Abstract
The new gene prbA encodes an esterase responsible for the hydrolysis of the ester bond of parabens in Enterobacter cloacae strain EM. This gene is located on the chromosome of strain EM and was cloned by several PCR approaches. The prbA gene codes for an immature protein of 533 amino acids, the first 31 of which represent a proposed signal peptide yielding a mature protein of a putative molecular mass of 54.6 kDa. This enzyme presents analogies with other type B carboxylesterases, mainly of eukaryotic origin. The cloning and expression of the prbA gene in a strain of Escherichia coli previously unable to hydrolyze parabens resulted in the acquisition of a hydrolytic capacity comparable to the original activity of strain EM, along with an increased resistance of the transformed strain to methyl paraben. The presence of homologues of prbA was tested in additional ubiquitous bacteria, which may be causative factors in opportunistic infections, including Enterobacter gergoviae, Enterobacter aerogenes, Pseudomonas agglomerans, E. coli, Pseudomonas aeruginosa, and Burkholderia cepacia. Among the 41 total strains tested, 2 strains of E. gergoviae and 1 strain of Burkholderia cepacia were able to degrade almost completely 800 mg of methyl paraben liter−1. Two strains of E. gergoviae, named G1 and G12, contained a gene that showed high homology to the prbA gene of E. cloacae and demonstrated comparable paraben esterase activities. The significant geographical distance between the locations of the isolated E. cloacae and E. gergoviae strains suggests the possibility of an efficient transfer mechanism of the prbA gene, conferring additional resistance to parabens in ubiquitous bacteria that represent a common source of opportunistic infections.
Bacterial hydrolysis of antimicrobial agents used as preservatives in common pharmaceutical, cosmetic, or food products is a significant medical and economic concern. The esters of 4-hydroxybenzoic acid, commonly named parabens, are important commercial preservatives. Parabens are often used by the pharmaceutical, cosmetic, and food industries due to their excellent stability, wide pH range, antimicrobial spectrum of activity, and low toxicity (8, 16, 20). The ability of various species of Enterobacter, Alcaligenes, and Pseudomonas to utilize parabens for growth has been demonstrated (3). Additionally, the resistance to these compounds by the hydrolysis of their ester linkage has been documented for Cladosporium resinae, Pseudomonas aeruginosa, and Burkholderia cepacia (4, 19, 21, 30). Although some of these studies speculated on the role of an esterase in the hydrolysis of parabens, the identification of such an enzyme has not been reported.
Many of the organisms cited in the literature as showing resistance to parabens are bacterial species that may become opportunistic pathogens. Numerous cases of nosocomial infections by various species of Enterobacter introduced through contaminated medical products have been reported (2, 24, 27). Recently, we described the Enterobacter cloacae strain EM, which was isolated from a contaminated batch of a commercial mineral supplement normally well stabilized with a mixture of 1,700 mg of methyl paraben liter−1 (11.2 mM) and 180 mg of propyl paraben liter−1 (1.0 mM). This strain demonstrated a very high resistance to the parabens attributed to the action of an esterase (26). Here, we report the cloning and sequencing of the gene named prbA coding for this esterase, which is responsible for the hydrolysis of the parabens, and the presence of close homologues of this enzyme in Enterobacter gergoviae strains, which also show a high resistance to parabens.
MATERIALS AND METHODS
Materials.
Culture media were obtained as follows: tryptic soy broth (TSB), tryptic soy agar (TSA), tryptone-peptone, and agar were purchased from Difco (Sparks, Md.). Yeast extract, glucose, and sodium dodecyl sulfate were obtained from ICN Biomedicals (Aurora, Ohio). Methyl paraben was obtained from Sigma-Aldrich (St. Louis, Mo.), polyethylene glycol was obtained from A & C American Chemicals (Fair Lawn, N.J.), and agarose was obtained from Life Technologies (Grand Island, N.Y.). DNA was purified from agarose with the Qiaex II gel extraction kit from Qiagen (Mississauga, Ontario, Canada), and plasmid DNA was further purified with the QIAprep spin miniprep kit (Qiagen). All restriction enzymes were purchased from Pharmacia Biotech (Baie d'Urfé, Quebec, Canada). Lysozyme was obtained from Roche (Laval, Quebec, Canada), and proteinase K was obtained from Qiagen. All reagents for PCRs, including Taq polymerase, were obtained from Pharmacia Biotech. All reagents for ligation, including T4 DNA ligase, were included in the pGEM-T Easy Vector System I from Promega (Madison, Wis.). Digoxigenin (DIG)-labeled DNA was constructed with the DIG DNA labeling kit from Roche according to the manufacturer's instructions. Nylon membranes were obtained from Roche, and Biomax MR Scientific Imaging Film was obtained from Kodak (Rochester, N.Y.).
Cloning of the prbA gene from E. cloacae strain EM.
The degenerate primers EcoN and EcoPep4 were used to amplify a 300-bp stretch of the N terminus of the esterase of strain EM. The sequence of the primer EcoN (5′-AT(T/C/A)GA(G/A)GGNGTNAA(G/A)AA(T/C)GA-3′) was derived from a stretch of 20 amino acids comprising the N terminus of the protein, as determined by Edman degradation of a sodium dodecyl sulfate-polyacrylamide gel electrophoresis band, and consisting of the amino acids QELSPVVQMSKGTIEGVKND. Because the N-terminal portion showed significant similarity to type B carboxylesterases, an alignment of the amino acid sequences of representative carboxylesterases revealed a conserved 9-amino-acid region (Fig. 1). The first 8 amino acids of this region (sequence PVMVWIHG) were suitable for the construction of the second primer, EcoPep4, because they contained the codons with minimum degeneracy at the third position necessary to maximize the chances of hybridization with the unknown prbA gene. The deduced sequence of the EcoPep4 primer was 5′-CC(A/G)TG(T/G/A)ATCCANACCATNACNGG-3′. Due to the degeneracy of the primers, a touchdown PCR approach was selected, to facilitate the hybridization of degenerate primers to their proper target (5). The amplification conditions were as follows: 80°C for 3 min and 94°C for 5 min (1 cycle); 60°C for 1 min, 72°C for 2 min, and 94°C for 40 s (3 cycles); repeats of the previous parameters at decreasing 2°C increments until 42°C for 3 cycles each; 40°C for 1 min, 72°C for 2 min, and 94°C for 40 s (14 cycles); and 40°C for 1 min, followed by 72°C for 10 min (1 cycle). A 300-bp stretch of DNA thus amplified was purified from agarose, ligated into pGEM-T Easy, and transformed into E. coli DH5α cells. Subsequently, the pGEM-T Easy plasmid containing the DNA of interest was purified, and the 300-bp fragment was sequenced.
FIG. 1.
Conserved amino acid region in type B carboxylesterases. The amino acid sequences of representative carboxylesterases were aligned with the Pileup program: Deinococcus radiodurans, GenBank accession no. AAF12163 and AAF09993; Delftia acidovorans, BAA76305; Streptomyces coelicolor, CAB55678 and CAA22794; Bacillus sp. strains BP-23 and BP-7, CAB42083 and CAB93516, respectively; Mycobacterium tuberculosis, CAA17259; Arthrobacter oxydans, AAA22078; Myzus persicae, CAA52649; Caenorhabditis elegans, CAB03277. Amino acid numbering refers to the D. radiodurans 1 sequence.
In order to amplify sections of the chromosome of strain EM containing the entire prbA gene, a DNA-DNA hybridization probe was constructed by labeling the purified 300-bp N-terminal region with DIG, yielding a probe of suitable size to ensure specificity. The chromosomal DNA of strain EM was subsequently fragmented by four common restriction enzymes. The fragments generated were transferred by Southern blotting to a hybridization membrane and tested with the 300-bp DIG probe at 65°C. EcoRI and HindIII produced DNA fragments of 3.0 and 2.8 kb, respectively, that hybridized with the probe. They were subsequently self-circularized, and inverse PCR was used to amplify the unknown sequences flanking the 300-bp fragment (25). The selected primers for inverse PCR, L1R and L2F, were constructed from the extremities of the only known portion of the esterase sequence, the 300-bp fragment of the N terminus. These primers were designed to hybridize at the opposite ends of the 300-bp portion of the gene and to direct amplification around the entire self-circularized fragments. The sequence of the reverse primer L1R was 5′-GGTTCGCTTTTAACGTGCCC-3′, and that of the forward primer L2F was 5′-CACCAAGCTCACTTTGGGTG-3′. The PCR conditions were as follows: 1 cycle of 80°C for 2 min, 94°C for 5 min, and 55°C for 5 min; and 30 cycles of 72°C for 2 min, 94°C for 40 s, and 55°C for 1 min; followed by 72°C for 10 min. The amplified 2.8- and 3.0-kb fragments were subsequently ligated into pGEM-T Easy, and the same procedure described above was used for sequencing the 300-bp fragment.
Expression of the prbA gene in E. coli DH5α.
Two primers were designed based on the complete sequence of the prbA gene in order to amplify the gene from the chromosomal DNA of strain EM. The sequence of the forward primer was 5′-AAGGGAAATAATGGAACTAC-3′ (nucleotides 238 to 257 [GenBank accession no. AY077721]). This sequence included 10 nucleotides upstream of the methionine start codon of prbA, and the transcription initiation site and promoter for the expression of the gene were those present upstream of the multiple cloning site of pGEM-T Easy. The sequence of the reverse primer was 5′-TACCCCTGCGAACTCATCAC-3′ (nucleotides 1824 to 1843). Due to potential primer instability, the reverse primer ended 3 nucleotides before the stop codon, and the amplified product was ligated in frame with a stop codon in the multiple cloning site of pGEM-T Easy. The PCR amplification protocol was the same as that used above for the amplification of the 2.8-kb and 3.0-kb fragments. The amplified gene was ligated into the pGEM-T Easy vector and transformed into competent E. coli DH5α cells. The hydrolysis of 600 mg of methyl paraben liter−1 (4.0 mM) in TSB growth medium was monitored at 37°C as previously described (26).
Resistance to parabens and presence of prbA homologues in related strains.
A series of Enterobacter strains were obtained from various sources, many of which were isolated from commercial products stabilized with parabens (Table 1). The MICs were determined by inoculating a culture pregrown in TSB to the stationary phase in TSB containing 200, 400, 800, 1,600, or 3,200 mg of methyl paraben liter−1 to an optical density at 600 nm (OD600) of 0.05, where an OD of 1.0 corresponded to 6 × 108 Enterobacter cells per ml, and incubating the culture for 24 h at 30°C with shaking. OD readings were taken at the moment of inoculation and after 24 h, and the MICs were defined as the minimal amount of methyl paraben that prevented an increase in the OD of the suspension. To determine the efficiency of the strains to degrade parabens, the cells of exponentially growing cultures at an OD of 2.0 were harvested by centrifugation and resuspended in TSB medium containing 800 mg (5.3 mM) of methyl paraben liter−1 for a 2-h incubation period at 30°C with shaking. At the beginning and end of the 2-h incubation, aliquots were removed and heated immediately to 80°C for 10 min in order to prevent further enzymatic degradation of the parabens. The amount of parabens remaining in the samples and the appearance of p-hydroxybenzoic acid was then analyzed by high-performance liquid chromatography as previously described (26). The presence of homologues of prbA in selected strains was determined by touchdown PCR with the primers and conditions specified above.
TABLE 1.
Origin of strains, MICs, and hydrolytic ability
| Strain designation | Origin | MIC (mg liter−1)f | Degradation (%)g |
|---|---|---|---|
| E. cloacae | |||
| EMa | Mineral supplement | >3,200 | 99.9 |
| ID 67037b | Clinical strain | 3,200 | 12.7 |
| ID 67284b | Clinical strain | 3,200 | 12.8 |
| LSPQ 3022 | Reference strain | 3,200 | 15.3 |
| LSPQ 3345 | Reference strain | 3,200 | 19.2 |
| E. gergoviae | |||
| G1c | Eye liner | >3,200 | 100 |
| G2c | Foundation | 3,200 | 10.3 |
| G3c | Exfoliating gel | 3,200 | 8.9 |
| G4c | Foundation | 3,200 | 8.5 |
| G5c | Clinical strain | 3,200 | 7.8 |
| G6c | Cream | 3,200 | 10.4 |
| G7c | Challenge test | 3,200 | 14.7 |
| G8c | Shampoo | 3,200 | 14.4 |
| G9c | Cream | 3,200 | 11.4 |
| G10c | Shampoo | 3,200 | 12.3 |
| G11c | Cream | 3,200 | 16.3 |
| G12c | Rinsing material | 3,200 | 97.9 |
| G13c | Clinical strain | 1,600 | 15.5 |
| G14c | Clinical strain | 3,200 | 20.0 |
| G15c | Clinical strain | 3,200 | 22.6 |
| GMa | Mineral supplement | 3,200 | 18.7 |
| WLd | Syrup | 3,200 | 14.8 |
| LSPQ 3347 | Reference strain | 3,200 | 14.0 |
| ATCC 33028 | Reference strain | 3,200 | 12.6 |
| ATCC 33426 | Reference strain | 3,200 | 14.7 |
| ATCC 33428 | Reference strain | 3,200 | 18.1 |
| E. aerogenes | |||
| AE1c | Clinical strain | 3,200 | 15.4 |
| AE1c | Clinical strain | 3,200 | 12.7 |
| AE1c | Clinical strain | 3,200 | 14.7 |
| LSPQ 3811 | Reference strain | 3,200 | 15.6 |
| ATCC 13048 | Reference strain | 3,200 | 12.9 |
| P. agglomerans | |||
| ID 60635b | Clinical strain | 3,200 | 10.1 |
| ID 65518b | Clinical strain | 3,200 | 14.4 |
| LSPQ 3353 | Reference strain | 1,600 | 14.5 |
| LSPQ 3825 | Reference strain | 800 | 13.5 |
| E. coli | |||
| ID 41839e | Clinical strain | 3,200 | 9.1 |
| ATCC 25922 | Reference strain | 1,600 | 12.6 |
| ATCC 35218 | Reference strain | 3,200 | 11.3 |
| B. cepacia IFO 15124 | Oil-in-water emulsion | 1,600 | 37.9 |
| P. aeruginosa | |||
| ID 33122e | Clinical strain | 3,200 | 12.4 |
| ATCC 33350 | Reference strain | 1,600 | 10.2 |
Provided by a private company in Quebec, Canada.
Provided by the “Laboratoire de Santé Publique” (LSPQ), Quebec, Canada.
Provided by C. Bollet, Faculty of Medicine, INSERM, Marseille, France.
Provided by a private company in Ontario, Canada.
Provided by the Maisonneuve-Rosemont Hospital, Montreal, Canada.
Expressed as the concentration of methyl paraben.
Expressed as the percentage of 800 ppm (5.7 mM) of methyl paraben degraded after 2 h of incubation by an exponentially growing culture.
Nucleotide sequence accession number.
The GenBank accession numbers for the complete sequence of prbA from E. cloacae EM and the partial sequences for prbA from E. gergoviae G1 and G12 are AY077721, AY077722, and AY077723.
RESULTS
Cloning of the prbA gene from strain EM.
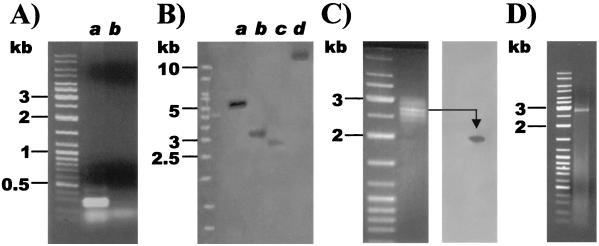
To amplify an initial segment of the N terminus of the esterase, the degenerate primers EcoN and EcoPep4 were hybridized to both the chromosomal and plasmid DNA of E. cloacae strain EM by touchdown PCR. A strong amplification signal of a 300-bp fragment was obtained with the chromosomal DNA of strain EM (Fig. 2A, lane a). No corresponding 300-bp fragment was found with the plasmid DNA of strain EM (Fig. 2A, lane b). The sequence of the amplified 300-bp fragment from the chromosomal DNA of strain EM contains at each extremity the sequence of the respective primers EcoN and EcoPep4, as well as 92 codons in frame between these sequences, and its deduced amino acid sequence showed homology to other type B carboxylesterases.
FIG. 2.
Cloning of the prbA gene. (A) Amplification of the 300-bp N-terminus region of strain EM by touchdown PCR. Lanes: a, chromosomal DNA; b, plasmid DNA. (B) Hybridization of chromosomal fragments of strain EM generated by BamHI (lane a), EcoRI (lane b), HindIII (lane c), PstI (lane d) with the digoxigenin-labeled N terminus of prbA. (C) Extraction of the chromosomal fragments between 2.5 and 3.0 kb generated by HindIII and hybridization with the digoxigenin probe. (D) Amplification of the 2.8-kb chromosomal fragment generated by HindIII containing the prbA gene by inverse PCR.
Sections of the chromosome of strain EM containing the esterase gene were identified with a DIG-labeled probe constructed from the 300-bp N terminus of the esterase (Fig. 2B). The probe hybridized to fragments of various sizes generated with all four of the selected restriction enzymes. The two fragments generated by EcoRI (lane b [3.0 kb]) and by HindIII (lane c [2.8 kb]) were targeted for separate extraction and cloning. To extract and amplify a sufficient quantity of the 2.8-kb fragment generated by HindIII (Fig. 2B, lane c), 100 μg of chromosomal DNA was cut with this enzyme, and all of the fragments migrating between 2.5 and 3.0 kb were purified (Fig. 2C). To ensure that the fragment of interest was present, the extracted fragments were transferred to a hybridization membrane by Southern blotting and tested with the 300-bp DIG probe. As shown in Fig. 2C, a fragment of the appropriate size hybridized with the probe, suggesting that the targeted 2.8-kb fragment containing the esterase gene had been extracted. The purified fragments were subsequently self-circularized, and the 2.8-kb fragment was amplified by inverse PCR. As shown in Fig. 2D, a single fragment of the appropriate size expected from a HindIII cut was amplified. The same procedure, as illustrated in Fig. 2C and D, was used to clone the fragment of 3.0 kb corresponding to the EcoRI cut (Fig. 2B, lane b), which also contained the esterase gene. To ensure the accuracy of the sequence and to fill in small gaps created by the cloning procedure, both the 2.8- and 3.0-kb fragments were sequenced on both the forward and reverse strands.
The nucleotide sequence of the esterase gene of E. cloacae strain EM, named prbA (for paraben A), has been annotated and deposited in the GenBank public database. The coding sequence is composed of 1,602 nucleotides, including the stop codon, constituting an immature protein of 533 amino acids with a theoretical molecular mass of 57.9 kDa (Protein General Sequence Analysis, UK HGMP Resource Centre) (29). The first 31-amino-acid stretch of the protein (nucleotides 248 to 340) is believed to represent a signal peptide, according to the signal peptide prediction tool SignalP v1.1 (Technical University of Denmark), which suggested a cleavage site between amino acids 31 (A) and 32 (Q) (14). Additionally, the prediction tool TMHMM v2.0 (Technical University of Denmark) for transmembrane helices in proteins suggested that a transmembrane helix exists between amino acids 12 (L) and 31 (A), with amino acids 1 to 11 (M—I) inside the cytosol and amino acids 32 to 533 outside the cell membrane (11). After cleavage of the signal peptide, the mature protein would contain 502 amino acids, with a theoretical molecular mass of 54.6 kDa (29). Analysis of the mature amino acid sequence with the prediction tool PredictProtein (Columbia University Bioinformatics Center) detected a carboxylesterase type B signature 2 motif between amino acids 78 and 88 (EDCLYLNVYTP) as well as a carboxylesterase type B serine active site between amino acids 176 and 191 (FGGDNHNVTLFGESAG). Additionally, secondary structure predictions with the same analysis tool suggested a compact globular protein, with 25% α-helices, 20% β-strands, and the remaining 55% as loop (or other) structures (9, 17, 18).
Expression of the prbA gene in E. coli DH5α.
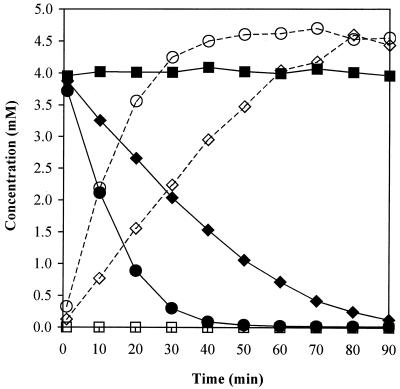
The cloning and expression of the prbA gene from E. cloacae strain EM into E. coli DH5α resulted in the acquisition of a paraben esterase activity comparable to that of the original E. cloacae strain (Fig. 3). E. coli DH5α containing the prbA gene was able to hydrolyze 49% of 600 mg of methyl paraben liter−1 (4.0 mM) within 30 min of inoculation and 97% after 90 min. In comparison, strain E. cloacae EM hydrolyzed 93% of methyl paraben after 30 min and 99.6% after 90 min (Fig. 3). The slightly slower rate of hydrolysis in E. coli compared to that in E. cloacae may be due to differences in expression of the protein between the two strains. No paraben esterase activity was present in E. coli DH5α transformed with the pGEM-T Easy vector alone as shown by the constant amount of methyl paraben remaining in solution and by the lack of production of p-hydroxybenzoic acid (Fig. 3), and similarly, no paraben esterase activity was found in E. coli DH5α cells alone (results not shown). Additionally, the MIC for methyl paraben increased from 3,200 ppm for E. coli DH5α cells without the prbA gene to >3,200 ppm for DH5α cells containing prbA. This increased resistance caused by the prbA gene is better illustrated by the large increase in the OD of the prbA-containing strain, which increased from 0.174 to 1.64 after a 24-h incubation, in comparison with the unmodified E. coli DH5α strain that presented only a small OD increase from 0.155 to 0.207 during the same period.
FIG. 3.
Hydrolysis of 600 mg of methyl paraben liter−1 (4.0 mM) (solid lines) and production of p-hydroxybenzoic acid (dotted lines) by E. coli DH5α containing the prbA gene cloned in the pGEM-T Easy vector (♦ and ⋄), by the E. cloacae strain EM (• and ○), and by E. coli DH5α containing the pGEM-T Easy vector alone (▪ and □).
Resistance to parabens in related strains.
The resistance to parabens of other strains closely related to E. cloacae EM was measured with 3 other E. cloacae strains, 21 E. gergoviae strains, 5 E. aerogenes strains, and 4 P. agglomerans strains obtained from various commercial or clinical sources or from bacterial collections (Table 1). Three E. coli and two Ps. aeruginosa strains were selected as ubiquitous bacteria, which may represent a health risk, and finally a strain of B. cepacia previously known to hydrolyze parabens was added (21). These strains were tested for their resistance to the most soluble of the parabens, methyl paraben, in liquid aerated culture. Their resistance was expressed as the MIC of methyl paraben (Table 1). Although the MIC does not provide indications of the mechanism of resistance, it shows the ability of the strains to grow in the presence of this preservative. As seen in Table 1, virtually all strains (except P. agglomerans LSPQ 3825) showed a high tolerance to this agent, with MICs of methyl paraben of 1,600 mg liter−1 (10.5 mM) or greater. In fact, the MIC for the majority of the strains, of either commercial or clinical origin, was 3,200 mg liter−1, regardless of the lack of previous known exposure to this biocide. The MIC for E. cloacae strain EM, containing the prbA gene, predictably was very high, greater than 3,200 mg liter−1, which is near the limit of solubility of this paraben and exceeds the concentration normally added to commercial products, which is generally less than 2000 mg liter−1 (7, 16). Interestingly, the MIC for a strain of E. gergoviae (G1) was also >3,200 mg liter−1, comparable to that of strain EM.
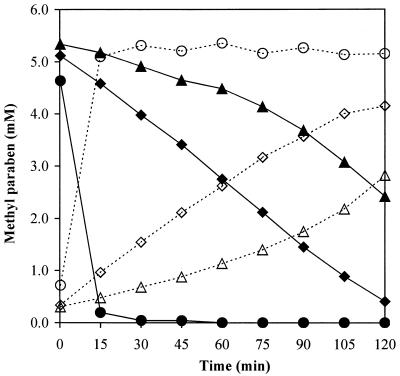
The ability of these strains to degrade methyl paraben was then tested as a potential indication of the presence of a gene similar to prbA. Degradation was measured by selecting a concentration of methyl paraben at which all the strains could grow (800 mg liter−1) and incubating exponentially growing cells at this concentration (Table 1). Despite their high resistance, as indicated by the MICs, 37 of the 41 strains showed a poor capacity to degrade the paraben, ranging from 8 to 23% degradation, with a median value of 13.5%. Among the remaining four strains, E. cloacae EM was able to hydrolyze 99.9% of the paraben, while E. gergoviae strain G1, which had shown a comparable MIC, degraded 100% of the paraben. Another E. gergoviae strain (G12) also showed a significant degradation capacity, removing 97.9% of the paraben, while the B. cepacia strain previously shown to have hydrolytic capacity (21) was able to degrade only 37.9% of the paraben. The ability of strains EM, G1, and G12 to hydrolyze the ester bond of methyl paraben was further confirmed by monitoring the appearance of the degradation product (p-hydroxybenzoic acid) concurrent with the disappearance of methyl paraben (Fig. 4). All three strains showed a high paraben esterase activity. Strain EM hydrolyzed 96% of the methyl paraben within 15 min of inoculation, while in the same period of time, strain G1 hydrolyzed 15%, and strain G12 hydrolyzed 3.5% of the paraben. After 120 min, strain EM had completely hydrolyzed 800 mg of methyl paraben liter−1, while strain G1 hydrolyzed 92%, and strain G12 hydrolyzed 55% of the paraben. The B. cepacia IFO strain did not substantially hydrolyze the paraben within these time periods. Therefore, in addition to strain EM, the E. gergoviae strains G1 and G12 were selected as potentially harboring a gene of similar function to prbA.
FIG. 4.
Paraben esterase activity in strains E. cloacae EM (• and ○) and E. gergoviae G1 (⧫ and ◊) and G12 (▵ and ▴). The amount of the degradation product p-hydroxybenzoic acid produced (dotted lines) is consistent with the amount of methyl paraben concurrently degraded (solid lines).
Presence of homologues of prbA in the other strains.
To determine if a homologue of the gene prbA was present in E. gergoviae strains G1 and G12 or B. cepacia IFO, a touchdown PCR approach was chosen to amplify the 300-bp N-terminus portion previously amplified in strain EM. The same primers as used with strain EM were able to anneal to the chromosomal DNA of strains G1 and G12 and showed an amplification signal of similar intensity that migrates accordingly to its expected size of 300 bp. This finding confirmed that a homologous esterase was present in E. gergoviae strains G1 and G12 and that their N termini were sufficiently similar to that of prbA from strain EM to hybridize to the same primers. No amplification was observed with the chromosomal or plasmid DNA of B. cepacia strain IFO, suggesting that the esterase that hydrolyzes the parabens in this strain was considerably different from those of the hydrolases in the Enterobacter strains. No significant amplification was detected on plasmid DNA of either of the E. gergoviae strains, suggesting that, as in the case of E. cloacae, the esterase is of chromosomal origin.
An alignment of the sequences of the N termini of the esterases present in E. gergoviae G1 and G12 and E. cloacae EM showed that the sequences of the N termini are virtually identical between the three strains, showing only 11 nucleotide substitutions, the majority of which are in the wobble position and do not affect the identity of the amino acid. Analysis of the nucleotide sequences with the alignment software BLAST 2 Sequences (National Center for Biotechnology Information, Bethesda, Md.) showed that the sequences of the G1 and G12 gene fragments were 97 and 96% identical to that of EM, respectively, while the sequences of G1 and G12 were 98% identical to each other (22).
DISCUSSION
Previously, we demonstrated that strain EM was able to rapidly hydrolyze the ester bond of parabens by the action of an esterase (26). The gene corresponding to this enzyme, located on the chromosome of strain EM, was named prbA and coded for a mature enzyme of 54.6 kDa. A putative signal peptide was found, suggesting that the enzyme may be targeted to the membrane and released into the periplasmic space after cleavage of the signal peptide. This is consistent with previous studies on the ability of whole cells and cell extracts of strain EM to hydrolyze the parabens, which indicated an intracellular or periplasmic location (26) and is consistent with the sequence of the N terminus of the mature protein determined by Edman degradation. Introduction of the prbA gene into an E. coli strain that did not initially have a paraben-degrading capability resulted in the hydrolysis of the paraben and the concurrent production of p-hydroxybenzoic acid and an increased MIC for methyl paraben. This transfer confirms that prbA encodes for a paraben esterase in E. cloacae strain EM. Recently, the salicylate esterase gene salE in Acinetobacter was shown to be part of an operon regulating salicylate catabolism (10). However, this gene participates in channeling salicylate esters into the β-ketoadipate pathway through a salicylate hydroxylase, while the p-hydroxybenzoic acid generated from parabens by prbA is not channeled through this pathway by E. cloacae EM, but instead is converted into phenol (26).
As observed previously (26), strain E. cloacae EM showed a higher resistance to parabens than the other E. cloacae strains (Table 1). This increase in resistance could be attributed to the ability to degrade parabens provided by the esterase encoded by the prbA gene. A similar increase in paraben resistance in strain E. gergoviae G1 could also be attributed to the presence of a homologue of prbA, allowing it to completely hydrolyze the same amount of methyl paraben as the E. cloacae strain EM in 2 h. The second strain of E. gergoviae (G12) also contained a homologue of prbA. However, the MIC for the second strain was similar to those for the other E. gergoviae strains, with the exception of strain G1. The fact that it did not show an increased resistance to paraben could be explained by the fact that its hydrolytic activity was smaller than those of EM and G1 (Fig. 4). Previous reports have indicated a MIC of methyl paraben for E. cloacae of 1,000 mg liter−1 (8). We report MICs of methyl paraben approximately threefold higher for almost all Enterobacter strains tested. However, the MICs in this report were determined in liquid aerated media, while previous determinations were made on solid media and with a lower inoculum size (106 to 107 cells/ml versus approximately 3 × 107 cells/ml in this report). In the present work, the majority of the enterobacterial strains tested demonstrated comparably high MICs without the accompanying hydrolysis of the paraben, suggesting an efficient mechanism of resistance to these compounds. A well-documented method of resistance in E. coli is the efflux of a variety of structurally distinct biocides through membrane proteins of a wide specificity serving as pumps (15). Although less well investigated, there are indications that similar efflux mechanisms exist in Enterobacteriaceae (6, 12), raising the possibility that the remaining species tested may have resistance to the parabens by a mechanism of efflux. Nevertheless, the increased resistance to methyl paraben of strains EM and G1 and of the E. coli DH5α strain containing the prbA gene shows that a high hydrolytic activity increases the resistance to methyl paraben, as shown by the increases in the MICs for these bacteria.
Analysis of the prbA gene from E. cloacae EM with the nucleotide database by using the nucleotide-nucleotide BLAST program showed a conserved stretch of 23 nucleotides (positions 1679 to 1701) corresponding to the sequence TACTGGACCAACTTTGCCAAAAC, translating to the amino acids YWTNFAK (positions 447 to 453), near the protein's C terminus (1). These nucleotides show 100% identity to a number of eukaryotic esterases. These esterases were predominantly of human origin (Homo sapiens) and represent either bile salt-dependent lipases or cholesterol esterases, although several others were carboxyl lipases or cholesterol esterases from the rat (Rattus norvegicus), the gorilla (Gorilla gorilla), or the rabbit (Oryctolagus cuniculus). A similar alignment of the amino acid sequence of PrbA by using protein-protein BLAST showed significant homology (greater than 200 bits) to approximately 100 other esterases. The great majority of the corresponding proteins were carboxylesterases of eukaryotic origin, mostly of rat, mouse, and human origin, as well as others from rabbit, hamster, mallard, monkey, dog, bovine, or pig origin. However, a few esterases were of prokaryotic origin, among them, carboxylesterases from Deinococcus radiodurans, Salmonella sp., Bacillus subtilis, and Delftia acidovorans. Some of these esterases showed very high homology scores to PrbA. Among the top 10 homologous esterases found by the BLAST search, four were of prokaryotic origin, including a type B carboxylesterase from D. radiodurans, a polyurethane esterase from D. acidovorans and two esterases from Salmonella sp. Additionally, the two proteins with the greatest amino acid identities to PrbA were the polyurethane esterase from D. acidovorans (38%) and the carboxylesterase from D. radiodurans (37%).
The sequences of the N termini of the esterases present in strains E. gergoviae G1 and G12 were almost identical to each other and to the esterase of strain EM. The sequences of strains G1 and G12 are closer to each other than to strain EM, the two former strains being from the same species and from a similar geographical location. Although the amino acid sequences of prbA between strains G1 and G12 are 100% identical, the differences in certain wobble positions of their nucleotide sequences suggest a certain evolution of the protein within the species. However, the minimal divergence between the sequences of the N termini of the esterases of both E. gergoviae strains, isolated in France, and of E. cloacae EM, originating in North America, suggests that the acquisition of this gene by either species was recent in evolutionary terms.
The analysis of the sequences of PrbA from E. cloacae EM and those of E. gergoviae G1 and G12 seems to indicate greater homology to a number of esterases of eukaryotic origin from animals that are normally hosts to enteric bacteria. Additionally, many of the homologous esterases were bile salt dependent or similarly localized to the intestinal tract of the host animals. A precedent exists for a eukaryotic-like lipase discovered in P. aeruginosa, which is postulated to have been acquired by horizontal transfer (28), and it is known that bile salts possess the ability to disrupt bacterial membranes (13, 23), hence possibly facilitating the acquisition of extraneous DNA. It is possible that the paraben-hydrolyzing esterase PrbA was acquired by Enterobacter sp. through horizontal transfer from a eukaryotic host. However, the significant homologies present between PrbA and a few prokaryotic esterases, which in some cases are from nonenteric species, indicate either convergent evolution or, alternatively, a more distant origin for the PrbA esterase. Because Enterobacter species are ubiquitous in nature, the presence of certain strains containing the esterase able to hydrolyze parabens raises health concerns. Such strains may be introduced in commercial products from the raw materials used during their preparation and thus contaminate and remain viable in the finished product. Under the appropriate settings, such as in persons with weakened immune systems or in hospital wards, these otherwise innocuous strains might become opportunistic pathogens of an infectious nature.
Acknowledgments
This work was funded in part by postgraduate fellowships from the Natural Sciences and Engineering Research Council (NSERC) and by the Fonds de la Recherche en Santé du Québec—Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (FRSQ-FCAR) program.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald, L. K., M. Ramos, M. J. Arduino, S. M. Aguero, C. Deseda, S. Banerjee, and W. R. Jarvis. 1998. Enterobacter cloacae and Pseudomonas aeruginosa polymicrobial bloodstream infections traced to extrinsic contamination of a dextrose multidose vial. J. Pediatr. 133:640-644. [DOI] [PubMed] [Google Scholar]
- 3.Beveridge, E. G., and A. Hart. 1970. The utilisation for growth and the degradation of p-hydroxybenzoate esters by bacteria. Int. Biodeterior. Bull. 6:9-12. [Google Scholar]
- 4.Close, J.-A., and P. A. Nielsen. 1976. Resistance of a strain of Pseudomonas cepacia to esters of p-hydroxybenzoic acid. Appl. Environ. Microbiol. 31:718-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Don, R. H., P. T. Cox, B. J. Wainwright, K. Baker, and J. S. Mattick. 1991. ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 19:4008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George, A. M., R. M. Hall, and H. W. Stokes. 1995. Multidrug resistance in Klebsiella pneumoniae: a novel gene, ramA, confers a multidrug resistance phenotype in Escherichia coli. Microbiology 141:1909-1920. [DOI] [PubMed] [Google Scholar]
- 7.Gottfried, N. S. 1962. Alkyl p-hydroxybenzoate esters as pharmaceutical preservatives. Am. J. Hosp. Pharm. 19:310-314. [Google Scholar]
- 8.Haag, T., and D. F. Loncrini. 1984. Esters of para-hydroxybenzoic acid. Cosmet. Sci. Technol. Ser. 1:63-77. [Google Scholar]
- 9.Hofmann, K., P. Bucher, L. Falquet, and A. Bairoch. 1999. The PROSITE database, its status in 1999. Nucleic Acids Res. 27:215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, R. M., V. Pagmantidis, and P. A. Williams. 2000. sal genes determining the catabolism of salicylate esters are part of a supraoperonic cluster of catabolic genes in Acinetobacter sp. strain ADP1. J. Bacteriol. 182:2018-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krogh, A., B. Larsson, G. von Heijne, and E. L. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 12.Mallea, M., J. Chevalier, C. Bornet, A. Eyraud, A. Davin-Regli, C. Bollet, and J. M. Pages. 1998. Porin alteration and active efflux: two in vivo drug resistance strategies used by Enterobacter aerogenes. Microbiology 144:3003-3009. [DOI] [PubMed] [Google Scholar]
- 13.Mallonee, D. H., and P. B. Hylemon. 1996. Sequencing and expression of a gene encoding a bile acid transporter from Eubacterium sp. strain VPI 12708. J. Bacteriol. 178:7053-7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 15.Nikaido, H., and H. I. Zgurskaya. 2001. AcrAB and related multidrug efflux pumps of Escherichia coli. J. Mol. Microbiol. Biotechnol. 3:215-218. [PubMed] [Google Scholar]
- 16.Rastogi, S. C., A. Schouten, N. de Kruijf, and J. W. Weijland. 1995. Contents of methyl-, ethyl-, propyl-, butyl- and benzylparaben in cosmetic products. Contact Dermatitis 32:28-30. [DOI] [PubMed] [Google Scholar]
- 17.Rost, B. 1996. PHD: predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol. 266:525-539. [DOI] [PubMed] [Google Scholar]
- 18.Rost, B., and C. Sander. 1993. Prediction of protein secondary structure at better than 70% accuracy. J. Mol. Biol. 232:584-599. [DOI] [PubMed] [Google Scholar]
- 19.Sokoloski, A. G., C. G. Chidester, and G. E. Honeywell. 1962. The hydrolysis of methyl p-hydroxybenzoate by Cladosporium resinae. Dev. Ind. Microbiol. 3:179-187. [Google Scholar]
- 20.Soni, M. G., G. A. Burdock, S. L. Taylor, and N. A. Greenberg. 2001. Safety assessment of propyl paraben: a review of the published literature. Food Chem. Toxicol. 39:513-532. [DOI] [PubMed] [Google Scholar]
- 21.Suemitsu, R., K. Horiuchi, S. Yanagawase, and T. Okamatsu. 1990. Biotransformation activity of Pseudomonas cepacia on p-hydroxybenzoates and benzalkonium chloride. J. Antibact. Antifung. Agents 18:579-582. [Google Scholar]
- 22.Tatusova, T. A., and T. L. Madden. 1999. Blast 2 sequences—a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174:247-250. [DOI] [PubMed] [Google Scholar]
- 23.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tresoldi, A. T., M. C. Padoveze, P. Trabasso, J. F. Veiga, S. T. Marba, A. von Nowakonski, and M. L. Branchini. 2000. Enterobacter cloacae sepsis outbreak in a newborn unit caused by contaminated total parenteral nutrition solution. Am. J. Infect. Control 28:258-261. [DOI] [PubMed] [Google Scholar]
- 25.Triglia, T., M. G. Peterson, and D. J. Kemp. 1998. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 16:8186.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valkova, N., F. Lépine, L. Valeanu, M. Dupont, L. Labrie, J.-G. Bisaillon, R. Beaudet, F. Shareck, and R. Villemur. 2001. Hydrolysis of 4-hydroxybenzoic acid esters (parabens) and their aerobic transformation into phenol by the resistant Enterobacter cloacae strain EM. Appl. Environ. Microbiol. 67:2404-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, S. A., R. B. Levine, L. A. Carson, M. J. Arduino, T. Killar, F. G. Grillo, M. L. Pearson, and W. R. Jarvis. 1999. An outbreak of gram-negative bacteremia in hemodialysis patients traced to hemodialysis machine waste drain ports. Infect. Control Hosp. Epidemiol. 20:746-751. [DOI] [PubMed] [Google Scholar]
- 28.Wilderman, P. J., A. I. Vasil, Z. Johnson, and M. L. Vasil. 2001. Genetic and biochemical analyses of a eukaryotic-like phospholipase D of Pseudomonas aeruginosa suggest horizontal acquisition and a role for persistence in a chronic pulmonary infection model. Mol. Microbiol. 39:291-303. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins, M. R., E. Gasteiger, A. Bairoch, J.-C. Sanchez, K. L. Williams, R. D. Appel, and D. F. Hochstrasser. 1999. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 112:531-552. [DOI] [PubMed] [Google Scholar]
- 30.Zedan, H. H., and F. M. Serry. 1984. Metabolism of esters of p-hydroxybenzoic acid by a strain of Pseudomonas aeruginosa. Egypt. J. Microbiol. 19:41-54. [Google Scholar]