Abstract
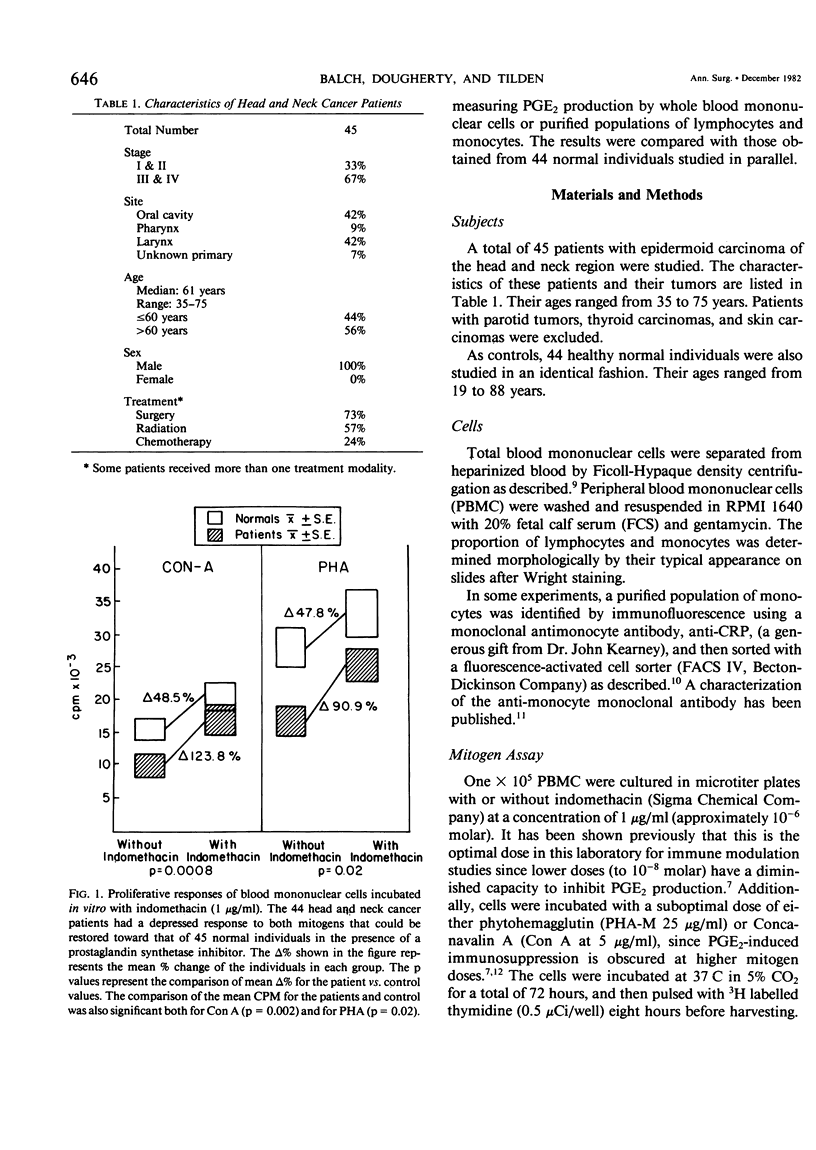
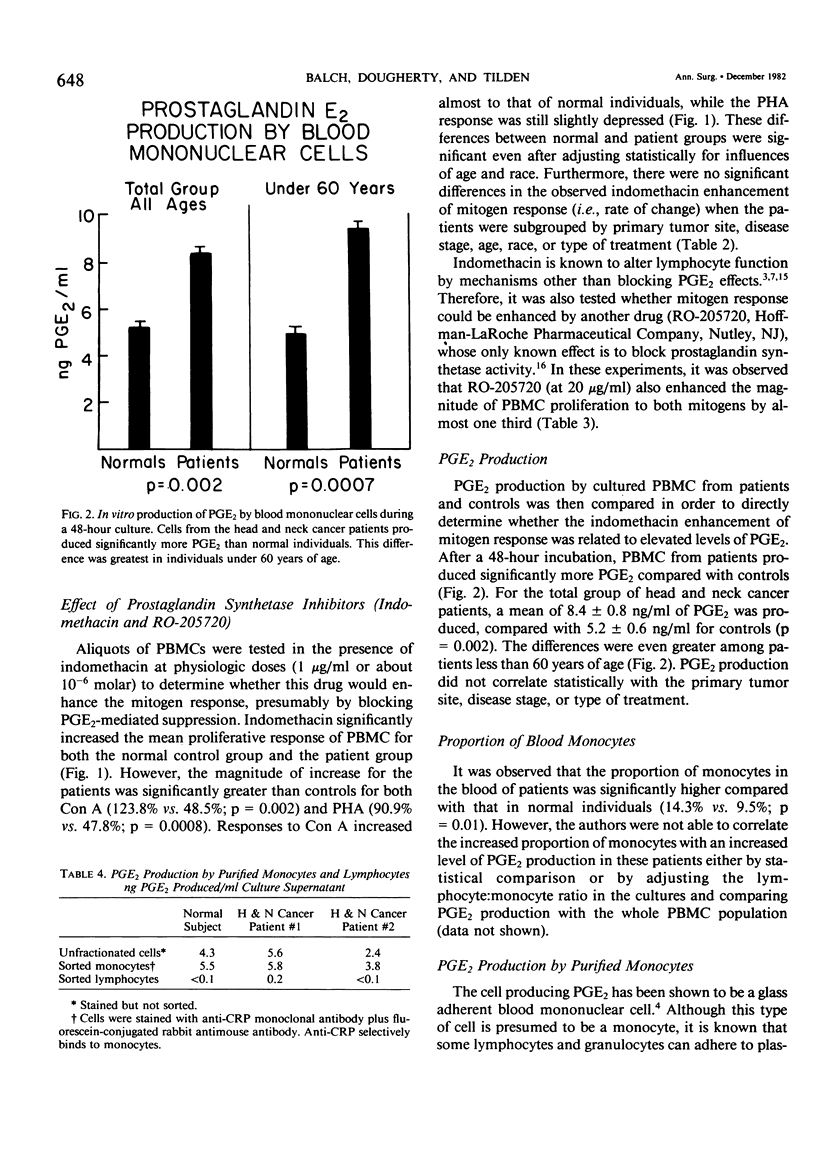
The proliferative response of peripheral blood mononuclear cells (PBMC) to the mitogens PHA and Con A significantly depressed in 86% of 45 head and neck cancer patients compared with 44 normal controls. This depression of immune competence was greatest in older patients and in those with more advanced disease stages. The abnormal mitogen responses could be restored toward normal (especially with Con A stimulation) by incubating the cells with either of two prostaglandin synthetase inhibitors (indomethacin or RO-205720). This augmentation of immune response was independent of other factors, including the primary tumor site, disease stage, treatment (surgery, radiation therapy, or chemotherapy) or the patients's age or race. The most likely explanation for this depressed level of immunocompetence was an excessive production of PGE2 by suppressor cells. This was confirmed by the finding that PBMC from patients produced more PGE2 than PBMC from normal individuals (8.4 ng/ml vs. 5.2 ng/ml, p=0.002). This difference was greatest among patients less than 60 years of age whose cultured PBMC produced 91% more PGE2 than controls (p less than 0.0007). Virtually all of the PGE2 was produced by a population of monocytes defined by a monoclonal antibody and purified with a fluorescence-activated cell sorter. Patients with epidermoid cancer of the head and neck thus have an abnormality of immunoregulatory monocytes that can contribute significantly to their depression of cellular immunity by elaborating prostaglandin E2. This abnormality could be partially corrected in vitro by incubating their PMBC with a prostaglandin synthetase inhibitor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch C. M., Ades E. W., Loken M. R., Shore S. L. Human "null" cells mediating antibody-dependent cellular cytotoxicity express T lymphocyte differentiation antigens. J Immunol. 1980 Apr;124(4):1845–1851. [PubMed] [Google Scholar]
- Balch C. M., Dougherty P. A., Dagg M. K., Diethelm A. G., Lawton A. R. Detection of human T cells using anti-monkey thymocyte antisera. Tissue distribution and evidence for antigenic heterogeneity. Clin Immunol Immunopathol. 1977 Nov;8(3):448–460. doi: 10.1016/0090-1229(77)90008-3. [DOI] [PubMed] [Google Scholar]
- Balch C. M. Recent advances in human cellular immunobiology. Surg Clin North Am. 1979 Apr;59(2):235–252. doi: 10.1016/s0039-6109(16)41783-4. [DOI] [PubMed] [Google Scholar]
- Braun W., Ishizuka M. Antibody formation: reduced responses after administration of excessive amounts of nonspecific stimulators. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1114–1116. doi: 10.1073/pnas.68.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunda M. J., Herberman R. B., Holden H. T. Inhibition of murine natural killer cell activity by prostaglandins. J Immunol. 1980 Jun;124(6):2682–2687. [PubMed] [Google Scholar]
- Droller M. J., Schneider M. U., Perlmann P. A possible role of prostaglandins in the inhibition of natural and antibody-dependent cell-mediated cytotoxicity against tumor cells. Cell Immunol. 1978 Aug;39(1):165–177. doi: 10.1016/0008-8749(78)90091-6. [DOI] [PubMed] [Google Scholar]
- Fulton A. M., Levy J. G. The possible role of prostaglandins in mediating immune suppression by nonspecific T suppressor cells. Cell Immunol. 1980 Jun;52(1):29–37. doi: 10.1016/0008-8749(80)90397-4. [DOI] [PubMed] [Google Scholar]
- Gaut Z. N., Baruth H., Randall L. O., Ashley C., Paulsrud J. R. Stereoisomeric relationships among anti-inflammatory activity, inhibition of platelet aggregation, and inhibition of prostaglandin synthetase. Prostaglandins. 1975 Jul;10(1):59–66. doi: 10.1016/0090-6980(75)90093-3. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Peake G. T. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978 Oct;62(4):753–760. doi: 10.1172/JCI109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P. Sensitivity of lymphocytes to prostaglandin E2 increases in subjects over age 70. J Clin Invest. 1979 Aug;64(2):434–439. doi: 10.1172/JCI109480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Han T., Takita H. Indomethacin-mediated enhancement of lymphocyte response to mitogens in healthy subjects and lung cancer patients. Cancer. 1980 Dec 1;46(11):2416–2420. doi: 10.1002/1097-0142(19801201)46:11<2416::aid-cncr2820461120>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Koopman W. J., Gillis M. H., David J. R. Prevention of MIF activity by agents known to increase cellular cyclic AMP. J Immunol. 1973 Jun;110(6):1609–1614. [PubMed] [Google Scholar]
- Plescia O. J., Smith A. H., Grinwich K. Subversion of immune system by tumor cells and role of prostaglandins. Proc Natl Acad Sci U S A. 1975 May;72(5):1848–1851. doi: 10.1073/pnas.72.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Pavlidis N. A., Stylos W. A., Chirigos M. A. Regulation of macrophage tumoricidal function: a role for prostaglandins of the E series. Science. 1978 Oct 20;202(4365):320–321. doi: 10.1126/science.694537. [DOI] [PubMed] [Google Scholar]
- Shen T. Y., Winter C. A. Chemical and biological studies on indomethacin, sulindac and their analogs. Adv Drug Res. 1977;12:90–245. [PubMed] [Google Scholar]
- Taffet S. M., Russell S. W. Macrophage-mediated tumor cell killing: regulation of expression of cytolytic activity by prostaglandin E. J Immunol. 1981 Feb;126(2):424–427. [PubMed] [Google Scholar]
- Tilden A. B., Balch C. M. Immune modulatory effects of indomethacin in melanoma patients are not related to prostaglandin E2-mediated suppression. Surgery. 1982 Sep;92(3):528–532. [PubMed] [Google Scholar]
- Tilden A. B., Balch C. M. Indomethacin enhancement of immunocompetence in melanoma patients. Surgery. 1981 Jul;90(1):77–84. [PubMed] [Google Scholar]
- Wanebo H. J., Jun M. Y., Strong E. W., Oettgen H. T-cell deficiency in patients with squamous cell cancer of the head and neck. Am J Surg. 1975 Oct;130(4):445–451. doi: 10.1016/0002-9610(75)90482-1. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Osheroff P. L. Antigen stimulation of prostaglandin synthesis and control of immune responses. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1300–1304. doi: 10.1073/pnas.73.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]


