Abstract
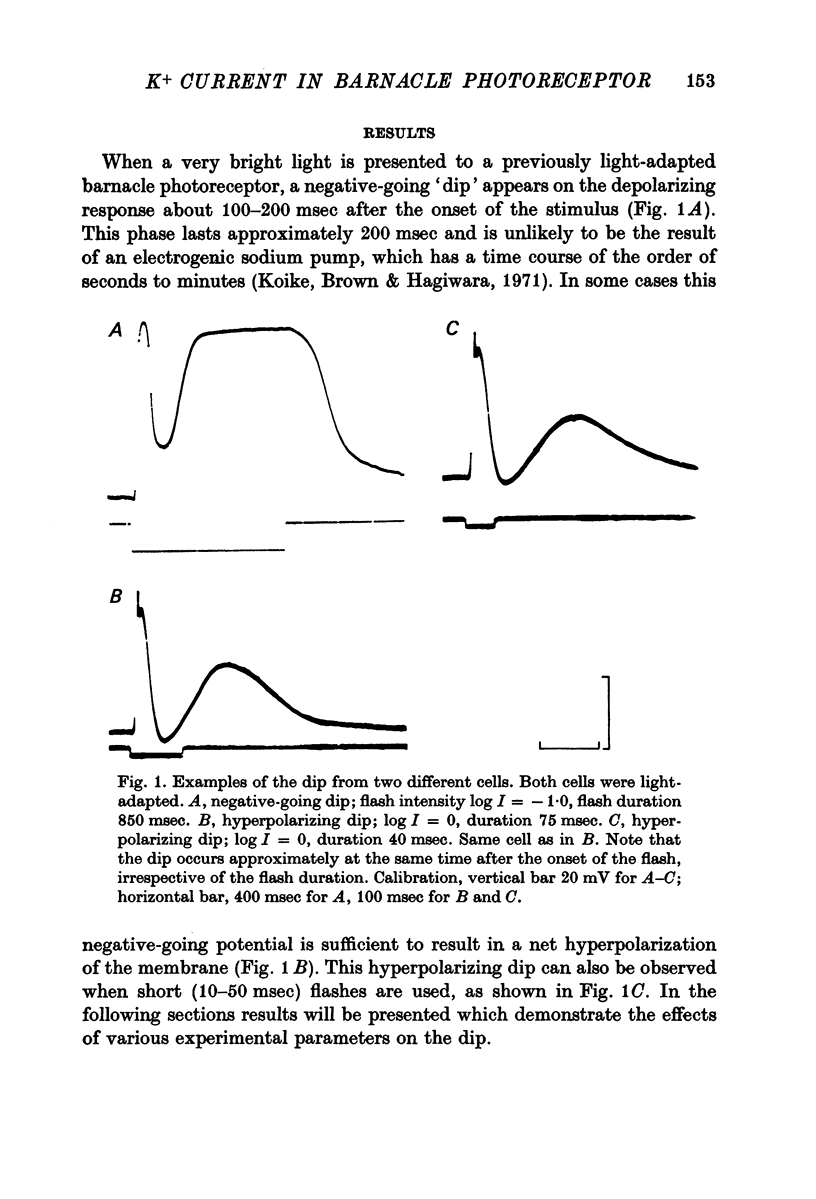
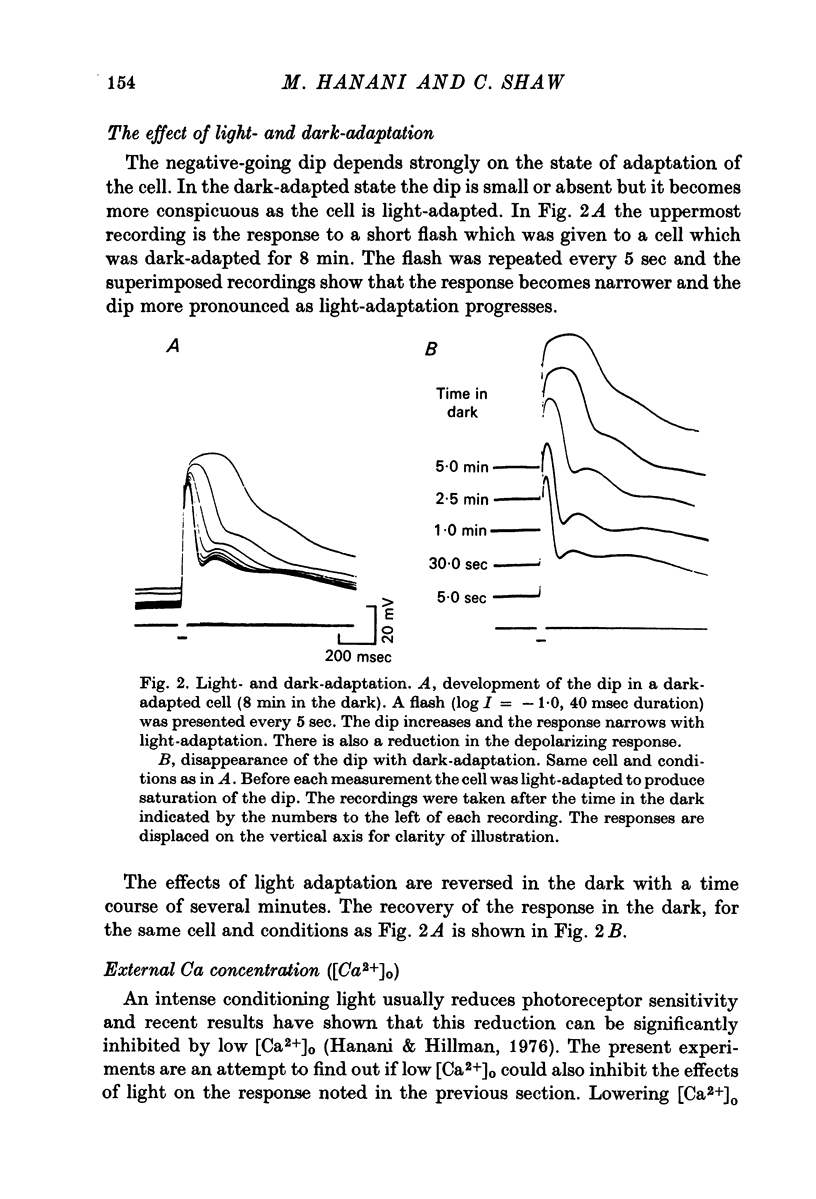
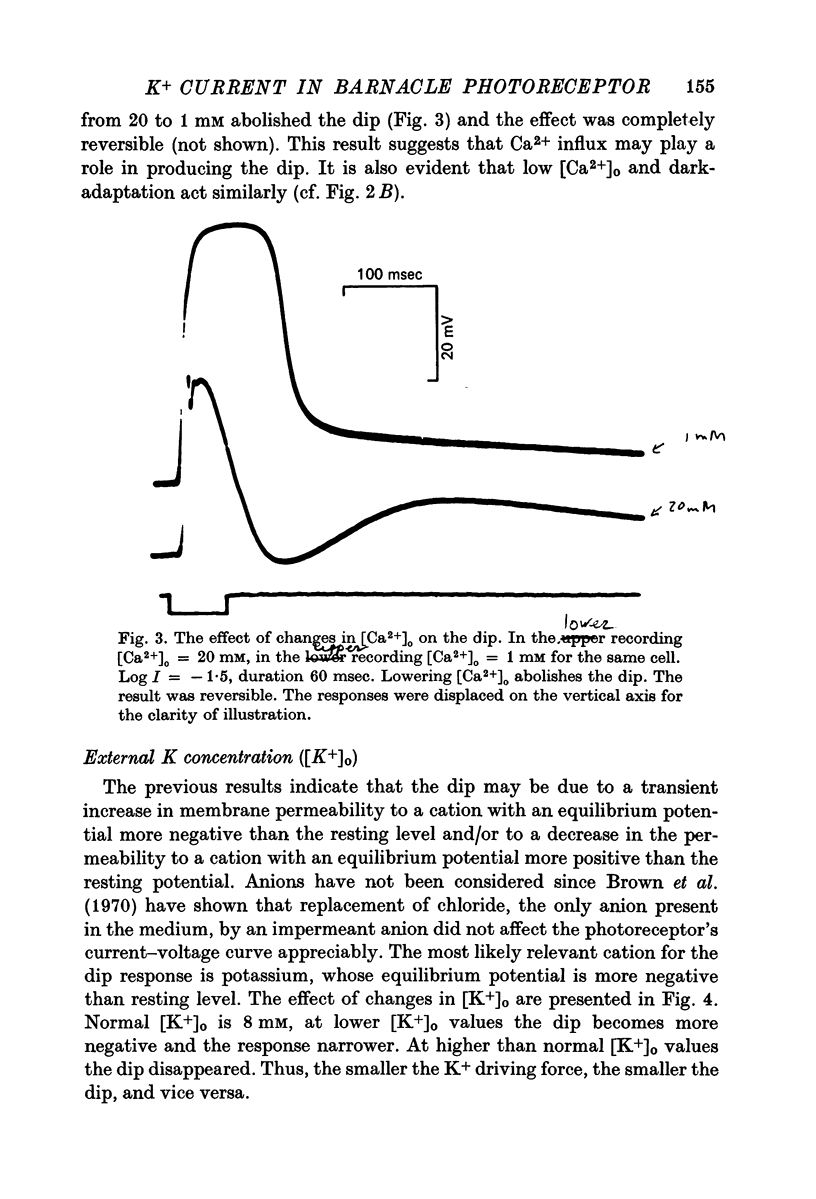
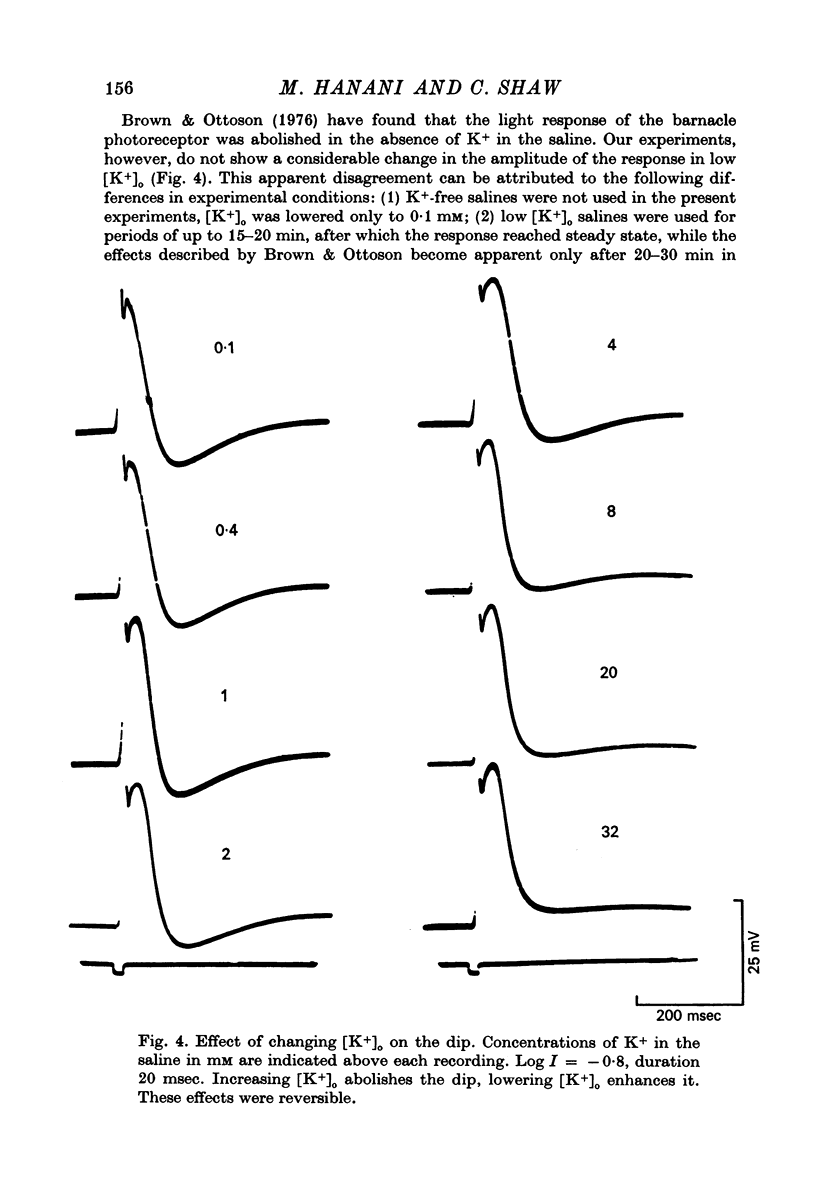
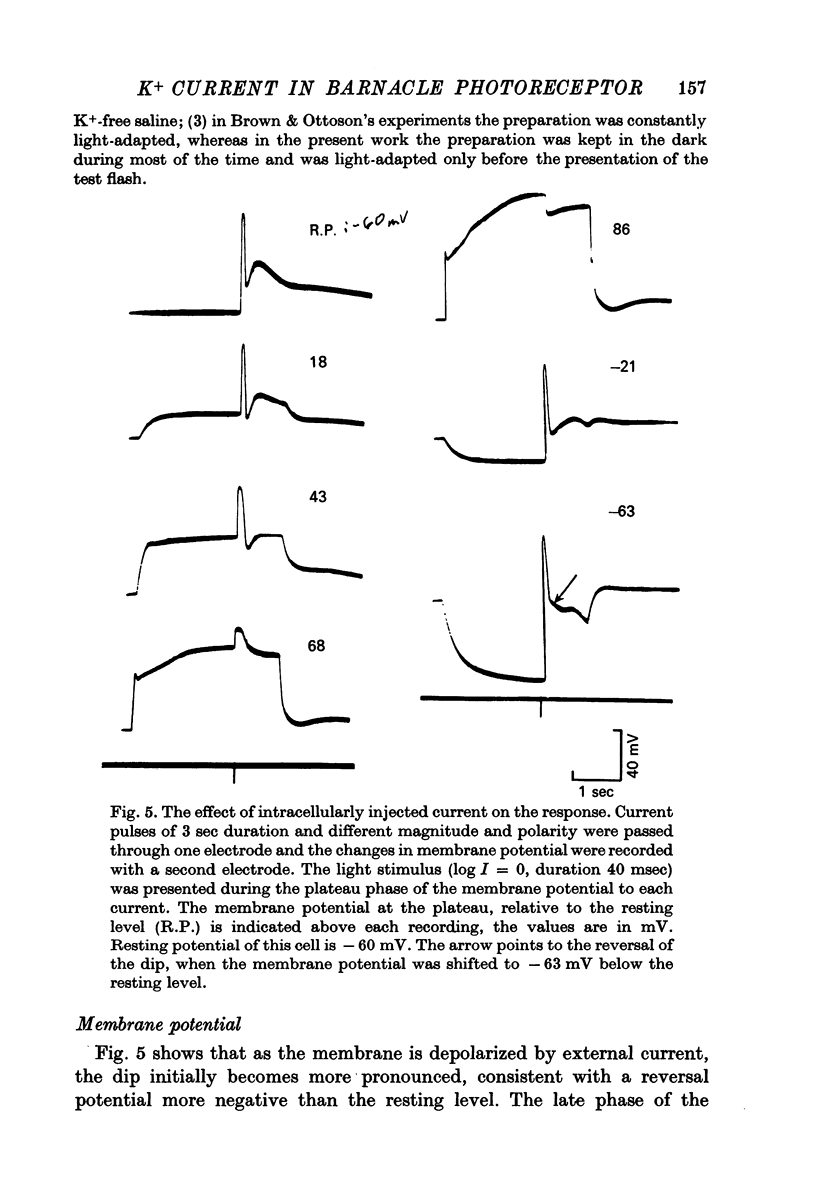
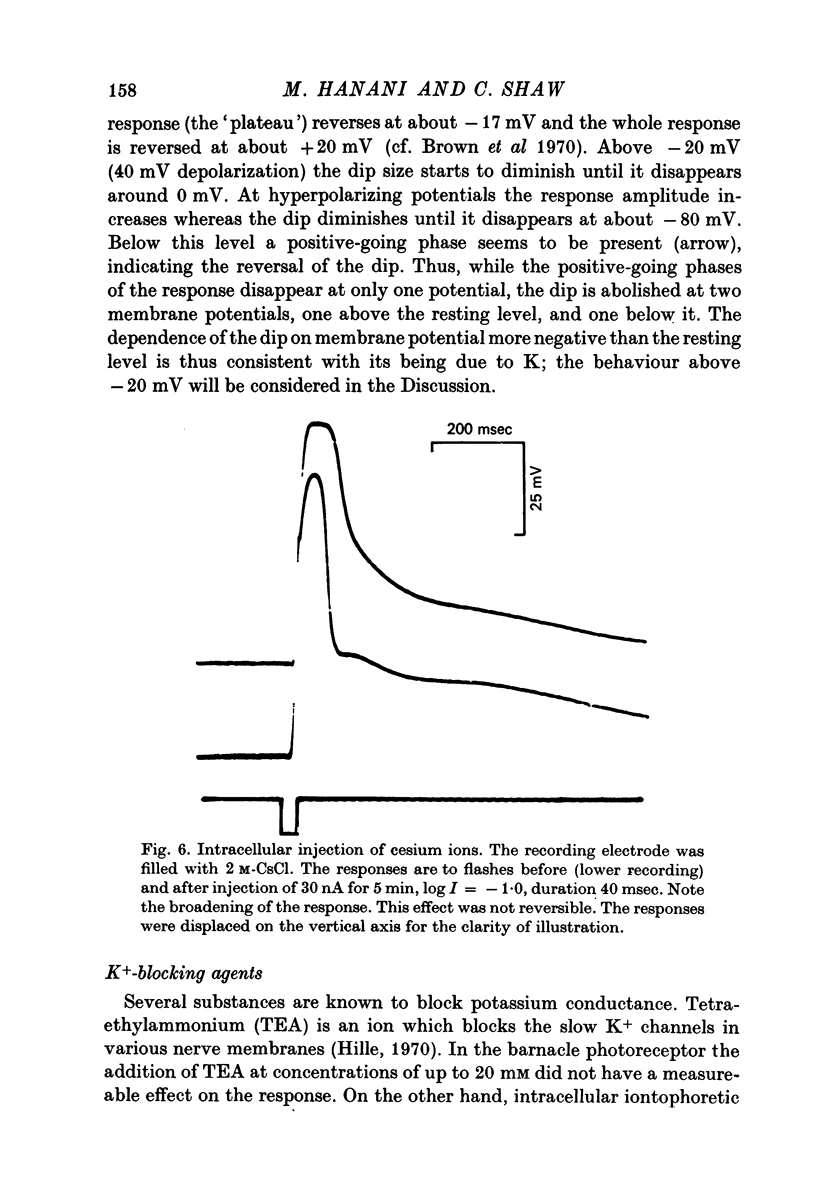
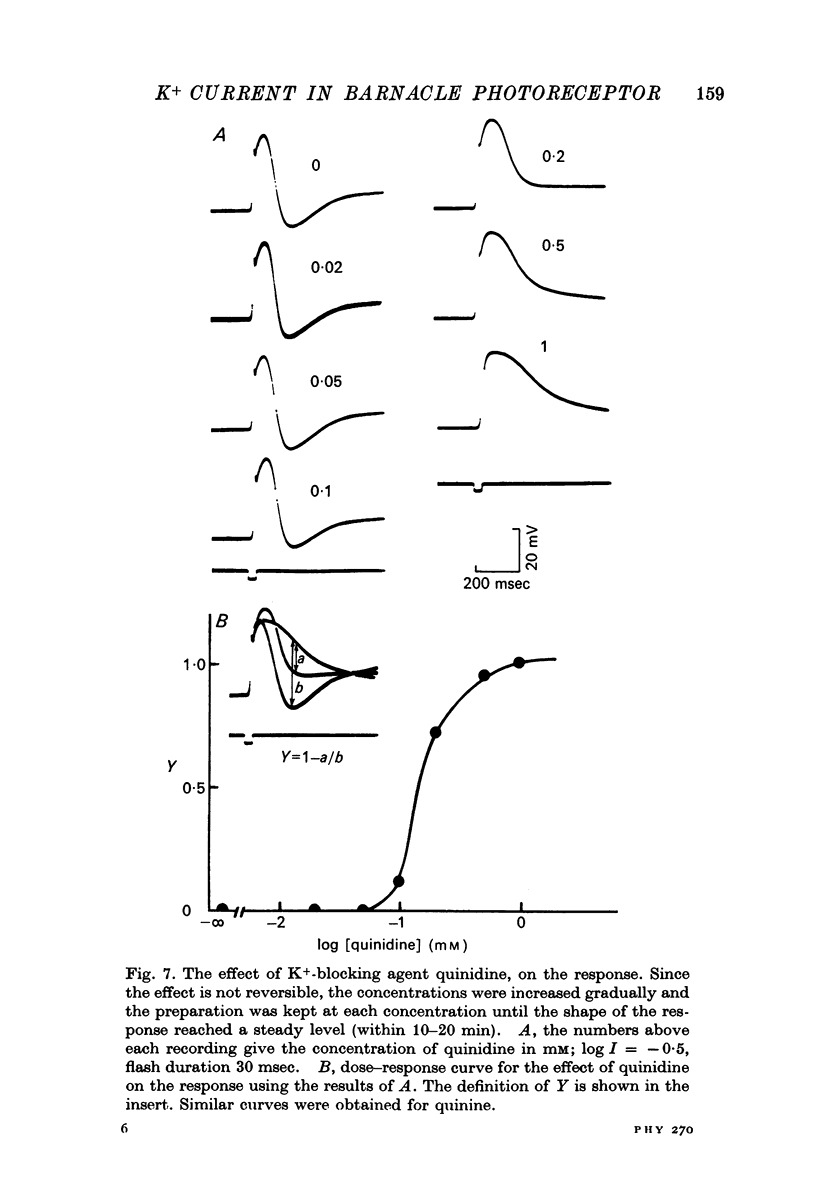
1. Intracellular recording from photoreceptors in the lateral eye of the barnacle show a brief negative-going 'dip' shortly after the onset of the late receptor potential. This phase can sometimes result in a hyperpolarization relative to the resting membrane potential. 2. The dip is prominent in light-adapted cells and is reduced by dark-adaptation. Low extracellular Ca2+ also reduces it. 3. The amplitude of the dip changes inversely with the K+ concentration in the saline. 4. The amplitude of the dip depends on the membrane potential, with a reversal potential near - 80 mV. 5. K+ blocking agents such as quinine and quinidine reduce or abolish the dip. 6. These observations indicate that the dip is due to a brief increase in K+ conductance which may be dependent on an influx of Ca ions. The fast decay of this phase may be brought about by a rapid uptake of Ca2+ by an intracellular mechanism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. M., Brown H. M. Light response of a giant Aplysia neuron. J Gen Physiol. 1973 Sep;62(3):239–254. doi: 10.1085/jgp.62.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Ottoson D. Dual role for potassium in Balanus photoreceptor: antagonist of calcium and suppression of light-induced current. J Physiol. 1976 May;257(2):355–378. doi: 10.1113/jphysiol.1976.sp011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Blinks J. R. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. Detection with aequorin. J Gen Physiol. 1974 Dec;64(6):643–665. doi: 10.1085/jgp.64.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Lisman J. E. Intracellular Ca modulates sensitivity and time scale in Limulus ventral photoreceptors. Nature. 1975 Nov 20;258(5532):252–254. doi: 10.1038/258252a0. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Mote M. I. Ionic dependence of reversal voltage of the light response in Limulus ventral photoreceptors. J Gen Physiol. 1974 Mar;63(3):337–350. doi: 10.1085/jgp.63.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B. Multiple light-evoked conductance changes in the photoreceptors of Hermissenda crassicornis. J Physiol. 1976 Apr;256(3):691–708. doi: 10.1113/jphysiol.1976.sp011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Knight B. W., Toyoda J. Voltage noise in Limulus visual cells. Science. 1968 Apr 5;160(3823):88–90. doi: 10.1126/science.160.3823.88. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Cesium induced rectifications in frog myelinated fibres. Pflugers Arch. 1975 Apr 2;355(4):361–364. doi: 10.1007/BF00579857. [DOI] [PubMed] [Google Scholar]
- FAHRENBACK W. H. THE MICROMORPHOLOGY OF SOME SIMPLE PHOTORECEPTORS. Z Zellforsch Mikrosk Anat. 1965 Apr 8;66(2):233–254. [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., McReynolds J. S. Control of membrane N+ permeability in a hyperpolarizing photoreceptor: similar effect of light and metabolic inhibitors. Science. 1974 Aug 16;185(4151):620–621. doi: 10.1126/science.185.4151.620. [DOI] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hanani M., Hillman P. Adaptation and facilitation in the barnacle photoreceptor. J Gen Physiol. 1976 Feb;67(2):235–276. doi: 10.1085/jgp.67.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C. E., Brown J. E. Ion fluxes in photoreception in Limulus polyphemus ventral eye. I. The response of potassium efflux to light. Biochim Biophys Acta. 1972 Jul 3;274(1):140–157. doi: 10.1016/0005-2736(72)90289-1. [DOI] [PubMed] [Google Scholar]
- Koike H., Brown H. M., Hagiwara S. Hyperpolarization of a barnacle photoreceptor membrane following illumination. J Gen Physiol. 1971 Jun;57(6):723–737. doi: 10.1085/jgp.57.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Lisiewicz A. Injections of calcium ions into spinal motoneurones. J Physiol. 1972 Sep;225(2):363–390. doi: 10.1113/jphysiol.1972.sp009945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevíc K., Puil E., Werman R. Evidence for Ca2+-activated K+ conductance in cat spinal motoneurons from intracellular EGTA injections. Can J Physiol Pharmacol. 1975 Dec;53(6):1214–1218. doi: 10.1139/y75-171. [DOI] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972 Jun;59(6):701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J. S., Gorman A. L. Ionic basis of hyperpolarizing receptor potential in scallop eye: increase in permeability to potassium ions. Science. 1974 Feb 15;183(4125):658–659. doi: 10.1126/science.183.4125.658. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Calcium ion distribution in cytoplasm visualised by aequorin: diffusion in cytosol restricted by energized sequestering. Science. 1975 Dec 19;190(4220):1204–1206. doi: 10.1126/science.1198106. [DOI] [PubMed] [Google Scholar]
- Stieve H., Malinowska T., Sonnemann D. Dependence of potassium ion and sodium ion exchange on light in the crayfish retina. Z Naturforsch C. 1974 Nov-Dec;29(11-12):745–753. doi: 10.1515/znc-1974-11-1216. [DOI] [PubMed] [Google Scholar]


