Abstract
From Streptomyces virginiae, in which production of streptogramin antibiotic virginiamycin M1 and S is tightly regulated by a low-molecular-weight Streptomyces hormone called virginiae butanolide (VB), which is a member of the γ-butyrolactone autoregulators, the hormone biosynthetic gene (barS1) was cloned and characterized by heterologous expression in Escherichia coli and by gene disruption in S. virginiae. The barS1 gene (a 774-bp open reading frame encoding a 257-amino-acid protein [Mr, 27,095]) is situated in the 10-kb regulator island surrounding the VB-specific receptor gene, barA. The deduced BarS1 protein is weakly homologous to β-ketoacyl-acyl carrier protein/coenzyme A reductase and belongs to the superfamily of short-chain alcohol dehydrogenase. The function of the BarS1 protein in VB biosynthesis was confirmed by BarS1-dependent in vitro conversion of 6-dehydro-VB-A to VB-A, the last catalytic step in VB biosynthesis. Of the four possible enantiomeric products from racemic 6-dehydro-VB-A as a substrate, only the natural enantiomer of (2R,3R,6S)-VB-A was produced by the purified recombinant BarS1 (rBarS1), indicating that rBarS1 is the stereospecific reductase recognizing (3R)-isomer as a substrate and reducing it stereospecifically to the (6S) product. In the ΔbarS1 mutant created by homologous recombination, the production of VB as well as the production of virginiamycin was lost. The production of virginiamycin by the ΔbarS1 mutant was fully recovered by the external addition of VB to the culture, which indicates that the barS1 gene is essential in the biosynthesis of the autoregulator VBs in S. virginiae and that the failure of virginiamycin production was a result of the loss of VB production.
Members of the filamentous, gram-positive bacterial genus Streptomyces are versatile producers of many secondary metabolites, including over two thirds of all known antibiotics used in human medicine and in agriculture (4). Many factors, such as nutrient limitations, have been known to affect the antibiotic production in the genus Streptomyces (5, 6); however, detailed knowledge of the mechanism and/or hierarchy of the regulatory machinery is generally lacking, which has hampered the rational design of a highly productive industrial strain.
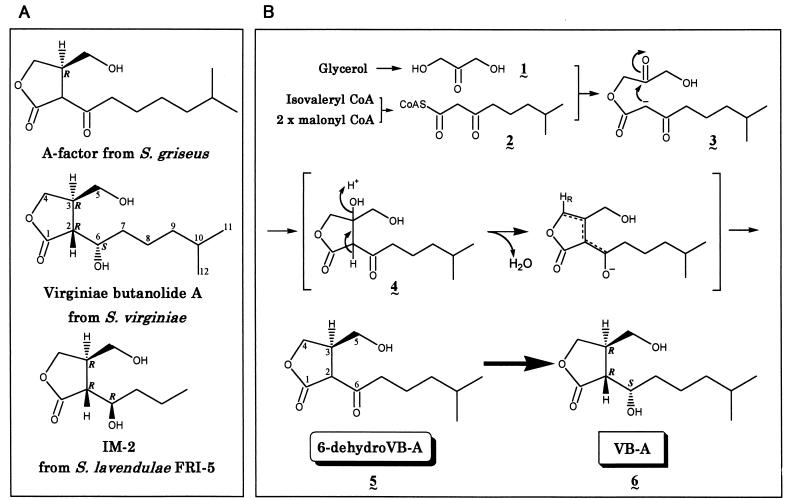
Among factors known to affect the behavior of Streptomyces species (10, 11, 41), γ-butyrolactone autoregulators are one of the most studied, and they have been shown for several Streptomyces species to trigger the onset of secondary metabolism in general and that of antibiotic production in particular (38, 40). All the known γ-butyrolactone autoregulators, which belong to one of three types (virginiae butanolide [VB] type, possessing a 6-α-hydroxy group [18, 39]; IM-2 type, possessing a 6-β-hydroxy group [30, 36]; and A-factor type, possessing a 6-keto group [14, 20] [Fig. 1A ]), possess type-specific receptor proteins that recognize the tiny structural differences among the three types of autoregulators. The effectiveness of the autoregulators at extremely low concentrations, usually at a concentration of a few nanomolars (23), as well as the presence of receptor proteins of high ligand specificity (24, 35, 37), implies that the γ-butyrolactone autoregulators should be regarded as Streptomyces hormones.
FIG. 1.
(A) Three types of γ-butyrolactone autoregulators from Streptomyces species. (B) A plausible biosynthetic pathway deduced from precursor feeding. (A) Absolute configurations of A-factor (20), VB-A (38), and IM-2 (38, 40) have been assigned to (3R), (2R,3R,6S), and (2R,3R,6R), respectively, as depicted. (B) The condensation to form a β-ketoacyl-CoA (2) occurs between an isovaleryl-CoA and two malonyl-CoAs in a process similar to that of polyketide biosynthesis, and β-ketoacyl-CoA (2) couples with a dihydroxyacetone-type C3 unit (1) derived from glycerol to create a β-keto ester (3), followed by intramolecular aldol condensation to form a γ-butyrolactone skeleton (4). Successive dehydration and reduction should lead to 6-dehydro-VB-A (5). Finally, reduction of the 6-carbonyl group (5) will result either in (2R,3R,6S)-VB-A (6) or its (6R)-epimer.
Streptomyces virginiae is one of the representative strains in which VB-A regulates the production of two structurally different antibiotics, virginiamycin M1 and virginiamycin S, by binding to the VB-specific receptor protein, BarA. With regard to the signal transduction pathway and/or regulation mechanism after the complex formation between VB-A and BarA, BarA's function as the VB-dependent transcriptional repressor has been clarified (16, 21, 22). However, almost nothing is known about the biosynthesis of VB itself. It is essential to obtain a gene(s) that catalyzes the VB biosynthesis in order to gain insight into how and when VB production is regulated. Furthermore, to clarify the mechanism of why one strain, such as S. virginiae, produces only the VB type of γ-butyrolactone autoregulators, and another strain, such as Streptomyces lavendulae FRI-5, solely produces the IM-2 type of autoregulators, it is important to biochemically characterize the enzyme that catalyzes the last reduction step of the autoregulator biosynthesis.
Regarding the biosynthetic pathway of the autoregulators, we have previously established a plausible pathway mainly by feeding precursors labeled with radioactive or stable isotopes (27, 28) (Fig. 1B). The estimated pathway suggested the presence of two reduction steps in autoregulator biosynthesis; namely, the NADH-dependent enoyl reductase-type reduction to form the A-factor-type precursor (6-dehydro-VB-A) and the final NADPH-dependent keto-reduction to form either VB-type compounds of (6S) absolute configuration or IM-2-type compounds of (6R) absolute configuration. In the course of our searching for genes responsible for the autoregulator biosynthesis a novel open reading frame (ORF) (orf4) (13) was identified upstream of the barA gene encoding the VB specific receptor, raising the possibility that the gene may participate in the VB biosynthetic pathway. In this study we focused on the orf4 gene and found that the Orf4 protein is the essential biosynthetic enzyme catalyzing the stereospecific reduction from A-factor-type precursor into VB-type compounds. This is, to our knowledge, the first report describing the isolation and characterization of a gene encoding an actual biosynthetic enzyme for the γ-butyrolactone autoregulators.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and plasmids.
S. virginiae (strain MAFF 10-06014; National Food Research Institute, Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Japan) was grown at 28°C as described previously (15, 39). For genetic manipulation in Escherichia coli, strain DH5α (7) was used. For expression of the cloned gene in E. coli, BL21(DE3)/pLysS (33) was used as the host. pET-3d (34) was used for construction of the expression plasmids. For conjugal transfer of DNA into S. virginiae, the methylation-deficient E. coli strain ET12567 (dam-13::Tn9 dcm-6 hsdM hsdS) (19) containing the RP4 derivative pUZ8002 (25) was used as the donor. The plasmid used for conjugal transfer was pKC1132 (3). DNA manipulation in E. coli was performed as described by Sambrook et al. (29).
Chemicals.
All the chemicals were of reagent or high-performance liquid chromatography (HPLC) grade and were purchased from either Nacalai Tesque, Inc. (Osaka, Japan), Takara Shuzo Co., Ltd. (Shiga, Japan), or Wako Pure Chemical Industrial, Ltd. (Osaka, Japan). β-NADPH, β-NADH, marker proteins for molecular sieve HPLC, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) materials were purchased from Oriental Yeast Co., Ltd. (Osaka, Japan), and Pharmacia Biotech K.K. (Osaka, Japan), respectively.
Construction of pET-barS1 and preparation of rBarS1.
A BamHI-BamHI (2.0 kb) fragment carrying barS1 was used as a template in the PCR. PCR was performed with primer 1 (5′-CATGCCATGGCTGATCGTCAGGGCCTTCTGACAGAC-3′) and primer 2 (5′-CGCGGATCCTGAAATCAGAGGATGGTGAACCCGCC-3′) to generate an NcoI site and a BamHI site at the 5′ and 3′ ends of the barS1 coding sequence, respectively (underlined). The amplified product was digested with NcoI and BamHI and was ligated into NcoI-BamHI-digested pET-3d, resulting in pET-barS1. The nucleotide sequence was confirmed by DNA sequencing. For preparing recombinant BarS1 (rBarS1), E. coli BL21(DE3)/pLysS harboring pET-barS1 was grown overnight at 37°C in Luria-Bertani medium containing both ampicillin (25 μg/ml) and chloramphenicol (25 μg/ml). Fresh Luria-Bertani medium (250 ml) in a 500-ml Sakaguchi flask was inoculated with 2.5 ml of the preculture and was cultivated at 37°C until the A600 reached 0.5, followed by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration of 1 mM) and 2 to 3 h of induction. Cells were harvested and resuspended (1 g [wet weight] of cells per 10 ml of buffer) in buffer A (0.02 M triethanolamine-HCl, pH 7.0, containing 20% [wt/vol] glycerol, 5 mM EDTA-Na2, 5 mM 2-mercaptoethanol, 1 mM dithiothreitol, 0.1 mM p-[amidinophenyl] methanesulfonyl fluoride hydrochloride, 10 μM leupeptin, and 0.3 μM pepstatin A) containing 0.5 M NaCl and were disrupted by sonication for 3 min at 50% duty cycle (Branson Sonifier 250) in an ice bath. Cell extracts were used for SDS-PAGE analysis and the assay of 6-dehydro-VB-A reductase activity. SDS-PAGE was performed with a precast 10 to 20% linear gradient gel (Daiichi Pure Chemical Co. Ltd., Tokyo, Japan) by using a minigel apparatus (Daiichi Pure Chemical Co.), and the gel was stained with Coomassie brilliant blue G-250.
For purification of rBarS1, the dialyzed supernatant after centrifugation (18,000 × g, 20 min) was adsorbed on a DEAE-Sephacel column (bed volume of 140 ml) preequilibrated with buffer A containing 0.05 M NaCl and 100 μM NADPH. After being washed with 560 ml of the same buffer the protein was successively eluted with 910 ml of buffer A containing 0.1 M NaCl and 100 μM NADPH, 700 ml of buffer A containing 0.15 M NaCl and 100 μM NADPH, and finally 840 ml of buffer A containing 0.2 M NaCl and 100 μM NADPH. The column was eluted at a flow rate of 45 ml/min, and 14-ml fractions were collected. Fractions 169 to 214 eluted at 0.2 M NaCl were pooled and concentrated to 61.5 ml by ultrafiltration (UK-20; Advantech Toyo). Fractions showing a single band on SDS-PAGE were stored at −80°C until use.
Determination of molecular weight.
The molecular weight of purified rBarS1 under nondenaturing conditions was estimated by gel filtration HPLC with a Superose 12 column on a SMART system (Amersham Pharmacia Biotech) with buffer A (without 2-mercaptoethanol) containing 0.3 M NaCl and 10 μM NADPH at a flow rate of 50 μl/min. A calibration curve was prepared with glutamate dehydrogenase (Mr, 290,000), lactate dehydrogenase (Mr, 142,000), yeast enolase (Mr, 67,000), yeast adenylate kinase (Mr, 32,000), and cytochrome c (Mr, 12,400).
Assay of 6-dehydro-VB-A reductase activity.
6-Dehydro-VB-A reductase activity was assayed at 25°C by using buffer A (without EDTA-Na2 [pH 7.5]) containing 0.5 M NaCl as described previously (31, 32). One unit of enzyme activity is defined as the amount of enzyme necessary to produce 1 μmol of VB-A per min at 25°C. Protein content was determined either by a Bio-Rad protein assay kit or by comparison of peak areas on HPLC charts with bovine serum albumin as a standard. (±)-6-Dehydro-VB-A was added to a final concentration of 766 μM. NADPH or NADH was added to a final concentration of 5 mM.
Enzyme reaction with commercial dehydrogenases was performed at 24°C in buffer A (pH 7.0) (without EDTA-Na2 [pH 7.5]) containing 0.5 M NaCl in the presence of 5 mM NADH and 5 mM NADPH. The reaction with 3-hydroxybutyrate dehydrogenase was carried out at 37°C. Glycerol was omitted from the buffer for assay with glycerol dehydrogenase.
For measuring the activity of a series of A-factor-type substrates, the amount of the resulting VB product was determined as the amount of dibenzoyl derivative on C18 reverse-phase HPLC as described below. Reaction progress was followed intermittently, and the catalytic rate was calculated from the linear part of each reaction. The enzyme reaction was initiated as described previously (31, 32) in a total volume of 575 μl and was terminated by adding 200 μl of 1% trifluoroacetic acid and 1,225 μl of cold water. Five micrograms of racemic VB-D was added as an internal standard. The mixture was diluted with water (9 ml) and applied to an OASIS HLB extraction cartridge (1 ml; Waters) followed by washing with 5 ml of water. The absorbed VB together with VB-D was eluted with 8 ml of 75% (vol/vol) CH3CN. The 75% CH3CN fraction was evaporated to dryness. The lyophilized sample was benzoylated with benzoyl cyanide and tri-n-butylamine as described previously (31, 32). The benzoylation mixture was extracted with 4 ml of hexane-methanol-water (3 ml-250 μl-750 μl), and the organic layer containing both VB dibenzoate and VB-D dibenzoate was evaporated to dryness. The amount of VB dibenzoate was measured by a method similar to that used for VB-A by comparing the peak area with an authentic VB dibenzoate standard. Dibenzoyl derivatives of VB-C4, VB-C5, VB-C6, VB-A, VB-D (VB-C7), VB-type isomer of SCB1, VB-C8, and VB-C9 were eluted at 9.78, 12.5, 16.5, 21.0, 23.0, 27.2, 30.4, and 44.1 min, respectively. (±)-6-Dehydro-VB-A, racemic A-factor C4∼C9, racemic A-factor, racemic VB-A, racemic VB-C4∼C9, and racemic VB-type isomer of SCB1 were chemically synthesized as described elsewhere (23, 27, 36). Racemic standards of VB dibenzoates were prepared similarly by benzoylation of corresponding racemic VBs, and their identities were confirmed by chemical ionization-mass spectrometry (CI-MS).
Isolation and chiral HPLC analysis of the rBarS1-catalyzed product.
VB-A produced by rBarS1 was purified as dibenzoate by C18 reverse-phase HPLC (32), and the chemical structure was verified by 600 MHz 1H-nuclear magnetic resonance and CI-MS with the data reported previously (27). The product was then analyzed by chiral HPLC to determine the optical purity. Authentic chiral standards of VB-A dibenzoate isomers were synthesized as described previously (32).
Preparation of crude cell extract from S. virginiae.
The S. virginiae culture was initiated by inoculating 2.1 ml of preculture into 70 ml of f medium (15) in a 500-ml baffled flask. After cultivation at 28°C on a reciprocating shaker (120 strokes per min), cells were harvested by centrifugation (3,000 × g, 10 min, 4°C). The cells suspended in a fivefold volume of buffer A (without EDTA-Na2 [pH 7.5]) containing 0.5 M NaCl and 100 μM NADPH were disrupted by sonication for 5 min as described above. After centifugation (18,000 × g, 20 min, 4°C), the dialyzed supernatant was stored at −80°C and was used as the source of native 6-dehydro-VB-A reductase of S. virginiae.
The ΔbarS1 mutant of S. virginiae.
A 5.0-kb fragment of Bst1107I-EcoT22I (varM, orf4, orf5, and barX; Fig. 2) was blunt ended and subcloned into the SmaI site of pUC19. From the resulting plasmid a 297-bp Psp1406I-BsmI fragment internal to the orf4 coding region was removed to create disruption plasmid pJK101, by which an in-frame deletion of 99 amino acids from 84Phe to 192Gly was introduced. The status of the in-frame deletion in the orf4 (barS1) gene on pJK101 was confirmed by DNA sequencing, and the deletion mutation was designated ΔbarS1.
FIG. 2.
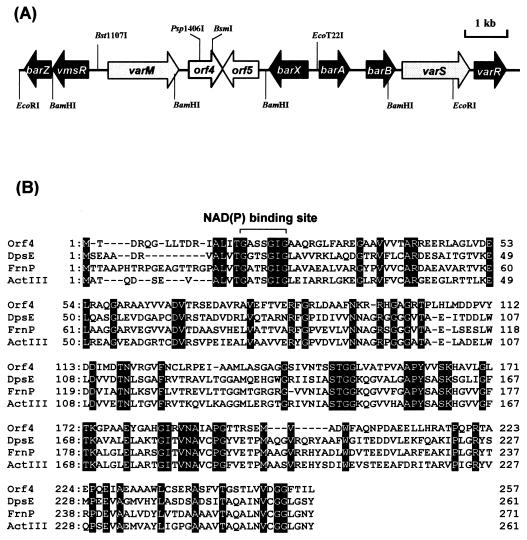
(A) Location of the orf4 gene in the 10-kb EcoRI-EcoRI fragment containing barA. (B) Amino acid alignment of the orf4 product with several β-ketoacyl-acyl carrier protein/CoA reductases. (A) Solid arrows, shaded arrows, and open arrows indicate plausible regulatory genes, resistance genes for virginiamycin, and genes for catalytic enzymes, respectively. (B) Identical residues are indicated by white letters in black boxes. Orf4, S. virginiae (this study); DpsE, S. peucetius (8); FrnP, S. roseofulvus (2); ActIII, S. coelicolor A3(2) (9).
To construct a conjugation plasmid for gene replacement in S. virginiae, a HindIII-EcoRI fragment (4.7 kb; varM-ΔbarS1-orf5-barX) from pJK101 was subcloned at the corresponding sites of pKC1132 (3), a conjugation plasmid containing oriT and an apramycin resistance cassette, resulting in pJK102. The conjugation protocol between E. coli ET12567/pUZ8002 containing pJK102 and S. virginiae spores was essentially similar to those for S. lavendulae FRI-5 (17) on ISP-2 solid medium with selection by overlaying nalidixic acid and apramycin.
Analyses on the production of virginiamycin and VB.
Spores of S. virginiae (108) were inoculated in 70 ml of f broth (7.5 g of Bacto Casitone [Difco Laboratories] per liter, 7.5 g of yeast extract [Difco Laboratories] per liter, 15 g of glycerol per liter, and 2.5 g of NaCl per liter [pH 6.5] [23]) and were reciprocally incubated for 24 h (140 strokes per min). Cells were harvested and resuspended in the same volume of f broth. The cell suspension was immediately frozen and kept at −80°C until use. The frozen culture was thawed at room temperature and was added to a 70-ml f broth, diluted to an A600 of 0.075. For conventional analysis of virginiamycin production, the culture was withdrawn periodically and supernatant (after centrifugation) was used for bioassay with Bacillus subtilis PCI219 as an indicator strain (23). Purified virginiamycins obtained from C.-K. Lee (Osaka University) were used as the standard.
The amount of VB produced during the growth in f broth was estimated by measuring the VB-dependent production of virginiamycin essentially according to the previously described method (23). Chemically synthesized VB-C6 (23) was used as the standard. One unit of VB activity is defined as the minimum amount of VB-A needed to induce virginiamycin production (0.6 ng/ml or 2.6 nM) (39).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper has been submitted to the DDBJ/EMBL/GenBank data bank as accession number AB035548.
RESULTS AND DISCUSSION
Nucleotide sequence of the orf4 gene.
Nucleotide sequencing of the 10-kb EcoRI-EcoRI fragment containing barA revealed the orf4 gene starting 2,986 bp upstream of the barA start codon in the same direction as barA (Fig. 2A). The resulting ORF is predicted to encode a 257-amino-acid protein of 27,095 Da which showed moderate similarity to several β-ketoacyl-acyl carrier protein/coenzyme A (CoA) reductases belonging to the short-chain alcohol dehydrogenase superfamily, such as DpsE from S. peucetius (36.0% identity), functioning as a reductase in the biosynthesis of daunorubicin (8); FrnP from S. roseofulvus (36.0% identity), participating in the biosynthesis of frenolicin (2); and ActIII from S. coelicolor A3 (2) (34.8% identity), functioning as a reductase of a β-keto group during the assembly of the actinorhodin polyketide chain (9). Multiple alignment of the deduced Orf4 product with these short-chain alcohol dehydrogenase proteins (Fig. 2B) revealed that the most significant identity exists at an amino-terminal region containing a typical βαβ fold as an NAD(P)H-binding motif, Gly-X-X-X-Gly-X-Gly, as well as at a middle region that includes Ser148, Tyr161, and Lys165 assigned as active-site residues (26).
Overexpression and purification of rOrf4.
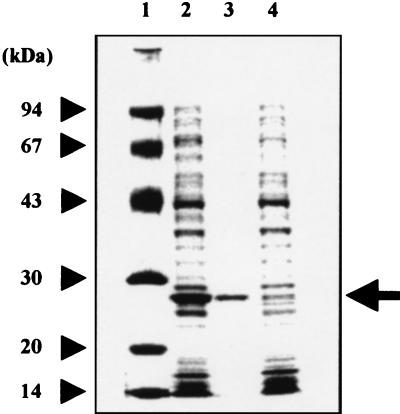
To examine the orf4 product in more detail, we expressed recombinant Orf4 (rOrf4) protein in E. coli by means of the T7 expression vector pET-3d. The coding region was amplified by PCR and placed under the control of the T7 RNA polymerase promoter. SDS-PAGE analysis (Fig. 3, lane 2) indicated that IPTG-induced E. coli BL21(DE3)/pLysS harboring pET-orf4 significantly overexpressed a 27-kDa protein, whose identity to the orf4 product was confirmed by analysis of its N-terminal amino acid sequence (data not shown).
FIG. 3.
SDS-PAGE analysis of rOrf4 expressed in and purified from E. coli. The rOrf4 protein is indicated by an arrow. Lane 1, molecular size markers; lane 2, crude extract from IPTG-induced E. coli BL21/pLysS harboring pET-orf4; lane 3, purified rOrf4 after DEAE-Sephacel anion-exchange chromatography; lane 4, crude extract from IPTG-induced E. coli BL21/pLysS harboring pET-3d. A sample containing 7 μg was separated on a 10 to 20% linear gradient gel, and the gel was stained with Coomassie brilliant blue G-250.
To clarify the actual enzymatic function of rOrf4, we tested its activity by using 6-dehydro-VB-A as a substrate (23, 24). Cell extracts prepared from IPTG-induced cells harboring pET-orf4 showed a high 6-dehydro-VB-A reductase activity for forming VB-A (268 mU/mg of protein) in the presence of NADPH, while cell extracts from the control cells harboring pET-3d showed no activity (Table 1). The overexpressed rOrf4 was purified to homogeneity in an activity yield of 32.8% on a DEAE-Sephacel column (Fig. 3, lane 3). The apparent molecular weight of 27,000 on SDS-PAGE agreed well with that calculated from the nucleotide sequence (Mr, 27,095). Under native conditions by molecular sieve HPLC, purified rOrf4 showed an apparent Mr of 54,000, indicating the dimeric nature of the enzyme. Enzymatic reaction of this purified enzyme with 6-dehydro-VB-A was carried out, and the product after benzoylation was purified by reverse-phase C18 HPLC. All the 600 MHz 1H-nuclear magnetic resonance and CI-MS spectra of the isolated product agreed well with those of synthetic VB-A dibenzoate (27), confirming that the catalytic product of the rOrf4 protein is VB-A.
TABLE 1.
Assay of 6-dehydro-VB-A reductase activity for crude and purified rOrf4 protein
| Protein | Results with the following substrates:
|
Amt of VB-A produced (pmol/min)c | ||
|---|---|---|---|---|
| 6-Dehydro-VB-Aa | NADPHb | NADHb | ||
| Crude (pET-orf4) | + | + | − | 3.51 × 102 |
| Crude (pET-3d) | + | + | − | 0 |
| Purified rOrf4 | + | + | − | 3.34 × 102 |
| Purified rOrf4 | + | − | + | 0 |
| Purified rOrf4 | + | − | − | 0 |
| Purified rOrf4 | − | + | − | 0 |
| No protein | + | + | − | 0 |
(±)-6-Dehydro-VB-A was added to a final concentration of 766 μM.
NADPH or NADH was added to a final concentration of 5 mM.
For assays with crude extracts, 1.31 μg of pET-orf4 protein and 14.6 μg of pET-3d protein were used. For assays with purified rOrf4, 3.0 μg of protein was used. The enzyme activity was measured within the linear range of activity after 0.5 to 20 h of incubation, with a 3-pmol detection limit per assay (31, 32).
In order to verify that the 6-dehydro-VB-A reductase activity is a specific feature of rOrf4, we tested several commercial dehydrogenases for their 6-dehydro-VB-A reductase activity. No detectable activity was found in any of the five dehydrogenases tested (alcohol dehydrogenase from baker's yeast, glycerol dehydrogenase from Bacillus megaterium, 3-hydroxybutyrate dehydrogenase from Rhodopseudomonas sphaeroides, 3α-hydroxysteroid dehydrogenase from Pseudomonas testosteroni, and β-hydroxysteroid dehydrogenase from P. testosteroni), even in the presence of 2,000 times the amount of rORF4 (1 U per assay) during a 20-h incubation (data not shown). From these results we concluded that the orf4 gene encodes the 6-dehydro-VB-A reductase catalyzing the reduction of 6-dehydro-VB-A to VB-A, and we designated it barS1 (for butyrolactone autoregulator synthesis).
Characterization of rBarS1.
To characterize the coenzyme specificity, purified rBarS1 was assayed in the presence of 5 mM NADH or NADPH. Clear activity was detected with 5 mM NADPH but none was detected with NADH, indicating that rBarS1 was NADPH specific (Table 1). The Km value for (±)-6-dehydro-VB-A and the Vmax value were determined to be 11.1 μM and 269 mU/mg of protein in the presence of 10 mM NADPH, respectively. The optimum pH was determined to be 7.5. The optimum temperature was narrow, with a maximum at 25°C, together with a narrow range of stability, as is evident from the 80% loss and the complete loss of activity after 30 min of incubation at 35°C and at 40 to 50°C, respectively.
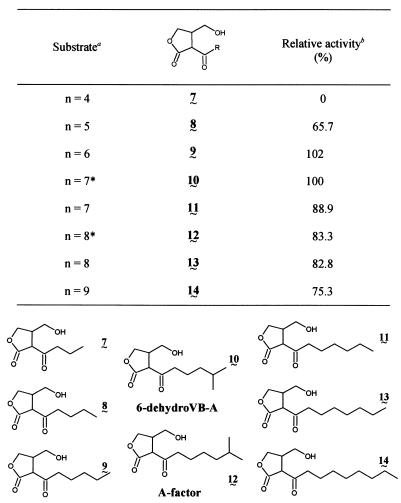
To determine the substrate specificity of the enzyme, especially the influence of the C-2 side chain structure, we performed a 6-dehydro-VB reductase assay in the presence of a series of synthetic A-factor-type analogues, i.e., A-factor-C4 to A-factor-C9 (linear side chain), A-factor (natural form), and 6-dehydro-VB-A (Table 2). The activity increased sharply with a chain length of 4 to 6 carbons, but any further increase in the side chain length only resulted in a moderate decrease in activity. This substrate specificity agreed well with the profiles of natural autoregulators produced by S. virginiae, namely, VBs having a linear or branched 6- to 7-carbon C-2 side chain (VB-A, -B, -C, and -D) are predominant, while the amount of a VB with a shorter side chain (VB-E) is scarce (18).
TABLE 2.
VB synthesizing activity of rBarS1 for a series of A-factor-type substrates
Total carbon number in the C-2 side chain is indicated by n, with an asterisk indicating the substrate having terminal isopropyl moiety.
Each reaction was performed with purified rBarS1 (1.54 μg) at a substrate concentration of 1 mM and with 10 mM NADPH at 25°C in buffer A (without EDTA-Na2 [pH 7.5]) containing 0.5 M NaCl and 100 μM NADPH.
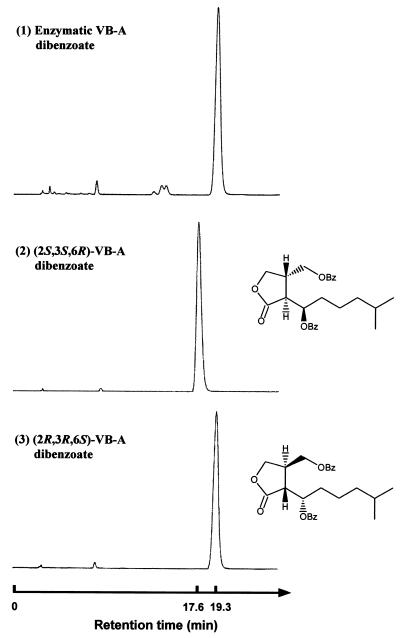
To study the stereospecificity of the reaction, we analyzed purified enzymatic VB-A dibenzoate by chiral HPLC (Fig. 4) (32) and found it to be an optically pure (2R,3R,6S)-form. Considering that no IM-2-type compound [(2R,3R,6R)- or (2S,3S,6S)-form] was detected by our highly sensitive procedure (data not shown), we concluded that rBarS1 accepts only (3R)-6-dehydro-VB-A as a substrate and catalyzes the stereospecific reduction to form natural (2R,3R,6S)-VB-A. To deduce the in vivo function of barS1 further, we investigated the time course of the 6-dehydro-VB-A reductase activity in the cell during the cultivation of S. virginiae for 6 to 16 h. Specific activity was almost constant (521 μU/mg of protein) during the entire cultivation, which agreed well with the constitutive expression of barS1 by reverse transcription-PCR (13).
FIG. 4.
Chiral HPLC profiles of synthetic VB-A dibenzoates and the benzoylated reaction product with rBarS1. HPLC was performed with a Chiralpak AD column (4.6 mm [inside diameter] by 25 cm) at 22°C, with hexane and isopropanol (90:10) as the mobile phase at a flow rate of 1.0 ml/min. (1) Enzymatic product dibenzoate; (2) synthetic (2S,3S,6R)-enantiomer dibenzoate; and (3) synthetic (2R,3R,6S)-enantiomer dibenzoate.
Disruption of barS1 by homologous recombination.
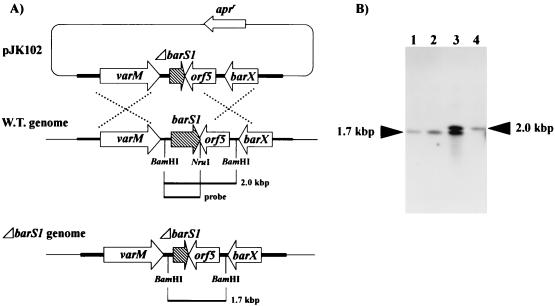
To know the in vivo function of the barS1 in either the production of VB and/or virginiamycin, the barS1 gene was disrupted by in-frame deletion of a 297-bp fragment internal to the barS1 gene. The disruption plasmid (pJK102; Fig. 5A) derived from a conjugation vector, pKC1132, was introduced into S. virginiae via conjugation from E. coli ET12567/pUZ8002. After selection of the single-crossover strain on solid ISP-2 containing apramycin (50 μg/ml), ΔbarS1 mutant strains of S. virginiae were obtained by three rounds of cultivation from spore to spore on solid ISP-2 without apramycin. The integration of pJK102 in the single-crossover strain and the replacement of the wild-type allele with the ΔbarS1-mutated allele in the ΔbarS1 mutant were confirmed by PCR (data not shown) and Southern blot hybridization (Fig. 5). Two of the representative double-crossover strains were selected for further study. No defect of growth either in liquid media or on solid media was observed for the barS1 disruptants compared to that with wild-type S. virginiae. In contrast, the barS1 disruption caused complete loss of the production of VB and virginiamycin (Tables 3 and 4). Usually, S. virginiae starts producing VB after 10.5 h of cultivation and the concentration of VB reaches up to 150 U per ml at around 12 h of cultivation, which in turn induces the production of virginiamycin after 13 h of cultivation. In the orf4 disruptant, no VB production was detected even after 24 h of cultivation and no virginiamycin production was observed. To clarify whether this defect in virginiamycin production was due to the loss of VB production, external VB was added to the culture of the barS1 disruptants at 8 h of cultivation, which restored the virginiamycin production to a level identical to that of the wild-type strain. These results indicate that the barS1 gene is the essential and only gene encoding the 6-dehydro-VB-A reductase catalyzing the last biosynthetic step of the autoregulator VB.
FIG. 5.
Construction of a barS1 disruption mutant. (A) Schematic representation of the strategy used for the disruption of barS1. The shaded arrow represents the barS1 gene, and open arrows represent the apramycin resistance gene (apr) and the varM, orf5, and barX genes. W.T., wild type. (B) Southern hybridization analysis of chromosomal DNA from the wild-type strain (lane 4), ΔbarS1 strains (lanes 1 and 2), and a single-crossover strain (lane 3) digested with BamHI. The probe used was the BamHI-NruI fragment shown as the probe in panel A. BamHI digestion results in a 2.0-kb fragment and a 1.7-kb fragment in the wild-type chromosome and the ΔbarS1 chromosome, respectively.
TABLE 3.
Concentration of VB and virginiamycin produced by two independent ΔbarS1 disruptants and by the wild-type strain in the absence of external VB addition
| Strain | Cultivation time and concn of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| VB (U/ml)
|
Virginiamycin (μg/ml)
|
|||||||
| 10 h | 13 h | 16 h | 24 h | 10 h | 13 h | 16 h | 24 h | |
| Wild type | <0.2 | ≥150 | ≥150 | ≥150 | 0 | 94 | 92 | 94 |
| ΔbarSI disruptant no. 1 | <0.2 | <0.2 | <0.2 | <0.2 | 0 | 0 | 0 | 0 |
| ΔbarSI disruptant no. 2 | <0.2 | <0.2 | <0.2 | <0.2 | 0 | 0 | 0 | 0 |
TABLE 4.
Concentration of virginiamycin produced by the ΔbarSI disruptants with external VB addition (VB-C6; 60 ng/ml) at 8 h of cultivation
| Strain | Cultivation time and virginiamycin concn (μg/ml)
|
|||
|---|---|---|---|---|
| 8 h | 10 h | 12 h | 14 h | |
| ΔbarSI disruptant no. 1 plus VB | 0 | 43 | 81 | 94 |
| ΔbarSI disruptant no. 2 plus VB | 0 | 40 | 76 | 81 |
Although S. griseus afsA has been proposed to encode one of the biosynthetic enzymes for A-factor (1, 12), the actual catalytic step or enzymatic function in the biosynthetic pathway has been left unexplained until now. Furthermore, contradictory information regarding the function of AfsA has become apparent recently in the findings that two close homologues of afsA, namely, S. virginiae barX and S. coelicolor scbA, act as pleiotropic regulatory proteins rather than as catalytic enzymes (13, 35). Successful cloning and analysis of barS1 as one of the genes in the autoregulator biosynthesis will promote the elucidation and understanding of the actual biosynthetic mechanism of γ-butyrolactone autoregulators at the molecular level.
REFERENCES
- 1.Ando, N., N. Matsumori, S. Sakuda, T. Beppu, and S. Horinouchi. 1997. Involvement of AfsA in A-factor biosynthesis as a key enzyme. J. Antibiot. 50:847-852. [DOI] [PubMed] [Google Scholar]
- 2.Bibb, M. J., D. H. Sherman, S. Ômura, and D. A. Hopwood. 1994. Cloning, sequencing and deduced functions of a cluster of Streptomyces genes probably encoding biosynthesis of the polyketide antibiotic frenolicin. Gene 142:31-39. [DOI] [PubMed] [Google Scholar]
- 3.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 4.Champness, W. 2000. Actinomycete development, antibiotic production, and phylogeny: questions and challenges, p. 11-31. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. ASM Press, Washington, D.C.
- 5.Chater, K. F., and M. J. Bibb. 1997. Regulation of bacterial antibiotic production, p. 57-105. In H. Kleinkauf and H. V. Döhren (ed.), Bio/Technology, vol. 7. VCH Press, Weinheim, Germany.
- 6.Demain, A. L., and A. Fang. 1995. Emerging concepts of secondary metabolism in actinomycetes. Actinomycetology 9:98-117. [Google Scholar]
- 7.Grant, S. G. N., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 87:4645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimm, A., K. Madduri, A. Ali, and C. R. Hutchinson. 1994. Characterization of the Streptomyces peucetius ATCC 29050 genes encoding doxorubicin polyketide synthase. Gene 151:1-10. [DOI] [PubMed] [Google Scholar]
- 9.Hallam, S. E., F. Malpartida, and D. A. Hopwood. 1988. Nucleotide sequence, transcription and deduced function of a gene involved in polyketide antibiotic synthesis in Streptomyces coelicolor. Gene 74:305-320. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs, G., A. I. C. Obanye, J. Petty, J. C. Mason, E. Barratt, D. C. J. Gardener, F. Flett, C. P. Smith, P. Broda, and S. G. Oliver. 1992. An integrated approach to studying regulation of production of the antibiotic methylenomycin by Streptomyces coelicolor A3(2). J. Bacteriol. 174:1487-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hood, D. W., R. Heidstra, U. K. Swoboda, and D. A. Hodgson. 1992. Molecular genetic analysis of proline and tryptophan biosynthesis in Streptomyces coelicolor A3(2): interaction between primary and secondary metabolism—a review. Gene 115:5-12. [DOI] [PubMed] [Google Scholar]
- 12.Horinouchi, S., H. Suzuki, M. Nishiyama, and T. Beppu. 1989. Nucleotide sequence and transcriptional analysis of the Streptomyces griseus gene (afsA) responsible for A-factor biosynthesis. J. Bacteriol. 171:1206-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawachi, R., T. Akashi, Y. Kamitani, A. Sy, U. Wangchaisoonthorn, T. Nihira, and Y. Yamada. 2000. Identification of an AfsA homologue (BarX) from Streptomyces virginiae as a pleiotropic regulator controlling autoregulator biosynthesis, virginiamycin biosynthesis and virginiamycin M1 resistance. Mol. Microbiol. 36:302-313. [DOI] [PubMed] [Google Scholar]
- 14.Khokhlov, A. S. 1980. Problems of studies of specific cell autoregulators (on the example of substances produced by some actinomycetes), p. 201-210. In S. N. Ananchenko (ed.), Frontiers of bioorganic chemistry and molecular biology. Pergamon Press, Oxford, United Kingdom.
- 15.Kim, H. S., T. Nihira, T. Tada, H. Yanagimoto, and Y. Yamada. 1989. Identification of binding protein of virginiae butanolide C, an autoregulator in virginiamycin production, from Streptomyces virginiae. J. Antibiot. 42:769-778. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita, H., T. Tsuji, H. Ipposhi, T. Nihira, and Y. Yamada. 1999. Characterization of binding sequences for butyrolactone autoregulator receptors in streptomycetes. J. Bacteriol. 181:5075-5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitani, S., Y. Yamada, and T. Nihira. 2001. Gene replacement analysis of the butyrolactone autoregulator receptor (FarA) reveals that FarA acts as a novel regulator in secondary metabolism of Streptomyces lavendulae FRI-5. J. Bacteriol. 183:4357-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo, K., Y. Higuchi, S. Sakuda, T. Nihira, and Y. Yamada. 1989. New virginiae butanolide from Streptomyces virginiae. J. Antibiot. 42:1873-1876. [DOI] [PubMed] [Google Scholar]
- 19.MacNeil, D. J., J. L. Occi, K. M. Gewain, T. MacNeil, P. H. Gibbons, C. L. Ruby, and S. J. Danis. 1992. Complex organization of the Streptomyces avermetilis genes encoding the avermectin polyketide synthase. Gene 115:119-125. [DOI] [PubMed] [Google Scholar]
- 20.Mori, K. 1983. Revision of the absolute configuration of A-factor. Tetrahedron 39:3107-3109. [Google Scholar]
- 21.Nakano, H., E. Takehara, T. Nihira, and Y. Yamada. 1998. Gene replacement analysis of the Streptomyces virginiae barA gene encoding the butyrolactone autoregulator receptor reveals that BarA acts as a repressor in virginiamycin biosynthesis. J. Bacteriol. 180:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano, H., C. K. Lee, T. Nihira, and Y. Yamada. 2000. A null mutant of the Streptomyces virginiae barA gene encoding the butyrolactone autoregulator receptor, and its pleiotropic and transcriptional analyses. J. Biosci. Biotechnol. 90:204-207. [DOI] [PubMed] [Google Scholar]
- 23.Nihira, T., Y. Shimizu, H. S. Kim, and Y. Yamada. 1988. Structure-activity relationships of virginiae butanolide C, an inducer of virginiamycin production in Streptomyces virginiae. J. Antibiot. 41:1828-1837. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto, S., K. Nakamura, T. Nihira, and Y. Yamada. 1995. Virginiae butanolide binding protein from Streptomyces virginiae. J. Biol. Chem. 270:12319-12326. [DOI] [PubMed] [Google Scholar]
- 25.Paget, M. S. B., L. Chamberlin, A. Atrih, S. J. Foster, and M. J. Buttner. 1999. Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 181:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persson, B., M. Krook, and H. Jörnvall. 1991. Characteristics of short-chain alcohol dehydrogenases and related enzymes. Eur. J. Biochem. 200:537-543. [DOI] [PubMed] [Google Scholar]
- 27.Sakuda, S., A. Higashi, S. Tanaka, T. Nihira, and Y. Yamada. 1992. Biosynthesis of virginiae butanolide A, a butyrolactone autoregulator from Streptomyces. J. Am. Chem. Soc. 114:663-668. [Google Scholar]
- 28.Sakuda, S., and Y. Yamada. 1998. Biosynthesis of butyrolactone and cyclopentanoid skeltons formed by aldol condensation, p. 139-158. In D. H. I. Barton, K. Nakanishi, and O. Meth-Cohn (ed.), Comprehensive natural products chemistry, vol. 1. Elsevier Science Ltd., Oxford, United Kingdom.
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Sato, K., T. Nihira, S. Sakuda, M. Yanagimoto, and Y. Yamada. 1989. Isolation and structure of a new butyrolactone autoregulator from Streptomyces sp. FRI-5. J. Ferment. Biotechnol. 68:170-173. [Google Scholar]
- 31.Shikura, N., T. Nihira, and Y. Yamada. 1999. Identification and characterization of 6-dehydroVB-A reductase from Streptomyces antibioticus. FEMS Microbiol. Lett. 171:183-189. [DOI] [PubMed] [Google Scholar]
- 32.Shikura, N., T. Nihira, and Y. Yamada. 2000. Identification of a plausible biosynthetic enzyme for the IM-2-type autoregulator in Streptomyces antibioticus. Biochim. Biophys. Acta 1475:329-336. [DOI] [PubMed] [Google Scholar]
- 33.Studier, F. W., and B. A. Moffat. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113.. [DOI] [PubMed] [Google Scholar]
- 34.Studier, F. W., and B. A. Moffat. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 35.Takano, E., R. Chakraburtty, T. Nihira, Y. Yamada, and M. J. Bibb. 2001. A complex role for the γ-butyrolactone SCB1 in regulating antibiotic production in Streptomyces coelicolor A3(2). Mol. Microbiol. 41:1015-1028. [DOI] [PubMed] [Google Scholar]
- 36.Takano, E., T. Nihira, Y. Hara, J. J. Jones, C. J. L. Gershater, Y. Yamada, and M. J. Bibb. 2000. Purification and structural determination of SCB1, a γ-butyrolactone that elicits antibiotic production in Streptomyces coelicolor A3(2). J. Biol. Chem. 275:11010-11016. [DOI] [PubMed] [Google Scholar]
- 37.Waki, M., T. Nihira, and Y. Yamada. 1997. Cloning and characterization of the gene (farA) encoding the receptor for an extracellular regulatory factor (IM-2) from Streptomyces sp. strain FRI-5. J. Bacteriol. 179:5131-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada, Y. 1999. Autoregulatory factors and regulation of antibiotic production in Streptomyces, p. 177-196. In R. England, G. Hobbs, N. Bainton, and D. L. McRoberts (ed.), Microbial signalling and communication. Society for General Microbiology, Cambridge University Press, Cambridge, United Kingdom.
- 39.Yamada, Y., K. Sugamura, K. Kondo, M. Yanagimoto, and H. Okada. 1987. The structure of inducing factors for virginiamycin production in Streptomyces virginiae. J. Antibiot. 40:496-504. [DOI] [PubMed] [Google Scholar]
- 40.Yamada, Y., and T. Nihira. 1998. Microbial hormones and microbial chemical ecology, p. 377-413. In D. H. R. Barton and K. Nakanishi (ed.), Comprehensive natural products chemistry, vol. 8. Elsevier Science Ltd., Oxford, United Kingdom.
- 41.Yang, K., L. Han, and L. C. Vining. 1995. Regulation of jadomycin B producing in Streptomyces venezuelae ISP5230: involvement of a repressor gene, jadR2. J. Bacteriol. 177:6111-6117. [DOI] [PMC free article] [PubMed] [Google Scholar]