Abstract
Two closely related signal transduction proteins, PII and GlnK, have distinct physiological roles in the regulation of nitrogen assimilation. Here, we examined the physiological roles of PII and GlnK when these proteins were expressed from various regulated or constitutive promoters. The results indicate that the distinct functions of PII and GlnK were correlated with the timing of expression and levels of accumulation of the two proteins. GlnK was functionally converted into PII when its expression was rendered constitutive and at the appropriate level, while PII was functionally converted into GlnK by engineering its expression from the nitrogen-regulated glnK promoter. Also, the physiological roles of both proteins were altered by engineering their expression from the nitrogen-regulated glnA promoter. We hypothesize that the use of two functionally identical PII-like proteins, which have distinct patterns of expression, may allow fine control of Ntr genes over a wide range of environmental conditions. In addition, we describe results suggesting that an additional, unknown mechanism may control the cellular level of GlnK.
Most studies of the regulation of gene expression are focused on elucidating the identities of regulatory factors, the mechanisms by which these factors are controlled, the nature of the targets of regulation, and the interactions of regulatory factors and their targets. Here, we focused on the role of genetic connectivities (i.e., the position within a complex genetic circuit) in determining the physiological function of a signal transduction protein.
The closely related PII and GlnK signal transduction proteins play distinct roles in the regulation of nitrogen assimilation in Escherichia coli (reviewed in reference 23). The PII protein, the product of glnB, is produced constitutively. It participates in regulating transcription of the glnA gene encoding glutamine synthetase (GS) and in regulating GS catalytic activity. In contrast, the GlnK protein, the product of glnK, is found only in nitrogen-limited cells and does not contribute significantly to regulation of glnA transcription. However, GlnK is required to regulate expression of Ntr genes other than glnA in cells that lack PII. In the absence of this regulation by GlnK, cells have a severe growth defect on defined medium that is correlated with overexpression of the Ntr regulon (6).
Ntr promoters, such as glnAp2, glnKp, and nacp, are expressed by RNA polymerase containing the minor sigma factor σ54 (reviewed in references 5 and 19). Expression from these promoters requires the activator NRI∼P (NtrC∼P), which binds to upstream enhancer sequences and interacts with the promoter-bound polymerase by formation of a DNA loop. The interaction of the activator with the polymerase is required for the polymerase to melt the DNA strands and form the open transcription complex competent for transcript initiation. Previous studies of glnAp2, nacp, and glnKp activation both in vivo and in vitro have indicated that expression from these promoters is regulated by the concentration of NRI∼P, which is amplified as cells are starved for ammonia (reference 2 and references therein). Of these promoters, glnAp2 is the most sensitive to activation by NRI∼P, while the other promoters require a higher concentration of NRI∼P for activation (reviewed in reference 24).
Phosphorylation and dephosphorylation of NRI are brought about by the product of the glnL (ntrB) gene, NRII (NtrB) (reviewed in reference 24). The glnL and glnG genes are adjacent to glnA, and under nitrogen-limiting conditions activation of the glnAp2 promoter leads to increased expression of NRI and NRII (27). Under these conditions NRII acts as a kinase and phosphorylates NRI. Thus, under nitrogen-limiting conditions, NRI and NRII positively regulate their own synthesis. Under nitrogen-excess conditions, NRI is dephosphorylated by NRII, and basal levels of NRI and NRII are maintained by expression from the glnLp promoter (38). This promoter is recognized by the main form of RNA polymerase, Eσ70, and is repressed by NRI and NRI∼P. Therefore, under nitrogen-excess conditions, NRI negatively regulates its own expression.
While the growth of E. coli on ammonia requires GS, it does not require NRI (29). Nevertheless, the rate of growth on ammonia is higher when E. coli contains NRI and is able to increase the level of GS by activation of glnAp2. In contrast, growth on certain poor nitrogen sources, such as arginine, not only requires NRI but also requires the ability to raise the intracellular concentration of NRI by activation of the glnAp2 promoter (27, 34). When this positive autoregulatory loop is disrupted by genetic manipulations that provide a low, constant level of NRI, the regulation of glnAp2 by nitrogen status is normal, but the cells are unable to use arginine and certain other nitrogen sources (6, 27).
The kinase and phosphatase activities of NRII are regulated by the interaction of NRII with the related PII and GlnK proteins (reviewed in references 23 and 24). Binding of PII or GlnK to NRII results in inhibition of the NRII kinase activity and activation of the NRII phosphatase activity, leading to dephosphorylation of NRI∼P. Signals of carbon and nitrogen status regulate the ability of PII and GlnK to either interact with or regulate NRII. One nitrogen signal that regulates PII activity is glutamine, which controls the antagonistic activities of the signal-transducing uridylyltransferase (UTase)/uridylyl-removing enzyme (UR), the product of glnD. When the intracellular concentration of glutamine is low, the UTase activity of UTase/UR brings about uridylylation of PII, which prevents PII binding to NRII. When the concentration of glutamine is high, the UR activity of UTase/UR converts PII∼UMP to PII, restoring its ability to bind to NRII. 2-Ketoglutarate, a signal of both carbon and nitrogen status, allosterically regulates PII activity. The trimeric PII protein contains three sites for this effector, which are bound with negative cooperativity. At low effector concentrations, one of the three sites in the PII trimer is occupied, resulting in a conformation of PII that binds to and regulates NRII. At high effector concentrations, the negative cooperativity is overcome, and three molecules of effector bind PII, resulting in a conformation of PII that is unable to regulate NRII. Studies of the purified GlnK protein have shown that it is similarly controlled by uridylylation and by binding of 2-ketoglutarate but that the deuridylylation of GlnK by the UR activity of UTase/UR is very slow (7).
PII and GlnK also participate in the regulation of GS activity by controlling the adenylyltransferase (ATase) (glnE product) that catalyzes the reversible adenylylation of GS. Studies with intact cells have indicated that PII and GlnK are each able to control the activities of ATase, although distinct regulatory profiles are obtained when cells contain only PII or only GlnK (6).
Working hypothesis and questions addressed in this work.
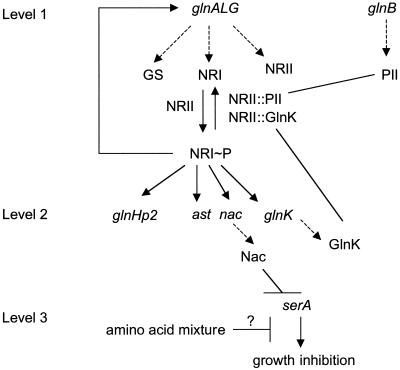
The current state of our knowledge is summarized in the circuit diagram in Fig. 1, in which the connectivities between genes and regulatory proteins are shown. We propose that there is a hierarchy of ntr gene expression in E. coli that is the result of NRI∼P accumulating as cells become starved for nitrogen (Fig. 1). Transcription from the glnAp2 promoter is at the first level of the hierarchy. This promoter is very sensitive to activation by NRI∼P. Expression from the glnHp2, glnK, nac, and ast promoters requires high intracellular concentrations of NRI∼P that occur only under nitrogen-limiting conditions and is therefore at the second level.
FIG. 1.
Hypothetical gene cascade regulating nitrogen assimilation in E. coli. The solid arrows indicate activation of genes by NRI∼P. The dashed arrows indicate the products of the genes. Lines ending in bars indicate hypothetical negative control interactions. For a discussion, see the text.
We propose a third level, containing at least one gene (designated serA), in Fig. 1. Our rationale for proposing this additional level in the cascade of Ntr gene expression is based on the following observation. Cells lacking both PII and GlnK have a severe growth defect on minimal medium due to the unregulated kinase activity of NRII and the absence of NRII phosphatase activity, which results in very high NRI∼P levels. This growth defect is ameliorated either by inclusion of a complex mixture of amino acids in the growth medium (casein hydrolysate) or by genetic manipulations that prevent the increase in the NRI concentration that typically results from the activation of glnAp2 (6). It has been shown elsewhere that serA is represssed by Nac and that this repression is responsible for the poor growth phenotype of cells lacking both PII and GlnK (9). Repression of serA, by limiting the production of glycine and C1 units necessary for purine biosynthesis, may provide an advantage to cells when they are exposed to extreme environmental stress. One role of GlnK and PII may be to prevent high levels of nac expression and repression of serA, which reduces growth, under favorable environmental conditions (9).
Within the context of this working hypothesis, there are several possible explanations for the apparent differences in the PII and GlnK functions. Structural differences in these proteins may result in alterations of their interactions with NRII (although no such differences were seen in vitro [7]). For example, some special feature of GlnK, such as sensitivity to a regulatory factor or metabolite present only in starved cells, may make it better suited for regulation of NRII under nitrogen-limiting conditions. Alternatively, the apparently distinct functions of PII and GlnK may result from their different positions within the gene cascade and the consequent differences in the timing of expression and the levels of accumulation. To examine this issue, we altered the positions of PII and GlnK within the Ntr gene cascade by engineering expression of these proteins from various promoters and examined the effects of these manipulations.
MATERIALS AND METHODS
Bacteriological techniques.
For preparation of Luria-Bertani (LB) broth and W-salts-based defined media, preparation of plasmid DNA, preparation of competent cells, transformation of cells with DNA, sequencing of plasmid DNA, PCR amplification of DNA, preparation of P1vir phage lysates, P1-mediated transduction, recombination of DNA on the bacterial chromosome, and long-term storage of strains we used standard techniques or previously described techniques (4, 6, 12, 15, 22, 33, 36). Site-specific mutagenesis was performed by using the Altered Sites mutagenesis system (Promega Corporation) according to the manufacturer's instructions. The bacterial strains, plasmids, and oligonucleotides used in this work are described in Table 1 bacterial cultures were measured with a Beckman DU65 spectrophotometer.
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant characteristics | Reference, construction, or source |
|---|---|---|
| Strains | ||
| YMC10 | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs | 8 |
| RB9060 | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 | 10 |
| K | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs Δmdl-glnK::Kan | 6 |
| BK | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 Δmdl-glnK::Kanr | 6 |
| BKc | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 Δmdl-glnK::Camr | 6 |
| BKΦ | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 Δmdl-glnK::CamrtrpDC700::putPA130 [Φ(glnKp-lacZ) Kanr Cams] | 6 |
| WCH30 | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnK1 (amtB+) | 1 |
| UNF3435 | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnK1 (amtB+) ΔglnB2306 | 1 |
| YMC15 | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 | 11 |
| BKgΦ | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnK1 (amtB+) ΔglnB2306 trpDC700::putPA130 [Φ(glnKp-lacZ) Kanr Cams] | BΦ × WCH30 P1vir |
| BΦ | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 trpDC700::putPA130 [Φ(glnKp-lacZ) Kanr Cams] | 6 |
| BA | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 glnA::Tn5 | 13 |
| TEΦ | recD1903::Tn10 trpDC700::putPA130 [Φ(glnKp-lacZ) Kanr Cams] | 6 |
| YMC10Φ | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs trpDC700::putPA130 [Φ(glnKp-lacZ) Kanr Cams] | 6 |
| YMC15Φ | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 trpDC700::putPA130 [Φ(glnKp-lacZ) Kanr Cams] | YMC15 × TEΦ P1vir |
| MAKc | recD Δmdl-glnK::Camr | 6 |
| Kc | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs Δmdl-glnK::Camr | YMC10 × MAKc P1vir |
| YMC15K | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 Δmdl-glnK::Camr | YMC15 × MAKc P1vir |
| YMC15B | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 ΔglnB2306 | BA × YMC15 P1vir |
| YMC15BΦ | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 ΔglnB2306 trpDC700::putPA1303 [Φ(glnKp-lacZ) Kanr Cams] | YMC15B × TEΦ P1vir |
| YMC15BKΦ | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 ΔglnB2306 Δmdl-glnK::CamrtrpDC700::putPA1303 [Φ(glnKp-lacZ) Kanr Cams] | YMC15BΦ × MAKc P1vir |
| YMC15KΦ | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 Δmdl-glnK::CamrtrpDC700::putPA1303 [Φ(glnKp-lacZ) Kanr Cams] | YMC15Φ × MAKc P1vir |
| Y15KgΦ | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 ΔglnK1 (amtB+) trpDC700::putPA1303 [Φ(glnKp-lacZ) Kanr Cams] | YMC15Φ × WCH30 P1vir |
| TE2680 | recD1903::Tn10 trpDC700::putPA1303 [Kans Camrlac] | 12 |
| MABpB | recD1903::Tn10 trpDC700::putPA1303 [Kanr CamsglnB8] | TE2680 × pglnB8 DNA |
| MAKpK | recD1903::Tn10 trpDC700::putPA1303 [Kanr CamsglnK91] | TE2680 × pglnK91 DNA |
| MAKpB2 | recD1903::Tn10 trpDC700::putPA1303 [Kanr Cams Φ(glnKp-glnB)] | TE2680 × pglnKpB2 DNA |
| MABpK2 | recD1903::Tn10 trpDC700::putPA1303 [Kanr Cams Φ(glnBp-glnK)] | TE2680 × pglnBpK2 DNA |
| MAApB2 | recD1903::Tn10 trpDC700::putPA1303 [Kanr Cams Φ(glnAp-glnB)] | TE2680 × pglnApB2 DNA |
| MAApK2 | recD1903::Tn10 trpDC700::putPA1303 [Kanr Cams Φ(glnAp-glnK)] | TE2680 × pglnApK2 DNA |
| BKcBpB | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 Δmdl-glnK::CamrtrpDC700::putPA1303 [Kanr CamsglnB8] | BKc × MABpB P1vir |
| BKcBpK2 | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 Δmdl-glnK::CamrtrpDC700::putPA1303 [Kanr Cams Φ(glnBp-glnK)] | BKc × MABpK2 P1vir |
| BKcKpK | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 Δmdl-glnK::CamrtrpDC700::putPA1303 [Kanr CamsglnK91] | BKc × MAKpK P1vir |
| BKcKpB2 | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 Δmdl-glnK::CamrtrpDC700::putPA1303 [Kanr Cams Φ(glnKp-glnB)] | BKc × MAKpB2 P1vir |
| BKcApB2 | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 Δmdl-glnK::CamrtrpDC700::putPA1303 [Kanr Cams Φ(glnAp-glnB)] | BKc × MAApB2 P1vir |
| BKcApK2 | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs ΔglnB2306 Δmdl-glnK::CamrtrpDC700::putPA1303 [Kanr Cams Φ(glnAp-glnK)] | BKc × MAApK2 P1vir |
| Y15KcBpB | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 Δmdl-glnK::CamrtrpDC700::putPA1303 [Kanr CamsglnB8] | YMC15K × MABpB P1vir |
| Y15KcKpK | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 Δmdl-glnK::CamrtrpDC700::putPA1303 [Kanr CamsglnK91] | YMC15K × MAKpK P1vir |
| Y15KcKpB | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 Δmdl-glnK::CamrtrpDC700::putPA1303 [Kanr Cams Φ(glnKp-glnB)] | YMC15K × MAKpB2 P1 vir |
| Y15BBpB | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 ΔglnB2306 trpDC700::putPA1303 [Kanr CamsglnB8] | YMC15B × MABpB P1vir |
| Y15BKpK | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 ΔglnB2306 trpDC700::putPA1303 [Kanr CamsglnK91] | YMC15B × MAKpK P1vir |
| Y15BKpB | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 ΔglnB2306 trpDC700::putPA1303 [Kanr Cams Φ(glnKp-glnB)] | YMC15B × MAKpB2 P1vir |
| Y15BKcBpB | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 Δmdl-glnK::Camr ΔglnB2306 trpDC700::putPA1303 [Kanr CamsglnB8] | Y15BBpB × MAKc P1vir |
| Y15BKcKpK | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 Δmdl-glnK::Camr ΔglnB2306 trpDC700::putPA1303 [Kanr CamsglnK91] | Y15BKpK × MAKc P1vir/PICK> |
| Y15BKcKpB | endA1 thi-1 hsdR17 supE44 ΔlacU169 hutCklebs glnL2302 Δmdl-glnK::Camr ΔglnB2306 trpDC700::putPA1303 [Kamr Cams Φ(glnKp-glnB)] | Y15BKpB × MAKc P1vir |
| Plasmids | ||
| pRS551 | lac fusion vector | 36 |
| pAlter-1 | Amps mutagenesis vector | Promega Corporation |
| pUC18N | pUC18, with NdeI site destroyed | 6 |
| pUC18NS | pUC18, with NdeI and SalI sites destroyed | |
| pglnB5 | glnB PCR product cloned as BamHI fragment into pAlter-1 | |
| pglnB6 | NdeI site introduced at glnB start codon of pglnB5 | |
| pglnB8 | BamHI fragment containing glnB cloned into pRS551 from pglnB6 | |
| pglnB10 | glnB cloned into pUCN as EcoRI/HindIII fragment from pglnB6 | |
| pglnK51 | glnK PCR product cloned as BamHI fragment into pAlter-1 | |
| pglnK71 | NdeI site introduced at glnK start codon of pglnK5 | |
| pglnK81 | BamHI fragment containing glnK cloned into pUCNS from pglnK71 | |
| pglnK91 | BamHI fragment containing glnK cloned into pRS551 from pglnK71 | |
| pglnKpB1 | glnK coding region of pglnK81 exchanged for the glnB coding region from pglnB10 as NdeI/SalI fragment | |
| pglnKpB2 | BamHI fragment containing glnKp-glnB fusion gene cloned into pRS551 | |
| pglnBpK1 | glnB coding region of pglnB10 exchanged for the glnK coding region of pglnK71 | |
| pglnBpK2 | BamHI fragment containing glnBp-glnK fusion gene cloned into pRS551 | |
| pglnApB1 | glnB promoter of pglnB10 exchanged for glnA promoter generated by PCR as a KpnI/NdeI fragment | |
| pglnApB2 | BamHI fragment containing glnAp-glnB fusion gene cloned into pRS551 | |
| pglnApK1 | glnB coding region of glnAp-glnB fusion gene exchanged for the glnK coding region of glnK71 as NdeI/HindIII fragment | |
| pglnApK2 | BamHI fragment containing glnAp-glnK fusion gene cloned into pRS551 | |
| Primers | ||
| 113D | GGGGATCCGCGGCCCGGTTGGCAACATGC, primer downstream of glnB for cloning (BamHI) | |
| 7158I | GCTGCAGGGATCCCTCAACTATTTGCGTAAGCTGCTGC, cloning primer located upstream of glnB promoter (PstI/BamHI) | |
| 7159I | GCTGCAGGGATCCCATTGAGCGCCTGAATAGCGC, cloning primer located upstream of glnk promoter (PstI/BamHI) | |
| 5048F | CGGATCCGTCGACTTCCTGTTGCTGTGTGCCAGAG, primer downstream of glnK for cloning and sequencing (SalI/BamHI) | |
| 4158J | CGGTACCGGATCCCCTCCGCAAACAAGTATTGCAGAGTCCC, cloning primer located upstream of glnA promoter (KpnI/BamHI) | |
| 4157J | CCATATGTTAACTCTCCTGGATTGGTCATGGTCGTCGTG downstream primer introduces NdeI site at glnA ATG start codon for cloning the glnA promoter region (NdeI) | |
| 9829I | GACCGGAGGGGACATATGAAGCTGGTGACC, internal primer for site-specific mutagenesis introducing an NdeI | |
| site at the glnK ATG start codon | ||
| 9251I | TTTTCAAGGAATCATATGAAAAAGATTGAT, internal primer for site-specific mutagenesis introducing an NdeI site at the glnB ATG start codon | |
| 4588E | GGTCATCAGCAATCGCCAC, internal glnK sequencing primer | |
| 5551E | CGTCAGTAAGGCGGCTTACAC, internal glnK sequencing primer | |
| 4929F | GATTATCACGGTCACCAGCTTC, internal glnK sequencing primer | |
| 2820 | ACGGTGACCGAAGTGAAAGGC, internal glnB sequencing primer | |
| 2866 | CAGCTCGGTATGGCCTTTC, internal glnB sequencing primer | |
| 3098 | CGATCCGGCAACCCTTGACGCGG, internal glnB sequencing primer |
Construction of glnBp, glnKp, and glnAp fusions to the glnB and glnK structural genes.
glnBp, glnKp, and glnAp fusions to the glnB and glnK structural genes were constructed in three steps. First, an NdeI site was introduced at the ATG used for translation initiation by site-specific mutagenesis of the wild-type genes by using primers designed for this purpose (Table 1). The mutagenized genes were subcloned into intermediate plasmids. Second, the desired promoters were amplified by PCR by using primers that also introduced an NdeI site at the site of translation initiation. The amplification products were cloned into the intermediate plasmids to form the promoter-structural gene fusions. At this point the fusions were sequenced to verify the constructions. In the final step, the fusions were subcloned into pRS551 (37) and recombined into the trp locus in a single copy as described previously (12). Several independent isolates were examined to ensure that they behaved like the isolates reported here. Fusions were then amplified by PCR and sequenced again to verify the constructions. The fusions were then moved into various genetic backgrounds by P1vir-mediated generalized transduction.
β-Galactosidase and GS assays.
β-Galactosidase levels are expressed in Miller units and were determined as described by Silhavy et al. (36). The γ-glutamyl transferase activity of GS was measured as described previously (32). The results are expressed below in nanomoles of glutamyl hydroximate formed per minute per milligram of cell protein. Protein contents were determined as described by Lowry et al. (21). For the GS assay, two cultures were used for each determination, and the experiments whose results are shown in Table 3 were repeated on three different occasions. For a given experiment, the values for duplicate cultures were within 10%, while the day-to-day reproducibility was ±20%. Total GS activity was determined by using reaction mixtures in which Mn2+ was the sole metal ion, and unadenylylated GS activity was determined by using reaction mixtures in which Mg2+ was present in large excess over Mn2+, as described previously (32). In the former case both adenylylated and unadenylylated GS should have been active, whereas in the latter case only unadenylylated GS should have been active. However, our experience suggests that for unknown reasons, experiments in which there is excess Mg2+ may slightly underreport the activity of unadenylylated GS (6). Thus, the values for GS adenylylation state reported here, especially when the extent of adenylylation was low, may be systematically overestimated by up to ∼20%.
TABLE 3.
GS expression in adapted cultures
| Strain | Relevant genotype | GS transferase activity ina:
|
|
|---|---|---|---|
| Ggtrypb | GNgtryp | ||
| YMC10 | Wild type | 1,478 (4.1)c | 221 (8.7) |
| RB9060 | ΔglnB | 1,889 (4.2) | 1,516 (11.4) |
| Kc | ΔglnK | 1,661 (4.2) | 247 (9.8) |
| BKcd | ΔglnB ΔglnK | 1,864 (11.7) | 2,270 (11.9) |
| BKcBpB | ΔglnB ΔglnK Φ(glnBp-glnB) | 1,462 (3.9) | 180 (9.0) |
| BKcKpK | ΔglnB ΔglnK Φ(glnKp-glnK) | 1,910 (4.5) | 1,421 (11.4) |
| BKcBpK2d | ΔglnB ΔglnK Φ(glnBp-glnK) | 1,775 (12) | 2,005 (11.6) |
| BKcKpB2 | ΔglnB ΔglnK Φ(glnKp-glnB) | 1,676 (4.2) | 1,559 (11.4) |
| BKcApB2 | ΔglnB ΔglnK Φ(glnAp-glnB) | 1,010 (4.0) | 191 (7.4) |
| BKcApK2 | ΔglnB ΔglnK Φ(glnAp-glnK) | 1,594 (3.8) | 379 (9.7) |
Cultures were grown overnight, diluted to an optical density at 600 nm of ∼0.02, and grown at 30°C to an optical density at 600 nm of ∼0.5.
The media used were glucose-glutamine-tryptophan medium (Ggtryp) and glucose-ammonia-glutamine-tryptophan medium (GNgtryp). In all cases, tryptophan was present at a concentration of 0.004% (wt/vol), glucose was present at a concentration of 0.4% (wt/vol), and the nitrogen source was present at a concentration of 0.2% (wt/vol).
The number in parentheses is the adenylylation state expressed as the average number of adenylylated subunits per GS dodecamer.
Strain grew very poorly, with a doubling time of 4 h.
Immunoblotting.
Crude rabbit anti-PII antibody, which cross-reacts with GlnK, was kindly provided by W. C. van Heeswijk (39). The crude antibody was adsorbed against an extract derived from strain BK (lacking both PII and GlnK) to remove most of the antibodies reacting with other cellular components. For this, 3.25 g (wet weight) of pelleted strain BK from a saturated overnight culture in LB medium containing 0.2% glutamine was resuspended in 50 ml of Tris-buffered saline and disrupted by sonication. Ten microliters of crude antibody was added to the extract and incubated for 1 h, after which the solution was clarified by centrifugation. For immunoblotting we used the Amersham ECL Western blotting system according to the manufacturer's directions.
RESULTS
GlnK is required to prevent excessive Ntr gene expression in cells lacking PII.
The glnK gene is part of the glnK amtB operon, and the glnK null mutation used previously was null for both of these genes (6). In previous work, it was deduced that either PII or GlnK was required to prevent excessive Ntr gene expression by performing complementation experiments in which the ability to grow well on minimal medium was restored to strains lacking PII, GlnK, and AmtB by multicopy plasmids that encoded only either PII or GlnK (6). The results of these experiments, along with the results of experiments showing no apparent phenotype for amtB in isolation or when it is combined with ΔglnB2306, suggested that it is the absence of GlnK, and not AmtB, that is responsible for the growth defect in cells lacking PII (6). However, in subsequent work a nonpolar null mutation in glnK was examined in combination with ΔglnB2306; it was claimed that this strain grew significantly better than the analogous strain containing the polar mutation in glnK and thus that amtB had some role in the phenotype (1). We therefore examined the phenotype of the nonpolar null mutation in glnK, kindly provided by W. C. van Heeswijk. We found that the combination of ΔglnB2306 and ΔglnK amtB+ resulted in the same growth defect that was observed when ΔglnB2306 was combined with ΔglnK ΔamtB (Fig. 2).
FIG. 2.
Absence of both GlnK and PII results in a severe growth defect on defined medium. Single-colony isolates from rich LB medium supplemented with 0.2% (wt/vol) glutamine were streaked to obtain single colonies on a nitrogen-excess plate containing glucose (0.4%), ammonium sulfate (0.2%), and glutamine (0.2%) and incubated at 37°C for 48 h. The relevant genotypes are as follows: YMC10, wild type; K, Δmdl-glnK::Kanr; BK, Δmdl-glnK::Kanr ΔglnB2306; WCH30, ΔglnK1 amtB+; and UNF3435, ΔglnK1 amtB+ ΔglnB2306.
GlnK regulates the expression of Ntr genes in cells containing constitutive glnL (ntrB) alleles.
The poor-growth phenotype of strains lacking both PII and GlnK (strains BK) is due to expression of one or more Ntr genes (6). The poor growth associated with high levels of Ntr expression in these strains suggests that all previous mutations causing constitutive expression of glnA did not result in full activation of the Ntr regulon. We examined the most intensively studied such mutation, glnL(ntrB)2302, which results in elevated glnA expression under all conditions (3). Studies with the purified NRII2302 protein have shown that under conditions under which the interaction of PII and wild-type NRII was clearly evident, no interaction between PII and NRII2302 could be detected (25), yet cells containing the glnL2302 mutation displayed only a subtle growth defect and grew well on minimal media (3). Thus, we hypothesized that Ntr gene expression was not fully activated in a strain with the glnL2302 mutation, perhaps as a result of GlnK regulation of NRII2302 activity in vivo.
To explore this possibility, we examined the expression of a single-copy glnKp-lacZ fusion (single copy located in a landing pad within trp [6]) in strains YMC15Φ and Y15KgΦ (glnL2302 and glnL2302 ΔglnK1 amtB+, respectively) when these strains were grown on nitrogen-rich medium supplemented with casein hydrolysate (Table 2). The glnL2302 mutation resulted in elevated expression of the glnKp-lacZ fusion on nitrogen-rich medium compared to the expression in wild-type strain YMC10Φ (Table 2). However, the glnKp-lacZ fusion was expressed at 2.5-fold-higher levels in Y15KgΦ than in YMC15Φ, indicating that GlnK acts to control Ntr gene expression levels in a glnL2302 strain. The level of glnKp-lacZ expression in Y15KgΦ was nearly as high as the level of expression in strain BKgΦ (ΔglnB2306 ΔglnK1 amtB+), so we examined the growth phenotype of strains containing glnL2302 Δmdl-glnK::Camr. These strains displayed poor growth on minimal medium that was restored by supplementation of the medium with casein hydrolysate, similar to the growth displayed by strains BK (see below). The poor growth displayed by strain YMC15K (glnL2302 Δmdl-glnK::Camr) compared to the growth of YMC15 (glnL2302), along with the high level of Ntr gene expression in strain Y15KgΦ (glnL2302 ΔglnK1 amtB+), is consistent with the hypothesis that the growth defect observed in a glnL2302 ΔglnK mutant results from very high levels of Ntr gene expression. GlnK appears to be capable of at least partial in vivo regulation of the constitutive NRII2302, preventing a severe defect in growth on minimal defined media. We observed that the growth defect shown by ΔglnB2306 Δmdl-glnK::Camr strains was reproducibly slightly more severe than that shown by glnL2302 Δmdl-glnK::Camr strains. The absence of GS adenylylation state control in ΔglnB2306 Δmdl-glnK::Camr strains may accentuate the results of elevated Ntr gene expression (6).
TABLE 2.
β-Galactosidase expression of Φ(glnKp-lacZ) in adapted culturesa
| Strain | Relevant genotype | β-Galactosidase activity (Miller units)b |
|---|---|---|
| YMC10Φ | Wild type, trp::Φ(glnKp-lacZ) | <5 |
| BKgΦ | ΔglnB2306 ΔglnK1 trp::Φ(glnKp-lacZ) | 3,010 |
| YMC15Φ | glnL2302 trp::Φ(glnKp-lacZ) | 820 |
| Y15KgΦ | glnL2302 ΔglnK1 trp::Φ(glnKp-lacZ) | 2,140 |
Cells were grown at 37°C in nutrient-rich defined medium containing 0.4% (wt/vol) glucose, 0.2% (wt/vol) ammonium sulfate, 0.004% (wt/vol) tryptophan, and 0.1% (wt/vol) casein hydrolysate. Overnight cultures were diluted to an optical density at 600 nm of ∼0.001, and β-galactosidase activity was monitored every 45 min until a steady-state level was achieved (usually before the optical density at 600 nm was ∼0.150).
Values are averages for four samples from two independent experiments. The standard deviation was less than 5% for all strains. For YMC10Φ, no activity was detected under conditions under which 5 Miller units of enzyme activity was readily detectable.
Altering the positions of PII and GlnK in the Ntr gene cascade.
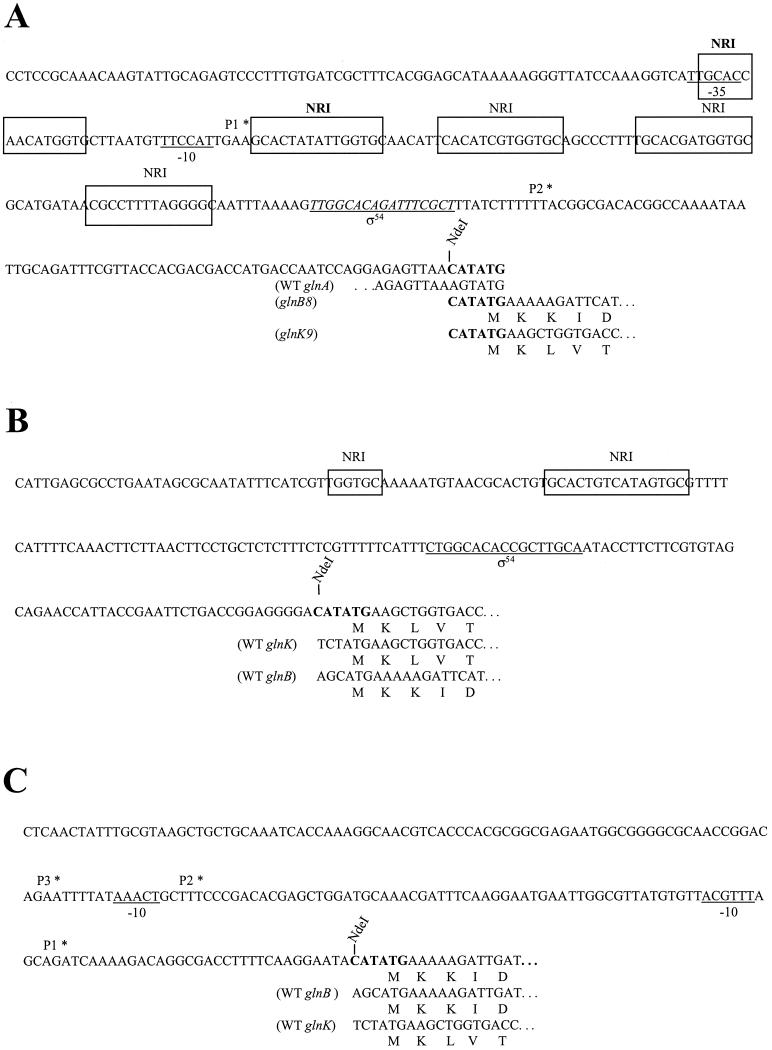
Given the different positions of GlnK and PII in the genetic cascade and the similar abilities of these proteins to regulate NRII in experiments with purified components (7; Q. Sun and A. J. Ninfa, unpublished data), we investigated whether the different physiological roles of GlnK and PII were indeed due to their different positions in the Ntr gene cascade. We formed fusions of the glnB structural gene to the glnA and glnK promoter regions by substitution of the structural gene sequence at the ATG codon used for translation initiation (Fig. 3) (see Materials and Methods). Similarly, we formed fusions of the glnK structural gene sequence to the glnB and glnA control regions by substitution of the structural gene sequence at the ATG codon used for translation initiation (Fig. 3) (see Materials and Methods). In each case, a few nucleotides immediately upstream from the translation initiation site were altered to engineer an NdeI restriction site overlapping the ATG translation initiation codon. The resulting fusions were integrated within trp as single copies and were examined by using cells lacking any other copies of glnB or glnK.
FIG. 3.
Design of promoter fusions. (A) Sequence of the Φ(glnAp-glnB) and Φ(glnAp-glnK) promoters. The mutation introducing the NdeI site to the start of the gene coding regions is indicated by boldface type. The transcription start site of the σ70 promoter, glnAp1, is labeled P1∗. The−10 and −35 RNA polymerase binding sites are underlined. The transcription start site of the σ54-dependent promoter, glnAp2, is labeled P2∗. The σ54 binding site is underlined and italicized. The NRI binding sites are enclosed in boxes. The two high-affinity NRI binding sites are indicated by boldface type. (B) Sequence of the Φ(glnKp-glnK) and Φ(glnKp-glnB) promoters. The putative σ54 binding site is underlined, and the putative NRI binding sites are enclosed in boxes. The mutation introducing the NdeI site to glnK91 is indicated by boldface type. The wild-type (WT) glnK and glnB DNA and amino acid sequences are shown at the bottom. (C) Sequence of the Φ(glnBp-glnB) and Φ(glnBp-glnK) promoters. The transcription start sites are labeled P1∗ to P3∗ (P1∗ is the major transcript). Putative −10 sequences for σ70 promoters are underlined. The mutation introducing the NdeI site to glnB8 is indicated by boldface type. The wild-type glnB and glnK DNA and amino acid sequences are shown at the bottom (20, 26, 31).
Altering the physiological roles of PII by altering the timing of its expression and the levels of accumulation.
When PII was expressed from glnAp, its expression was regulated by ammonia, as assessed by immunoblot analysis (Fig. 4A). The level of PII provided by expression from glnAp was slightly higher than the level provided by expression from glnBp in nitrogen-replete cells and was considerably higher than the level provided by expression from glnBp in nitrogen-limited cells (Fig. 4A). Thus, both the level and the timing of PII expression were altered. Cells containing PII expressed from glnAp as their sole PII-like protein grew well on minimal medium lacking casein hydrolysate (Fig. 5), but they were unable to grow on minimal medium containing arginine as the sole source of nitrogen (data not shown). The expression of GS in such cells was regulated by ammonia, but the level of GS was lower than the level in wild-type cells growing under the same conditions (Table 3). Thus, placing glnB into the gene cascade at the level of glnAp appeared to result in a reduction in the maximum level of NRI∼P, such that the threshold needed to activate arginine utilization genes was not obtained and glnA transcription could be only partially activated.
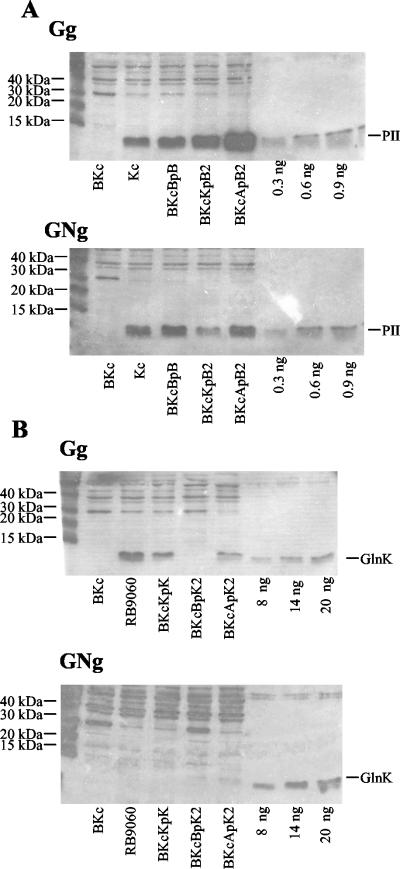
FIG. 4.
Immunoblot analysis of PII and GlnK expression from various promoters. Cells were grown on different media (Gg, glucose-glutamine medium [nitrogen limiting]; GNg, glucose-ammonia-glutamine medium [nitrogen excess]) to an optical density at 600 nm of 0.5. An aliquot of each culture was assayed for GS activity; results similar to those shown in Table 3 were obtained (data not shown). Cells from 1 ml of culture were pelleted, frozen at −20°C, resuspended in 0.9% NaCl, and disrupted by sonication. Protein concentrations were determined by the method of Lowry et al., and 6 μg (A) or 10 μg (B) of each extract was used. Electrophoresis was on sodium dodecyl sulfate-16% polyacrylamide gels. (A) Expression of PII from various promoters in cells lacking GlnK. (B) Expression of GlnK from various promoters in cells lacking PII. The source of each extract is shown at the bottom. The leftmost lanes contained electrophoresis size standards; the three rightmost lanes on each blot contained different amounts of purified PII (A) or GlnK (B). The positions of the PII and GlnK bands are indicated on the right.
FIG. 5.
Growth phenotypes of strains containing PII or GlnK expressed from various promoters in cells containing wild-type NRII. Cells were incubated at 37°C for 42 h on defined minimal medium containing 0.4% (wt/vol) glucose, 0.2% (wt/vol) ammonium sulfate, glutamine, and 0.004% (wt/vol) tryptophan (GNgt) or on the same medium containing in addition 0.04% Casamino Acids (GNgtCAA). The strains used (and their relevant genotypes) were as follows: 1, BKc (ΔglnB2306 Δmdl-glnK::Camr); 2, BKcBpB (ΔglnB2306 Δmdl-glnK::Camr trp::glnB8); 3, BKcKpK (ΔglnB2306 Δmdl-glnK::Camr trp::glnK91); 4, YMC10 (wild type); 5, RB9060 (ΔglnB2306); 6, Kc (Δmdl-glnK::Camr); 7, BKcBpK2 [ΔglnB2306 Δmdl-glnK::Camr trp::Φ(glnBp-glnK)]; 8, BKcKpB2 [ΔglnB2306 Δmdl-glnK::Camr trp::Φ(glnKp-glnB)]; 9, BKcApB2 [ΔglnB2306 Δmdl-glnK::Camr trp::Φ(glnAp-glnB)]; and 10, BKcApK2 [ΔglnB2306 Δmdl-glnK::Camr trp::Φ(glnAp-glnK)].
In contrast, when PII was expressed from glnKp, its expression was also nitrogen regulated (Fig. 4A), but the cells phenotypically resembled the ΔglnB2306 glnK+ strain; that is, the expression of glnA was not regulated by ammonia (Table 3), the cells grew well on minimal medium lacking casein hydrolysate (Fig. 5), and the cells were able to utilize arginine as a sole nitrogen source (data not shown). The regulation of glnA and other nitrogen-regulated genes seemed to be the same regardless of whether PII or GlnK was expressed from glnKp. Indeed, even the GS adenylylation state was regulated similarly when PII or GlnK was expressed from glnKp (Table 3). Thus, PII was functionally converted into GlnK by engineering its expression from the glnK promoter. When PII was expressed from glnKp, the level of PII was slightly elevated in nitrogen-limited cells and slightly decreased in nitrogen-replete cells compared to the level of PII when it was expressed from the glnB promoter (Fig. 4A). The decrease in the concentration of PII when it was expressed from glnKp compared to when it was expressed from glnBp (Fig. 4A) must account for the dramatic differences in regulation that were observed in nitrogen-replete cells (Table 3).
As a control experiment, we examined the expression of PII from glnBp using our engineered construct localized to the trp landing pad (Fig. 3). This experiment probed the effect of engineering an NdeI site near the translation initiation site (Fig. 3), as well as the effect of a different chromosomal location. The constitutive expression of PII from glnBp in our engineered construct was similar to the expression obtained with the natural arrangement (Fig. 4A), and the expression of GS and the control of its adenylylation state were similar to those seen with the natural glnBp-glnB strain (Table 3). Finally, the engineered glnBp-glnB construct restored the ability to grow on minimal medium to strain BKc (Fig. 5).
Altering the physiological roles of GlnK by altering the timing of its expression and the levels of accumulation.
GlnK, unlike PII, could not be efficiently expressed from glnBp in a single copy. When glnK was expressed from the glnB promoter, very low levels of GlnK were produced, as indicated by immunoblot analysis (Fig. 4B) and by the inability of the cells to grow on minimal medium lacking casein hydrolysate (Table 3 and Fig. 5). GS expression was unregulated and GS was highly adenylylated in strain BKc containing the glnBp-glnK fusion as the sole PII-like protein, as it was in strain BKc (Table 3). The glnBp-glnK construct that had been placed on the chromosome was amplified by PCR and subjected to DNA sequencing (as were the other fusions). This revealed that the entire construct was exactly as designed. Since both the strain construction and physiological results appeared to be unambiguous, we concluded that glnK, for whatever reason, could not be efficiently expressed from glnBp in a single copy in our experiments. These results prevented us from functionally converting GlnK into PII by expressing it from glnBp in a single copy.
When present on a multicopy plasmid, the glnBp-glnK fusion described above did express glnK. We also observed expression when the glnK open reading frame was inserted into pUC18 such that it was expressed from plasmid promoters (see above). The expression of GS and the regulation of its adenylylation state in strain BKc containing the multicopy plasmid pglnK12 resembled the expression of GS and the regulation of its adenylylation state in a glnB+ Δmdl-glnK::Camr strain (Table 4); that is, GlnK could be functionally converted to PII by expressing it from a multicopy plasmid. Strain BKc containing plasmid pglnBpK1 had a lower level of GS expression, resembling the level of GS expression of a strain overproducing PII.
TABLE 4.
GS expression in adapted cultures
| Strain | Relevant genotype | GS transferase activity ina:
|
|
|---|---|---|---|
| Ggb | GNg | ||
| YMC10 | Wild type | 1,280 (4.1)c | 210 (7.7) |
| Kc | ΔglnK | 1,450 (3.9) | 230 (8.2) |
| RB9060 | ΔglnB | 1,790 (4.2) | 1,090 (11) |
| BKc/pglnK12 | ΔglnB ΔglnK/pUC18-glnK | 1,500 (4.2) | 110 (6.6) |
| BKc/pglnBpK1 | ΔglnB ΔglnK/pUC18-Φ(glnBp-glnK) | 250 (4.5) | 50 (6.9) |
Cultures were grown overnight, diluted to an optical density at 600 nm of ∼0.02, and grown at 30°C to an optical density at 600 nm of ∼0.5.
The media used were glucose-glutamine medium (Gg) and glucose-ammonia-glutamine medium (GNg). In all cases, glucose was present at a concentration of 0.4% (wt/vol) and the nitrogen source was present at a concentration of 0.2% (wt/vol).
The number in parentheses is the adenylylation state expressed as the average number of adenylylated subunits per GS dodecamer.
Unlike the situation observed when the glnB promoter was used, the glnK gene was efficiently expressed from the glnK promoter when it was placed in a single copy into the trp landing pad, although it was not expressed quite as well as it was in the natural context (Fig. 4B). It was also expressed from glnAp when it was placed on the chromosome (Fig. 4B). Both of these constructs permitted strain BKc to grow without casein hydrolysate supplementation (Table 3 and Fig. 5). When expressed from glnAp, GlnK was nitrogen regulated, but the levels of GlnK were lower than expected when they were compared to the levels of expression of PII from glnAp (compare Fig. 4A and B). Immunoblot analysis suggested that the levels of GlnK produced from glnKp and glnAp were similar in nitrogen-starved cells (Fig. 4B), with a slightly higher level of expression obtained with glnKp. In nitrogen-replete cells, a slightly higher level of GlnK was expressed from glnAp than from glnKp (Fig. 4B).
Although cells expressing GlnK from glnAp and glnKp showed similar patterns of GlnK accumulation in the log phase, strains containing these constructs as the sole PII-like proteins had distinct phenotypes. When GlnK was expressed from glnKp (placed on the chromosome within the trp landing pad), both GS expression and adenylylation state control were the same as they were in RB9060 (that is, the same as when GlnK was expressed from its own promoter in the natural context) (Table 3). In contrast, cells expressing GlnK from glnAp had a pattern of GS expression and adenylylation state control that were more similar to the pattern of GS expression and adenylylation state control of cells that contained PII and lacked GlnK (strain Kc) (Table 3). Unlike cells containing the analogous glnAp-glnB fusion, cells containing the glnAp-glnK fusion were able to utilize arginine as a sole source of nitrogen. Together, the results show that the functions of GlnK were altered by expressing GlnK from glnAp and that the altered timing of expression and levels of accumulation resulted in a novel cellular phenotype.
Regulation of NRII2302 by PII.
As shown above, GlnK provides regulation of Ntr gene expression in cells containing NRII2302 in place of wild-type NRII. This may be accounted for by structural differences between GlnK and PII or by the altered levels and timing of expression of the two proteins. To distinguish between these hypotheses, we examined whether PII could interact with NRII2302 by using the various engineered promoter-structural gene fusions described above.
We observed that upon overexpression from a multicopy plasmid, PII restored the ability of strain YMC15K to grow on minimal medium (data not shown). More importantly, we observed that when PII was expressed from the glnK promoter in strain Y15KcKpB, it restored the ability to grow on minimal medium (Fig. 6). Strain Y15KcKpB is glnL2302 Δmdl-glnK::Camr and contains two copies of the glnB gene encoding PII, the natural copy and the transgene within the trp landing pad that is expressed from the glnK promoter. YMC15B, containing glnL2302 and lacking PII, has no significant growth defect, suggesting that GlnK alone is able to regulate NRII2302, when it is expressed from its own promoter (Fig. 6).
FIG. 6.
Growth phenotypes of strains containing PII or GlnK expressed from various promoters in cells containing NRII2302. Strains were incubated at 37°C for 42 h on defined minimal medium containing 0.4% (wt/vol) glucose, 0.2% (wt/vol) ammonium sulfate, glutamine, and 0.004% (wt/vol) tryptophan (GNgt) or on the same medium containing in addition 0.1% Casamino Acids (GNgtCAA). The strains used (and their relevant geneotypes) were as follows: 1, YMC15 (glnL2302); 2, YMC15B (glnL2302 ΔglnB2306); 3, YMC15K (glnL2302 Δmdl-glnK::Camr); 4, Y15KcKpK (glnL2302 Δmdl-glnK::Camr trp::glnK91); 5, Y15KcBpB (glnL2302 Δmdl-glnK::Camr trp::glnB8); 6, Y15KcKpB [glnL2302 Δmdl-glnK::Camr trp::Φ(glnKp-glnB)]; 7, YMC10 (wild type); 8, YMC15BKΦ [glnL2302 ΔglnB2306 Δmdl-glnK::Camr trp::Φ(glnKp-lacZ)]; 9, Y15BKcKpK (glnL2302 ΔglnB2306 Δmdl-glnK::Camr trp::glnK91); 10, Y15BKcBpB (glnL2302 ΔglnB2306 Δmdl-glnK::Camr trp::glnB8); and 11, Y15BKcKpB [glnL2302 ΔglnB2306 Δmdl-glnK::Camr trp::Φ(glnKp-glnB)].
Because one copy of the glnB gene did not provide enough PII to regulate the NRII2302 protein in strain YMC15K, we examined the effects of two copies. Strain Y15KcBpB containing glnL2302, Δmdl-glnK::Camr, the natural glnB+, and a transgene located in trp consisting of the glnB+ gene behind its own promoter had no significant growth defect. Thus, in wild-type cells, the level of PII obtained from the single natural copy of the glnB gene is just a little too low to regulate NRII2302. Together, these results show that GlnK was not specifically needed to prevent the poor-growth phenotype in a glnL2302 background; just a sufficient concentration of a PII-like protein was required.
DISCUSSION
Levels and timing of expression determine activity of PII and GlnK.
Our results showed that the functions of PII and GlnK were mainly due to the timing of expression and the levels of accumulation of these two proteins. We could convert PII to GlnK simply by expressing PII from the glnK promoter, and we could convert GlnK to PII by expressing the glnK structural gene from a plasmid. Also, we obtained novel phenotypes by making various alterations in the levels of expression and timing of expression of the two proteins. Overexpression of either protein resulted in an inability to use arginine as the sole nitrogen source and an inability to fully activate glnA expression in the absence of ammonia. Expression of GlnK from the glnA promoter as the sole PII-like protein, which did not significantly alter the level of accumulation of the protein but presumably altered the timing of its expression (2), resulted in a phenotype that was somewhat reminiscent of the phenotype of cells that contain PII and lack GlnK. These results support the hypothesis that the distinct functions of PII and GlnK, at least for the functions measured in our experiments, result mainly from the expression profiles. They seem to exclude the possibility that the distinct functions of PII and GlnK result from unique structural features of these proteins. This conclusion is consistent with earlier studies showing that PII and GlnK are similar in their abilities to regulate NRII activities in vitro (7).
Our studies of the constitutive glnL2302 allele support this conclusion. We observed that GlnK plays an important role in regulating Ntr gene expression in cells containing the glnL2302 allele. However, PII could fulfill this role if its expression was modestly increased. Recently, strain YMC15 (glnL2302) was used for genome-wide expression analysis to identify members of the Ntr regulon (40). At the time, it was believed that the mutation in this strain was constitutive for Ntr expression and thus that it would be useful for identification of all Ntr genes. Our results suggest that the levels of Ntr gene expression may have been underestimated and that some nitrogen-regulated genes may have been missed in the earlier genome-wide expression analysis (40).
The glnL2302 mutation results in conversion of alanine 129 of NRII to threonine (3). Recent work suggests that residue 129 of NRII is part of the central domain of NRII, which forms a four-helix bundle in the dimer (18). This portion of NRII has both the phosphotransferase and phosphatase activities of NRII (18), yet PII does not bind to this domain but rather binds to the C-terminal ATP-binding domain of NRII (28). Thus, the NRII2302 protein contains an intact PII-binding site and presumably binds PII normally. A hypothesis to explain these observations is that the NRII2302 protein is partially defective in NRII phosphatase activity, such that a higher fraction of the protein must be complexed with PII in order to get physiologically significant levels of the NRII phosphatase activity. Our experiments suggest that the level of PII resulting from its expression from its natural promoter is insufficient for physiologically significant levels of the phosphatase activity from the NRII2302 protein, that double this level of PII results in sufficient phosphatase activity to limit Nac expression, and that the high level of PII that results from its expression from the glnK promoter is sufficient to bring about a level of phosphatase activity that limits Nac expression, permitting the cells to grow well on minimal medium. We are currently testing this hypothesis by quantifying the interaction of PII and GlnK with purified NRII2302 protein and reexamining the abilities of these proteins to activate the NRII2302 phosphatase activity.
The PII and GlnK proteins act through NRII to control the concentration of NRI∼P in response to signals of nitrogen and carbon availability (6). The position of PII outside the regulated gene circuitry is reminiscent of the position of LacI outside the regulated lac circuitry. In both systems, low basal levels of expression of the gene circuitry and the ability to attain a high state of induction may require that the negative regulator be present at a constant concentration. The presence of negative regulators inside (glnK) and outside (glnB) the gene cascade may reflect the fact that NRI and NRII are expressed from multiple promoters also located both inside (glnAp2) and outside (glnLp) the Ntr gene circuitry. A low constitutive level of expression of either protein resulted in effective regulation of glnA expression by ammonia. Thus, the role of PII appears to be control of the kinase and phosphatase activities of NRII under conditions under which nitrogen starvation is not severe. Under these conditions, the concentration of NRII is low, reflecting limited activation of glnAp2, and the level of PII expressed constitutively from glnBp is sufficient to maintain precise control of NRII. However, upon activation of glnAp2, the intracellular concentration of NRII and NRI rises about 5- to 10-fold (2, 4, 30). If the system is fine-tuned, as seems likely based on a comparison of Fig. 4 and Table 3, this increase in the NRII concentration may prevent effective control of NRII by PII. We hypothesize that the role of GlnK is to control the intracellular concentration of NRI∼P under nitrogen starvation conditions, when the cells have elevated levels of NRII and NRI. This control by GlnK prevents the NRI∼P concentration from reaching the maximum level observed in the absence of both PII and GlnK and by so doing prevents expression of high levels of nac and the attendant repression of serA, which limits growth on defined medium lacking casein hydrolysate (9). The use of both PII and GlnK by E. coli may be necessary to permit fine control of the NRI∼P concentration over a broad range.
Whether GlnK has another role in addition to regulation of NRII, such as acting on a receptor that is not subject to control by PII, was not addressed by our studies. This possibility is suggested by the specific role of GlnK in the regulation of nitrogen fixation in the related organism Klebsiella pneumoniae (16, 17). Our conclusions concerning the functional identity of PII and GlnK are limited to the regulation of NRII and ATase, as studied in this work. Our experiments also did not resolve the question of whether GlnK and PII act solely through NRII (or NRII2302) to regulate expression of Ntr genes. Since strain YMC15K displays the poor-growth phenotype characteristic of Ntr overexpression yet contains PII, it seems that PII's ability to prevent this phenotype in wild-type cells is due to its ability to elicit the NRII phosphatase activity. It is certainly possible that GlnK also acts through another cellular receptor to regulate Ntr gene expression and permit growth of the cells under nitrogen-limiting conditions. However, if this is so, then PII is also able to regulate the (unknown) receptor(s), as PII was able to serve as GlnK when it was expressed from the glnK promoter.
Additional mechanism(s) for the regulation of GlnK expression.
Our experiments in which the glnK structural gene was fused to glnBp and glnAp revealed that for both fusions, the level of GlnK activity was lower than expected. Immunoblotting also showed that the level of expression of glnK from glnAp was lower than expected. Indeed, our synthetic fusion of the glnK structural gene to glnKp, in which an NdeI site had been engineered to overlap the natural ATG start codon for glnK, expressed noticeably lower levels of GlnK than the natural arrangement expressed (Fig. 4B). Since our fusions were produced by substitution of the structural genes at the ATG translational initiation codon, these results raise the possibility that an additional mechanism may regulate the intracellular concentration of GlnK and the possibility that a negative regulatory signal may be contained within the glnK structural gene. By analogy with phage antitermination systems (35), we speculate that such a negative regulatory element may limit GlnK expression when it is separated from an antagonistic element present in the glnK promoter or leader mRNA. Mutation of the region flanking the natural translational start site for glnK, by engineering an NdeI restriction site overlapping the translational start codon, may diminish the effectiveness of this regulatory mechanism in the otherwise natural context.
GlnK provides effective regulation of ATase in vivo.
Previous biochemical studies of the regulation of ATase by PII and GlnK suggested that these two proteins were distinct in terms of the ability to activate the ATase activities (7). However, in this study, using intact cells, we observed that regulation of the GS adenylylation state was the same when either PII or GlnK was expressed from the glnK promoter (Table 3). At present, the molecular basis for this discrepancy is unknown, and several hypotheses may be offered. For example, the cells may at all times contain a factor required for GlnK activity, which was lacking in vitro. Alternatively, GlnK may be partially inactivated by the purification methods described so far, such that it retains the ability to interact with NRII but loses the ability to interact with ATase in vitro.
Additional complexities of the experimental system.
Recent results of Weiss and colleagues (14) have shown that under certain conditions PII and GlnK may form heterotrimers in vivo. For this reason, the key experiments in our study were conducted with cells containing only a single PII-like protein. Nevertheless, it is possible that heterotrimer formation influenced the results of our control experiments with wild-type cells and resulted in additional complexities in the control of PII and GlnK function, which were not addressed in our studies.
Acknowledgments
This work was supported by Public Health Service grant GM57393 to A.J.N.
REFERENCES
- 1.Arcondeguy, T., W. C. van Heeswijk, and M. Merrick. 1999. Studies on the roles of GlnK and GlnB in regulating Klebsiella pneumoniae NifL-dependent nitrogen control. FEMS Microbiol. Lett. 180:263-270. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, M. R., T. A. Blauwkamp, V. Bondarenko, V. Studitsky, and A. J. Ninfa. 2002. Activation of the glnA, glnK, and nac promoters as Escherichia coli undergoes the transition from nitrogen excess growth to nitrogen starvation. J. Bacteriol. 184:5349-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson, M. R., and A. J. Ninfa. 1992. Characterization of mutations in the glnL gene of Escherichia coli affecting nitrogen regulation. J. Bacteriol. 174:4538-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson, M. R., and A. J. Ninfa. 1993. Mutational analysis of the bacterial signal-transducing protein kinase/phosphatase nitrogen regulator II (NRII or NtrB). J. Bacteriol. 175:7016-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson, M. R., and A. J. Ninfa. 1994. Mechanism and regulation of transcription from sigma-54 dependent promoters, p. 323-342. In R. Conaway and J. Conaway (ed.), Transcription mechanisms and control. Raven Press, New York, N.Y.
- 6.Atkinson, M. R., and A. J. Ninfa. 1998. Role of the GlnK signal transduction protein in the regulation of nitrogen assimilation in Escherichia coli. Mol. Microbiol. 29:431-447. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson, M. R., and A. J. Ninfa. 1999. Characterization of the GlnK protein of Escherichia coli. Mol. Microbiol. 32:301-313. [DOI] [PubMed] [Google Scholar]
- 8.Backman, K., Y.-M. Chen, and B. Magasanik. 1981. Physical and genetic characterization of the glnA-glnG region of the Escherichia coli chromosome. Proc. Natl. Acad. Sci. USA 78:3743-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blauwkamp, T. A., and A. J. Ninfa. 2002. Nac-mediated repression of the serA promoter of Escherichia coli. Mol. Microbiol. 45:351-363. [DOI] [PubMed] [Google Scholar]
- 10.Bueno, R., G. Pahel, and B. Magasanik. 1985. Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J. Bacteriol. 164:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, Y.-M., K. Backman, and B. Magasanik. 1982. Characterization of a gene, glnL, the product of which is involved in the regulation of nitrogen utilization in Escherichia coli. J. Bacteriol. 150:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliot, T. 1992. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-pfrA operon. J. Bacteriol. 174:245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, J., M. R. Atkinson, W. McCleary, J. B. Stock, B. L. Wanner, and A. J. Ninfa. 1992. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J. Bacteriol. 174:6061-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forchhammer, K., A. Hedler, H. Stobel, and V. Weiss. 1999. Heterotrimerization of PII-like signalling proteins: implications for PII-mediated signal transduction systems. Mol. Microbiol. 33:338-349. [DOI] [PubMed] [Google Scholar]
- 15.Hattori, M., and Y. Sakaki. 1986. Dideoxy sequencing using denatured plasmid templates. Anal. Biochem. 152:232-238. [DOI] [PubMed] [Google Scholar]
- 16.He, L., E. Soupene, A. J. Ninfa, and S. Kustu. 1998. Physiological role for the GlnK protein of enteric bacteria: relief of NifL inhibition under nitrogen-limiting conditions. J. Bacteriol. 180:6661-6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack, R., M. De Zamaroczy, and M. Merrick. 1999. The signal transduction protein GlnK is required for NifL-dependent nitrogen control of nif gene expression in Klebsiella pneumoniae. J. Bacteriol. 181:1156-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, P., M. R. Atkinson, S. Chatchawan, Q. Sun, and A. J. Ninfa. 2000. Functional dissection of the dimerization and enzymatic activities of Escherichia coli nitrogen regulator II and their regulation by the PII protein. Biochemistry 44:13433-13449. [DOI] [PubMed] [Google Scholar]
- 19.Kustu, S., E. Santero, J. Keener, D. Popham, and D. Weiss. 1989. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol. Rev. 53:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J., and B. Magasanik. 1993. The glnB region of the Escherichia coli chromosome. J. Bacteriol. 175:7441-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 22.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Ninfa, A. J., and M. R. Atkinson. 2000. PII signal transduction proteins. Trends Microbiol. 8:172-179. [DOI] [PubMed] [Google Scholar]
- 24.Ninfa, A. J., P. Jiang, M. R. Atkinson, and J. A. Peliska. 2000. Integration of antagonistic signals in the regulation of nitrogen assimilation in Escherichia coli. Curr. Top. Cell. Regul. 36:31-75. [DOI] [PubMed] [Google Scholar]
- 25.Ninfa, A. J., and B. Magasanik. 1986. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc. Natl. Acad. Sci. USA 83:5909-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ninfa, A. J., L. J. Reitzer, and B. Magasanik. 1987. Initiation of transcription at the bacterial glnAp2 promoter by purified E. coli components is facilitated by enhancers. Cell 50:1039-1046. [DOI] [PubMed] [Google Scholar]
- 27.Pahel, G., D. M. Rothstein, and B. Magasanik. 1982. Complex glnA-glnL-glnG operon of Escherichia coli. J. Bacteriol. 150:202-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pioszak, A. A., P. Jiang, and A. J. Ninfa. 2000. The Escherichia coli PII signal transduction protein regulates the activities of the two-component system transmitter protein NRII by direct interaction with the kinase domain of the transmitter module. Biochemistry 44:13450-13461. [DOI] [PubMed] [Google Scholar]
- 29.Reitzer, L. J. 1996. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine, p. 391-407. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 30.Reitzer, L. J., and B. Magasanik. 1983. Isolation of the nitrogen assimilation regulator, NRI, the product of the glnG gene of Escherichia coli. Proc. Natl. Acad. Sci. USA 80:5554-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitzer, L. J., and B. Magasanik. 1985. Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc. Natl. Acad. Sci. USA 82:1979-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee, S. G., P. B. Chock, and E. R. Stadtman. 1985. Glutamine synthetase from Escherichia coli. Methods Enzymol. 113:213-241. [DOI] [PubMed] [Google Scholar]
- 33.Saiki, R. K. 1990. Amplification of genomic DNA, p. 13-20. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols, a guide to methods and applications. Academic Press, San Diego, Calif.
- 34.Schneider, B. L., A. K. Kiupakis, and L. J. Reitzer. 1998. Arginine catabolism and the arginine succinyltransferase pathway in Escherichia coli. J. Bacteriol. 180:4278-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen, R., R. A. King, and R. A. Weisberg. 2001. Modification of the properties of elongating RNA polymerase by persistent association with antiterminator RNA. Mol. Cell 7:993-1001. [DOI] [PubMed] [Google Scholar]
- 36.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions, p. 107-111. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single copy and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 38.Ueno-Nishio, S., S. Mango, L. J. Reitzer, and B. Magasanik. 1984. Identification and regulation of the glnL operator-promoter of the complex glnALG operon of Escherichia coli. J. Bacteriol. 160:379-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Heeswijk, W. C., S. Hoving, D. Molenaar, B. Stegeman, D. Kahn, and H. V. Westerhoff. 1996. An alternative PII protein in the regulation of glutamine synthetase in Escherichia coli. Mol. Microbiol. 21:133-146. [DOI] [PubMed] [Google Scholar]
- 40.Zimmer, D. P., I. Soupene, H. L. Lee, V. F. Wendisch, A. B. Khodursky, B. J. Peter, R. A. Bender, and S. Kustu. 2000. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc. Natl. Acad. Sci. USA 97:14674-14679. [DOI] [PMC free article] [PubMed] [Google Scholar]