Abstract
The entire pathway for the biosynthesis of the phycobiliviolin-bearing His-tagged holo-α subunit of the cyanobacterial photosynthetic accessory protein phycoerythrocyanin was reconstituted in Escherichia coli. Cyanobacterial genes encoding enzymes required for the conversion of heme to 3Z-phycocyanobilin, a precursor of phycobiliviolin (namely, heme oxygenase 1 and 3Z-phycocyanobilin:ferredoxin oxidoreductase), were expressed from a plasmid under the control of the hybrid trp-lac (trc) promoter. Genes for the apo-phycoerythrocyanin α subunit (pecA) and the heterodimeric lyase/isomerase (pecE and pecF), which catalyzes both the covalent attachment of phycocyanobilin and its concurrent isomerization to phycobiliviolin, were expressed from the trc promoter on a second plasmid. Upon induction, recombinant E. coli used endogenous heme to produce holo-PecA with absorbance and fluorescence properties similar to those of the same protein produced in cyanobacteria. About two-thirds of the apo-PecA was converted to holo-PecA. No significant bilin addition took place in a similarly engineered E. coli strain that lacks pecE and pecF. By using immobilized metal affinity chromatography, both apo-PecA and holo-PecA were isolated as ternary complexes with PecE and PecF. The identities of all three components in the ternary complexes were established unambiguously by protein and tryptic peptide analyses performed by matrix-assisted laser desorption ionization-time of flight mass spectrometry.
Cyanobacteria produce macromolecular light-harvesting complexes made up largely of phycobiliproteins, called phycobilisomes. Each phycobiliprotein is an oligomer of a heterodimer, either a trimer [(αβ)3] or a hexamer [(αβ)6]. Two of the major phycobiliproteins, allophycocyanin (λmax, 650 nm) and phycocyanin (λmax, ∼620 nm), are found in all cyanobacterial phycobilisomes. Either phycoerythrocyanin (λmax, 570 nm, with a shoulder at 595 nm) or phycoerythrin (λmax, ∼560 nm) may be present as well (12). Phycoerythrocyanin occurs mainly in heterocystous cyanobacteria, and it has not been found together with phycoerythrin (4).
Different linear tetrapyrrole prosthetic groups (bilins), covalently attached through thioether linkages to cysteinyl residues, endow the phycobiliproteins with their distinctive light-harvesting properties. Phycocyanin carries one phycocyanobilin (PCB) on the α subunit (CpcA) at αCys84 and two PCBs on the β subunit at βCys82 and βCys155. Phycoerythrocyanin carries one phycobiliviolin (PXB) on its α subunit (PecA) at αCys84 and, like phycocyanin, two PCBs on the β subunit (PecB) at βCys82 and βCys155 (3, 20). The first step in the pathway of bilin biosynthesis is the conversion of heme to biliverdin IXα (BVD) by the heme oxygenase encoded by hox1 (8). Recently, Frankenberg et al. (11) have shown that cyanobacterial pcyA genes encode bilin reductases that catalyze the four-electron reduction of BVD to 3Z-PCB. A limited amount of information is available on the genes that encode the proteins required for the covalent attachment of particular bilins at specific residues to apo-phycobiliprotein polypeptides. Only two bilin lyases have been characterized: a heterodimeric phycocyanin α subunit PCB lyase encoded by the genes cpcE and cpcF (9, 10) and a phycoerythrocyanin α subunit PCB lyase/isomerase encoded by the genes pecE and pecF (14, 21).
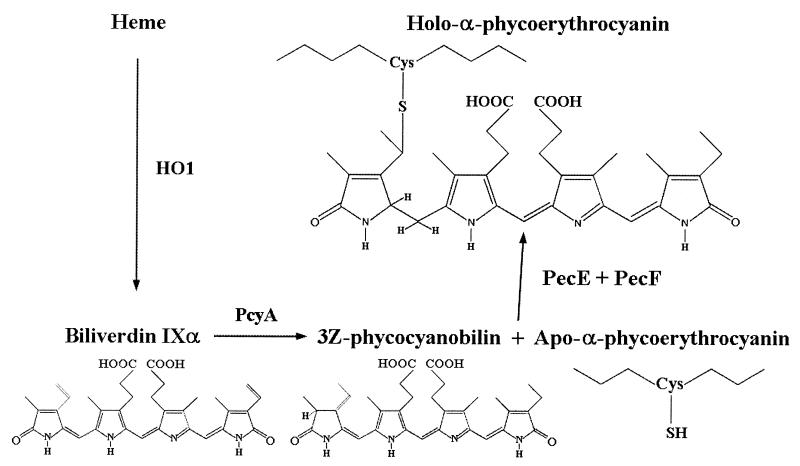
The structures of BVD, PCB, and PXB are shown in Fig. 1. Protein-linked PCB and PXB are isomers that differ as follows: there is a single bond between C-2 and C-3 in PCB and there is a double bond in PXB, and the C5 methine bridge in PCB is reduced in PXB. In vitro studies by Zhao et al. (21) have shown that incubation of recombinant apo-PecA with PCB in the presence of PecE and PecF leads to the formation of holo-PecA and that PecE and PecF together catalyze both the addition of PCB to apo-PecA and its isomerization to PXB.
FIG. 1.
Engineered biosynthetic pathway for the production of PCB from heme and its addition to the phycoerythrocyanin apo-α subunit and isomerization to PXB.
Recently, we reported the reconstitution of the pathway for the synthesis of holo-CpcA in Escherichia coli (19). In an E. coli strain, engineered with Synechocystis sp. strain PCC6803 genes, hox1 (encoding heme oxygenase 1) and pcyA (encoding 3Z-PCB:ferredoxin oxidoreductase) were expressed from one plasmid, and cpcA, cpcE, and cpcF were expressed from a second plasmid. The engineered E. coli strain expressed holo-CpcA with spectroscopic properties corresponding to those of the native CpcA produced in cyanobacteria.
Here we describe the in vivo production of holo-PecA in an appropriately engineered strain of E. coli and compare the results with the outcomes of the in vitro experiments of Zhao et al. (21) and Storf et al. (18) for PCB addition to apo-PecA. Our studies also demonstrate the formation of a ternary complex of His-tagged Pec-A (HT-PecA) with PecE and PecF.
MATERIALS AND METHODS
Materials.
Enzymes for DNA manipulation were obtained from New England Biolabs and Life Technologies (Rockville, Md.), and antibiotics were obtained from Sigma. Agar and organic nutrients for Luria-Bertani medium were obtained from Difco, and other chemicals were obtained from Sigma or Fisher Scientific. Superflow Ni2+-nitrilotriacetic acid (NTA) agarose for isolation of His-tagged proteins was purchased from Qiagen (Chatsworth, Calif.).
Cultures and strains.
E. coli strain DH5α (Life Technologies) grown in Luria-Bertani medium was used in all experiments. Plasmids conferring resistance to spectinomycin were selected with 100 μg of spectinomycin dihydrochloride per ml, and plasmids conferring resistance to kanamycin were selected with 50 μg of kanamycin sulfate per ml. For expression of Ptrc-controlled genes, isopropyl-β-d-thiogalactoside (IPTG) was added to a final concentration of 0.5 mM to exponentially growing cells. Induced cells were grown for 6 to 8 h at 30°C with shaking. Cultures were harvested, and pellets were stored at −20°C until they were used.
Cloning of relevant Anabaena sp. strain PCC7120 genes.
Standard procedures were used for most DNA manipulations. Gene sequences were obtained from GenBank (accession no. AF178757) and were compared with the complete genome sequence from the Kazusa DNA Research Institute CyanoBase (15, 17; http://www.kazusa.or.jp/cyano/index.html). By using primers described below, all genes were amplified from Anabaena sp. strain PCC7120 genomic DNA by PCR. The fidelity of all PCR-generated fragments was verified by direct nucleotide sequencing. A more typical E. coli ribosomal binding site was engineered upstream of the pecE and pecF open reading frames. DNA sequence analysis was performed with the program Editbase (Purdue Research Foundation and U.S. Department of Agriculture Agricultural Research Service), and predicted amino acid sequences were deduced by using LASERGENE (DNAstar Inc., Madison, Wis.).
Cloning of the gene encoding Anabaena sp. strain PCC7120 phycoerythrocyanin α subunit.
The primers used to amplify the pecA gene were 5′-GAG ATT AGG AGA CAT ATG AAA ACA CCT TTG ACC GAA GC-3′ and 5′-CAA GAC CGA ATT CGA GTC TCT TAA CTT AAA GCG TTA ATT GCA TAG TTC AGG TA-3′. The resulting 0.5-kb product was digested with restriction enzymes NdeI and EcoRI and cloned into NdeI- and EcoRI-digested cloning vector pBS350V (6), giving plasmid pBS430V. The PecA construct expressed from pBS430V consists of PecA fused at the N terminus to a 24-amino-acid sequence that includes a six-His tag (6).
Cloning of the genes encoding the Anabaena sp. strain PCC7120 phycoerythrocyanin α subunit PCB/PXB lyase/isomerase.
The primers used to amplify the pecE gene were 5′-AAT TTT GTC GAC AGG AGG AAA GCC ATA TGA CTG CTG AAC CAA TTC TTT CTC CAG-3′ and 5′-ATT CAG GCG GCC GCT TTA AAG TTG AAT TAA TAA ATC ATC AAT TGC TCC AAA TAA TAA AGC-3′. The resulting 0.8-kb PCR fragment was digested with restriction enzymes SalI and NotI and cloned into SalI- and NotI-digested pBS350V, giving plasmid pBS433V. The primers used to amplify the pecF gene were 5′-ATT GTT CGG CCG AGG AGG AAC ATA TGA ATC AAG CTT CAT TGA GCG TAG ACG-3′ and 5′-CCT GGA TCC GAG TCC CTT AAC TCA AGG CGA TCG CCA TAC GTG-3′. The resulting 0.55-kb product was digested with restriction enzymes EagI and BamHI and cloned into EagI- and BamHI-digested pBS350V, giving plasmid pBS434V.
Design and construction of the in vivo expression vector pBS437V containing the pecA-pecE-pecF cassette.
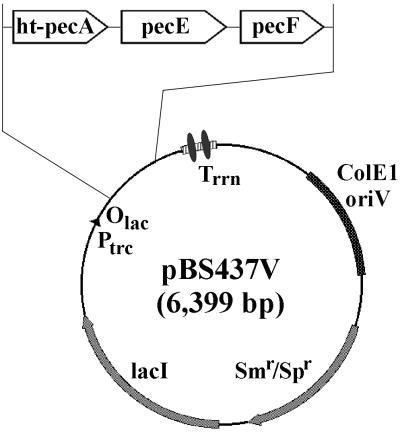
In order to engineer a strain of E. coli that could produce the phycoerythrocyanin holo-α subunit in vivo, an expression vector containing the genes involved in the production of holo-PecA (namely, ht-pecA, pecE, and pecF, where “ht” indicates the base sequence encoding the His tag) was designed. The aim was to introduce this plasmid, pBS437V, along with plasmid pAT101 (containing the Synechocystis sp. strain PCC6803 hox1-pcyA cassette [19]) into E. coli and thereby produce all of the catalytic functions and components believed to be required for the formation of holo-PecA. The pecE gene, as a 0.8-kb SalI-NotI fragment from pBS433V, was cloned into SalI- and NotI-digested pBS430V containing ht-pecA, giving plasmid pBS436V. Subsequently, the pecF gene, as a 0.55-kb EagI-BamHI fragment from pBS434V, was cloned into EagI- and BamHI-digested pBS436V containing ht-pecA and pecE, giving the final cassette plasmid pBS437V (Fig. 2).
FIG. 2.
Physical map of the pBS437V expression vector used for in vivo bilin addition to Anabaena sp. strain PCC7120 His-tagged phycoerythrocyanin apo-α subunit (HT-PecA) in E. coli. Vector pBS437V carries ht-pecA, the gene encoding HT-PecA, and the phycoerythrocyanin α subunit PCB lyase/isomerase genes pecE and pecF. Plasmid pBS437V contains the ColE1 oriV for replication in E. coli and the aadA gene conferring resistance to spectinomycin and streptomycin. Expression is controlled by the trc promoter, the lac operator, and the lacIq gene encoding the Lac repressor. Two strong, bidirectional, ρ-independent transcriptional terminator structures from the E. coli rrnB gene are located downstream of the expression cassette. Plasmid pBS430V (see text) is equivalent to plasmid pBS437V, except that the insert contains only ht-pecA.
Isolation of HT-PecA and associated proteins by immobilized metal affinity chromatography.
Cell pellets were thawed and resuspended in 20 ml of cold (0 to 4°C) buffer 0 (20 mM Tris-HCl [pH 8.0], 50 mM NaCl, 50 mM KCl).
Phenylmethylsulfonyl fluoride and 2-mercaptoethanol were added to final concentrations of 1 and 10 mM, respectively, immediately before breakage of cells by passage through a French pressure cell three times at 18,000 lb/in2. Cell debris was removed by centrifugation at 4°C in a Beckman JA20 rotor at 30,000 × g for 1 h. The supernatant solution was mixed with 2 to 3 ml of Ni2+-NTA agarose at 4°C for 15 min before the agarose was loaded onto a column. The agarose was then washed with 10 column volumes each of cold buffer A1 (20 mM Tris-HCl [pH 8.0], 50 mM NaCl, 50 mM KCl, 20 mM imidazole, 5% [vol/vol] glycerol), buffer B (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 500 mM KCl), and buffer A2 (20 mM Tris-HCl [pH 8.0], 50 mM NaCl, 50 mM KCl, 30 mM imidazole). His-tagged proteins were eluted from the agarose with buffer C (20 mM Tris-HCl [pH 8.0], 50 mM NaCl, 50 mM KCl, 200 mM imidazole) and were then dialyzed overnight against 50 mM Na phosphate (pH 7.0).
Absorbance and fluorescence spectrometry.
Absorbance spectra were acquired with a computer-controlled, dual-beam λ6 UV/VIS spectrophotometer (Perkin-Elmer Corp., Norwalk, Conn.). Corrected fluorescence spectra were obtained with an FP-750 spectrofluorometer (Jasco Inc., Easton, Md.). The excitation and emission slits were set at 5 nm for all measurements.
SDS-PAGE.
Proteins were precipitated in 10% (wt/vol) trichloroacetic acid and resolubilized in sodium dodecyl sulfate (SDS) loading buffer (2% SDS, 50 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol, 0.1% bromophenol blue, 10% [vol/vol] glycerol). SDS-polyacrylamide gel electrophoresis (PAGE) (16) was performed by using 10% acrylamide stacking and 14% acrylamide separating gels with a monomer/bis ratio of 37.5:1. Bilin-bearing polypeptides were visualized as fluorescent bands by UV (>312-nm) illumination after staining with 10 mM zinc acetate (1). Polypeptides were also visualized by staining with Coomassie brilliant blue. Protein molecular weight standards were purchased from Life Technologies.
Mass spectrometry.
For matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (13a), proteins (0.1 to 0.2 mg), purified by metal affinity chromatography, were dialyzed extensively against 5 mM ammonium acetate prior to lyophilization. The proteins were then redissolved in water. An 0.5-μl aliquot of this solution was mixed with of an equal volume of a saturated solution of 3,5-dimethoxy-4-hydroxycinnamic acid in 30% acetonitrile-70% 0.1% aqueous trifluoroacetic acid and allowed to crystallize on the MALDI target plate. Analysis was performed with a Bruker Reflex III MALDI-TOF mass spectrometer.
For peptide analyses, protein bands were excised from gels, suspended in 25 mM ammonium bicarbonate, and reduced; the cysteinyl residues were converted to the carboxamidomethyl derivatives by reaction with iodoacetamide, and the proteins were digested with trypsin as described elsewhere (13a). The tryptic peptide mixtures were each combined with an equal volume of matrix solution, which contained 10 g of α-cyano-4-hydroxycinnamic acid per liter dissolved in 50% acetonitrile-50% 0.1% aqueous trifluoroacetic acid, and were analyzed as described above. The MALDI-TOF spectra were internally calibrated with peptides having known masses to obtain masses for the tryptic peptides with a precision of <20 ppm. Peptides were identified by comparing their masses with those calculated for tryptic peptides predicted from the amino acid sequences of HT-PecA, PecE, and PecF obtained from the known DNA sequences.
RESULTS
Construction of E. coli expression strains.
In an earlier study, we introduced into an E. coli strain the gene encoding a His-tagged version of the apo-α subunit of phycocyanin (cpcA), as well as the genes encoding the components of the biosynthetic pathway in cyanobacteria leading from heme to PCB (hox1 and pcyA) and the genes encoding the lyase (cpcE and cpcF) required for the formation of the site-specific cysteinyl adduct of PCB with CpcA. The engineered E. coli produced holo-CpcA with spectroscopic properties characteristic of the native protein (19).
The genes (pecE and pecF) encoding the heterodimeric phycoerythrocyanin α subunit PXB lyase have been characterized (14). Moreover, Zhao et al. (21) and Storf et al. (18) have shown that in vitro addition of PCB to a mixture of recombinant HT-PecA, PecE, and PecF leads to the production of holo-PecA carrying a PXB chromophore. Consequently, PecE and PecF must a catalyze reaction(s) in which PCB is both attached to PecA and isomerized to PXB, although it is not clear whether the isomerization involves the thioether-bound or free PCB.
The information described above leads to the prediction that an E. coli strain engineered to contain hox1, pcyA, ht-pecA, pecE, and pecF should produce holo-HT-PecA with spectroscopic properties characteristic of the native holo subunit by the pathway illustrated in Fig. 1. The His tag on PecA is not expected to cause any difficulties. HT-PecA expressed in Anabaena sp. strain PCC7120 has native spectroscopic properties and is recovered by immobilized metal affinity chromatography as a 1:1 complex with holo-PecB that is efficiently incorporated into the phycobilisome (Y. A. Cai and A. N. Glazer, unpublished data).
To test the prediction described above, two strains of E. coli, each carrying two expression vectors, were generated. To maintain both plasmids simultaneously, double transformants were selected for resistance to both spectinomycin and kanamycin and were grown with both spectinomycin and kanamycin. E. coli strain DH5α(437V,101) carries plasmids pBS437V (ht-pecA-pecE-pecF cassette, Spr) and pAT101 (ht-hox1-pcyA cassette, Kmr) and expresses all the known genes believed to be involved in PCB biosynthesis and in addition and isomerization of PCB to apo-PecA (Fig. 2). Another E. coli strain, DH5α(430V,101), carries plasmid pBS430V (expressing HT-PecA but lacking PecE and PecF, Spr) and plasmid pAT101. In each case, the two plasmids contain different origins of replication so that both are maintained in E. coli. Comparison of the properties of the HT-PecA proteins recovered from these two E. coli strains tested the stringency of the requirement for PecE and PecF for PCB addition and isomerization to PXB in vivo.
Heterologous expression and characterization of holo-HT-PecA.
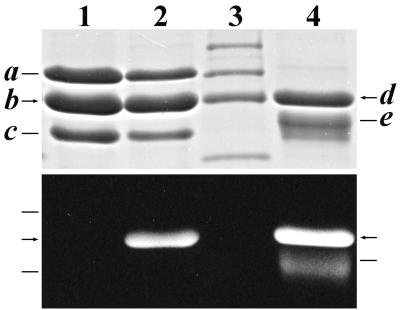
Expression of the gene cassettes in the two strains of E. coli led to two very different phenotypes. Upon induction with IPTG, the E. coli strain DH5α(437V,101) culture acquired a pronounced pink tint. This color change was not seen in the culture of DH5α(430V,101). HT-PecA was purified from strain DH5α(437V,101) by affinity chromatography. Analysis of Coomassie brilliant blue-stained gels after SDS-PAGE of the purified protein showed that there were three distinct bands (Fig. 3, lane 2, upper panel), band a at ∼26 kDa, band b at ∼20 kDa, and band c at ∼17.5 kDa. The sizes of these bands are compatible with those calculated for PecE, holo-HT-PecA, and PecF, respectively (see below). Upon exposure to Zn2+ (1) and UV illumination, only band b was fluorescent, indicating that it contained covalently attached bilin (Fig. 3, lane 2, lower panel). The mobility of band b was the same as that of band d, which is holo-HT-PecA expressed in Anabaena sp. strain PCC7120. HT-HO1 was not seen in the gel due to its low expression levels.
FIG. 3.
SDS-PAGE analysis of Anabaena sp. strain PCC7120 His-tagged proteins expressed in various E. coli strains and purified by immobilized metal affinity chromatography. Proteins were visualized by Coomassie brilliant blue staining (upper panel) and by the UV-excited fluorescence of bilin-bearing polypeptides in the presence of Zn2+ (1) (lower panel). Lane 1, PecE, HT-PecA, and PecF (bands a, b, and c, respectively) expressed in E. coli strain DH5α(437V); lane 2, PecE, HT-PecA, and PecF expressed in strain DH5α(437V,101), which also expresses HT-HO1 (band not in gel) and PcyA; lane 3, molecular mass standards (from the top, 30, 25, 20, and 15 kDa); lane 4, holo-HT-PecA (band d) and PecB (band e) from Anabaena sp. strain PCC7120 (see text).
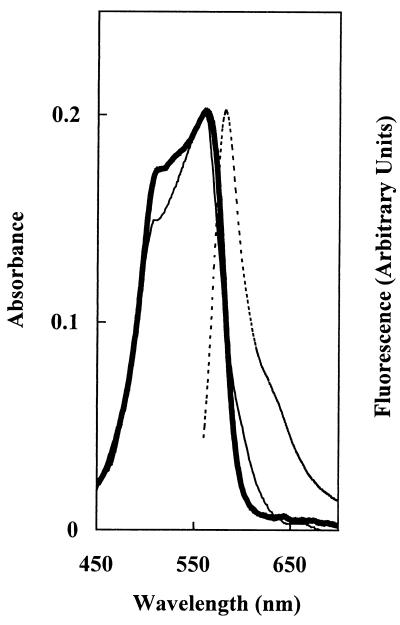
The spectroscopic properties of holo-HT-PecA were determined with the protein fraction purified by immobilized metal affinity chromatography after dialysis against 50 mM Na phosphate (pH 7.0). The absorbance spectrum of holo-HT-PecA had a λmax at 561 nm and a 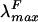 at 582 nm (Fig. 4). The spectrum corresponded closely to that of the native holo-PecA obtained from Anabaena sp. strain PCC7120 (5).
at 582 nm (Fig. 4). The spectrum corresponded closely to that of the native holo-PecA obtained from Anabaena sp. strain PCC7120 (5).
FIG. 4.
Spectroscopic properties of Anabaena sp. strain PCC7120 holo-HT-PecA expressed in E. coli. Shown are the absorbance (λmax, 561 nm) (thick solid line), control Anabaena sp. strain PCC7120 holo-PecA (from the study of Bryant et al. [5]) (thin solid line), and fluorescence emission ( 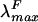 , 582 nm; λexc, 550 nm) (dashed line) spectra for HT-PecA purified from E. coli by affinity chromatography. The spectra were normalized at 561 nm to the absorbance spectrum of holo-PecA from E. coli.
, 582 nm; λexc, 550 nm) (dashed line) spectra for HT-PecA purified from E. coli by affinity chromatography. The spectra were normalized at 561 nm to the absorbance spectrum of holo-PecA from E. coli.
Storf et al. (18) reported an ɛM of 77,000 M−1cm−1 at 567 nm for holo-HT-PecA produced in vitro by the addition of PCB to a mixture of HT-PecA, PecE, and PecF in 100 mM phosphate buffer at pH 7.0. By using this ɛM and a molecular weight of 20,920 for holo-HT-PecA, it can be calculated that ∼0.05 mg of purified holo-HT-PecA was recovered per g (wet weight) of E. coli cells under the expression conditions described here.
Very little soluble HT-PecA was recovered upon cell breakage of induced E. coli strain DH5α(430V,101) (ht-pecA, ht-hox1-pcyA). A similar result was seen with E. coli strain DH5α(430V), in which only HT-PecA is expressed. However, when the ht-pecA-pecE-pecF gene cassette was expressed in E. coli from plasmid pBS437V, apo-HT-PecA was purified from the soluble fraction by affinity chromatography with a yield similar to that seen with E. coli strain DH5α(437V,101).
E. coli strains producing PecA, PecE, and PecF show approximately one-third slower growth than E. coli strains expressing similar, but different, phycobiliprotein cassettes (data not shown).
Characterization of a holo-HT-PecA-PecE-PecF ternary complex.
As described above, analysis of affinity-purified holo-HT-PecA produced in E. coli strain DH5α(437V,101) by SDS-PAGE showed the presence of three protein components whose sizes were compatible with those calculated for PecE, holo-HT-PecA, and PecF (Fig. 3, lane 2). Analysis of affinity-purified HT-PecA produced in E. coli strain DH5α(437V) likewise showed the presence of three protein components with the same electrophoretic mobilities on SDS-PAGE gels, except that the HT-PecA did not carry bilin (Fig. 3, lane 1).
The protein fractions purified from the two E. coli strains by immobilized metal affinity chromatography were subjected to analysis by MALDI-TOF mass spectrometry. For the fraction purified from induced E. coli strain DH5α(437V), three peaks with similar intensities were obtained, at 27,975 Da (calculated mass of PecE, 28,176.6 Da; difference between the calculated and observed masses [Δcalc. vs obs.], 0.72%), at 20,127 Da (calculated mass of apo-HT-PecA-Met+H+, 20,201.6 Da; Δcalc. vs obs., 0.37%), and at 18,383.3 Da (calculated mass of PecF-Met+H+, 18,337.2 Da; Δcalc. vs obs., −0.25%). The analyses showed nearly quantitative posttranslational removal of the N-terminal Met in HT-PecA and partial removal in PecE and PecF. The masses support identification of bands a, b, and c (Fig. 3, lane 1) as PecE, apo-HT-PecA, and PecF. The small Δcalc. vs obs. differences are most likely due to the presence of different amounts of two forms of each polypeptide, one with the N-terminal Met and the other lacking the N-terminal Met.
Similar results were obtained with the fraction purified from induced E. coli strain DH5α(437V,101), except that two overlapping peaks with an area ratio of ∼1:2 were obtained for HT-PecA. The first peak corresponded to apo-HT-PecA (see above), and the other peak corresponded to holo-HT-PecA (data not shown).
To identify the three proteins conclusively, the bands for the polypeptides were cut out of an SDS-PAGE gel and subjected to tryptic digestion (see Materials and Methods). MALDI-TOF mass spectrometry analyses of the peptides from each of the protein bands yielded multiple peptides whose masses matched the calculated masses of tryptic peptides predicted from the amino acid sequences of PecE, apo-HT-PecA, and PecF in every case to within <20 ppm. The results of the peptide analyses are presented in Fig. 5. These results, in addition to the data on the intact proteins, provided unambiguous identification of the three bands as PecE, HT-PecA, and PecF.
FIG. 5.
Amino acid sequences of HT-PecA, PecE, and PecF, showing in boldface type the residues in peptides identified by mass spectrometry of tryptic digests of proteins in gel slices from bands b, a, and c, respectively, cut out from gels such as the gel shown in Fig. 3, lanes 1 and 2.
DISCUSSION
The studies described here documented successful reconstitution of the predicted pathway for the biosynthesis of the phycoerythrocyanin holo-α subunit (Fig. 1) in E. coli. The results of our experiments also support the conclusion reached by Zhao et al. (21) and Storf et al. (18) on the basis of in vitro experiments that the heterodimeric lyase/isomerase (PecE/PecF) catalyzes both the covalent attachment of PCB and the concurrent isomerization of the molecule to PXB.
Storf et al. (18) also reported in vitro nonenzymatic formation of a PCB adduct with apo-HT-PecA. We saw no addition of PCB to HT-PecA in E. coli in the absence of PecE and PecF. A caveat is that little of the PecA is in a soluble form in the absence of PecE and PecF. Storf et al. (18) noted that, “The overexpressed proteins are deposited mainly in inclusion bodies, but can be well solubilized by extended sonication.” It is possible that the protein solubilized by sonication is in an altered conformation that allows PCB addition.
We show here that the presence of PecE and PecF markedly increases the solubility of apo-HT-PecA and that the three proteins form a complex that can be isolated by affinity chromatography. A similar result is seen with holo-HT-PecA (Fig. 3, lanes 1 and 2). In contrast, when holo-HT-CpcA was purified from E. coli that also expressed CpcE and CpcF, the latter did not copurify with holo-HT-CpcA during affinity chromatography (19). PecF has four consecutive His residues at positions 19 to 22 (Fig. 5), and it is possible that added interaction of these residues with Ni2+-NTA agarose may contribute to the stability of the HT-PecA-PecE-PecF complex during chromatographic purification.
Jung et al. (14) established that in Anabaena sp. strain PCC7120 interposon mutagenesis of pecE, pecF, or pecE and pecF eliminates the formation of holo-PecA. They showed that ∼30% of the wild-type level of PecB was present in the phycobilisomes of all of the mutants. In contrast, holo-PecA was barely detectable in the pecE and pecF mutants, but it was present in the pecEF deletion mutant as a PCB-adduct in a 1:1 ratio with the PecB. Jung et al. confirmed the identity of this unnatural adduct by isolation of the subunit and amino-terminal sequencing.
Jung et al. (14) commented on these results as follows: “A testable hypothesis is that when the cognate lyase is missing, the phycocyanin α subunit lyase ′rescues' the phycoerythrocyanin α subunit by adding PCB. How is the absence of such a PCB adduct of PecA in the PecE and PecF mutants explained in the context of this hypothesis? When only PecE or PecF is present either of these components (or a hybrid PecE/CpcF or PecF/CpcE lyase) may form a complex with the Pec apo-α subunit which is thereby made unavailable to the CpcEF lyase.” Our demonstration of the formation of a PecA-PecE-PecF complex provides support for this speculation.
Holo-HT-PecA expressed in Anabena sp. strain PCC7120 and purified by immobilized metal affinity chromatography is recovered as a 1:1 complex with holo-PecB (Cai and Glazer, unpublished results). It is thus possible that in cyanobacteria release of holo-HT-PecA from the PecA-PecE-PecF complex is mediated by the formation of the holo-PecA-holo-PecB heterodimer.
The ability to express in heterologous hosts phycobiliprotein subunits with distinctive spectroscopic properties, such as holo-PecA (λmax, 561 nm; 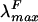 , 582 nm) and holo-CpcA (λmax, 625 nm;
, 582 nm) and holo-CpcA (λmax, 625 nm; 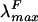 , 641 nm) (19), provides the potential for new fluorescent labels, which are valuable in cell biology, whose spectroscopic properties extend the range of wavelengths accessible to the widely used green fluorescent protein fusions (7, 13) and to the Discosoma red fluorescent protein (2).
, 641 nm) (19), provides the potential for new fluorescent labels, which are valuable in cell biology, whose spectroscopic properties extend the range of wavelengths accessible to the widely used green fluorescent protein fusions (7, 13) and to the Discosoma red fluorescent protein (2).
Acknowledgments
We are grateful to Arnold Falick and Sharleen Zhou of the HHMI Mass Spectrometry Laboratory of the Department of Molecular and Cell Biology at the University of California, Berkeley, for performing the mass spectrometric analyses. We thank Yuping A. Cai for helpful discussions and Cynthia Voong for her assistance.
This work was supported in part by a grant from the Lucille P. Markey Charitable Trust.
REFERENCES
- 1.Berkelman, T. R., and J. C. Lagarias. 1986. Visualization of bilin-linked peptides and proteins in polyacrylamide gels. Anal. Biochem. 156:194-201. [DOI] [PubMed] [Google Scholar]
- 2.Bevis, B. J., and B. S. Glick. 2002. Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed). Nat. Biotechnol. 20:83-87.11753367 [Google Scholar]
- 3.Bishop, J. E., H. Rapoport, A. V. Klotz, C. F. Chan, A. N. Glazer, P. Fuglistaller, and H. Zuber. 1987. Chromopeptides from phycoerythrocyanin. Structure and linkage of the three bilin groups. J. Am. Chem. Soc. 10:875-881. [Google Scholar]
- 4.Bryant, D. A. 1982. Phycoerythrocyanin and phycoerythrin: properties and occurrence in cyanobacteria. J. Gen. Microbiol. 128:835-844. [Google Scholar]
- 5.Bryant, D. A., A. N. Glazer, and F. A. Eiserling. 1976. Characterization and structural properties of the major biliproteins of Anabaena sp. Arch. Microbiol. 110:60-75. [DOI] [PubMed] [Google Scholar]
- 6.Cai, Y. A., J. T. Murphy, G. J. Wedemayer, and A. N. Glazer. 2001. Recombinant phycobiliproteins. Recombinant C-phycocyanins equipped with affinity tags, oligomerization, and biospecific recognition domains. Anal. Biochem. 290:186-204. [DOI] [PubMed] [Google Scholar]
- 7.Chan, F. K.-M., R. M. Siegel, D. Zacharias, R. Swofford, K. L. Holmes, R. Y. Tsien, and M. J. Lenardo. 2001. Fluorescence resonance energy transfer analysis of cell surface receptor interactions and signaling using spectral variants of the green fluorescent protein. Cytometry 44:361-368. [DOI] [PubMed] [Google Scholar]
- 8.Cornejo, J., R. D. Willows, and S. I. Beale. 1998. Phytobilin biosynthesis: cloning and expression of a gene encoding soluble ferredoxin-dependent heme oxygenase from Synechocystis sp. PCC6803. Plant J. 15:99-107. [DOI] [PubMed] [Google Scholar]
- 9.Fairchild, C. D., J. Zhao, J. Zhou, S. E. Colson, D. A. Bryant, and A. N. Glazer. 1992. Phycocyanin α subunit phycocyanobilin lyase. Proc. Natl. Acad. Sci. USA 89:7017-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairchild, C. D., and A. N. Glazer. 1994. Oligomeric structure, enzyme kinetics, and substrate specificity of the phycocyanin α subunit phycocyanobilin lyase. J. Biol. Chem. 269:8686-8694. [PubMed] [Google Scholar]
- 11.Frankenberg, N., K. Mukuogawa, T. Kohchi, and J. C. Lagarias. 2001. Functional genomic analysis of the Hy2 family of ferredoxin-dependent bilin reductases from oxygenic photosynthetic organisms. Plant Cell 13:965-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glazer, A. N. 1988. Phycobiliproteins. Methods Enzymol. 167:291-303. [DOI] [PubMed] [Google Scholar]
- 13.Heim, R., and R. Y. Tsien. 1996. Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr. Biol. 6:178-182. [DOI] [PubMed] [Google Scholar]
- 13a.Jimenez, C. R., L. Huang, Y. Qiu, and A. L. Burlingame. 1998. Mass spectrometry. In J. E. Coligan, B. M. Dunn, H. L. Ploegh, D. W. Speicher, and P. T. Wingfield (ed.), Current protocols in protein science. [on line.] Wiley Interscience, New York, N.Y. http://www3.interscience.wiley.com.
- 14.Jung, L. J., C. F. Chan, and A. N. Glazer. 1995. Candidate genes for the phycoerythrocyanin α subunit lyase. Biochemical analysis of pecE and pecF interposon mutants. J. Biol. Chem. 270:12877-12884. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko, T., Y. Nakamura, C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, M. Iriguchi, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, M. Kohara, M. Matsumoto, A. Matsuno, A. Muraki, N. Nakazaki, S. Shimpo, M. Sugimoto, M. Takazawa, M. Yamada, M. Yasuda, and S. Tabata. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC7120. DNA Res. 31:205-213. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura, Y., T. Kaneko, and S. Tabata. 2000. CyanoBase, the genome database for Synechocystis sp. strain PCC6803: status for the year 2000. Nucleic Acids Res. 28:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storf, M., A. Parbel, M. Meyer, B. Strohmann, H. Scheer, M.-G. Deng, M. Zheng, M. Zhou, and K.-H. Zhao. 2001. Chromophore attachment to biliproteins: specificity of PecE/PecF, a lyase-isomerase for the photoactive 31-Cys-α84-phycoviolobilin chromophore of phycoerythrocyanin. Biochemistry 40:12444-12456. [DOI] [PubMed] [Google Scholar]
- 19.Tooley, A. J., Y. A. Cai, and A. N. Glazer. 2001. Biosynthesis of a fluorescent cyanobacterial C-phycocyanin holo-α subunit in a heterologous host. Proc. Natl. Acad. Sci. USA 98:10560-10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams, V. P., and A. N. Glazer. 1978. Structural studies on phycobiliproteins. I. Bilin-containing peptides of C-phycocyanin. J. Biol. Chem. 253:202-211. [PubMed] [Google Scholar]
- 21.Zhao, K.-H., M.-G. Deng, M. Zheng, M. Zhou, A. Parbel, M. Storf, M. Meyer, B. Strohmann, and H. Scheer. 2000. Novel activity of a phycobiliprotein lyase: both the attachment of phycocyanobilin and the isomerization to phycobiliviolobilin are catalyzed by the proteins PecE and PecF encoded by the phycoerythrocyanin operon. FEBS Lett. 469:9-13. [DOI] [PubMed] [Google Scholar]