Abstract
The analysis of the different amino acid sequences deduced from the complete genome sequence of the gram-positive bacterium Staphylococcus aureus suggested the presence of two eukaryotic-protein-like low-molecular-mass phosphotyrosine protein phosphatases, which are usually found in gram-negative bacteria. To check this prediction, the corresponding genes were cloned and overexpressed in an Escherichia coli system. Two distinct proteins with an apparent molecular mass of 23 kDa each, PtpA and PtpB, were produced and then purified by affinity chromatography and assayed for enzymatic properties. As expected, they both exhibited phosphatase activity in vitro, with a maximum value at a pH of around 6.2 and at a temperature of 40°C. In addition, their kinetic constants, their specificity for phosphotyrosine residues, and their sensitivity to two phosphatase inhibitors, N-ethylmaleimide and orthovanadate, matched those of acid low-molecular-mass phosphotyrosine protein phosphatases.
Protein phosphorylation on tyrosine has long been considered a posttranslational modification specific to eukaryotes. In these organisms, it has been shown to play a key role in a series of fundamental biological functions, including signal transduction, growth control, metabolism, and malignant transformation (10, 12). In recent years, this modification has been demonstrated to occur also in bacteria, both gram-negative and gram-positive species (for a review, see reference 7). However, its biological significance remains unclear even if, in a few cases, a possible functional role has been proposed. Thus, it has been reported that protein tyrosine phosphorylation could be involved in the mechanism of pigment production in Streptomyces coelicolor (29). Alternatively, it could participate in the control of biosynthesis and/or transport of exopolysaccharides in Escherichia coli (13, 30, 31), Streptococcus pneumoniae (3, 18), and Sinorhizobium meliloti (21). Since exopolysaccharides have an important part in the interactions of bacteria with their environment, namely, with host cells, as well as in the process of adherence and in the resistance to host immunity, they are commonly considered essential virulence factors in many pathogens (24). On this basis, it has been suggested that protein tyrosine phosphorylation might be connected to bacterial pathogenicity (3, 13).
A major characteristic of protein phosphorylation in general is the fact that it is a reversible reaction. Indeed, the extent of phosphorylation of proteins results from the relative activity of two types of opposing enzymes, protein kinases and protein phosphatases. The differential functioning of these enzymes thus constitutes a regulatory device that the cell may utilize for controlling its metabolism and physiology. To discover the biological role(s) of protein tyrosine phosphorylation, it is therefore important to study not only the protein tyrosine kinases responsible for phosphorylation on tyrosine but also the phosphotyrosine protein phosphatases that catalyze dephosphorylation.
Two main classes of phosphotyrosine protein phosphatases have been characterized so far based on the type of bacteria in which they are found (14). First, in gram-negative species, phosphatase activity is borne by eukaryotic-protein-like enzymes of low molecular mass which all contain two conserved active-site sequence motifs, D-P-Y and C-X4-C-R (9, 26, 27). Second, in gram-positive bacteria, phosphatase activity is generally harbored by phosphoesterases of the PHP (polymerase and histidinol phosphatase) family, which have in common four conserved motifs, termed domains I to IV, essential for metal binding and dephosphorylating activity (2, 19). In particular, the presence of an enzyme of the latter type, called phosphatase CapC, has been predicted in Staphylococcus aureus (19), a bacterium that represents one of the main causes of community-acquired and hospital-acquired infections and that has become resistant to practically all antibiotics. This phosphatase is a homologue of protein CpsB, which has been demonstrated to possess PHP activity in Streptococcus pneumoniae (19).
In this work, we took advantage of the recent availability of the complete genome sequence of S. aureus to perform an exhaustive analysis of its phosphotyrosine protein phosphatase content. We present for the first time evidence that S. aureus contains two other phosphotyrosine protein phosphatases besides CapC. Interestingly, these two enzymes exhibit the structural and biochemical features specific to the low-molecular-mass phosphatases usually found in gram-negative bacteria rather than in gram-positive species.
Prediction of two low-molecular-mass phosphatases.
The starting point of this study was the comparative analysis of the different amino acid sequences deduced from the complete genome sequence of serotype 5 S. aureus available in The Institute for Genomic Research database (www.tigr.org) and the amino acid sequences of certain phosphotyrosine protein phosphatases previously characterized in other organisms. Besides the occurrence of CapC, a phosphotyrosine protein phosphatase of the PHP type, which was previously suggested by other authors (19), we detected a striking sequence similarity between two putative proteins of S. aureus (Reynolds strain) and various prokaryotic and eukaryotic phosphotyrosine protein phosphatases of low molecular mass (around 20 kDa), including Wzb from E. coli (30), EpsP from Pseudomonas solanacearum (11), AmsI from Erwinia amylovora (4), SA-PTPase from Schizosaccharomyces pombe (17), and BLACP1 from bovine heart (32) (Fig. 1). In particular, these two proteins appeared to contain the essential catalytic-site residue D within the conserved motif D-P-Y (amino acids 120 to 122) as well as the essential active-site residues C-X4-C-R (amino acids 8 to 14) in the phosphate binding loop, which are considered two signatures specific to this class of protein phosphatases (9, 27). These two proteins were termed PtpA and PtpB to recall their homology with phosphotyrosine phosphatases. The molecular masses calculated from the nucleotide sequences of the relevant genes were 17,493 Da for PtpA and 15,790 Da for PtpB. Further analysis using The Institute for Genomic Research database showed that the ptpA and ptpB genes are distant from each other (separated by 221,093 nucleotides), which suggested that they are not located on the same operon, even though no information on the nature of the surrounding genes is currently available. Interestingly, the presence of a pair of genes that were 100% identical in sequence to ptpA and ptpB was also observed in each of several different strains of S. aureus, including N315, COL, EMRSA, NTC 8325, Mu50, and MSSA, and even in Staphylococcus epidermidis. It therefore appeared that these two genes are widely conserved across the Staphylococcus species.
FIG. 1.
Comparison of PtpA and PtpB with different prokaryotic and eukaryotic low-molecular-mass phosphotyrosine protein phosphatases. The alignment was made by using the CLUSTAL W program (28). Asterisks indicate the amino acid residues identical in all proteins, and underlining indicates similarity. The typical signatures of phosphotyrosine protein phosphatases are framed (C-X4-C-R and D-P-Y). The amino acid numbering is based on the amino acid sequence of protein PtpA.
Cloning and overproduction of phosphatases.
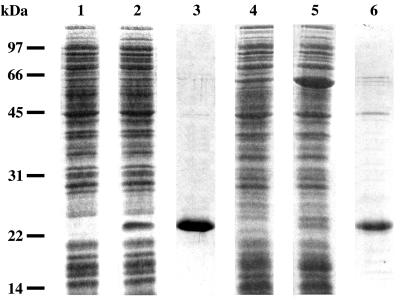
Based on this predictive analysis, a series of experiments was undertaken to demonstrate the presence of proteins PtpA and PtpB in S. aureus cells and then to study their biochemical properties. First, the ptpA and ptpB genes, with appropriate restriction sites at both ends, were prepared by using total DNA from S. aureus (25) as a template in PCR amplification and specific oligonucleotide primers. The locations, directions, and DNA sequences of the various primers used are available from the authors on request. Amplified fragments were digested with appropriate restriction enzymes and inserted separately into the pET15b vector previously opened with the same enzymes to yield the expression plasmids pET15b-ptpA and pET15b-ptpB. Each plasmid could thus synthesize a fusion protein with a six-histidine tag at its N terminus. After transformation of competent cells from the BL21(DE3) strain of E. coli at 37°C (1, 8), the pET15b-ptpA construct could efficiently overproduce, in the soluble fraction of cells, a 23-kDa protein, PtpA, upon induction by isopropyl-β-d-thiogalactopyranoside (IPTG) (Fig. 2, lane 2). By contrast, the pET15b-ptpB plasmid was unable to direct high-yield production of soluble protein PtpB in the same BL21(DE3) strain of E. coli. In fact, a significant amount of total protein was produced, but most of it was found in inclusion bodies, i.e., in insoluble form. Therefore, a different strain of E. coli, BL21(DE3) (pREP4-groESL), was used. This strain overproduces the two chaperone proteins GroES and GroEL and is suitable for obtaining proteins in a soluble form when they possess a high degree of hydrophobicity and, consequently, a tendency to aggregate. In these conditions, pET15b-ptpB could then overproduce a relatively large amount of a 23-kDa soluble protein, His-tagged PtpB, upon induction by IPTG (Fig. 2, lane 5). Still, PtpB appeared to be essentially soluble in cells grown at around 20°C and became insoluble when the temperature was raised to 37°C, even in the presence of chaperones GroES and GroEL.
FIG. 2.
Overproduction and purification of PtpA and PtpB. The soluble fraction from BL21(DE3) (pET15b-ptpA) cells grown for 2 h at 37°C in the absence (lane 1) or in the presence (lane 2) of 1 mM IPTG was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with Coomassie blue. Protein PtpA was purified by affinity chromatography on Ni-NTA agarose (lane 3). The soluble fraction from BL21(DE3) (pREP4-groESL) (pET15b-ptpB) cells grown for 3 h at 20°C in the absence (lane 4) or in the presence (lane 5) of 1 mM IPTG was analyzed under the same conditions. Protein PtpB was purified by affinity chromatography on Ni-NTA agarose (lane 6).
Purification of proteins PtpA and PtpB.
E. coli DH5α cells were transformed with plasmid pET15b-ptpA or plasmid pET15b-ptpB. Then, plasmid pET15b-ptpA was transferred from E. coli DH5α cells to E. coli BL21(DE3) cells. Cells from this strain were used to inoculate 100 ml of Luria-Bertani medium supplemented with ampicillin (100 μg ml−1) and were incubated at 37°C with shaking until the A600 reached 0.5. IPTG was added at a final concentration of 1 mM. Growth was continued for 2 h at 37°C with shaking. Plasmid pET15-ptpB was transferred from E. coli DH5α cells to E. coli BL21(DE3) (pREP4-groESL) cells. Cells from this strain were used to inoculate 200 ml of Luria-Bertani medium supplemented with ampicillin (100 μg ml−1) and kanamycin (25 μg ml−1) and were incubated at 37°C with shaking until the A600 reached 0.5. Ampicillin and IPTG were then added at final concentrations of 100 μg ml−1 and 1 mM, respectively. Growth was continued for 3 h at 20°C with shaking.
Both types of cells were further treated under the same conditions. Cells were harvested by centrifugation at 3,000 × g for 10 min and suspended in 5 ml of buffer A (50 mM Tris-HCl [pH 7.4], 300 mM NaCl, 10% [vol/vol] glycerol) containing DNase I and RNase A at a final concentration of 2 μg ml−1 each. Cells were disrupted in a French pressure cell at 16,000 lb/in2. The resulting suspension was centrifuged at 4°C for 30 min at 30,000 × g. The supernatant was incubated for 1 h at 4°C with 750 μl of Ni-nitrilotriacetic acid (NTA) agarose (Qiagen) suitable for purification of fusion protein carrying a polyhistidine tag. The matrix was then packed and washed with 100 ml of buffer A containing 10 mM imidazole. Protein elution was monitored at 280 nm, and eluted fractions were analyzed by electrophoresis (15). His-tagged PtpA and PtpB were eluted at a concentration of 200 mM imidazole. Fractions containing purified PtpA or PtpB were dialyzed against buffer B (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 10% [vol/vol] glycerol) and stored at −20°C. A typical electrophoretic pattern of each purified phosphatase is shown in Fig. 2 (lanes 3 and 6). In each case, besides the major band corresponding to the phosphotyrosine protein phosphatase, a few contaminating proteins could also be detected in the upper part of the gel in the form of two to three minor bands. It was unlikely, however, that these proteins could harbor a phosphatase activity, namely, one arising from the E. coli host in which PtpA and PtpB were produced. Indeed, they had molecular masses ranging from 45 to 75 kDa, whereas the two phosphotyrosine protein phosphatases present in E. coli, Wzb and Etp, are known to have masses of only 16,709 and 16,386 Da, respectively (30). In addition, these two E. coli phosphatases could hardly copurify, and consequently comigrate, with PtpA and PtpB since only the latter enzymes were His tagged and could be adsorbed on a Ni2+-agarose column.
Phosphatase activity of PtpA and PtpB.
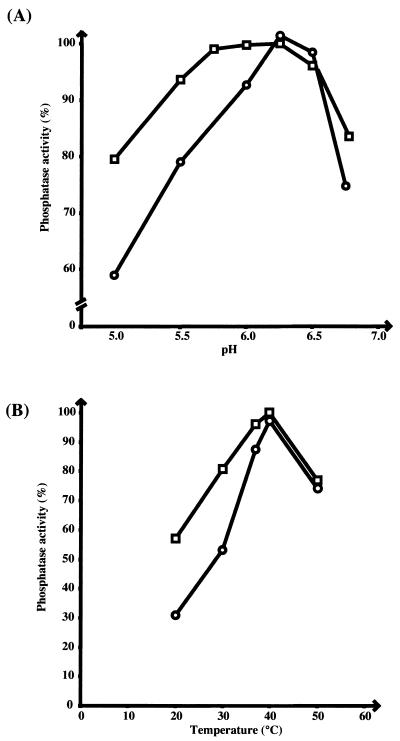
The phosphatase activity of these two proteins was assayed in vitro by using a method based on the detection of p-nitrophenol (PNP) formed from p-nitrophenyl phosphate (PNPP) cleavage. Tests were performed in a 1-ml reaction volume containing 100 mM sodium citrate, 1 mM EDTA, and PNPP at various concentrations from 1 to 40 mM. The amount of PNP produced was determined by the addition of 500 μl of 4 M NaOH to the reaction mixture. Phosphatase activity was estimated at 405 nm by using a PNP concentration range (22). The assay was optimized with respect to protein concentration, time, and pH. Two series of assays were performed to evaluate the effects of pH and temperature. Maximum activity was observed at pH 6.2 for PtpA and at a pH value ranging from 5.7 to 6.5 for PtpB (Fig. 3A), i.e., at acidic pH for both enzymes. Measurement of the phosphatase activity as a function of temperature showed that, for each enzyme, maximum activity was obtained at around 40°C (Fig. 3B). All subsequent enzyme assays were carried out at pH 6.2 and 37°C, which is a more physiological temperature. In these conditions, the kinetic constants were measured. The Km values for PNPP were found to be similar for both enzymes (1.2 mM for PtpA and 1.5 mM for PtpB), whereas the specific activity of PtpA was much higher than that of PtpB (33.6 and 1.4 μmol min−1 mg−1, respectively). These values were consistent with those previously reported for other acid low-molecular-mass protein phosphatases (23, 33).
FIG. 3.
Phosphatase activity of PtpA and PtpB as a function of pH and temperature. The phosphatase activity was measured by using 40 mM PNPP as a substrate and 0.4 μg of PtpA (○) or PtpB (□) per assay. (A) pH varying from 5.0 to 6.75; (B) temperature varying from 20 to 50°C.
Substrate specificity of phosphatases.
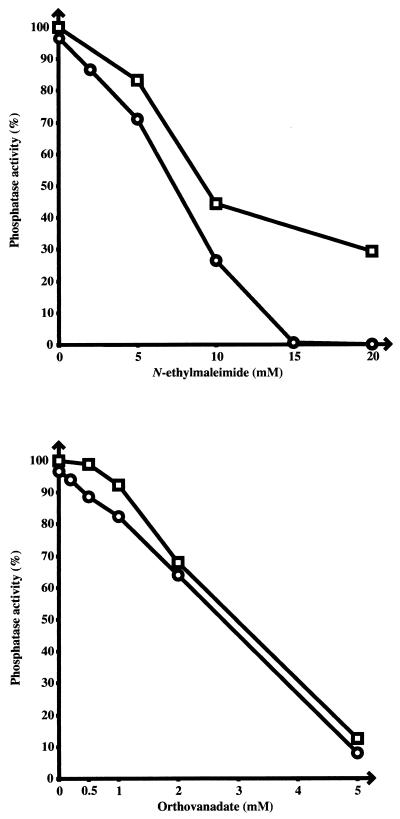
To gain more information on the substrate specificity of PtpA and PtpB, their in vitro activity on individual phosphorylated amino acids was assayed at 37°C in a 50-μl reaction volume containing 10 mM O-phosphoserine, O-phosphothreonine, or O-phosphotyrosine as a substrate, 100 mM sodium citrate (pH 6.25), 1 mM EDTA, and 0.4 μg of purified PtpA or PtpB. After 15 min of incubation, the reaction was stopped by adding 150 μl of 25% (wt/vol) trichloroacetic acid and then 50 μl of bovine serum albumin (10 mg ml−1). The precipitated protein was removed by centrifugation, and the supernatant was used for measuring released inorganic phosphate by using 1 volume of a mixture containing 1.2 M sulfuric acid, 0.5% ammonium molybdate, and 2% ascorbic acid. Samples were heated at 56°C for 15 min, and the absorbance was measured at 750 nm (5, 20). It was found that PtpA and PtpB released 140 and 40 μmol, respectively, of inorganic phosphate from phosphotyrosine but had no effect on either phosphoserine or phosphothreonine. The specificity of PtpA and PtpB for phosphotyrosine was confirmed by studying the effect of two different molecules known to inhibit phosphotyrosine protein phosphatases, N-ethylmaleimide and sodium orthovanadate (6, 34). Assays were carried out by using the same method as that described above for the phosphatase assay, with a buffer containing 100 mM sodium citrate (pH 6.25), 1 mM EDTA, 40 mM PNPP, purified PtpA or PtpB, and various concentrations of inhibitors. Both N-ethylmaleimide and sodium orthovanadate were found to strongly inhibit the phosphatase activity of PtpA and PtpB (Fig. 4). More precisely, the concentrations required for blocking the enzyme activity by 50% were around 7 mM for N-ethylmaleimide and 3 mM for sodium orthovanadate, with, in general, PtpA having a slightly higher sensitivity to inhibitors than PtpB.
FIG. 4.
Effect of phosphatase inhibitors on the activity of PtpA and PtpB. The phosphatase activity was measured by using 40 mM PNPP as a substrate and 0.4 μg of PtpA (○) or PtpB (□) per assay. Two different inhibitors of phosphotyrosine phosphatases were used at the indicated concentrations. The results are expressed in each case as a percentage of the phosphatase activity measured in the absence of inhibitor (taken as 100%).
Conclusion.
Together, these data concurred in showing that S. aureus harbors two distinct enzymes, PtpA and PtpB, which have similar structural and functional properties and which belong to the family of acid low-molecular-mass phosphotyrosine protein phosphatases. A series of previous observations (26) has shown that, in general, this type of phosphatase is found in gram-negative bacteria rather than in gram-positive species. The present results provide evidence that low-molecular-mass phosphatases are also present in gram-positive bacteria and thus confirm a similar observation made with another gram-positive bacterium, Streptomyces coelicolor (16), which had been considered an exception until now. Given the previous suggestion that S. aureus also contains a phosphotyrosine phosphatase, CapC, of the PHP family that is homologous to the CpsB protein from Streptococcus pneumoniae (19), it would therefore appear, for the first time, that one bacterial species can simultaneously harbor two different classes of phosphotyrosine protein phosphatases. Further experiments are now needed to characterize the protein substrates of these various phosphatases and to assess their differential roles in the physiology, and possibly in the pathogenicity, of bacteria.
Acknowledgments
We thank I. Martin-Verstraete (Pasteur Institute, Paris, France) for providing E. coli strain BL21(DE3) (pREP4-groESL).
This work was supported by grants from the Ministère de la Recherche (contract FNS 2000 Microbiologie), the Société Ezus-Lyon (contract 482.022), and the Institut Universitaire de France.
REFERENCES
- 1.Amrein, K. E., B. Takacs, M. Stieger, J. Molnos, N. A. Flint, and P. Burn. 1995. Purification and characterization of recombinant human p50csk protein-tyrosine kinase from an Escherichia coli expression system overproducing the bacterial chaperones GroES and GroEL. Proc. Natl. Acad. Sci. USA 92:1048-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravind, L., and E. V. Koonin. 1998. Phosphoesterase domains associated with DNA polymerases of diverse origins. Nucleic Acids Res. 26:3746-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, M. H., and J. Yother. 2001. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J. Biol. Chem. 276:47966-47974. [DOI] [PubMed] [Google Scholar]
- 4.Bugert, P., and K. Geider. 1995. Molecular analysis of the ams operon required for exopolysaccharide synthesis of Erwinia amylovora. Mol. Microbiol. 15:917-933. [DOI] [PubMed] [Google Scholar]
- 5.Chen, P. S., T. Y. Toribara, and H. Warner. 1956. Microdetermination of phosphorus. Anal. Chem. 28:1756-1758. [Google Scholar]
- 6.Cirri, P., P. Chiarugi, G. Camici, G. Manao, G. Raugei, G. Cappugi, and G. Ramponi. 1993. The role of Cys12, Cys17 and Arg18 in the catalytic mechanism of low-Mr cytosolic phosphotyrosine protein phosphatase. Eur. J. Biochem. 214:647-657. [DOI] [PubMed] [Google Scholar]
- 7.Cozzone, A. J. 1993. ATP-dependent protein kinases in bacteria. J. Cell. Biochem. 51:7-13. [DOI] [PubMed] [Google Scholar]
- 8.Dagert, M., and S. D. Ehrlich. 1979. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene 6:23-28. [DOI] [PubMed] [Google Scholar]
- 9.Davis, J. P., M. M. Zhou, and R. L. Van Etten. 1994. Kinetic and site-directed mutagenesis studies of the cysteine residues of bovine low molecular weight phosphotyrosyl protein phosphatase. J. Biol. Chem. 269:8734-8740. [PubMed] [Google Scholar]
- 10.Fantl, W. J., D. E. Johnson, and L. T. Williams. 1993. Signalling by receptor tyrosine kinases. Annu. Rev. Biochem. 62:453-481. [DOI] [PubMed] [Google Scholar]
- 11.Huang, J., and M. Schell. 1995. Molecular characterization of the eps gene cluster of Pseudomonas solanacearum and its transcriptional regulation at a single promoter. Mol. Microbiol. 16:977-989. [DOI] [PubMed] [Google Scholar]
- 12.Hunter, T. 1995. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80:225-236. [DOI] [PubMed] [Google Scholar]
- 13.Ilan, O., Y. Bloch, G. Frankel, H. Ullrich, K. Geider, and I. Rosenshine. 1999. Protein tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. EMBO J. 18:3241-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennelly, P. J., and M. Potts. 1999. Life among the primitives: protein O-phosphatases in prokaryotes. Front. Biosci. 4:372-385. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Li, Y., and W. R. Strohl. 1996. Cloning, purification, and properties of a phosphotyrosine protein phosphatase from Streptomyces coelicolor A3(2). J. Bacteriol. 178:136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondesert, O., S. Moreno, and P. Russell. 1994. Low molecular weight protein-tyrosine phosphatases are highly conserved between fission yeast and man. J. Biol. Chem. 269:27996-27999. [PubMed] [Google Scholar]
- 18.Morona, J. K., J. C. Paton, D. C. Miller, and R. Morona. 2000. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 35:1431-1442. [DOI] [PubMed] [Google Scholar]
- 19.Morona, J. K., R. Morona, D. C. Miller, and J. C. Paton. 2002. Streptococcus pneumoniae capsule biosynthesis protein CpsB is a novel manganese-dependent phosphotyrosine-protein phosphatase. J. Bacteriol. 184:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mustelin, T., K. M. Coggeshall, and A. Altman. 1989. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc. Natl. Acad. Sci. USA 86:6302-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niemeyer, D., and A. Becker. 2001. The molecular weight distribution of succinoglycan produced by Sinorhizobium meliloti is influenced by specific tyrosine phosphorylation and ATPase activity of the cytoplasmic domain of the ExoP protein. J. Bacteriol. 183:5163-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson, C., R. Nordfelth, A. Holmstrom, S. Hakansson, R. Rosqvist, and H. Wolf-Watz. 1995. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol. 18:135-150. [DOI] [PubMed] [Google Scholar]
- 23.Pot, D. A., and J. E. Dixon. 1992. A thousand and two protein tyrosine phosphatases. Biochim. Biophys. Acta 1136:35-43. [DOI] [PubMed] [Google Scholar]
- 24.Roberts, I. S. 1996. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu. Rev. Microbiol. 50:285-315. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Shi, L., M. Potts, and P. J. Kennelly. 1998. The serine, threonine, and/or tyrosine-specific protein kinases and protein phosphatases of prokaryotic organisms: a family portrait. FEMS Microbiol. Rev. 22:229-253. [DOI] [PubMed] [Google Scholar]
- 27.Su, X. D., N. Taddei, M. Stefani, G. Ramponi, and P. Nordlund. 1994. The crystal structure of a low-molecular-weight phosphotyrosine protein phosphatase. Nature (London) 370:575-578. [DOI] [PubMed] [Google Scholar]
- 28.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umeyama, T., Y. Tanabe, B. D. Aigle, and S. Horinouchi. 1996. Expression of the Streptomyces coelicolor A3(2) ptpA gene encoding a phosphotyrosine protein phosphatase leads to overproduction of secondary metabolites in S. lividans. FEMS Microbiol. Lett. 144:177-184. [DOI] [PubMed] [Google Scholar]
- 30.Vincent, C., P. Doublet, C. Grangeasse, E. Vaganay, A. J. Cozzone, and B. Duclos. 1999. Cells of Escherichia coli contain a protein-tyrosine kinase, Wzc, and a phosphotyrosine-protein phosphatase, Wzb. J. Bacteriol. 181:3472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 32.Wo, Y. Y., M. M. Zhou, P. Stevis, J. P. Davis, Z. Y. Zhang, and R. L. Van Etten. 1992. Cloning, expression, and catalytic mechanism of the low molecular weight phosphotyrosyl protein phosphatase from bovine heart. Biochemistry 31:1712-1721. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Z. Y., and R. L. Van Etten. 1990. Purification and characterization of a low-molecular-weight acid phosphatase—a phosphotyrosyl-protein phosphatase from bovine heart. Arch. Biochem. Biophys. 282:39-49. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, Z. Y., G. Zhou, J. M. Denu, L. Wu, X. Tang, O. Mondesert, P. Russell, E. Butch, and K. L. Guan. 1995. Purification and characterization of the low molecular weight protein tyrosine phosphatase, Stp1, from the fission yeast Schizosaccharomyces pombe. Biochemistry 34:10560-10568. [DOI] [PubMed] [Google Scholar]