Abstract
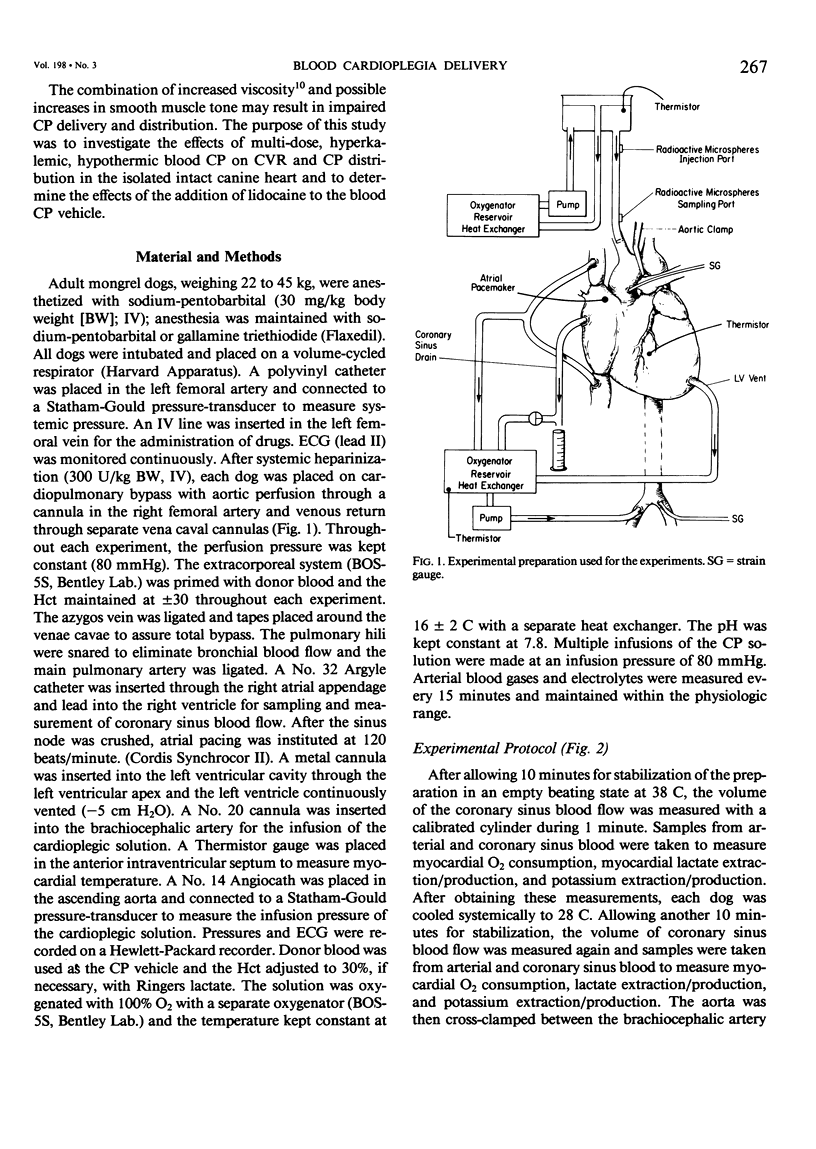
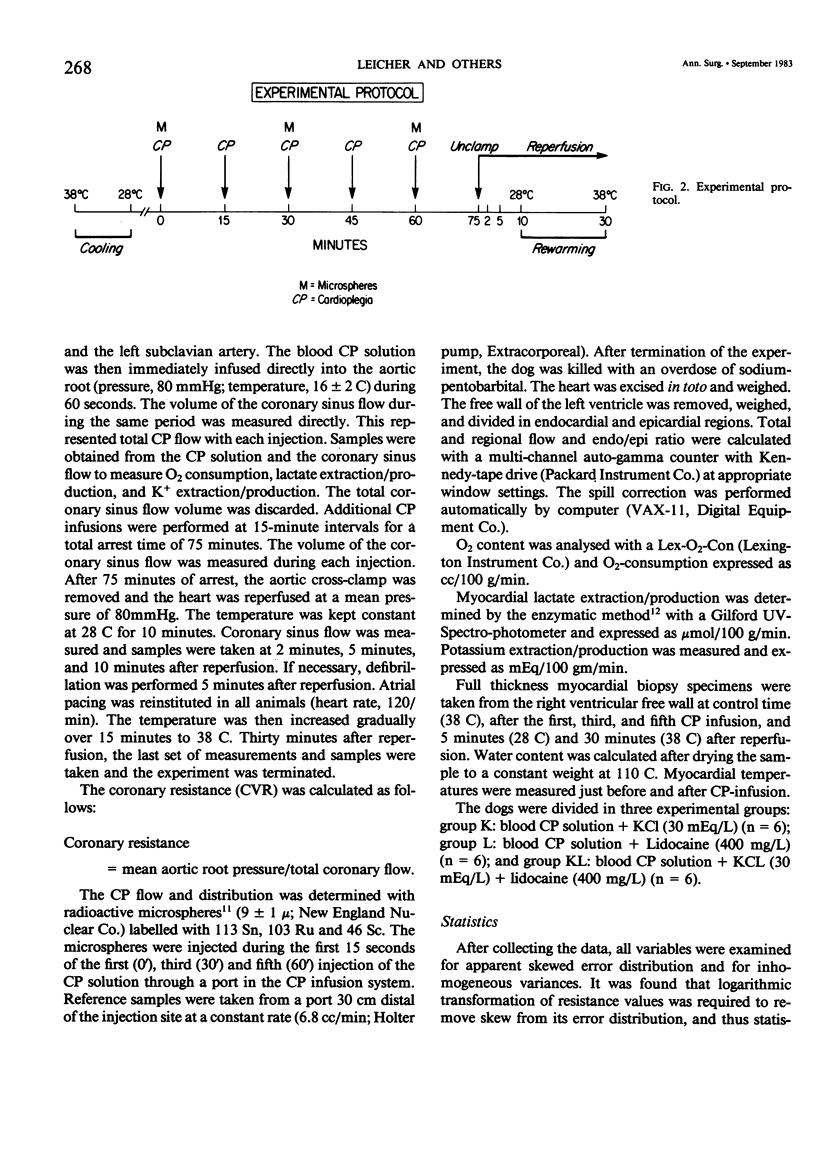
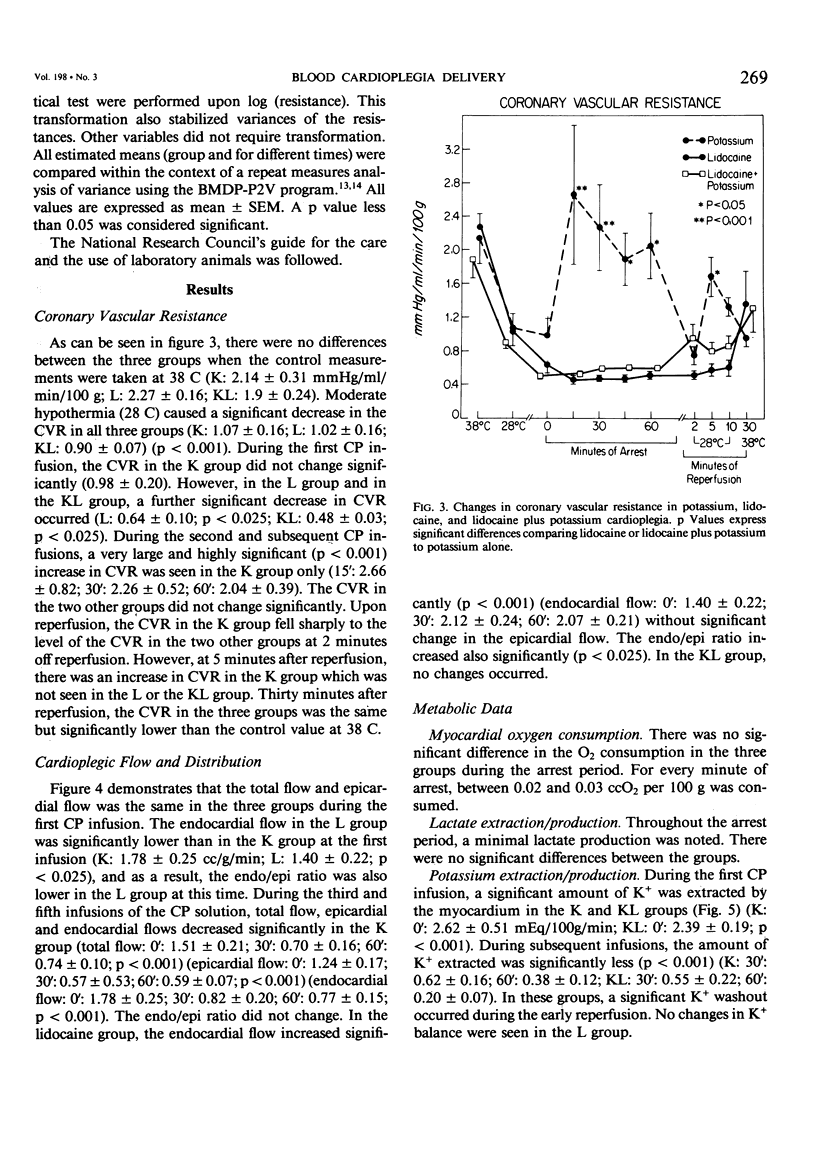
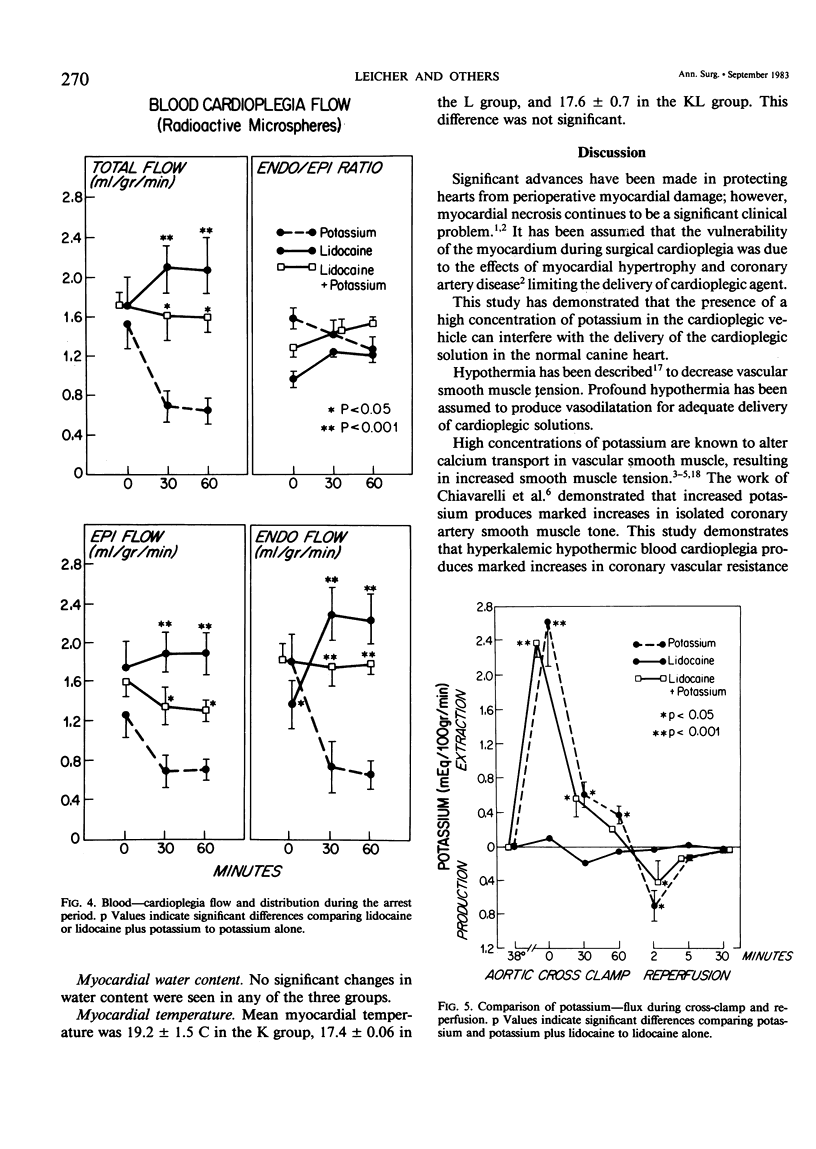
Delivery of cardioplegic (CP) solutions to all regions of the myocardium is critical for optimal myocardial protection during cardiac surgery. However, there are little data regarding the effects of CP agents upon coronary vascular resistance (CVR) and CP delivery. Accordingly, we evaluated blood CP (Hct 30) delivery and CVR during 75 minutes of multi-dose hypothermic blood CP arrest in an in vivo isolated dog heart preparation. Three groups of dogs were studied: K(K+ = 30 mEq/L; n = 6), L (Lidocaine = 400 mg/L; K+ = 4 mEq/L; n = 6), and KL (K+ = 30 mEq/L, Lidocaine 400 mg/L; n = 6) during total cardiopulmonary bypass and moderate systemic hypothermia (28 C). Basal CVR was calculated by measuring total coronary flow (HR 120/min; mean aortic pressure = 80 mmHg) in the empty beating heart. After aortic cross-clamping, the blood CP solution was infused into the aortic root at a constant pressure (80 mmHg) and constant temperature (16 +/- 2 C) for 60 seconds at 15 minute intervals for a total arrest time of 75 min. Total CP flow, CVR, O2 consumption, lactate extraction/production, and K+ balance during 75 minutes of arrest and 30 minutes of reperfusion were determined. The distribution of the CP solution in the left ventricle was measured with radioactive microspheres (9 +/- 1 mu). Biopsy specimens were taken to measure wet to dry ratios. Values are mean +/- SEM. Data were analyzed by BMDP-P2V. During the first CP infusion, after aortic cross-clamping, no differences in CVR or CP distribution were found among the three groups. However, CVR was increased significantly in the K group during the second CP infusion (O': 0.98 +/- 0.20 mmHg/ml/min/100 g; 15': 2.66 +/- 0.82; p less than 0.001). The CVR remained high for the remainder of the arrest period. Moreover, total, epi- and endocardial flow decreased significantly (54%, p less than 0.001). In groups L and KL, no significant changes in CVR were seen. Groups K and KL showed a significant K+ extraction during the first CP infusion. During the early reperfusion period, K+ washout occurred in these two groups, which was not seen in the L group. There was no significant difference between the three groups in myocardial O2 consumption, lactate metabolism, and water content during the arrest and the reperfusion period. In conclusion, high concentrations of K+ (30 mEq/L) can markedly increase CVR and impair blood CP delivery and distribution. These effects can be prevented by lidocaine. These findings warrant reassessment of the various additives to CP solutions and their effects on CVR and CP distribution during multi-dose hypothermic CP arrest.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Best P. M. Effects of tetracaine on displacement currents and contraction of frog skeletal muscle. J Physiol. 1976 Nov;262(3):583–611. doi: 10.1113/jphysiol.1976.sp011611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H., Vinten-Johansen J., Buckberg G. D., Follette D. M., Robertson J. M. Critical importance of ensuring cardioplegic delivery with coronary stenoses. J Thorac Cardiovasc Surg. 1981 Apr;81(4):507–515. [PubMed] [Google Scholar]
- Buckberg G. D. Left ventricular subendocardial necrosis. Ann Thorac Surg. 1977 Oct;24(4):379–393. doi: 10.1016/s0003-4975(10)63418-2. [DOI] [PubMed] [Google Scholar]
- Chiavarelli M., Toscano M., Chiavarelli R., Carpi A., Marino B. Effects of cardioplegic solutions on conductive coronary arteries. J Thorac Cardiovasc Surg. 1982 Jul;84(1):23–27. [PubMed] [Google Scholar]
- Chiu R. C., Blundell P. E., Scott H. J., Cain S. The importance of monitoring intramyocardial temperature during hypothermic myocardial protection. Ann Thorac Surg. 1979 Oct;28(4):317–322. doi: 10.1016/s0003-4975(10)63128-1. [DOI] [PubMed] [Google Scholar]
- Ekroth R., Berggren H., Südow G., Wojciechowski J., Zackrisson B. F., William-Olsson G. Thermographic demonstration of uneven myocardial cooling in patients with coronary lesions. Ann Thorac Surg. 1980 Apr;29(4):341–345. doi: 10.1016/s0003-4975(10)61482-8. [DOI] [PubMed] [Google Scholar]
- Ekroth R., Berggren H., Südow G., Wojciechowski J., Zackrisson B. F., William-Olsson G. Thermographic demonstration of uneven myocardial cooling in patients with coronary lesions. Ann Thorac Surg. 1980 Apr;29(4):341–345. doi: 10.1016/s0003-4975(10)61482-8. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., Mavroudis C., Gardner C., Turley K., Ullyot D., Ebert P. A. Relationship between atrioventricular arrhythmias and the concentration of K+ ion in cardioplegic solution. J Thorac Cardiovasc Surg. 1980 Oct;80(4):517–526. [PubMed] [Google Scholar]
- Engelman R. M., Spencer F. C., Boyd A. D., Chandra R. The significance of coronary arterial stenosis during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1975 Nov;70(5):869–879. [PubMed] [Google Scholar]
- Follette D. M., Mulder D. G., Maloney J. V., Buckberg G. D. Advantages of blood cardioplegia over continuous coronary perfusion or intermittent ischemia. Experimental and clinical study. J Thorac Cardiovasc Surg. 1978 Nov;76(5):604–619. [PubMed] [Google Scholar]
- HARDIN R. A., SCOTT J. B., HADDY F. J. Effect of cardiac cooling on coronary vascular resistance in normothermic dogs. Am J Physiol. 1960 Jul;199:163–166. doi: 10.1152/ajplegacy.1960.199.1.163. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Konold P., Gebert G., Brecht K. The mechanical response of isolated arteries to potassium. Experientia. 1968 Mar 15;24(3):247–248. doi: 10.1007/BF02152797. [DOI] [PubMed] [Google Scholar]
- Kostis J. B., Goodking M. J., Gotzoyannis S., Gerber N. H., Kuo P. T. Effect of lidocaine on the atrial fibrillation threshold. Am Heart J. 1977 Dec;94(6):764–768. doi: 10.1016/s0002-8703(77)80218-4. [DOI] [PubMed] [Google Scholar]
- Lubbe W. F., Bricknell O. L., Marzagao C. Ventricular fibrillation threshold and vulnerable period in the isolated perfused rat heart. Cardiovasc Res. 1975 Sep;9(5):613–620. doi: 10.1093/cvr/9.5.613. [DOI] [PubMed] [Google Scholar]
- O'Neill M. J., Jr, Francalancia N., Wolf P. D., Parr G. V., Waldhausen J. A. Resistance differences between blood and crystalloid cardioplegic solutions with myocardial cooling. J Surg Res. 1981 Apr;30(4):354–360. doi: 10.1016/0022-4804(81)90171-2. [DOI] [PubMed] [Google Scholar]
- Roberts A. J. Perioperative myocardial infarction and changes in left ventricular performance related to coronary artery bypass graft surgery. Ann Thorac Surg. 1983 Feb;35(2):208–225. doi: 10.1016/s0003-4975(10)61464-6. [DOI] [PubMed] [Google Scholar]
- Ross R. S. The next 30 years--will the progress continue? Circulation. 1980 Jul;62(1):1–7. doi: 10.1161/01.cir.62.1.1. [DOI] [PubMed] [Google Scholar]
- Scherphof G. L., Scarpa A., van Toorenenbergen A. The effect of local anesthetics on the hydrolysis of free and membrane-bound phospholipids catalyzed by various phospholipases. Biochim Biophys Acta. 1972 Jun 19;270(2):226–240. doi: 10.1016/0005-2760(72)90234-2. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]


