the CTnDOT recombination distinct from the lambda recombination system. After the CTnDOT integration is finished in vivo, the coupling sequences are believed to be resolved by DNA replication or DNA mismatch repair systems. During the in vitro reactions, the coupling sequences are not resolved and heteroduplex DNA sequences are still present in both the attL and attR junctions. This suggests that the mechanism of CTn integration is novel despite its evident evolutionary relationship to the lambda family recombinases. The availability of the in vitro integration system should facilitate future work on the reaction mechanism.
Based on the in vivo mini-excision system we developed (4), we are trying to determine which gene products are important for excision in order to construct an in vitro excision system. So far, it appears that here, too, the CTnDOT element will be unique. The Xis equivalent for CTnDOT, in contrast to the Xis of Tn916, which resembles the lambda Xis in many ways, is a much larger protein that has topoisomerase activity (Y. Sutanto, submitted for publication). This is a property that has not been previously reported for excisionases of phage or other CTns.
Acknowledgments
We thank Steve Goodman for providing purified IHF and Anca Segall for purified HU. We thank Kimberly Taylor for assistance in the cloning and protein purification. We also thank George Chaconas for valuable suggestions.
This collaborative study was supported by grants AI22383 (to A. A. Salyers) and GM28717 (to J. F. Gardner) from the National Institutes of Health.
REFERENCES
- 1.Chalmers, R. M., and N. Kleckner. 1994. Tn10/IS10 transposase purification, activation, and in vitro reaction. J. Biol. Chem. 269:8029-8035. [PubMed] [Google Scholar]
- 2.Chambers, S. P., S. E. Prior, D. A. Barstow, and N. P. Minton. 1988. The pMTL nic- cloning vectors: improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene 68:139-149. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, Q., B. J. Paszkiet, N. B. Shoemaker, J. F. Gardner, and A. A. Salyers. 2000. Integration and excision of a Bacteroides conjugative transposon, CTnDOT. J. Bacteriol. 182:4035-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, Q., Y. Sutanto, N. B. Shoemaker, J. F. Gardner, and A. A. Salyers. 2001. Identification of genes required for the excision of CTnDOT, a Bacteroides conjugative transposon. Mol. Microbiol. 41:625-632. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, A. J., A. P. Kalinowski, N. B. Shoemaker, and A. A. Salyers. 1997. Construction and characterization of a Bacteroides thetaiotaomicron recA mutant: transfer of Bacteroides integrated conjugative elements is RecA independent. J. Bacteriol. 179:6221-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craigie, R., and K. Mizuuchi. 1986. Role of DNA topology in Mu transposition: mechanism of sensing the relative orientation of two DNA segments. Cell 45:793-800. [DOI] [PubMed] [Google Scholar]
- 7.Goodman, S. D., S. C. Nicholson, and H. A. Nash. 1992. Deformation of DNA during site-specific recombination of bacteriophage lambda: replacement of IHF protein by HU protein or sequence-directed bends. Proc. Natl. Acad. Sci. USA 89:11910-11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goryshin, I. Y., and W. S. Reznikoff. 1998. Tn5 in vitro transposition. J. Biol. Chem. 273:7367-7374. [DOI] [PubMed] [Google Scholar]
- 9.Hoopes, B. C., and W. R. McClure. 1981. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 9:5493-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, E. C., R. I. Gumport, and J. F. Gardner. 1990. Genetic analysis of bacteriophage lambda integrase interactions with arm-type attachment site sequences. J. Bacteriol. 172:1529-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metcalf, W. W., W. Jiang, and B. L. Wanner. 1994. Use of the rep technique for allele replacement to construct new Escherichia coli hosts for maintenance of R6Kv origin plasmids at different copy numbers. Gene 138:1-7. [DOI] [PubMed] [Google Scholar]
- 12.Mizuuchi, K. 1992. Transpositional recombination: mechanistic insights from studies of Mu and other elements. Annu. Rev. Biochem. 61:1011-1051. [DOI] [PubMed] [Google Scholar]
- 13.Mizuuchi, M., and K. Mizuuchi. 1989. Efficient Mu transposition requires interaction of transposase with a DNA sequence at the Mu operator: implications for regulation. Cell 58:399-408. [DOI] [PubMed] [Google Scholar]
- 14.Moore, W. E., E. P. Cato, and L. V. Holdeman. 1978. Some current concepts in intestinal bacteriology. Am. J. Clin. Nutr. 31:S33-S42. [DOI] [PubMed] [Google Scholar]
- 15.Nash, H. A. 1975. Integrative recombination of bacteriophage lambda DNA in vitro. Proc. Natl. Acad. Sci. USA 72:1072-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nash, H. A., and C. A. Robertson. 1981. Purification and properties of the Escherichia coli protein factor required for lambda integrative recombination. J. Biol. Chem. 256:9246-9253. [PubMed] [Google Scholar]
- 17.Pargellis, C. A., S. E. Nunes-Duby, L. M. de Vargas, and A. Landy. 1988. Suicide recombination substrates yield covalent lambda integrase-DNA complexes and lead to identification of the active site tyrosine. J. Biol. Chem. 263:7678-7685. [PubMed] [Google Scholar]
- 18.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 19.Shoemaker, N. B., C. Getty, J. F. Gardner, and A. A. Salyers. 1986. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J. Bacteriol. 165:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoemaker, N. B., C. Getty, E. P. Guthrie, and A. A. Salyers. 1986. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J. Bacteriol. 166:959-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoemaker, N. B., H. Vlamakis, K. Hayes, and A. A. Salyers. 2001. Evidence for extensive resistance gene transfer among Bacteroides spp. and between Bacteroides and other genera in the human colon. Appl. Environ. Microbiol. 67:561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoemaker, N. B., G. R. Wang, and A. A. Salyers. 2000. Multiple gene products and sequences required for excision of the mobilizable integrated Bacteroides element NBU1. J. Bacteriol. 182:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson, J. F., L. M. de Vargas, S. E. Skinner, and A. Landy. 1987. Protein-protein interaction in a higher-order structure direct lambda site-specific recombination. J. Mol. Biol. 195:481-493. [DOI] [PubMed] [Google Scholar]
- 24.Ton-Hoang, B., P. Polard, and M. Chandler. 1998. Efficient transposition of IS911 circles in vitro. EMBO J. 17:1169-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, J., N. B. Shoemaker, G. R. Wang, and A. A. Salyers. 2000. Characterization of a Bacteroides mobilizable transposon, NBU2, which carries a functional lincomycin resistance gene. J. Bacteriol. 182:3559-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]