Abstract
The protein RamC is required for the production of the spore-forming cells called aerial hyphae by the filamentous bacterium Streptomyces coelicolor. We showed that RamC, which contains several weakly predicted membrane-spanning sequences, is located exclusively in the S. coelicolor membrane. By constructing site-directed mutants in the cloned ramC gene and complementing a ramC null mutant, we showed that protein kinase-like sequence motifs in the amino-terminal half of the protein are required for function in vivo. These data suggest that RamC is a membrane-associated receptor kinase.
Growth of the filamentous bacterium Streptomyces coelicolor commences with spore germination and the propagation of substrate hyphae, which elongate and branch to form a substrate mycelium. Aerial hyphae then form on the surface of the substrate mycelium and metamorphose into chains of spores. While the aerial hyphae are producing spores, the substrate hyphae produce secondary metabolites, many of which have antibiotic activity (5).
One gene that is required for the formation of aerial hyphae is ramC (7). This gene is expressed in substrate hyphae but shut off in aerial hyphae during sporulation, a pattern that depends on the genes ramR, cprA, and bldD. RamR, a response regulator protein, binds directly to the ramC promoter region and is believed to activate ramC transcription early in the S. coelicolor life cycle (7).
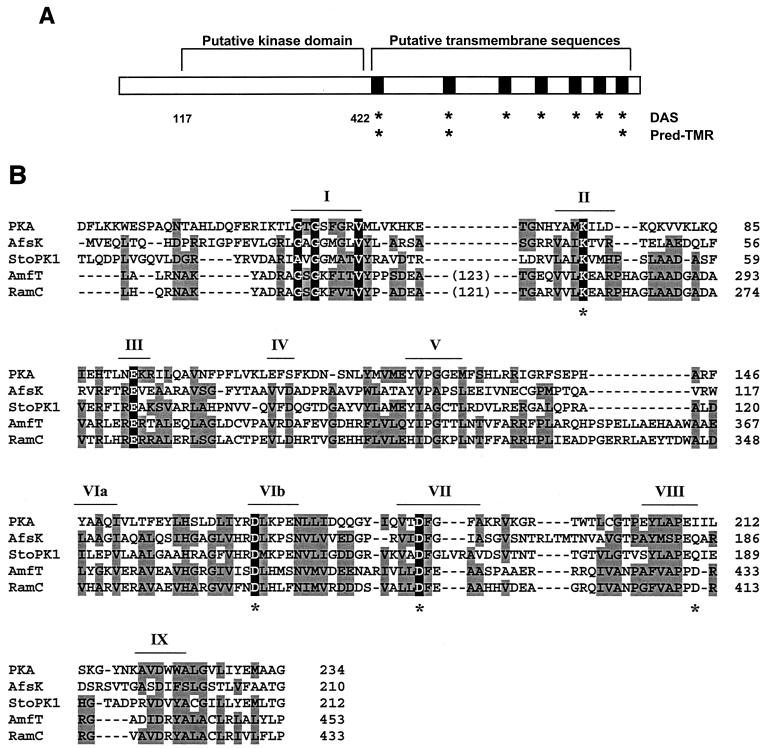
The most obvious functional motifs in RamC are summarized in Fig. 1A. We analyzed RamC by use of computer programs for identifying putative membrane-spanning sequences, and the results suggested that RamC might be a membrane protein. The dense alignment surface (DAS) program (1) predicted seven transmembrane sequences spanning residues 422 to 439, 563 to 575, 652 to 659, 707 to 714, 762 to 770, 809 to 813, and 866 to 879, while the Pred-TMR algorithm (8) predicted three such sequences overlapping those predicted by the DAS program at residues 420 to 440, 536 to 556, and 867 to 888. In contrast, the TMHMM program (9) did not predict any transmembrane segments in RamC. BLAST searches and COG (clusters of orthologous groups) analysis (10, 11) of the amino-terminal half of RamC (in particular, residues 117 to 422) suggested that this portion of RamC might be a serine/threonine protein kinase.
FIG. 1.
(A) Sequence analysis of the RamC polypeptide. Locations of the membrane-spanning sequences predicted by the DAS and Pred-TMR programs are indicated by asterisks. The putative kinase is bounded by residues 117 and 422. (B) Sequence alignment of RamC with a cAMP-dependent protein kinase (PKA), the S. coelicolor kinase AfsK, the Streptomyces toyocaensis kinase StoPK1, and the S. griseus RamC homologue AmfT. Residues shaded in grey denote similarity to RamC; those shaded in black are predicted, on the basis of well-known model kinases, to be involved in catalysis of phosphorylation. Conserved kinase-like sequence motifs (“Hanks” motifs) are indicated by roman numerals according to convention (3). Asterisks indicate the point mutations introduced into RamC.
To study RamC, we prepared a polyclonal antiserum against the 422 amino-terminal residues of the protein. DNA encoding these residues was amplified by PCR and cloned into the vectors pGEX-5x-1 (Amersham Biosciences) and pET21a (Novagen), which produce glutathione S-transferase (GST) and His6 fusion proteins, respectively. We purified the GST fusion protein under native conditions and used it to immunize New Zealand White rabbits. After five injections of 250 μg of protein at 4-week intervals, serum was prepared and affinity purified with denatured, purified His6 fusion protein coupled to Affigel beads (Bio-Rad) (4). The resulting antibodies exhibited selective binding to a ∼97-kDa polypeptide that was present in wild-type S. coelicolor but absent in a ramC null mutant (data not shown). A description of the strains, plasmids, and primers used in this study and their sources are given in Table 1.
TABLE 1.
Strains, plasmids, and primers used
| Strain, plasmid, or primer | Description | Background | Reference or source |
|---|---|---|---|
| Strains | |||
| S. coelicolor | |||
| M145 | Prototroph SCP1− SCP2− | Lab stock | |
| N373 | ramC::acc(3)IV SCP1− SCP2− | 7 | |
| E. coli | |||
| BL21 | F−dcm ompT hsdS(rB− mB−) gal met | Novagen | |
| BL21(DE3)plysS | F−dcm ompT hsdS(rB− mB−) gal λ(DE3) (pLysS Camr) | Novagen | |
| XL-1 Blue | recA1 endA1 gyrA96 thi-1 glnV44 hsdR17 supE44 relA1 lac [F′ proAB lac1q ZΔM15 Tn10(Tetr)] | Stratagene | |
| Er2-1 | F′ lacI leuB6 thi-1 fhuA31 lacY1 tsx-78 galK2 galT22 supE44 hisG4 rpsL136(Strr) xyl-5 mtl-1 dam-13::Tn9(Camr) dcm-6 mcrB1 mcrA hsdR2(rk− mk+) | J. McCormick | |
| Plasmids | |||
| E. coli | |||
| pET21a | T7 promoter based expression plasmid (Ampr) | Novagen | |
| pGEX-5x-1 | N-terminal GST fusion expression vector (Ampr) | Amersham Pharmacia | |
| pEMH-3 | RamC N terminus-GST fusion (Ampr) | pGEX-5X-1 | This study |
| pEMH-4 | RamC N terminus-His6 fusion (Ampr) | pET21a | This study |
| S. coelicolor | |||
| pSETΩ | aac(3)IV aadA+ (Spcr) | pSET152 | 7 |
| pTO8 | ramC (Spcr) | pSETΩ | 7 |
| pTO8-K259A | ramC K259A (Spcr) | pTO8 | This study |
| pTO8-D369A | ramC D369A (Spcr) | pTO8 | This study |
| pTO8-D387A | ramC D387A (Spcr) | pTO8 | This study |
| pTO8-D421A | ramC D421A (Spcr) | pTO8 | This study |
| Oligonucleotides | |||
| JJ5 | 5′-GGAGCACATATGAACAAGGGCTACGCCGTG-3′ | ||
| JJ31 | 5′-ACACGACAATTCTCACCGGTCCACGGCCACCCC-3′ | ||
| JJ32 | 5′-TGTGCTGAATTCACGGCTCCTGGTGGGGCC-3′ | ||
| K259ATop | 5′-ACCGGTGCGCGGGTCGTGCTAGCGGAGGCCCGT-3′ | ||
| K259ABot | 5′-ACGGGCCTCCGCTAGCACGACCCGCGCACCGGT-3′ | ||
| D369ATop | 5′-CCGGGGTGTGGTCTTCAACGCGTTGCACCTGTTC-3′ | ||
| D369ABot | 5′-GAACAGGTGCAACGCGTTGAAGACCACACCCCGG-3′ | ||
| D387ATop | 5′-ACGACAGCGTCGCCCTGCTAGCCTTCGAG-3′ | ||
| D387ABot | 5′-CTCGAAGGCTAGCAGGGCGACGCTGTCGT-3′ | ||
| D421ATop | 5′-TTCGTGGCGCCTCCCGCGCGCCGCGGG-3′ | ||
| D421ABot | 5′-CCCGCGGCGCGCGGGAGGCGCCACGAA-3′ |
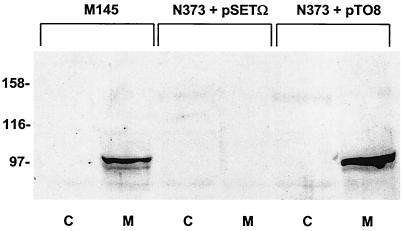
To determine the subcellular location of RamC, we examined the RamC content of cytoplasmic and membrane fractions of S. coelicolor cells grown for 36 h on solid R2YE medium (5). Cells were harvested, treated with lysozyme, and subjected to centrifugation at 3,000 × g for 5 min to pellet insoluble material. The resulting supernatant was then subjected to centrifugation at 100,000 × g for 1 h to pellet membranes and leave cytoplasmic proteins in the supernatant. Equivalent amounts of membrane and cytoplasmic proteins from wild-type cells, the ramC null mutant N373, and N373 containing the ramC complementation plasmid pTO8 were run on a sodium dodecyl sulfate-polyacrylamide gel, electroblotted onto a polyvinylidene difluoride membrane, and analyzed by Western blotting with anti-RamC. A band of the predicted size for RamC was observed in the membrane fraction but not the cytoplasmic fraction of the wild-type cells (Fig. 2, lanes M145). This band was absent from both fractions of the ramC null mutant N373 cells (Fig. 2, lanes N373+pSETΩ) but was present, again exclusively in the membrane fraction of N373 cells complemented with pTO8 (Fig. 2, lanes N373+pTO8). We conclude that the RamC protein is located in the S. coelicolor membrane.
FIG. 2.
Subcellular localization of RamC. Western analysis with affinity-purified anti-RamC of cytoplasmic fractions (lanes C) and membrane fractions (lanes M) from M145, the ramC::acc(3)IV strain N373 with the control plasmid pSETΩ, and N373 complemented in trans with pTO8.
Most serine/threonine kinases exhibit conservation of 12 sequence motifs that fold into the substrate binding and catalytic centers (3). Sequence alignment with the known serine/theonine kinases AfsK (14) and StoPK1 (6) and the cyclic AMP (cAMP)-dependent protein kinase (PKA) from Mus musculus (Fig. 1B) illustrates similarity in RamC to 10 of these motifs. The Streptomyces griseus homologue of RamC, AmfT (13), also contains these motifs. This putative kinase domain also contains additional sequences not found in any known kinase. These include an amino-terminal extension of 117 residues and a 121-residue insertion between the first and second blocks of sequence homology, both of which are conserved in AmfT. Importantly, however, we identified residues within the kinase-like motifs that were identical to invariant residues that play essential roles in catalysis in known serine/threonine kinases. For example, residues K259 and D387 in RamC align well with K72 and D184 in PKA. In known kinases, these residues orient ATP through interactions with the α-, β-, and γ-phosphates (2, 15). D369 aligns well with D166, which is thought to deprotonate the target hydroxyl on target amino acids, thereby stimulating its nucleophilic attack on the γ-phosphate (15, 16). Finally, D412 in RamC could correspond to E208 in PKA, a residue that forms important stabilizing interactions with residues in the C terminus of the kinase domain (12).
To determine whether these protein kinase-like motifs were important for RamC function, we altered several of them in the context of the ramC complementation plasmid pTO8. As shown in Fig. 3A, N373 containing pTO8 was able to produce a white layer of aerial hyphae during growth on solid R2YE medium but the same strain containing the parent vector pSETΩ was not. Similarly, alleles of ramC bearing mutations that after predicted catalytic residues K259, D369, and D387 failed to induce formation of aerial hyphae (Fig. 3A). A mutation altering the acidic residue in motif VIII (D412) impaired but did not eliminate ramC function.
FIG. 3.
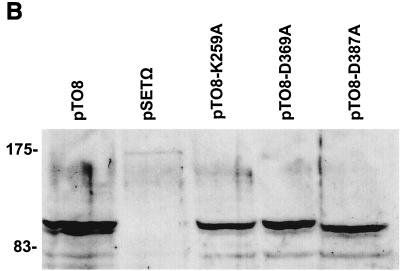
Mutations in kinase-like motifs in RamC block function in vivo. (A) Growth of strains on solid R2YE medium. The plates show the S. coelicolor ramC null mutant N373 containing the ramC complementation plasmid pTO8 (top left), control plasmid pSETΩ (top middle), and pTO8 containing mutant alleles expressing RamC with mutations in predicted catalytic residues, including K259A (top right), D369A (bottom left), D387A (bottom middle), and D412A (bottom right). (B) Western analysis of membrane fractions of N373 containing pTO8 and pTO8 expressing the K259A, D369A, and D387A mutations in RamC.
To determine whether these mutations had caused destabilization in vivo or a failure of targeting of RamC to S. coelicolor membranes, we produced membrane fractions from the N373 strains containing the three defective alleles and compared their RamC contents to that of N373 containing pTO8 (Fig. 3B) by Western analysis with anti-RamC. All three mutants accumulated RamC polypeptide to levels similar to that of the wild type, and the RamC protein was correctly targeted to the membrane.
These observations suggest that the protein kinase-like motifs in RamC are important for protein function. We have attempted to demonstrate in vitro protein kinase activity for RamC expressed in Escherichia coli by both autophosphorylation and the phosphorylation of heterologous proteins (histone H1, myelin basic protein, and several peptide substrates). Any activity that we found by use of these assays was extremely weak and of borderline reproducibility (data not shown). We surmise either that RamC expressed in E. coli lacks an important subunit or activating signaling molecule and hence is not able to carry out this reaction on its own or that RamC phosphorylates other molecules such as lipids or polysaccharides, possible explanations that we are currently pursuing. In sum, however, the results presented here are consistent with our prediction that RamC is a membrane-associated kinase. Such a protein could act as a receptor for developmental signaling molecules.
Acknowledgments
We thank Gerry Wright, John Neu, and Brenda Leskiw for their assistance.
This work was supported by an Ontario Graduate Scholarship to Michael Hudson and a grant (MT-15108) and New Investigator award from the Canadian Institutes for Health Research to Justin Nodwell.
REFERENCES
- 1.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673-676. [DOI] [PubMed] [Google Scholar]
- 2.Grant, B. D., W. Hemmer, I. Tsigelny, J. A. Adams, and S. S. Taylor. 1998. Kinetic analyses of mutations in the glycine-rich loop of cAMP-dependent protein kinase. Biochemistry 37:7708-7715. [DOI] [PubMed] [Google Scholar]
- 3.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 4.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 5.Keiser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 6.Neu, J. M., S. V. MacMillan, J. R. Nodwell, and G. D. Wright. 2002. StoPK-1, a serine/threonine protein kinase from the glycopeptide antibiotic producer Streptomyces toyocaensis NRRL 15009 affects oxidative stress response. Mol. Microbiol. 44:417-430. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor, T., P. Kanellis, and J. R. Nodwell. Cell type-specific expression and requirement for morphogenesis of the ramC gene of Streptomyces coelicolor. Mol. Microbiol., in press. [DOI] [PubMed]
- 8.Pasquier, C., V. J. Promponas, G. A. Palaios, J. S. Hamodrakas, and S. J. Hamodrakas. 1999. A novel method for predicting transmembrane segments in proteins based on a statistical analysis of the SwissProt database: the PRED-TMR algorithm. Protein Eng. 12:381-385. [DOI] [PubMed] [Google Scholar]
- 9.Sonnhammer, E. L., G. von Heijne, and A. Krogh. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6:175-182. [PubMed] [Google Scholar]
- 10.Tatusov, R. L., E. V. Koonin, and D. J. Lipman. 1997. A genomic perspective on protein families. Science 278:631-637. [DOI] [PubMed] [Google Scholar]
- 11.Tatusov, R. L., D. A. Natale, I. V. Garkavtsev, T. A. Tatusova, U. T. Shankavaram, B. S. Rao, B. Kiryutin, M. Y. Galperin, N. D. Fedorova, and E. V. Koonin. 2001. The COG database: new developments in phylogenetic classification of proteins. Nucleic Acids Res. 29:22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor, S. S., D. R. Knighton, J. Zheng, L. F. ten Eyck, and J. M. Sowadski. 1992. Structural framework for the protein kinase family. Annu. Rev. Cell Biol. 8:429-462. [DOI] [PubMed] [Google Scholar]
- 13.Ueda, K., K. Miyake, S. Horinouchi, and T. Beppu. 1993. A gene cluster involved in aerial mycelium formation in Streptomyces griseus encodes proteins similar to the response regulators of two-component regulatory systems and membrane translocators. J. Bacteriol. 175:2006-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umeyama, T., P. C. Lee, K. Ueda, and S. Horinouchi. 1999. An AfsK/AfsR system involved in the response of aerial mycelium formation to glucose in Streptomyces griseus. Microbiology 145:2281-2292. [DOI] [PubMed] [Google Scholar]
- 15.Zheng, J., D. R. Knighton, L. F. ten Eyck, R. Karlsson, N. Xuong, S. S. Taylor, and J. M. Sowadski. 1993. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MgATP and peptide. Biochemistry 32:2154-2161. [DOI] [PubMed] [Google Scholar]
- 16.Zhou, J., and J. A. Adams. 1997. Is there a catalytic base in the active site of cAMP-dependent protein kinase? Biochemistry 36:2977-2984. [DOI] [PubMed] [Google Scholar]