Abstract
TonB-gated transporters have β-barrels containing an amino-terminal globular domain that occludes the interior of the barrel. Mutations in the globular domain prevent transport of ligands across the outer membrane. Surprisingly, FepA with deletions of the globular domain (amino acids 3 to 150 and 17 to 150) was previously reported to retain significant sensitivity to colicins B and D and to use ferric enterochelin, all in a TonB-dependent fashion. To further understand TonB interaction with the β-barrel, in the present study, proteins with deletions of amino acids 1 to 152, 7 to 152, 20 to 152, and 17 to 150 in fepA were constructed and expressed in a ΔfepA strain. In contrast to previous studies of fepA globular domain deletions, constructs in this study did not retain sensitivity to colicin B and conferred only marginal sensitivity to colicin D. Consistent with these observations, they failed to bind colicin B and detectably cross-link to TonB in vivo. To address this discrepancy, constructs were tested in other strains, one of which (RWB18-60) did support activity of the FepA globular domain deletion proteins constructed in this study. The characteristics of that strain, as well as the strain in which the ΔFhuA globular domain mutants were seen to be active, suggests the hypothesis that interprotein complementation by two individually nonfunctional proteins restores TonB-dependent activity.
TonB-gated transporters are located in the outer membranes of gram-negative bacteria, where they mediate the active transport of iron siderophores and vitamin B12 across the outer membrane. The energy for this process is transduced from the cytoplasmic membrane by a complex of cytoplasmic membrane proteins—TonB, ExbB, and ExbD (for reviews, see references 6 and 26). The crystal structures of TonB-gated transporters (also known as outer membrane receptors) reveal that they consist of a β-barrel that is occluded by an amino-terminal globular domain (also known as the “cork” or “plug” domain) (7, 10, 21). TonB protein physically interacts with the transporters (29), with at least one contact occurring between TonB and a region near the amino terminus of the globular domain, termed the TonB box (8, 23). Mutations within the TonB box prevent function of the transporter (3, 12, 22, 25). Recently, it has been reported that the amino-terminal globular domains of FhuA and FepA can be deleted without significant reduction in their activities and without alleviating their requirements for TonB (5, 28). In particular, deletion of the FepA globular domain (amino acids 17 to 150) resulted in a protein termed Fepβ, reported to support binding of ferric enterochelin (also known as ferric enterobactin), weak growth with ferric enterochelin as a sole source of iron, and significant sensitivity to colicins B and D (28). The latter three activities were also dependent upon TonB. The authors interpreted these data and data from hybrid transporters to suggest that the globular domain is not important for ligand recognition and that TonB does not function by interaction with the internal globular domain. Thus, TonB must interact within the β-barrel itself. To further examine TonB-barrel interactions, various deletions removing the globular domain of FepA were constructed for the present study.
The fepA gene was amplified as a BspHI fragment by PCR and cloned into the NcoI site of pBAD24 to create pKP515. Deletions in fepA were constructed in pKP515 by extra-long PCR as described previously (15) with primer sequences that are available upon request. DNA sequences of all plasmids were determined, and the absence of unintended base changes was confirmed. Deletion of amino acids 1 to 152 removed the entire globular domain up to an aromatic anchoring residue (trp) preceding the first β strand (7). Deletion of amino acids 7 to 152 was constructed to mimic the FhuA deletion (FhuAΔ5-160) characterized previously (5), with a less extensive deletion (residues 20 to 152) constructed to leave the TonB box (a region through which TonB demonstrably interacts [8]) intact. During these studies the work on Fepβ was reported, and so the identical protein FepAΔ17-150 was engineered as a control. Arabinose was added to the media for expression of wild-type FepA and three deletion proteins at chromosomal levels (final concentration, 0.25 μg/ml). FepAΔ1-152 required a final concentration of 10 μg of arabinose/ml to reach chromosomal levels (Fig. 1).
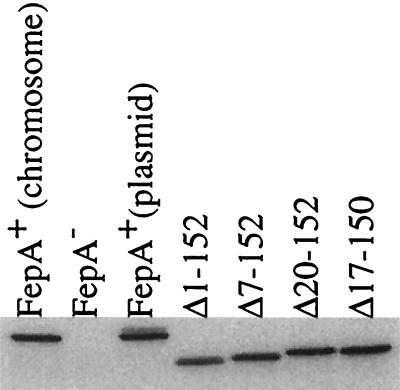
FIG. 1.
Steady-state levels of FepA and FepA with globular domain deletions. Immunoblot of samples resolved on a sodium dodecyl sulfate-11% polyacrylamide gel developed with α-FepA polyclonal antibody at 1:5,000 is shown. FepA+ (chromosome), W3110; FepA−, KP1394; FepA+ (plasmid), KP1394/pKP515; fepAΔ1-152, KP1394/pKP516; fepAΔ7-152, KP1394/pKP517; fepAΔ20-152, KP1394/pKP518; and fepAΔ17-150, KP1394/pKP519. KP1394 was constructed by P1vir transduction of recA::cat from KP1286 (11) into KP1270 (20). Strains were induced with arabinose as described in the text.
To determine if the constructs were correctly localized, strains expressing the FepA variants in KP1394 (fepA::kan recA::cat) were fractionated on sucrose density gradients as previously described (20). All of the mutant FepA derivatives localized as efficiently to the outer membrane as their wild-type parent (Fig. 2).
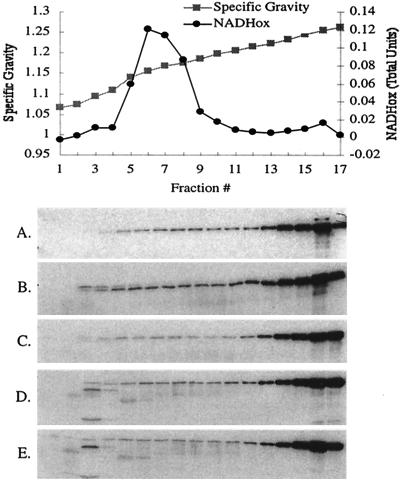
FIG. 2.
FepA globular domain deletions fractionate with the outer membrane. Specific gravity and NADH oxidase (NADHox) activity are provided for fractions from the strain expressing wild-type FepA; the mutants had similar profiles (not shown). Immunoblots of sodium dodecyl sulfate-11% polyacrylamide gels developed with α-FepA polyclonal antibody (13) at 1:5,000 are shown. (A) KP1394/pKP515 (FepA wild type); (B) KP1394/pKP516 (FepAΔ1-152); (C) KP1394/pKP517 (FepAΔ7-152); (D) KP1394/pKP518 (FepAΔ20-152); and (E) KP1394/pKP519 (FepAΔ17-150).
The ability of the deletion-containing FepA proteins to use ferric enterochelin as the sole iron source was evaluated. Ferric enterochelin was freshly obtained from culture supernatants of KP1344 (W3110, tonB::blaM) (20). While wild-type FepA could support ferric enterochelin-dependent growth (with zone sizes similar to those observed previously [28]), none of the FepA globular domain deletion proteins could support growth beyond that observed for KP1411 (W3110, fepA::kan recA::cat aroB) (Table 1). The slight amount of growth conferred upon KP1411 suggests the presence in the sterile culture supernatants of additional siderophore-type molecules that enter via a different TonB-gated transporter, since the aroB tonB strain KP1406 is completely unable to grow in the presence of the culture supernatant.
TABLE 1.
FepA globular domain deletion mutants do not support enterochelin-dependent growth
| Strain | Genotype | Growtha |
|---|---|---|
| KP1411b/pBAD24 | aroBfepA | 12 ± 0.5 |
| KP1406 | aroBtonB | No growth |
| KP1411/pKP515 | aroB, pfepA+ | 21 ± 0 |
| KP1411/pKP516 | aroB, pfepAΔ1-152 | 9 ± 1.8 |
| KP1411/pKP517 | aroB, pfepAΔ7-152 | 8 ± 0.9 |
| KP1411/pKP518 | aroB, pfepAΔ20-152 | 11 ± 0.5 |
| KP1411/pKP519 | aroB, pfepAΔ17-150 | 12 ± 0.3 |
Size of growth zones due to ferric enterochelin supplied from KP1344-conditioned medium (in millimeters; disk size is 6 mm). Supernatants from KP1406 (W3110, tonB::blaM aroB) supported no growth for any of the strains (data not shown).
Since FepA and TonB are both required for sensitivity of Escherichia coli to colicins B and D, the sensitivity conferred by various fepA plasmids to colicins B and D was determined. Surprisingly, none of the deletions supported sensitivity to colicin B (Table 2). All of the deletions showed marginal sensitivity to one fivefold dilution of colicin D, with a barely visible zone or clearing. The activity levels of these colicin preparations were, if anything, of slightly higher titer than those used in characterization of Fepβ (28).
TABLE 2.
FepA globular domain deletion mutants are resistant to colicin B (ColB) and marginally sensitive to colicin D (ColD)
| Strain | Genotype | Sensitivitya to
|
|
|---|---|---|---|
| ColB | ColD | ||
| KP1411/pBAD24 | aroB fepA− | Rd | UDb |
| KP1411/pKP515 | aroB fepA+ | 3.9 × 10−5 | 7.8 × 10−4 |
| KP1411/pKP516 | aroB fepAΔ1-152 | R | 0.2c |
| KP1411/pKP517 | aroB fepAΔ7-152 | R | 0.2c |
| KP1411/pKP518 | aroB fepAΔ20-152 | R | 0.2c |
| KP1411/pKP519 | aroB fepAΔ17-150 | R | 0.2c |
Results are expressed as the highest dilution at which clearing of the bacterial lawn was observed.
Undiluted colicin.
Almost undetectable zones of killing.
R, resistant.
The ability of the FepA with globular domain deletions to bind colicin B was measured in vivo. The strain expressing wild-type FepA clearly bound colicin B. However, consistent with the sensitivity assays, none of the strains expressing globular domain deletions bound more colicin B than isogenic fepA controls (Fig. 3).
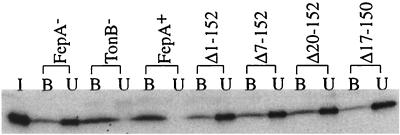
FIG. 3.
FepA globular domain deletions do not bind colicin B. KP1411 (W3110, fepA::kan, recA::cat, aroB) expressing wild-type or mutant FepA was collected at room temperature, washed once in λ-calcium buffer (10 mM Tris-HCl, pH 7.8, 20 mM MgSO4, and 5 mM CaCl2), and suspended in 0.1 volume of λ-calcium buffer. Purified colicin B (estimated at 3.5 × 1013 colicin B molecules/μl) was diluted and added to the bacteria at a ratio of 1 colicin B to 13 FepA, with FepA calculations based on recent per-cell determinations (13). After incubation at 37°C for 30 min, bound colicin B was collected by centrifugation at 16,000 × g for 5 min at 4°C. Unbound colicin B was collected from the resultant supernatants by precipitation with trichloroacetic acid. Immunoblots of samples from an entire experiment were resolved on sodium dodecyl sulfate-11% polyacrylamide gels and developed with anti-colicin B antibody at 1:5,000. I, initial amount of colicin B; U, unbound colicin B; and B, bound colicin B.
FepA can cross-link to TonB in vivo (29). This cross-linking is significantly enhanced by the presence of ligand (14). To determine if the FepA globular domain played a role in that process, the various fepA plasmids in KP1394 (fepA::kan and recA::cat) were cross-linked as described previously. None of the fepA deletion mutants was able to cross-link to TonB, although wild-type FepA expressed from either the chromosome or a plasmid could form that complex (Fig. 4). This difference could be due to lack of the ligand occupancy signal transmitted through the globular domain (7, 21) or due to loss of a TonB interaction domain (8, 18, 23, 29) or both. It is important to remember that these results do not exclude a further interaction of TonB with the barrel, one that is not detected by cross-linking. Consistent with that idea, we were able to detect very weak levels of coimmunoprecipitation of TonB by both wild-type FepA and the globular domain deletion derivatives (data not shown).
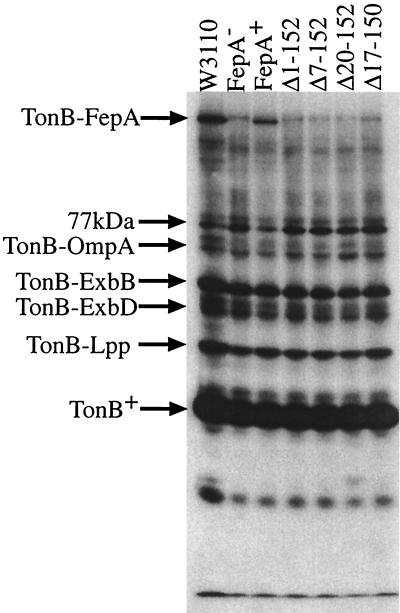
FIG. 4.
FepA globular domain mutants do not cross-link to TonB. Immunoblot of cross-linked samples on a sodium dodecyl sulfate-11% polyacrylamide gel developed with anti-TonB monoclonal antibody 4F1 (19) at 1:5,000 is shown. W3110, fepA+ (chromosome); FepA−, KP1394; FepA+ (plasmid), KP1394/pKP515; fepAΔ1-152, KP1394/pKP516; fepAΔ7-152, KP1394/pKP517; fepAΔ20-152, KP1394/pKP518; and fepAΔ17-150, KP1394/pKP519.
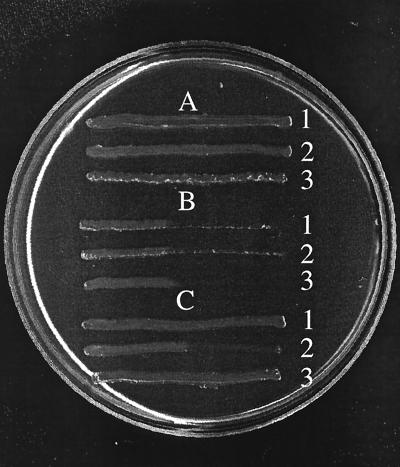
The above results are in direct contrast with the previous results of Scott et al., who demonstrated TonB specific activity for their Fepβ protein (28). The major difference between that study and this work is in the genetic backgrounds of the strains examined. Here the activity of FepAΔ17-150 was assessed in a W3110-derived background, whereas the identical Fepβ was assayed in strain KDF541 (F− thi entA proC trp rpsL recA fepA fhuA cir). KDF541 is derived from RWB18-60 (27) and was isolated from it by a sequential selection for resistance to colicin Ia and bacteriophage T5 to recover mutations in cir and fhuA, respectively. To our surprise, examination of RWB18-60 revealed that the presence of any of our plasmid-encoded ΔfepA constructs rendered it sensitive to undiluted colicins B and D in cross-streaks (Fig. 5 and data not shown). Because one of the strain differences involved the way in which enterochelin synthesis was disrupted (entA for RWB18-60, aroB in our case), we repeated the colicin sensitivity assays in RW193/MT912-59 (F− thi trpE proC leuB lacY mtl xyl rpsL azi tsx supA ΔfepA::kan entA403 ΔrecA srl::Tn10 [1]), which also carries fepA, entA, and recA mutations. In contrast to RWB18-60, RW193/MT912-59 carrying any one of the ΔfepA constructs was completely resistant to undiluted colicins B and D in cross-streaks, excluding the aroB/entA difference as the source of variation, and confirming the other data presented in this paper (Fig. 5).
FIG. 5.
Different strains support different levels of colicin B sensitivity for FepA globular domain deletions. Strains 1394 (1), RWB18-60 (2), and RWB193-MT912-59 (3) with pBAD24 (vector) (A), pKP515 (fepA+) (B), or pKP519 (fepAΔ17-150) (C) were cross streaked against undiluted colicin B on Luria-Bertani agar containing 100 μg of ampicillin per ml. Colicin B was applied as a vertical line down the center of the plate. Similar results were obtained for colicin D cross-streaks. All the strains were sensitive to colicins Ia and M (data not shown).
RWB18-60 and RWB193-MT912-59 share the same genetic background, RW193 (F− thi proC leu trp entA403 and tsx [2, 16]). One of the key differences between them, however, is in the chromosomally encoded fepA mutant allele. The strains that do not support any activity in the plasmid-encoded ΔfepA mutants (including RWB193-MT912-59) contain a fepA deletion of codons 55 to 359 replaced by a kan gene (1). In contrast, RWB18-60 contains an uncharacterized fepA mutation (16) and is also a lambda lysogen (M. A. McIntosh, personal communication).
The mutant FepA expressed by RWB193-MT912-59 does not contain the globular domain, whereas the mutant FepA encoded by RWB18-60 could potentially do so. If the FepA protein of RWB18-60 contains a globular domain, then one possibility for the difference between the two strains might be due to interprotein complementation. The globular domain from the inactive chromosomally encoded FepA might insert into the empty β-barrel of the plasmid-encoded globular domain deletions. This possiblity is strengthened by the fact that a similar situation exists in the case of the ΔFhuA globular domain analysis (5). In that case, the fhuA412 mutant background used to test the ΔfhuA globular domain mutations actually expresses a full-length FhuA412 protein carrying 5 different amino acid substitutions (17), which might functionally complement the plasmid-encoded ΔFhuA globular domain deletions. Failure to detect FhuA412 (or mutant FepA) protein in the plasmid-less strains could be explained if they were unstable, except in the presence of the plasmid-encoded globular domain deletion proteins.
Consistent with this hypothesis, FepA has been cross-linked into trimers in vivo by formaldehyde (29), suggesting that these transporters can function as a multimeric complex. In addition, only 10% of the FepA molecules in the previous study were active (28), suggesting a low level of complementation or low level of stability of the chromosomally encoded FepA mutant protein. Furthermore, it has recently been demonstrated that FhuA barrels could combine with non-FhuA globular domains to form active proteins, although, in that case, they were encoded on the same gene (17). This hypothesis also weakens the only existing strong evidence for TonB interaction with the β-barrel of the transporters (5, 28): the TonB-dependent activities might have arisen from in vivo reconstitution of intact transporters. If they did arise in that manner, it suggests that the internal globular domain does indeed exit the barrel.
Finally, the present results are also most consistent with the significant body of data reported elsewhere stating that the amino-terminal globular domains of TonB-gated transporters are indeed important for ligand transport, binding, and interaction with TonB (4, 8, 9, 12, 21, 24, 30). Given the complexity of the system, it is not unreasonable to expect that both the internal globular domain and the β-barrel are required for activity of the TonB-gated transporters and that each might still interact independently with TonB.
Acknowledgments
We thank Wendy Shuttleworth and Greg Chen for purification of colicin B, Penelope Higgs and Greg Chen for production of anti-colicin B antibodies, Ray Larsen for construction of KP1406 (to be described elsewhere) and critical reading of the manuscript, and Mark McIntosh for strains RWB18-60 and RW193/MT912-59.
This work was supported by National Science Foundation research grant MCB97-24049 and National Institutes of Health research grant GM42146 (to K.P.).
REFERENCES
- 1.Armstrong, S. K., C. L. Francis, and M. A. McIntosh. 1990. Molecular analysis of the Escherichia coli ferric enterobactin receptor FepA. J. Biol. Chem. 265:14536-14543. [PubMed] [Google Scholar]
- 2.Barnard, T. J., M. E. Watson, Jr., and M. A. McIntosh. 2001. Mutations in the Escherichia coli receptor FepA reveal residues involved in ligand binding and transport. Mol. Microbiol. 41:527-536. [DOI] [PubMed] [Google Scholar]
- 3.Bell, P. E., C. D. Nau, J. T. Brown, J. Konisky, and R. J. Kadner. 1990. Genetic suppression demonstrates direct interaction of TonB protein with outer membrane transport proteins in Escherichia coli. J. Bacteriol. 172:3826-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonhivers, M., M. Desmadril, G. S. Moeck, P. Boulanger, A. Colomer-Pallas, and L. Letellier. 2001. Stability studies of FhuA, a two-domain outer membrane protein from Escherichia coli. Biochemistry 40:2606-2613. [DOI] [PubMed] [Google Scholar]
- 5.Braun, M., H. Killmann, and V. Braun. 1999. The β-barrel domain of FhuAΔ5-160 is sufficient for TonB-dependent FhuA activities of Escherichia coli. Mol. Microbiol. 33:1037-1049. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V., and H. Killmann. 1999. Bacterial solutions to the iron-supply problem. Trends Biochem. Sci. 24:104-109. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 8.Cadieux, N., and R. J. Kadner. 1999. Site-directed disulfide bonding reveals an interaction site between energy-coupling protein TonB and BtuB, the outer membrane cobalamin transporter. Proc. Natl. Acad. Sci. USA 96:10673-10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van Der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 11.Held, K., and K. Postle. 2002. ExbB and ExbD do not function independently in TonB-dependent energy transduction. J. Bacteriol. 184:5170-5173. [DOI] [PMC free article] [PubMed]
- 12.Heller, K. J., R. J. Kadner, and K. Günter. 1988. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene 64:147-153. [DOI] [PubMed] [Google Scholar]
- 13.Higgs, P. I., R. A. Larsen, and K. Postle. 2002. Quantitation of known components of the Escherichia coli TonB-dependent energy transduction system: TonB, ExbB, ExbD, and FepA. Mol. Microbiol. 44:271-281. [DOI] [PubMed] [Google Scholar]
- 14.Higgs, P. I., T. E. Letain, K. K. Merriam, N. S. Burke, H. Park, C. Kang, and K. Postle. 2002. TonB interacts with nonreceptor proteins in the outer membrane of Escherichia coli. J. Bacteriol. 184:1640-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgs, P. I., P. S. Myers, and K. Postle. 1998. Interactions in the TonB-dependent energy transduction complex: ExbB and ExbD form homomultimers. J. Bacteriol. 180:6031-6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollifield, W. C., and J. B. Neilands. 1978. Ferric enterobactin transport system in Escherichia coli K12. Extraction, assay, and specificity of the outer membrane receptor. Biochemistry 17:1922-1928. [DOI] [PubMed] [Google Scholar]
- 17.Killmann, H., M. Braun, C. Herrmann, and V. Braun. 2001. FhuA barrel-cork hybrids are active transporters and receptors. J. Bacteriol. 183:3476-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen, R. A., D. Foster-Hartnett, M. A. McIntosh, and K. Postle. 1997. Regions of Escherichia coli TonB and FepA proteins essential for in vivo physical interactions. J. Bacteriol. 179:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen, R. A., P. S. Myers, J. T. Skare, C. L. Seachord, R. P. Darveau, and K. Postle. 1996. Identification of TonB homologs in the family Enterobacteriaceae and evidence for conservation of TonB-dependent energy transduction complexes. J. Bacteriol. 178:1363-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen, R. A., M. G. Thomas, and K. Postle. 1999. Protonmotive force, ExbB and ligand-bound FepA drive conformational changes in TonB. Mol. Microbiol. 31:1809-1824. [DOI] [PubMed] [Google Scholar]
- 21.Locher, K. P., B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771-778. [DOI] [PubMed] [Google Scholar]
- 22.Mende, J., and V. Braun. 1990. Import-defective colicin B derivatives mutated in the TonB box. Mol. Microbiol. 4:1523-1533. [DOI] [PubMed] [Google Scholar]
- 23.Merianos, H. J., N. Cadieux, C. H. Lin, R. J. Kadner, and D. S. Cafiso. 2000. Substrate-induced exposure of an energy-coupling motif of a membrane transporter. Nat. Struct. Biol. 7:205-209. [DOI] [PubMed] [Google Scholar]
- 24.Moeck, G. S., P. Tawa, H. Xiang, A. A. Ismail, J. L. Turnbull, and J. W. Coulton. 1996. Ligand-induced conformational change in the ferrichrome-iron receptor of Escherichia coli K12. Mol. Microbiol. 22:459-471. [DOI] [PubMed] [Google Scholar]
- 25.Pilsl, H., C. Glaser, P. Gross, H. Killmann, T. Olschlager, and V. Braun. 1993. Domains of colicin-M involved in uptake and activity. Mol. Gen. Genet. 240:103-112. [DOI] [PubMed] [Google Scholar]
- 26.Postle, K. 1999. Active transport by customized beta-barrels. Nat. Struct. Biol. 6:3-6. [DOI] [PubMed] [Google Scholar]
- 27.Rutz, J. M., J. Liu, J. A. Lyons, J. Goranson, S. K. Armstrong, M. A. McIntosh, J. B. Feix, and P. E. Klebba. 1992. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science 258:471-475. [DOI] [PubMed] [Google Scholar]
- 28.Scott, D. C., Z. Cao, Z. Qi, M. Bauler, J. D. Igo, S. M. Newton, and P. E. Klebba. 2001. Exchangeability of N termini in the ligand-gated porins of Escherichia coli. J. Biol. Chem. 276:13025-13033. [DOI] [PubMed] [Google Scholar]
- 29.Skare, J. T., B. M. M. Ahmer, C. L. Seachord, R. P. Darveau, and K. Postle. 1993. Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J. Biol. Chem. 268:16302-16308. [PubMed] [Google Scholar]
- 30.Usher, K. C., E. Ozkan, K. H. Gardner, and J. Deisenhofer. 2001. The plug domain of FepA, a TonB-dependent transport protein from Escherichia coli, binds its siderophore in the absence of the transmembrane barrel domain. Proc. Natl. Acad. Sci. USA 98:10676-10681. [DOI] [PMC free article] [PubMed] [Google Scholar]