Abstract
The diphtheria toxin repressor (DtxR) uses Fe2+ as a corepressor and inhibits transcription from iron-regulated promoters (IRPs) in Corynebacterium diphtheriae. A new IRP, designated IRP6, was cloned from C. diphtheriae by a SELEX-like procedure. DtxR bound to IRP6 in vitro only in the presence of appropriate divalent metal ions, and repression of IRP6 by DtxR in an Escherichia coli system was iron dependent. The open reading frames (ORFs) downstream from IRP6 and previously described promoter IRP1 were found to encode proteins homologous to components of ATP-binding cassette (ABC) transport systems involved in high-affinity iron uptake in other bacteria. IRP1 and IRP6 were repressed under high-iron conditions in wild-type C. diphtheriae C7(β), but they were expressed constitutively in C7(β) mutant strains HC1, HC3, HC4, and HC5, which were shown previously to be defective in corynebactin-dependent iron uptake. A clone of the wild-type irp6 operon (pCM6ABC) complemented the constitutive corynebactin production phenotype of HC1, HC4, and HC5 but not of HC3, whereas a clone of the wild-type irp1 operon failed to complement any of these strains. Complementation by subclones of pCM6ABC demonstrated that mutant alleles of irp6A, irp6C, and irp6B were responsible for the phenotypes of HC1, HC4, and HC5, respectively. The irp6A allele in HC1 and the irp6B allele in HC5 encoded single amino acid substitutions in their predicted protein products, and the irp6C allele in HC4 caused premature chain termination of its predicted protein product. Strain HC3 was found to have a chain-terminating mutation in dtxR in addition to a missense mutation in its irp6B allele. These findings demonstrated that the irp6 operon in C. diphtheriae encodes a putative ABC transporter, that specific mutant alleles of irp6A, irp6B, and irp6C are associated with defects in corynebactin-dependent iron uptake, and that complementation of these mutant alleles restores repression of corynebactin production under high-iron growth conditions, most likely as a consequence of restoring siderophore-dependent iron uptake mediated by the irp6 operon.
Corynebacterium diphtheriae is the gram-positive bacterium that causes diphtheria. Most isolates of C. diphtheriae from patients with respiratory diphtheria produce diphtheria toxin (DT), which catalyzes the NAD-dependent ADP ribosylation of elongation factor 2 and causes inhibition of protein synthesis and death in cells from humans or susceptible animals. DT is encoded by temperate phages, which are present in all toxin-producing strains of C. diphtheriae.
The diphtheria toxin repressor (DtxR), originally identified as a repressor of the gene that encodes DT, is now known to function as global regulator of metabolism in C. diphtheriae. Representative functions that are negatively regulated by iron are production of DT, synthesis of a siderophore (corynebactin), corynebactin-dependent iron uptake, and utilization of iron from heme. Although the physiological role of DtxR in C. diphtheriae is similar to that of the ferric uptake regulator protein (Fur) in several gram-negative bacteria, DtxR differs from Fur in structure and cannot substitute for Fur in function. DtxR is therefore the prototype for a novel class of metal-activated transcriptional regulators. Homologs of DtxR are present in a diverse and rapidly growing group of bacteria, as summarized by Feese et al. (5).
DtxR is synthesized as a 226-amino-acid (aa) polypeptide that forms dimers, utilizes Fe2+ as a corepressor, and binds to specific operator sequences in iron-regulated promoters (IRPs), thereby inhibiting transcription of downstream genes. Seven DtxR-specific, iron-regulated promoters in C. diphtheriae, including the tox promoter, IRP1, IRP2, IRP3, IRP4, IRP5, and the hmuO promoter, have been reported to date (10, 11, 21, 25). IRP1 to IRP5 were isolated by shotgun cloning of genomic fragments into promoterless lacZ reporter plasmid pQF50, followed by screening for promoters that are repressed by iron in the presence of DtxR in an Escherichia coli system. The open reading frame (ORF) immediately downstream from IRP1 encodes a lipoprotein that is homologous with FhuD, the ferrichrome binding protein from Bacillus subtilis, which is involved in ferrichrome transport (26, 28). The ORF downstream from IRP3 encodes a homolog of AraC-type transcriptional activators, which suggests that a multilevel network of iron-dependent regulation may occur in C. diphtheriae (11). The products of ORFs downstream from promoters IRP2, IRP4, and IRP5 do not show significant homologies to known proteins (11, 25). The hmuO gene, which encodes a heme oxygenase, was isolated by complementation of heme utilization in C. diphtheriae and Cornynebacterium ulcerans mutants (20, 21). The hmuO promoter is negatively regulated by iron and DtxR and positively regulated by heme (21).
C. diphtheriae, like many other pathogens, is capable of utilizing various forms of iron in host environments where the concentration of free iron ions is extremely low. Heme uptake (HmuTUV) (3) and utilization (HmuO) (20, 21, 22) systems are required for C. diphtheriae to acquire iron from heme or hemoglobin, and corynebactin is required for C. diphtheriae to assimilate nonheme iron during growth under low-iron conditions (17, 18). Partially purified corynebactin from C. diphtheriae did not react in chemical tests for catechol- and hydroxymate-type siderophores (18), and the structures of corynebactin and the components of the corynebactin-dependent iron uptake system have not been determined. C. diphtheriae can also utilize aerobactin from Shigella flexneri for siderophore-dependent iron uptake (17, 18). Another siderophore from Corynebacterium glutamicum, also called corynebactin, was analyzed by nuclear magnetic resonance spectroscopy and shown to be a catechol-type siderophore (1), but the structural relationships between the corynebactins from C. diphtheriae and C. glutamicum are not yet established.
Two types of mutants that are defective in corynebactin-dependent iron uptake were isolated after chemical mutagenesis of C. diphtheriae C7(β) (2, 18). The first mutant type, represented by strain HC6, does not produce corynebactin even under low-iron conditions and appears to be deficient in siderophore production (18). Interestingly, the PW8 strain of C. diphtheriae, used commercially to produce DT for conversion to diphtheria toxoid for vaccines, also lacks the ability to produce corynebactin (17). In contrast, mutants of the second type, represented by strains HC1, HC3, HC4, and HC5, overproduce siderophore even under high-iron growth conditions and exhibit much lower rates of corynebactin-dependent 59Fe3+ uptake under low-iron conditions than does the parental strain, C7(β) (2). Therefore, the utilization of iron from ferric corynebactin appears to be impaired in these mutants. Attempts to complement the mutant phenotype of strains HC1, HC3, HC4, and HC5 with a plasmid clone containing the whole irp1 operon were unsuccessful (26), suggesting that mutations in the irp1 operon are not responsible for the defects in corynebactin-dependent iron uptake in C. diphtheriae strains HC1, HC3, HC4, and HC5.
Here, we describe the cloning and characterization of a new DtxR-dependent IRP from C. diphtheriae, called IRP6, by using a SELEX (systematic evolution of ligands by exponential enrichment)-like selection method that is similar to procedures used successfully with other organisms for isolation of specific target sequences in DNA (13, 33). The gene products of the corresponding irp6 operon were found to be homologous with components of ATP-binding cassette (ABC) transporters. We show by complementation analysis that the wild-type irp6 operon corrects the defects in repression of siderophore production by iron in C. diphtheriae strains HC1, HC4, and HC5 and that strain HC3 has mutations in both the irp6 operon and the dtxR gene. We also completed the sequencing of the irp1 operon and demonstrated that it encodes another putative ABC transport system in C. diphtheriae, whose function is currently unknown.
MATERIALS AND METHODS
Bacterial strains, plasmids, growth conditions, and β-galactosidase assays.
Strains of E. coli and C. diphtheriae and plasmids used in this study are listed in Table 1. E. coli strains were routinely cultured in Luria-Bertani (LB) broth (19), and C. diphtheriae strains were routinely grown in heart infusion broth (Difco, Detroit, Mich.) with 0.2% Tween 80 (HITW). C. diphtheriae strains were grown in the modified PGT medium described by Tai et al. for siderophore assay experiments (30). All bacterial strains in broth media were grown at 37°C with shaking. Antibiotics or other supplements were added at the following concentrations: ampicillin, 100 mg/liter; kanamycin, 25 mg/liter; 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), 40 mg/liter; chloramphenicol, 10 mg/liter for E. coli and 2 mg/liter for C. diphtheriae. To create iron-limiting conditions for C. diphtheriae strains in HITW, iron chelator ethylenediamine-di(o-hydroxylphenyl) acetic acid (EDDA) was added at a final concentration of 100 μg/ml. For β-galactosidase activity assays with E. coli strains, LB broth was first deferrated by treatment with Chelex-100 (Bio-Rad, Hercules, Calif.) at 10 g/liter and then either FeCl3 at 10 μM or EDDA at 200 μg/ml was added to create high-iron or low-iron growth conditions, respectively. For β-galactosidase activity assays with C. diphtheriae strains, untreated HITW was used for high-iron growth conditions and HITW supplemented with EDDA at 100 μg/ml was used for low-iron growth conditions. HITW with added FeCl3 at 20 μM (HITW-Fe) was also used in some experiments to examine the effects of excess iron on the activity of IRPs in mutant strains of C. diphtheriae.
TABLE 1.
Strains and plasmids used in this study
| Strain(s) or plasmid | Description | Reference or source |
|---|---|---|
| C. diphtheriae strains | ||
| C7 | Wild-type strain, nonlysogenic, nontoxinogenic | 7 |
| C7(β) | Wild-type reference strain, tox+, lysogenic for phage β | 7 |
| HC1, HC3, HC4, and HC5 | Siderophore uptake mutants derived from C7(β) | 2 |
| Plasmids | ||
| pQF50 | Ampr; E. coli reporter vector containing a promoterless lacZ gene | 4 |
| pQF1 | pQF50 derivative containing IRP1 promoter | 25 |
| pQF6 | pQF50 derivative containing IRP6 promoter | This study |
| pQF1- | Reverse orientation of promoter fragment versus pQF1 | This study |
| pQF6- | Reverse orientation of promoter fragment versus pQF6 | This study |
| pSK6a | Library clone containing a 1.9-kb EcoRI insert of irp6 region | This study |
| pSK6e | Library clone containing a 2.2-kb EcoRI insert of irp6 region | This study |
| pCM2.6 | Shuttle vector for C. diphtheriae | 23 |
| pCM6ABC | pCM2.6 derivative containing a 3.6-kb SalI-BamHI insert of the irp6 operon | This study |
| pCM6AB | pCM2.6 derivative containing a 2.9-kb SalI-BamHI insert containing the irp6A-irp6B region of the irp6 operon | This study |
| pCM6A | pCM2.6 derivative carrying a 1.9-kb SalI-BamHI insert containing the irp6A region of the irp6 operon | This study |
| pNGR-6ABC | Kmr; shuttle plasmid containing the dtxR gene and the irp6 operon | This study |
| pWR382 | Ampr; contains an 8.2-kb EcoRI insert of irp1 operon | 26 |
| pDSK29 | Kmr; contains the dtxR gene | 23 |
Assays for β-galactosidase activities were performed as described previously (23, 25), with some modifications for C. diphtheriae strains. Briefly, approximately 2 × 107 cells from overnight C. diphtheriae cultures were collected and resuspended in 1 ml of buffer Z (0.06 M Na2HPO4 · 7H2O, 0.04 M NaH2PO4 · H2O, 0.01 M MgSO4 · 7H2O, 0.05 M β-mercaptoethanol, pH 7.0). Cells were permeabilized by mixing them with 40 μl of 0.1% sodium dodecyl sulfate-80 μl of chloroform on a vortexer for 10 s. Reactions were initiated by adding 200 μl of o-nitrophenyl-β-d-galactopyranoside (ONPG; 4 mg/ml). When yellow was visible, the incubation times were recorded and the reactions were stopped by adding 500 μl of 1 M Na2CO3. For samples that did not turn yellow, reactions were terminated after 1 h. The clear aqueous phase was collected by centrifugation and used for A420 measurements. Miller units were used for enzymatic activity.
DNA preparation, cloning, and sequencing.
Chromosomal DNA from C. diphtheriae strains was purified by cesium chloride gradient ultracentrifugation. The sequence of IRP6 was determined at the automated DNA sequencing facility in the Department of Biochemistry at Colorado State University, Fort Collins, Colo. The sequences of ORFs downstream from IRP6 and IRP1 and of mutant alleles of genes irp6A, irp6B, and irp6C in the irp6 operon were determined at the University of Colorado Cancer Center DNA Sequencing and Analysis Core Facility, Denver, Colo. Clone Manager software was used to identify ORFs, and BLAST searches were used to search for proteins in the National Center for Biotechnology Information database that are homologous to predicted proteins encoded by ORFs.
Cycle selection procedure.
Chromosomal DNA of C. diphtheriae strain C7 was partially digested with HhaI or HpaII, and DNA fragments of approximately 150 to 600 bp were isolated by 1.5% agarose gel electrophoresis. Single-stranded oligonucleotides for linkers (Table 2) containing an HhaI- or HpaII-compatible protruding end, a SpeI site, and an additional BamHI or XbaI cloning site were prepared by GIBCO-BRL (Gaithersburg, Md.). After the annealing of the complementary oligonucleotides and phosphorylation, 40 pmol of the appropriate linker was ligated to 4 pmol of HhaI- or HpaII-digested chromosomal DNA. After digestion with SpeI to eliminate multimeric linkers, the resulting DNA fragments were gel purified and amplified by PCR using linker-specific primer PAS-A or PAS-B (Table 2), as appropriate. A single PCR primer was used in each reaction because the complementary sequence was present on both ends of the template DNA fragments. To isolate DNA fragments capable of binding to DtxR, 2 μg of each purified PCR product was mixed with 500 μl of binding buffer (20 mM Na2HPO4 [pH 7.0], 50 mM NaCl, 5 mM MgCl2, 100 μg of bovine serum albumin/ml, 10 μg of sonicated salmon sperm DNA/ml, 10% glycerol, and 300 μM CoSO4) and purified DtxR at 200 nM. After incubation of the reaction mixture for 30 min at room temperature, 30 μl of rabbit anti-DtxR serum or a monoclonal antibody against the third domain of DtxR was added, and incubation was continued for another 30 min at 37°C. The mixture was then loaded onto a HiTrap protein G column (Pharmacia, Uppsala, Sweden) equilibrated with the binding buffer. The column was washed with 3 ml of binding buffer, and the bound DNA-DtxR-antibody complexes were eluted with 1.5 ml of 0.1 M glycine-HCl buffer (pH 2.5). Fractions of 200 μl were collected and immediately neutralized by the addition of 20 μl of 1 M Tris-HCl (pH 9.0). The DNA-containing fractions, detected by PCR amplification using primers specific for appropriate linker sequences, were combined. The amplified DNA fragments were then subjected to two more rounds of DtxR binding and PCR amplification, digested with BamHI or XbaI as appropriate, and cloned into the BamHI or XbaI site of vector pQF50 (4).
TABLE 2.
Linkers and primers used for cycle selection
| Method | Sequence of linker or primera |
|---|---|
| HhaI selection | |
| Linker | BamHISpeI |
| 5′CGGATCCACTCTTGACCTCGACTAGTGCA3′ | |
| 3′GCGCCTAGGTGAGAACTGGAGCTGATCACGT5′ | |
| (HhaI) | |
| PCR primer PAS-A | 3′TAGGTGAGAACTGGAGCT5′ |
| HpaII selection | |
| Linker | (HpaII) XbaISpeI |
| 5′CGGATCTAGACTTGACCTCGACTAGTGCA3′ | |
| 3′CTAGATCTGAACTGGAGCTGATCACGT5′ | |
| PCR primer PAS-B | 3′TAGATCTGAACTGGAGCT5′ |
Boldface letters indicate the identical sequence between the linker and its specific PCR primer. Underlined letters represent target sites for indicated restriction enzymes, and protruding ends of linkers are compatible with DNA fragments generated by restriction enzymes shown in parentheses.
Gel mobility shift and footprinting assays.
DNA fragments carrying the IRP1 or IRP6 promoter/operator were end-labeled with [α-32P]dCTP by using Klenow fragments (GIBCO-BRL). The labeled DNA fragments at approximately 0.5 nM were incubated with various concentrations of purified DtxR in 10 μl of binding buffer (described in “Cycle selection procedure”). For mobility shift assays, the reaction mixtures were incubated for 10 to 15 min at room temperature and then subjected to electrophoresis on 5% nondenaturing polyacrylamide gels at 4°C in 20 mM Na2HPO4 (pH 7.0) buffer for 1 to 1.5 h at 70 V. For footprinting assays, 1 μl of DNase I (GIBCO-BRL) at 10 ng/ml was added to each DtxR-DNA mixture at the end of the room temperature incubation. The reaction mixtures were incubated for another 1 to 3 min, at which time the DNase I treatment was terminated by phenol-chloroform extraction. The resulting DNA samples were subjected to electrophoresis on a sodium dodecyl sulfate-8% polyacrylamide gel at 70 to 90 V for 2 to 2.5 h at room temperature, and the gels were then dried and analyzed by autoradiography.
Siderophore assays.
Overnight cultures of C. diphtheriae strains grown in modified PGT medium with or without 10 μM FeCl3 were harvested by centrifugation, and the supernatants were used for siderophore assays. Chrome azurol S (CAS) assays were carried out by a slight modification of a previously described method (30). Each 500-μl sample of appropriately diluted culture supernatant was mixed with an equal volume of CAS assay buffer. The reaction mixtures were incubated for 2 h at room temperature, and their absorbance values at 630 nm were measured. A linear standard curve was constructed by testing 500-μl samples containing EDDA at concentrations of 5, 10, 20, and 40 μM. A sample of supernatant contained 1 U of siderophore/ml if its absorbance at 630 nm was equal to that of test sample containing 1 μM EDDA.
Inverse PCR.
C. diphtheriae strain C7 chromosomal DNA was completely digested with HindIII and purified by phenol-chloroform extraction. Approximately 1.2 μg of HindIII DNA fragments was self-ligated with 5 U of T4 ligase in a final reaction volume of 250 μl, and a 0.2-μl sample of the ligation mixture was mixed with appropriate primers (Table 3) and then used for each inverse PCR using Elongase enzyme mixture (GIBCO-BRL) with appropriate Mg2+ concentrations as recommended by the manufacturer.
TABLE 3.
Primers used for PCR or inverse PCR in irp6 cloning and sequencing
| Primer | Sequence (5′-3′) |
|---|---|
| 6C2 | GCCGATGGAGGCTGCGAC |
| 6C3 | AGACCTCGACATGCTCGG |
| 6D2 | GGACTTCCGCCAGAAGCC |
| 6E1 | CCTGCGCCATCAGGTGGA |
| 3Q2 | CGGATCCCTCCTAGGGAT |
| 6A | AGGATCCGGCCCCACCACAGTGG |
| 6AB | CGGATCCCAGTGGCACTGGGCCA |
Nucleotide sequence accession numbers.
The GenBank accession number for the nucleotide sequence of the irp6 operon is AY061890. The sequence that contains an additional three ORFs downstream from the original irp1 ORF was submitted to GenBank, and the accession number is AF176902.
RESULTS
Isolation of IRP6 from the chromosome of C. diphtheriae by a SELEX-like procedure.
As part of an ongoing effort in our laboratory to identify and characterize the DtxR-regulated genes of C. diphtheriae, DNA fragments with potential DtxR binding sites were isolated from C. diphtheriae chromosomal DNA by a SELEX-like procedure. DNA fragments that bind to purified holo-DtxR can be enriched exponentially, in principle, by forming DNA-DtxR complexes, purifying the complexes by use of anti-DtxR antibodies and a protein G column, using PCR to amplify the DNA fragments isolated from the complexes, and repeating the cycle several times.
DNA fragments collected after three cycles of this SELEX-like procedure in several independent experiments were approximately 200 bp. They were digested with BamHI or XbaI, as appropriate, and cloned into pQF50 upstream from the promoterless lacZ gene. The resulting plasmid clones were transformed into E. coli strain DH5α containing pDSK29 (dtxR+). To identify promoters that were negatively regulated by DtxR under high-iron conditions, transformants were first plated on low-iron LB agar medium containing X-Gal plus ampicillin and kanamycin to maintain positive selection for the two compatible plasmids. Colonies showing various degrees of blue, indicating that they contained cloned promoters that were active under low-iron conditions, were then picked and transferred onto high-iron LB agar plates containing X-Gal plus ampicillin and kanamycin. Colonies that were white under these high-iron conditions were considered to contain putative DtxR-regulated, iron-repressible promoters. Over 2,000 separate transformants were tested, and approximately 100 colonies that exhibited some degree of blue only under low-iron growth conditions were examined further by β-galactosidase assays after subculturing them in LB broth under high-iron and low-iron conditions. Isolates with promoters that were not consistently repressible by DtxR and iron were eliminated, as were clones that gave positive signals in Southern blots with DNA probes for the previously known IRPs. Two clones containing newly identified IRPs were identified in this manner. One of these, pQF6, containing an approximately 200-bp BamHI fragment, designated IRP6, is the subject of the present study.
IRP6 promoter activity and its regulation by DtxR and iron in E. coli.
Expression of β-galactosidase from pQF6 in E. coli DH5α containing or lacking dtxR+ plasmid pDSK29 and after growth in high-iron or low-iron LB broth was examined (Table 4). Vector pQF50 and plasmid pQF1, which contained previously reported iron-regulated promoter/operator IRP1, were included as negative and positive controls, respectively. The β-galactosidase activity from pQF50 was barely detectable under any of the conditions tested and was considered to represent the baseline value for the assay system. The promoter strength of IRP6 was comparable to that of IRP1, as shown by the similar β-galactosidase activities from pQF6 and pQF1 in the absence of DtxR under low-iron or high-iron growth conditions. The activity of IRP6 was repressed to the baseline level by iron and DtxR, and the ratio of β-galactosidase activity from pQF6 under derepressing (low-iron) conditions to that under repressing (high-iron) conditions was 56. IRP6 was fully derepressed under low-iron conditions in the presence of DtxR (78.8 U of β-galactosidase activity), but IRP1 was only slightly derepressed under similar conditions (6.8 U of β-galactosidase activity). These findings raised the possibility that IRP6 has significantly lower affinity for holo-DtxR than does IRP1. To determine whether the inserts in pQF1 and pQF6 contained unidirectional or bidirectional promoters, they were excised and recloned in the opposite orientation into pQF50, generating plasmids pQF1- and pQF6-, respectively. The β-galactosidase activities from pQF1- or pQF6- in the presence or absence of pDSK29 (dtxR+) and under high-iron or low-iron growth conditions were all comparable to the baseline level for the assay system, demonstrating that DNA fragments containing both IRP1 and IRP6 have unidirectional promoter activity.
TABLE 4.
Promoter activities of IRP6 in the presence or absence of DtxR in an E. coli systema
| Plasmid | Iron level | β-Galactosidase activity (Miller units)
|
|
|---|---|---|---|
| − dtxR | + dtxR | ||
| pQF50 | Low | 0.9 ± 0.2 | 1.2 ± 0.3 |
| High | 0.7 ± 0.1 | 1.0 ± 0.2 | |
| pQF6 | Low | 87.7 ± 9.1 | 78.8 ± 8.6 |
| High | 83.0 ± 0.3 | 1.4 ± 0.2 | |
| pQF6- | Low | 1.4 ± 0.3 | 1.6 ± 0.6 |
| High | 1.2 ± 0.3 | 0.9 ± 0.0 | |
| pQF1 | Low | 84.7 ± 8.7 | 6.8 ± 2.4 |
| High | 69.7 ± 6.4 | 0.8 ± 0.2 | |
| pQF1- | Low | 0.5 ± 0.1 | 0.8 ± 0.2 |
| High | 0.5 ± 0.0 | 0.5 ± 0.2 | |
Cells were grown in LB medium treated with Chelex-100 resin, and overnight cultures were used for β-galactosidase activity assays. Low iron, addition of 200 μg of EDDA/ml; high iron, addition of 10 μM FeCl3. Values represent the means ± standard deviations of assays performed on cultures grown in triplicate. −dtxR, without a plasmid that carries dtxR; +dtxR, with a plasmid that carries dtxR.
Binding of DtxR to IRP6.
The DtxR-binding activity of IRP6 was investigated by gel mobility shift assays and DNase I protection assays using previously characterized DtxR-regulated promoter/operator IRP1 as a control. As shown in Fig. 1A, retardation of DNA fragments containing IRP1 and IRP6 required both DtxR at 0.1 or 0.5 μM and Co2+ at 300 μM. To compare the relative affinities of IRP6 and IRP1 for DtxR, additional gel mobility shift assays were performed with DtxR at concentrations ranging from 0 to 2,000 nM in the presence of 300 μM Co2+ (Fig. 1B). For IRP6, the lowest DtxR concentration required to show a clearly detectable mobility shift was 100 nM. In contrast, the DNA fragment containing IRP1 showed a clearly detectable mobility shift in the presence of as little as 5 nM DtxR. These findings indicate that DtxR has significantly higher affinity for IRP1 than for IRP6 in vitro, consistent with the results of the β-galactosidase activity assays for the E. coli system.
FIG. 1.
(A) DtxR binding of IRP6 requires a divalent transitional metal ion, such as Co2+. (B) Effect of DtxR concentration on formation of DtxR-DNA complexes in gel mobility shift assays. Detectable shifts of IRP6 occurred at 100 nM DtxR, while detectable shifts of IRP1 started at 5 nM DtxR. Co2+ was present in all samples at 300 μM.
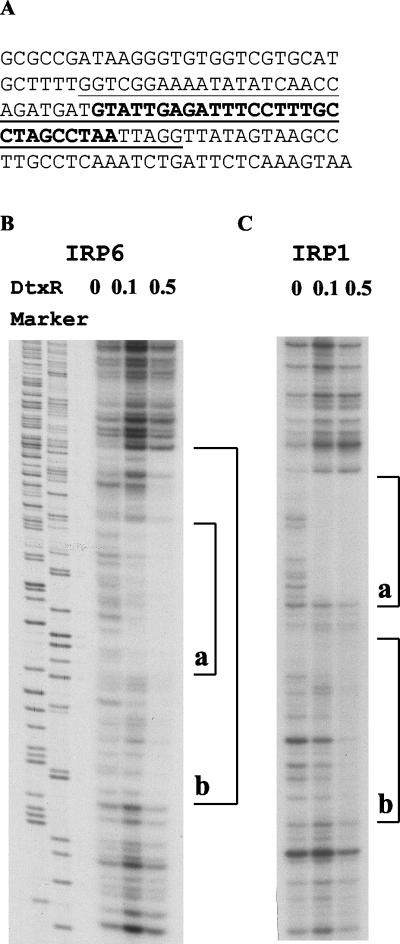
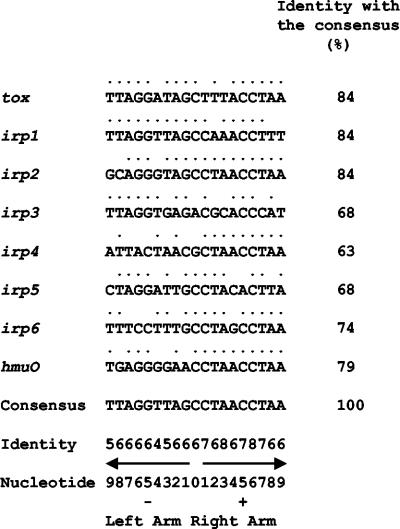
To identify the DtxR-binding region of IRP6, the nucleotide sequence of IRP6 was determined (Fig. 2A ) and DtxR footprinting experiments were performed with DNase I (Fig. 2B). Purified DtxR at 100 nM protected a 28-bp region in IRP6 from digestion by DNase I, but with DtxR at 500 nM the protected region extended further in both directions to include a total of 60 bp. With IRP1, DtxR at 100 nM protected a 32-bp sequence, and at 500 nM DtxR the protected region extended further in one direction only to include a total of 63 bp. These are the first reported examples of DtxR footprints that vary in size as a function of the concentration of DtxR. The putative 19-bp core sequence of the primary, high-affinity DtxR binding site in IRP6 was compared with the previously reported DtxR-specific operators from tox, IRP1, IRP2, IRP3, IRP4, IRP5, and hmuO (12) (Fig. 3). The core sequence of the IRP6 operator matched the 19-bp consensus sequence at 14 positions (74% identity), and the consensus sequence for the core DtxR binding site, based on all eight of the currently known DtxR-regulated promoters/operators in Fig. 3, was identical with the previously deduced consensus sequence. The nucleotides in the right arm of the core region were slightly more conserved than those in the left arm, and cytidylic acid at position +6 was the only invariant nucleotide within the core regions of these eight DtxR-regulated promoters/operators. No sequences that were highly homologous with the consensus DtxR-binding sequence were found within the sequences of IRP1 and IRP6 that were protected only in the presence of high concentrations of DtxR, and the characteristics that define low-affinity DtxR binding sites have not yet been determined.
FIG. 2.
(A) Nucleotide sequence of IRP6. The primary DtxR binding site defined by DNase I footprinting assays with DtxR at 0.1 μM is indicated by boldface. The longer nucleotide sequence protected from DNase I by 0.5 μM DtxR is underlined and includes the primary DtxR binding site. (B and C) DNase I footprinting assays. All the fragments were 3′ end labeled with [α-32P]dCTP on one strand and incubated in the presence of Co2+ (300 μM) and DtxR (0, 0.1, or 0.5 μM). Brackets indicate the sequences protected by DtxR from DNase I digestion. (B) With IRP6, a longer 60-bp sequence (b) was protected by 0.5 μM DtxR and a shorter 28-bp sequence (a) that is contained within sequence b was significantly protected by 0.1 μM DtxR. Although the total radioactivity loaded in the lane with 0.1 μM DtxR was greater than that in the other lanes, the intensity of the bands within the 28-bp (a) sequence was decreased significantly both in comparison withbands in other regions of the same lane and with bands in the same 28-bp region in the lane without DtxR. (C) In IRP1, two contiguous regions (a and b) were protected by 0.5 μM DtxR but only region a was protected by 0.1 μM DtxR.
FIG. 3.
Compilation of the 19-mer core sequences of DtxR binding sites and the consensus sequence. Dots above nucleotides indicate that the nucleotide matches the nucleotide of the consensus sequence. The identity score indicates the numbers of times that nucleotide was found among all the aligned DtxR binding sites. Arrows, inverted repeats within the core consensus sequence. The identity of each DtxR binding site with the consensus is indicated.
Identification of an operon downstream from IRP6.
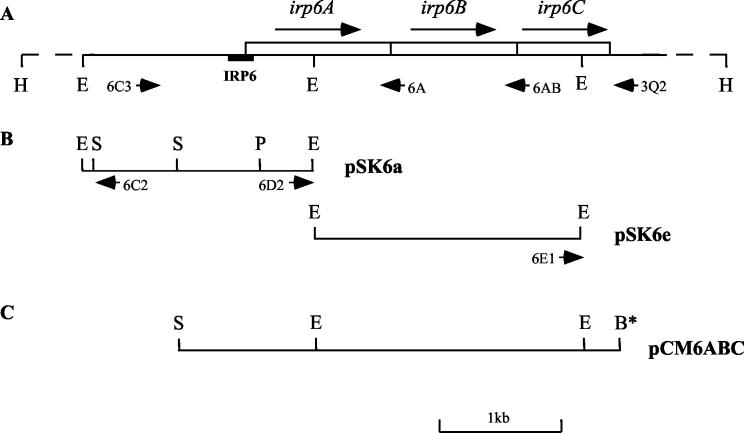
Southern hybridization experiments (data not shown) indicated that the cloned insert in pIRP6 was located on a 6.8-kb HindIII fragment from the chromosome of C. diphtheriae (Fig. 4A). Plasmid pSK6a (Fig. 4B), containing a 1.9-kb EcoRI fragment, was recovered from a λZapII genomic library of C. diphtheriae because it hybridized with an IRP6 probe, and the region that is homologous with IRP6 was further localized to the 680-bp SalI-PstI fragment within pSK6a (Fig. 4B). Sequencing this SalI-PstI fragment confirmed the presence of IRP6 and revealed an incomplete ORF downstream from it. To obtain more information about the genomic DNA downstream from this incomplete ORF, inverse PCR was performed using a HindIII-digested and self-ligated preparation of genomic DNA as the template (see Materials and Methods) and the primer pair 6C2 and 6D2 (Fig. 4B) to amplify the 5.6-kb segment of the circularized 6.8-kb HindIII chromosomal fragment located between the primers. The resulting 5.6-kb PCR product was also used as a probe to screen the λZapII genomic library of C. diphtheriae for clones containing sequences downstream from the partial ORF in pSK6a. Plasmid pSK6e, which carries a 2.2-kb EcoRI insert contiguous with and immediately downstream from the insert in pSK6a (Fig. 4B), was obtained by this method and sequenced. Sequences further downstream from this 2.2-kb EcoRI fragment in pSK6e were obtained by directly sequencing the 3.6-kb inverse PCR product, which was amplified by using primer pair 6C2 and 6E1 (Fig. 4B).
FIG. 4.
Organization of irp6 operon. (A) Restriction map of the 6.8-kb chromosomal HindIII fragment containing the irp6 operon. Long arrows, orientations of the three ORFs; short arrows, locations and orientations of the primers used to amplify the entire irp6 region, or regions containing only the first ORF (irp6A) or the first two ORFs (irp6AB) of the irp6 operon; black bar, location of IRP6 promoter fragment isolated by the SELEX-like procedure. (B) Restriction map of library clone pSK6a, containing the 1.9-kb EcoRI fragment that hybridized to an IRP6 probe, and library clone pSK6e, containing the 2.2-kb EcoRI fragment that is adjacent to pSK6a on the chromosome. Short arrows, locations of the primers (6C2 and 6D2) used in inverse PCR to amplify the flanking sequence on the 6.8-kb HindIII fragment. (C) Restriction map of the 3.6-kb SalI-BamHI insert containing the irp6 region in plasmid pCM6ABC. The asterisk indicates that the BamHI site was generated by primer 3Q2. Restriction enzyme abbreviations: B, BamHI; E, EcoRI; H, HindIII; S, SalI; P, PstI.
The nucleic acid sequence of a 3,650-bp segment of the irp6 chromosomal region, determined by sequencing the two λZapII clones and the inverse PCR products described above, revealed contiguous ORFs irp6A, irp6B, and irp6C (Fig. 4A). BLAST searches were conducted with the amino acid sequences deduced from the three ORFs. Predicted protein Irp6A is a 395-aa polypeptide with relatively low homology to several periplasmic binding protein components of bacterial ABC transport systems, such as hypothetical protein TM0189 from Thermotoga maritima (27% identity; GenBank accession no. E72406). Irp6A has a typical N-terminal signal peptide and a predicted signal peptidase II recognition sequence (L-T-A-C-S-N), which suggests that the 27-aa N-terminal signal sequence is cleaved and that the mature form of the protein is tethered to the exterior of the plasma membrane by fatty acyl groups at the modified N-terminal cysteine residue indicated by boldface in the predicted recognition sequence shown above (14). The predicted size for the processed Irp6A protein is 372 aa. The predicted Irp6B protein is a 347-aa polypeptide with nine transmembrane segments predicted by HMMTOP analysis (http://www.enzim.hu/hmmtop). It shows homology with integral membrane components of ABC transport systems, with the highest homology being to heme uptake protein HmuU from C. diphtheriae (34% identity; GenBank accession no. AF109162). The irp6C ORF is predicted to encode a cytoplasmic protein with 252 aa residues that contains an ABC domain with well-conserved Walker A and B motifs as well as the ABC signature sequence, which is conserved among ABC transporters (8, 12, 27). Irp6C is most similar to FepC from E. coli (36% identity; GenBank accession no. P23878), the ATP-binding component of the ferric enterobactin transport system. ORFs irp6A, irp6B, and irp6C are tightly linked, with no interrupting nucleotides between the first two and only 1 bp separating the last two. The nucleotide sequence extending more than 400 bp downstream from irp6C did not show any significant homology to other proteins in the database. A potential stem-loop structure (with free energy level at −13.4 kcal) found 34 bp downstream from the stop codon (TAG) of irp6C probably functions as a transcriptional terminator. Therefore, we hypothesized that genes irp6A, irp6B, and irp6C form a single transcriptional unit, that the irp6 operon encodes an ABC transporter possibly involved in high-affinity iron uptake in C. diphtheriae, and that the Irp6A protein might function as the lipoprotein receptor for a ferric siderophore complex.
Complementation of C. diphtheriae iron uptake mutants by genes of the irp6 operon.
C. diphtheriae mutant strains HC1, HC3, HC4, and HC5 (2) were derived by chemical mutagenesis from wild-type strain C7(β) and produced large amounts of corynebacterial siderophore even under high-iron (repressing) conditions (30). 59Fe transport studies showed that even though these strains were capable of producing siderophore, they were defective in siderophore-mediated ferric iron uptake. Attempts to complement the mutations in these strains with plasmid pWS382, which was thought to contain the entire irp1 operon (26), were unsuccessful. We therefore tested whether the irp6 operon could complement the functional defect(s) in these strains. To obtain a clone containing the entire irp6 operon, primer pair 6C3 and 3Q2 (Table 3) was used to amplify an approximately 4.0-kb fragment (Fig. 4A and C) from chromosomal DNA of C. diphtheriae strain C7 using a Pfu-Taq enzyme mixture system (Elongase; GIBCO-BRL). The PCR product was then digested with BamHI and SalI, and the resulting 3.6-kb BamHI-SalI fragment (Fig. 4C) was cloned into the same sites in E. coli-C. diphtheriae shuttle vector pCM2.6, generating plasmid pCM6ABC. pCM6ABC was introduced into strains HC1, HC3, HC4, and HC5 by electroporation (24), and the transformants were tested for siderophore production under low- and high-iron conditions. Vector pCM2.6 was also introduced into these strains as a control. In wild-type strain C7(β) carrying either plasmid pCM6ABC or vector pCM2.6, siderophore production was repressed by iron to levels that were approximately 15-fold less than those under low-iron growth conditions (Table 5). All mutant strains carrying vector pCM2.6 produced much greater amounts of siderophore than wild-type strain C7(β) under high-iron conditions (≥180 U/ml) and showed slight increases in siderophore production (twofold or less) under low-iron conditions, in agreement with previous studies of these mutant strains (2, 30). When plasmid pCM6ABC was introduced into strains HC1, HC4, and HC5, production of siderophore under high-iron conditions was repressed to ≤63 U/ml, while under low-iron conditions siderophore production was derepressed to ≥236 U/ml (Table 4). These findings demonstrate that plasmid pCM6ABC was able to reverse the siderophore overproduction phenotype of strains HC1, HC4, and HC5, presumably due to the introduction of a wild-type copy of the putative siderophore uptake system encoded by the irp6 operon. In contrast, plasmid pCM6ABC did not reverse the mutant phenotype of strain HC3.
TABLE 5.
Suppression of siderophore overproduction under high-iron growth conditions by complementation of mutant alleles of the irp6 operon in C. diphtheriaea
| Strain | Plasmid | Siderophore level (U/ml) in:
|
|
|---|---|---|---|
| Low iron | High iron | ||
| C7(β) | pCM2.6 | 451 ± 30 | 26 ± 3 |
| pCM6ABC | 396 ± 3 | 26 ± 1 | |
| HC1 | pCM2.6 | 514 ± 12 | 386 ± 40 |
| pCM6ABC | 452 ± 19 | 53 ± 3 | |
| pCM6AB | 372 ± 35 | 43 ± 0 | |
| pCM6A | 332 ± 29 | 60 ± 8 | |
| HC3 | pCM2.6 | 576 ± 54 | 187 ± 10 |
| pCM6ABC | 585 ± 54 | 236 ± 58 | |
| HC4 | pCM2.6 | 499 ± 11 | 353 ± 109 |
| pCM6ABC | 594 ± 6 | 63 ± 10 | |
| pCM6AB | 411 ± 61 | 285 ± 18 | |
| pCM6A | 333 ± 26 | 460 ± 141 | |
| HC5 | pCM2.6 | 291 ± 10 | 194 ± 29 |
| pCM6ABC | 236 ± 35 | 44 ± 5 | |
| pCM6AB | 111 ± 14 | 3 ± 0 | |
| pCM6A | 109 ± 21 | 111 ± 8 | |
Supernatants from overnight cultures grown in modified PGT medium (mPGT) (low iron) or mPGT plus 10 μM FeCl3 (high iron) were used for siderophore assays. Values represent means ± standard deviations of assays performed on cultures grown in triplicate.
Plasmids pCM6A and pCM6AB were constructed by cloning PCR products encoding the first one or two ORFs of the irp6 operon with primer pair 6C3 and 6A or 6C3 and 6AB (Table 3), respectively (Fig. 4). These two constructs were introduced into strains HC1, HC4, and HC5, and siderophore production was measured in supernatants of cultures grown under low- and high-iron conditions. For strain HC1, introduction of either plasmid pCM6AB or pCM6A improved growth in low-iron PGT medium (not shown) and complemented the defect in repression of siderophore synthesis under high-iron conditions (Table 4), indicating that strain HC1 contains a mutant allele of irp6A. In strain HC4, however, neither subclone complemented the mutant phenotype, suggesting that strain HC4 has a mutant allele of irp6C. Finally, plasmid pCM6AB, but not pCM6A, enhanced the growth of strain HC5 in low-iron PGT medium and restored repression of siderophore synthesis under high-iron conditions, indicating that strain HC5 contains a mutant allele of irp6B. The nucleotide sequencing of the irp6A allele from strain HC1 showed that it has a G-to-A substitution at position 1280, causing a Gly240Asp substitution. The sequencing of the irp6C allele from strain HC4 revealed that a G-to-A substitution at position 2925 resulted in a Gly45Asp substitution in the conserved Walker A motif. The sequencing of the irp6B allele from strain HC5 revealed base substitutions in three different codons; two of them were silent, but a G-to-A substitution at position 2325 introduced a TGA codon that resulted in chain termination at the codon for Trp193.
In mutant strain HC3, the defect in siderophore-mediated iron uptake was not complemented by the wild-type irp6 operon alone. Since strain HC3 was produced from C7(β) by chemical mutagenesis with ethyl methane sulfonic acid ester, it was possible that HC3 harbored multiple point mutations. We observed that HC3 exhibited high sensitivity to the toxic effects of heme in HITW and that this property was shared by C7(β)hm723, a strain that harbors a mutant dtxR allele (24). In contrast, wild-type C7(β) and strains HC1, HC4, and HC5 were not highly sensitive to added heme in HITW. We determined the sequence of the dtxR allele in strain HC3, and we found that a G-to-A substitution in the codon for Trp104 caused premature chain termination of the DtxR protein. We constructed plasmid pNGR-6ABC, containing both the wild-type dtxR gene and the wild-type irp6 operon, and introduced it into strain HC3. We found that strain HC3 carrying this plasmid grew well under low-iron conditions, tolerated high concentrations of heme in HITW [up to 100 μM, which is comparable to the tolerance of wild-type C7(β)], and did not overproduce siderophore and DT under high-iron conditions. Further sequence analysis showed that HC3 also has a G-to-A substitution at position 2041 in the irp6B gene that results in a Glu99Lys substitution in the Irp6B protein.
Activities of the IRP1 and IRP6 promoters in wild-type and mutant strains of C. diphtheriae.
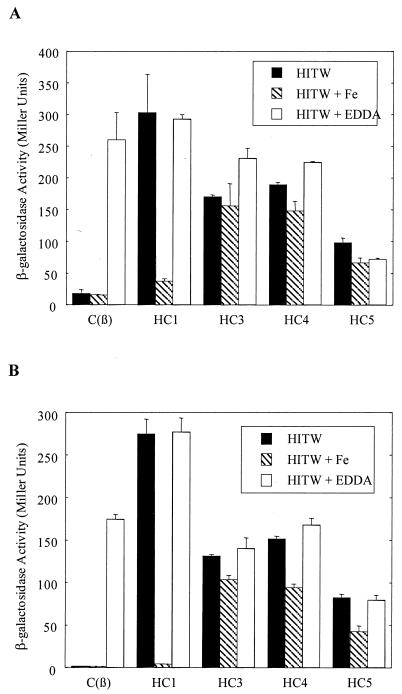
DNA fragments containing the IRP1 and IRP6 promoters were cloned into shuttle vector pCM502 (21) in front of the promoterless lacZ gene, and the two resulting transcriptional fusion constructs, pCM1 and pCM6, respectively, were introduced into wild-type C. diphtheriae C7(β) and into mutant strains HC1, HC3, HC4, and HC5. Activities of β-galactosidase in cultures grown under low- and high-iron conditions were measured. In wild-type C7(β), the β-galactosidase activities from both constructs were repressed under high-iron conditions (HITW or HITW-Fe) and derepressed in cultures grown under low-iron conditions in the presence of iron chelator EDDA (Fig. 5). As seen previously in E. coli (Table 4), the IRP1 promoter appeared to be more stringently repressed than the IRP6 promoter in response to iron. In all four of the mutant strains, the IRP6 and IRP1 promoter activities were derepressed when the cells were grown in HITW. Growth in HITW-Fe medium restored repression of the IRP6 and IRP1 promoters in strain HC1, but this effect was not seen with strains HC3, HC4, and HC5. These phenotypic differences between mutant strains HC1, HC3, HC4, and HC5, all of which are deficient in siderophore-mediated iron uptake, are discussed below.
FIG. 5.
β-Galactosidase activities of C. diphtheriae strains C7(β), HC1, HC3, HC4, and HC5 carrying promoter fusion constructs pCM6 (A) and pCM1(B) cultured in HITW, HITW-Fe, and HITW with 100 μg of EDDA/ml. Overnight cultures were used for β-galactosidase activity assays, and the activity values were the averages of samples from cultures grown in triplicate. The standard deviations are shown by the error bars.
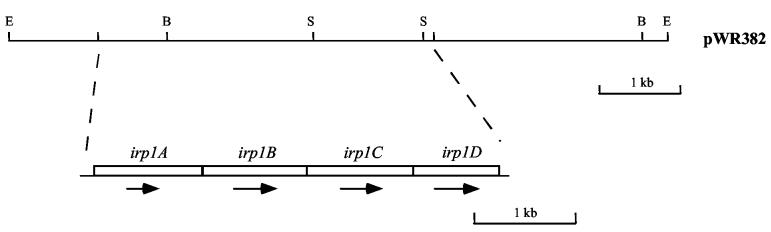
Complete sequence of the irp1 operon.
Previously, an ORF named irp1 was identified downstream of promoter IRP1 (26). More sequence downstream of irp1 was obtained from plasmid pWR382, which contains an 8-kb EcoRI insert (Fig. 6). Three additional ORFs were identified. The ORF originally called irp1 was therefore renamed irp1A, and the three genes that immediately follow it were named irp1B, irp1C, and irp1D (Fig. 6). There are only 2 bp separating the first two ORFs; the stop codon of irp1B overlaps with the start codon of irp1C (ATG). And the last two ORFs are only separated by 11 bp. Therefore, these four ORFs are tightly linked and most likely form a single transcriptional unit.
FIG. 6.
Gene organization of irp1 operon. Dashed lines indicate the location of the four ORFs (irp1A, irp1B, irp1C, and irp1D) in the 8-kb EcoRI insert in plasmid pWR382. Restriction enzyme abbreviations: B, BamHI; E, EcoRI; S, SalI.
Like the irp6 operon, the irp1 operon also showed homology with other ABC transport systems. As shown previously (26), irp1A encodes a 355-aa lipoprotein that was considered to be a candidate receptor for a siderophore uptake system in C. diphtheriae. There is no significant homology, however, between lipoprotein receptors Irp6A and Irp1A, even in the N-terminal signal sequences. The irp1B and irp1C genes are predicted to encode 343-aa and 351-aa polypeptides, respectively, and both have 10 transmembrane segments according to HMMTOP. Irp1B and Irp1C are most similar to the transmembrane components of a probable iron-siderophore uptake system in Streptomyces coelicolor (GenBank accession no. T36890 [41% identity] and T36890 [39% identity], respectively). There is extensive homology between membrane proteins Irp1B and Irp1C (31% identity), and the Irp1B and Irp1C proteins each have 30% homology with Irp6B. The irp1D gene is predicted to encode a 283-aa polypeptide which has homology with ATP-binding proteins of bacterial ABC transporters, and the Walker A and B motifs as well as the ABC signature sequence are well conserved. Irp1D is most homologous to permease protein FecE from the iron(III) dicitrate transport system in Synechocystis spp. (accession no. D90899, 53% identity). ATP-binding proteins Irp6C and Irp1D have 34% homology. Therefore, the irp1 operon seems to encode another ABC transporter in C. diphtheriae. By comparison with the subunit organizations of other ABC transporters (8, 27), the irp1 system most likely utilizes a heterodimer consisting of Irp1B and Irp1C as its transmembrane component, whereas the irp6 system most likely employs a homodimer consisting of two Irp6B polypeptides as its transmembrane component.
DISCUSSION
SELEX is a powerful tool for isolation of target nucleic acid sequences when a purified, sequence-specific oligonucleotide-binding protein, such as DtxR, is available (33). To obtain biologically relevant DNA fragments, we used a SELEX-like procedure followed by in vivo screening with an E. coli reporter system to isolate DtxR-binding and iron-regulated promoter fragments from C. diphtheriae. As a result, IRP6 was obtained, and it showed DtxR-dependent iron-regulated activities in both the E. coli system and C. diphtheriae C7(β).
High-affinity uptake of iron complexes such as ferrisiderophore across the cytoplasmic membrane usually involves a binding protein-dependent ABC transport system, the acronym for a family of transporters containing ABC domains (8). In gram-positive bacteria, a binding protein-dependent ABC transport system usually consists of an extracellular lipoprotein receptor, an integral membrane protein, and an intracellular ATP binding peripheral membrane protein (31). In gram-negative bacteria, due to the existence of an outer membrane (OM), an OM porin-like receptor in conjunction with the TonB/ExbBD energy-transducing system is often used to facilitate the active transport of iron complexes through the OM (6). The ABC uptake apparatus that transports ferrisiderophore complexes across the inner membrane in gram-negative bacteria is similar to that of gram-positive bacteria, except that gram-negative bacteria employ periplasmic binding proteins as ferrisiderophore receptors and gram-positive bacteria utilize homologous membrane-attached lipoproteins as ferrisiderophore receptors. Both the irp1 and irp6 operons exhibit gene organization typical of ABC transport systems for gram-positive bacteria. Studies of several sequenced bacterial genomes indicate that genes encoding ABC transport systems are abundant and constitute one of the major gene families. For example, there are an estimated 57 ABC transporters in E. coli (12) and 78 in B. subtilis (15). Several ABC transport systems have been isolated in corynebacteria, including the gluABCD system for glutamate uptake in C. glutamicum (9), a recently characterized ABC transport system involved in heme uptake in C. diphtheriae (3), and an ABC export system for tetracycline in Cornynebacterium striatum (32). The genome sequence of C. diphtheriae NCTC 13129 has very recently been completed by investigators at the Sanger Centre. We performed a BLAST search of the putative proteins encoded by the C. diphtheriae genome using Irp6C, the ABC protein encoded by the irp6 operon, as the probe, and we found at least 58 putative ATP-binding proteins with conserved Walker A and B motifs as well as the ABC signature sequence, which is present in ABC transport systems (8, 12, 27).
Our sequence analysis and mutant complementation experiments provided strong evidence that the irp6 operon encodes an ABC transport system for siderophore uptake in C. diphtheriae. Mutations in each of the three components of this uptake system were associated with previously documented abnormalities in siderophore-mediated iron uptake (18) that indirectly affected other intracellular processes regulated by DtxR and iron. Therefore, it is apparent that siderophore-mediated iron uptake plays an important role in this organism. Siderophore overproduction under high-iron conditions was also observed in a Rhizobium leguminosarum mutant strain defective in the membrane component of the siderophore-dependent iron uptake system (29). The proposed role of the irp6 transport system in C. diphtheriae is also consistent with previous biochemical evidence implicating ATP in ferrisiderophore uptake in C. diphtheriae, which was shown to be sensitive to arsenate, a phosphate homolog that inhibits ATP formation (16). Additional studies will be needed, however, to demonstrate directly that Irp6A can function as a specific binding protein for ferricorynebactin.
Previous studies showed that C. diphtheriae can use aerobactin from S. flexneri, as well as its own siderophore, corynebactin, but corynebactin does not function as a siderophore for S. flexneri (17, 18). Corynebactin and aerobactin must therefore be different, but the structure of corynebactin from C. diphtheriae has not been determined. We demonstrated that strains HC1, HC4, and HC5 could use aerobactin as a siderophore to stimulate bacterial growth in the presence of iron only when the wild-type irp6 operon was present on the complementing pCM6ABC plasmid (data not shown), indicating that the irp6 transporter of C. diphtheriae is required for both corynebactin-dependent and aerobactin-dependent iron uptake. Iron assimilation by C. diphtheriae can also occur by siderophore-independent pathways. C. diphtheriae has a high-affinity system for acquiring iron from heme or hemoglobin (3). In addition, the fact that siderophore-deficient strains such as C. diphtheriae PW8 and C7(β) mutant HC6 can grow under high-iron conditions, but not under low-iron conditions, in heart infusion medium suggests that C. diphtheriae also has siderophore-independent, low-affinity iron uptake activity.
Analysis of β-galactosidase activities of our IRP1 and IRP6 promoter fusion constructs in wild-type C. diphtheriae strain C7(β) showed that both of these promoters were repressed during growth in HITW (high-iron conditions) and derepressed during growth in HITW broth plus 100 μg of EDDA/ml (low-iron conditions). In mutant strains HC1, HC3, HC4, and HC5, which are deficient in siderophore-dependent iron uptake, expression of the β-galactosidase reporter gene from the IRP1 and IRP6 promoter fusion constructs was derepressed during growth in HITW. For strain HC1, which expresses a Gly240Asp variant of putative ferrisiderophore receptor Irp6A, growth in HITW-Fe restored the iron- and DtxR-dependent repression of the reporter gene. In contrast, for strain HC4, which has a defect in putative ATP-binding protein Irp6C, and for strain HC5, which has a defect in putative membrane protein Irp6B, growth in HITW-Fe did not restore repression of the reporter gene. One possible explanation for these observations is that the Gly240Asp variant of Irp6A retains a low affinity for ferrisiderophores. If so, and if the concentration of siderophore produced during growth of strain HC1 in HITW exceeded the concentration of available iron, then addition of more iron to the medium might increase the concentration of ferrisiderophore complexes and permit enough siderophore-dependent iron uptake by strain HC1 to activate DtxR and cause the repression of the reporter genes in the IRP1 and IRP6 fusion constructs that we observed in Fig. 5.
When the siderophore-deficient strains HC6 and PW8 of C. diphtheriae were grown in HITW, however, DtxR-regulated functions were fully repressed, presumably as a consequence of iron uptake by low-affinity, siderophore-independent pathways (data not shown). The lack of repression of DtxR-regulated functions in strains HC1, HC3, HC4, and HC5, but not in strains PW8 and HC6, during growth in HITW raises the possibility that the ABC transporter encoded by the irp6 operon may also have a role in low-affinity, siderophore-independent iron uptake in C. diphtheriae. Another possible explanation for the observed repression of the IRP1 and IRP6 promoters of strain HC1, but not of strains HC3, HC4, and HC5, during growth in HITW-Fe is that Irp6A is less important than Irp6B and Irp6C for low-affinity, siderophore-independent iron uptake in C. diphtheriae. Furthermore, the relatively high and easily measured baseline expression of the IRP6 promoter that was observed in the wild-type strain during growth in both HITW and HITW-Fe is consistent with a possible physiological role for the irp6 operon in low-affinity, siderophore-independent iron uptake by C. diphtheriae under high-iron growth conditions. Additional work will be needed to investigate these hypotheses further.
Strains C(β)hm723 and HC3 both have point mutations in their dtxR alleles which are known to cause decreased activity or inactivation of DtxR (24, 34). We showed that strains HC3 and C(β)hm723 grew poorly in HITW supplemented with 100 μM heme, probably as a consequence of derepression of the heme uptake pathway and accumulation of toxic levels of iron or heme degradation products in the intracellular milieu. When a wild-type dtxR allele was introduced into strain HC3, it still overproduced siderophore under high-iron conditions (data not shown), indicating that the Glu99Lys substitution in Irp6B resulted in inactivation of siderophore-dependent iron transport.
Complementation of strain HC3 with both the wild-type dtxR allele and the complete wild-type irp6 operon restored normal regulation of functions controlled by DtxR and iron. These findings demonstrated that the abnormal phenotypes of strain HC3 with respect to iron uptake and DtxR-dependent regulation were caused by mutations in dtxR and in the irp6 operon and did not involve additional chemically induced mutations in other genes.
In the present study, the complete sequence of the irp1 operon was determined and shown to encode four proteins that comprise a putative ABC transporter. Although the lipoprotein encoded by the irp1 operon, here renamed Irp1A, is homologous to FhuD from B. subtilis and was previously proposed as a candidate ferrisiderophore receptor, the irp1 operon could not complement the defects in siderophore-dependent iron transport in the HC1, HC3, HC4, and HC5 variants described above (25, 26). No mutants with defects in the irp1 operon have been described, and the function of the irp1 operon remains unknown. It is interesting that a search of the genome of C. diphtheriae NCTC 13129 did not reveal the irp1 operon from C. diphtheriae C7(β), indicating that this ABC transport system is not present in all isolates of C. diphtheriae. Since most studies of iron-dependent gene regulation in C. diphtheriae have been performed with strains C7(-), C7(β), and mutants derived from them, however, we have included our findings on the irp1 operon in the present report.
In summary, we conclude that siderophore production and siderophore-dependent iron uptake are central aspects of the DtxR regulatory circuit during growth of C. diphtheriae in media that do not contain heme as a source of iron. These processes directly influence intracellular iron concentrations and affect the activity of DtxR, which functions as a global effector for iron-regulated cellular processes. This study demonstrates that the irp6 operon is required for high-affinity, siderophore-dependent iron uptake in C. diphtheriae and that it may also have a role in low-affinity, siderophore-independent iron uptake in this pathogenic bacterium. This study also shows the effects of mutations in the irp6A, irp6B, and irp6C genes on the regulation of both the IRP1 and IRP6 promoters and on siderophore and toxin production (2, 30).
Acknowledgments
This work was supported in part by grant number R01 AI14107 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The University of Colorado Cancer Center DNA Sequencing and Analysis Core Facility was supported in part by NIH/NCI Cancer Support Grant CA 46934.
We thank Michael G. Jobling for assistance in preparing the figures for publication.
REFERENCES
- 1.Budzikiewicz, H., A. Bössenkamp, K. Taraz, A. Pandey, and J.-M. Meyer. 1997. Corynebactin, a cyclic catecholate siderophore from Corynebacterium glutamicum ATCC 14067 (Brevibacterium sp. DSM 20411). Z. Naturforsch. Sect. C 52:551-554. [Google Scholar]
- 2.Cryz, S. J., L. M. Russell, and R. Holmes. 1983. Regulation of toxinogenesis in Corynebacterium diphtheriae: mutations in the bacterial genome that alter the effects of iron on toxin production. J. Bacteriol. 154:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drazek, E. S., C. A. Hammack, Sr., and M. P. Schmitt. 2000. Corynebacterium diphtheriae genes required for acquisition of iron from haemin and haemoglobin are homologous to ABC haemin transporters. Mol. Microbiol. 36:68-84. [DOI] [PubMed] [Google Scholar]
- 4.Farinha, M. A., and A. M. Kropinski. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J. Bacteriol. 172:3496-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feese, M. D., E. Pohl, R. K. Holmes, and W. G. J. Hol. 2001. Iron-dependent regulators, p. 850-863. In A. Messerschmidt, R. Huber, T. Poulos, and K. Wieghardt (ed.), Handbook of metalloproteins. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 6.Guerinot, M. L. 1994. Microbial iron transport. Annu. Rev. Microbiol. 48:743-772. [DOI] [PubMed] [Google Scholar]
- 7.Holmes, R. K., and L. Barksdale. 1969. Genetic analysis of tox+ and tox bacteriophages of Corynebacterium diphtheriae. J. Virol. 3:586-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, P. M., and A. M. George. 1999. Subunit interactions in ABC transporters: towards a functional architecture. FEMS Microbiol. Lett. 179:187-202. [DOI] [PubMed] [Google Scholar]
- 9.Kronemeyer, W., N. Peekhaus, R. Krämer, H. Sahm, and L. Eggeling. 1995. Structure of the gluABCD cluster encoding the glutamate uptake system of Corynebacterium glutamicum. J. Bacteriol. 177:1152-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, J. H., and R. K. Holmes. 2000. Characterization of specific nucleotide substitutions in DtxR-specific operators of Corynebacterium diphtheriae that dramatically affect DtxR binding, operator function, and promoter strength. J. Bacteriol. 182:432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, J. H., T. Wang, K. Ault, J. Liu, M. P. Schmitt, and R. K. Holmes. 1997. Identification and characterization of three new promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. Infect. Immun. 65:4273-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linton, K. J., and C. F. Higgins. 1998. The Escherichia coli ATP-binding protein cassette (ABC) proteins. Mol. Microbiol. 28:5-13. [DOI] [PubMed] [Google Scholar]
- 13.Ochsner, U. A., and M. Vasil. 1996. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc. Natl. Acad. Sci. USA 93:4409-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quentin, Y., G. Fichant, and F. Denizot. 1999. Inventory, assembly and analysis of Bacillus subtilis ABC transporter systems. J. Mol. Biol. 287:467-487. [DOI] [PubMed] [Google Scholar]
- 16.Russell, L., and R. Holmes. 1983. Initial characterization of the ferric iron transport system of Corynebacterium diphtheriae. J. Bacteriol. 155:1439-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell, L., and R. Holmes. 1985. Highly toxigenic but avirulent Park-Williams 8 strain of Corynebacterium diphtheriae does not produce siderophore. Infect. Immun. 47:575-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell, L. M., S. J. Cryz, Jr., and R. K. Holmes. 1984. Genetic and biochemical evidence for a siderophore-dependent iron transport system in Corynebacterium diphtheriae. Infect. Immun. 45:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 20.Schmitt, M. P. 1997. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J. Bacteriol. 179:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt, M. P. 1997. Transcription of the Corynebacterium diphtheriae hmuO gene is regulated by iron and heme. Infect. Immun. 65:4634-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt, M. P. 1999. Identification of a two-component signal transduction system from Corynebacterium diphtheriae that activates gene expression in response to the presence of heme and hemoglobin. J. Bacteriol. 181:5330-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt, M. P., and R. K. Holmes. 1991. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect. Immun. 59:1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt, M. P., and R. K. Holmes. 1991. Characterization of a defective diphtheria toxin repressor (dtxR) allele and analysis of dtxR transcription in wild-type and mutant strains of Corynebacterium diphtheriae. Infect. Immun. 59:3903-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt, M. P., and R. K. Holmes. 1994. Cloning, sequence, and footprint analysis of two promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. J. Bacteriol. 176:1141-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt, M. P., B. G. Talley, and R. K. Holmes. 1997. Characterization of lipoprotein IRP1 from Corynebacterium diphtheriae, which is regulated by the diphtheria toxin repressor (DtxR) and iron. Infect. Immun. 65:5364-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider, E., and S. Hunke. 1998. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol. Rev. 22:1-20. [DOI] [PubMed] [Google Scholar]
- 28.Schneider, R., and K. Hantke. 1993. Iron-hydroxymate uptake system in Bacillus subtilis: identification of a lipoprotein as part of a binding protein-dependent transport system. Mol. Microbiol. 8:111-121. [DOI] [PubMed] [Google Scholar]
- 29.Stevens, J. B., R. A. Carter, H. Hussain, K. C. Carson, M. J. Dilworth, and A. W. Johnston. 1999. The fhu genes of Rhizobium leguminosarum, specifying siderophore uptake proteins: fhuDCB are adjacent to a pseudogene version of fhuA. Microbiology 145:593-601. [DOI] [PubMed] [Google Scholar]
- 30.Tai, S.-P. S., A. E. Krafft, P. Nootheti, and R. Holmes. 1990. Coordinate regulation of siderophore and diphtheria toxin production by iron in Corynebacterium diphtheriae. Microb. Pathog. 9:267-273. [DOI] [PubMed] [Google Scholar]
- 31.Tam, R., and M. H. Saier, Jr. 1993. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 57:320-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tauch, A., S. Krieft, A. Pühler, and J. Kalinowski. 1999. The tetAB genes of the Corynebacterium striatum R-plasmid pTP10 encode an ABC transporter and confer tetracycline, oxytetracycline and oxacillin resistance in Corynebacterium glutamica. FEMS Microbiol. Lett. 173:203-209. [DOI] [PubMed] [Google Scholar]
- 33.Tuerk, C., and L. Gold. 1990. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505-510. [DOI] [PubMed] [Google Scholar]
- 34.Wang, Z., M. P. Schmitt, and R. K. Holmes. 1994. Characterization of mutations that inactivate the diphtheria toxin repressor gene (dtxR). Infect. Immun. 62:1600-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]