Abstract
The modified nucleosides 2′-O-methylguanosine, present at position 18 (Gm18), 5-methyluridine, present at position 54 (m5U54), and pseudouridine, present at position 55 (Ψ55), are located in the D and T arms of tRNAs and are close in space in the three-dimensional (3D) structure of this molecule in the bacterium Escherichia coli. The formation of these modified nucleosides is catalyzed by the products of genes trmH (Gm18), trmA (m5U54), and truB (Ψ55). The combination of trmH, trmA, and truB mutations resulting in lack of these three modifications reduced the growth rate, especially at high temperature. Moreover, the lack of three modified nucleotides in tRNA induced defects in the translation of certain codons, sensitivity to amino acid analog 3,4-dehydro-dl-proline, and an altered oxidation of some carbon compounds. The results are consistent with the suggestion that these modified nucleosides, two of which directly interact in the 3D structure of tRNA by forming a hydrogen bond between Ψ55 and Gm18, stabilize the structure of the tRNA. Moreover, lack of Ψ55 in tRNA of human pathogen Shigella flexneri leads to a reduced expression of several virulence-associated genes.
In cells of all organisms there are several species of stable RNA, of which the most prominent are rRNA and tRNA. Most of the stable RNAs contain modified nucleosides, which are derivatives of the four ordinary nucleosides. In a global survey, 81 of 95 modified nucleosides were found in tRNA (49). Pseudouridine (Ψ) and 2′-O-methylated nucleosides are the most abundant modified residues in RNAs (33, 41). In the tRNA of the bacterium Escherichia coli, formation of Ψ occurs at positions 32 (48, 60), 38 to 40 (26), and 55 (39). The Ψ55 is present in the TΨC loop, which is present in all tRNA species of this bacterium. Deletion of the truB gene, which is responsible for the modification of Ψ55, has no major effect on growth rate, but a strain lacking the truB gene was outcompeted by wild-type cells in a growth competition experiment (23). Another modified nucleoside which is also present in the TΨC loop of all tRNA species in E. coli and whose function remains obscure is 5-methyluridine (ribothymidine, m5U[T]54). The trmA::cat insertion relatively early in the trmA gene (codon 161 out of 366), which is responsible for the formation of m5U54, is lethal for E. coli (44). However, several trmA point mutants lacking the methyl group in tRNA and producing full-length TrmA proteins (44, 57) have no apparent growth defects. The only observed disadvantage for cells lacking m5U54 is that they are outcompeted by wild-type cells in a mixed-population experiment (4).
2′-O-Methylguanosine (Gm18) is present in the conserved D loop in 13 out of the 46 E. coli tRNA species sequenced (54). Gm18 methylating activity has been established (19), and the trmH gene, the product of which is responsible for its synthesis, has been identified (45). Absence of Gm18 in tRNA of E. coli has no effect on the activity of the supF amber suppressor tRNA or on the growth rate of cells (45).
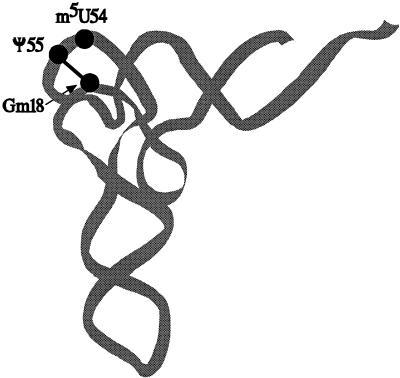
Nucleosides Gm18 and Ψ55 interact in the tRNA tertiary structure by hydrogen bonding, thereby stabilizing the L shape of tRNA (30, 46) (Fig. 1). Generally, 2′-O methylation of the ribose stabilizes the nucleoside in the C3′-endo form, thereby causing conformational rigidity (27, 28). The 2′-O methylation of Gm18 may contribute to the rigidity of G18, stabilizing Gm18-Ψ55 base pairing. Both 5-methylation of U54 and the isomerization of U55 into Ψ55 stabilize the tRNA, probably by enhancing base stacking (12, 13, 59). Also, a water-mediated bridge between Ψ55 and the phosphate backbone provides an additional stabilizing effect (1). Therefore, we expected that a combination of mutations that lead to the absence of Gm18, m5U54, and Ψ55 in tRNA of E. coli would destabilize the tRNA, resulting in a possible effect on the efficiency of translation, the growth rate, and/or the metabolism. The present work addresses this question, and indeed this expectation was verified. Moreover, we also show that expression of virulence genes in Shigella flexneri was affected by lack of Ψ55 and that the Pseudomonas aeruginosa orp mutant, deficient in the expression of virulence genes, lacks Ψ55-modifying activity.
FIG. 1.
Schematic model of three-dimensional structure of the yeast tRNAPhe molecule, adapted from reference 29. The locations of the modified nucleosides in positions 18, 54, and 55 are demonstrated. Black line, hydrogen bonding between nucleosides Gm18 and Ψ55.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used are listed in Tables 1 and 2.
TABLE 1.
Bacterial strains used
| Strain | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli | ||
| MW100 | Hfr P4X | M. Wikström |
| GOB113 | Hfr P4X sdr+truB2422::mini-Tn10Cm; donor for P1-mediated transduction | 7 |
| G11-5-18 | ilvA trmA5; donor for P1-mediated transduction | L. Isaksson |
| GRB1882 | Hfr P4X trmA5 | This work |
| CF4666 | ΔtrmH::kan (ΔspoU3::kan); donor for P1-mediated transduction | M. Cashel |
| CAG 12185 | argE86::Tn10; donor for P1-mediated transduction. | C. Gross |
| GRB1756 | Hfr P4X ΔtrmH::kan | This work |
| GRB1737 | Hfr P4X truB2422::mini-Tn10Cm ΔtrmH::kan | This work |
| GRB1887 | Hfr P4X truB2422::mini-Tn10Cm trmA5 | This work |
| GRB1814 | Hfr P4X ΔtrmH::kan trmA5 | This work |
| GRB1777 | Hfr P4X truB2422::mini-Tn10Cm trmH::Km trmA5 | This work |
| GOB083 | Hfr P4X sdr-43 truB2422::mini-Tn10Cm | 7 |
| GT527 | MC4100 with Φ(trhA1-lacZ)hyb2-1 | 51 |
| GRB1536 | MC4100 with Φ(trhA1-lacZ)hyb2-1truB2422::mini-Tn10Cm | This work |
| GBEC384 (-CSH41) | Δ(lac-pro) galE | Cold Spring Harbor Laboratory |
| GRB1490 | Δ(lac-pro) galE truB2422::mini-Tn10Cm | This work |
| GRB1886 | Δ(lac-pro) galE trmA5 | This work |
| GRB1739 | Δ(lac-pro) galE ΔtrmH::kan | This work |
| GRB1741 | Δ(lac-pro) galE truB2422::mini-Tn10Cm ΔtrmH::kan | This work |
| GRB 1890 | Δ(lac-pro) galE truB2422::mini-Tn10Cm trmA5 | This work |
| GRB1817 | Δ(lac-pro) galE ΔtrmH::kan trmA5 | This work |
| GRB1780 | Δ(lac-pro) galE truB2422::mini-Tn10Cm ΔtrmH::kan trmA5 | This work |
| GRB1655 | Δ(lac-pro) galE sdr-43 truB2422::mini-Tn10Cm | This work |
| UB585 | argE(UAG) ara Δ(lac-pro) gyrA rpoB tni tyrT (supF) metB | L. Isaksson |
| GRB1734 | argE(UAG) ara Δ(lac-pro) gyrA rpoB tni tyrT (supF) metB truB2422::mini-Tn10Cm | This work |
| GRB1743 | argE(UAG) ara Δ(lac-pro) gyrA rpoB tni tyrT (supF) metB trmA5 | This work |
| GRB1893 | argE(UAG) ara Δ(lac-pro) gyrA rpoB tni tyrT (supF) metB ΔtrmH::kan | This work |
| GRB1894 | argE(UAG) ara Δ(lac-pro) gyrA rpoB tni tyrT (supF) metB truB2422::mini-Tn10Cm trmA5 | This work |
| GRB1754 | argE(UAG) ara Δ(lac-pro) gyrA rpoB tni tyrT (supF) metB truB2422::mini-Tn10Cm ΔtrmH::kan | This work |
| GBR1819 | argE(UAG) ara Δ(lac-pro) gyrA rpoB tni tyrT (supF) metB ΔtrmH::kan trmA5 | This work |
| GBR1783 | argE(UAG) ara Δ(lac-pro) gyrA rpoB tni tyrT (supF) metB truB2422::mini-Tn10Cm ΔtrmH::kan trmA5 | This work |
| S. flexneri | ||
| 2457T | 2a, truB+ | 17 |
| GBOB8 | 2a, truB2422::mini-Tn10Cm | This work |
| BS184 | 2a, vir-83::MudI1734 (mxi-lacZ) | 34 |
| GBOB4 | 2a, vir-83::MudI1734 (mxi-lacZ) truB2422::mini-Tn10Cm | This work |
| YSH6200 | Avirulent 2a strain | 53 |
| P. aeruginosa | ||
| PAO1 | Prototroph, chl-3 | 50 |
| Tn5T1 | orp (truB)::Tn5Tc | 50 |
TABLE 2.
Plasmids used
| Plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| pCGA9 | Contains nine consecutive CGA codons translationally coupled to lacZ | 11 |
| pCGU9 | Contains nine consecutive CGU codons translationally coupled to lacZ | 11 |
| pCPF4 | Contains UUU-UAU frameshifting window fused to lacZ | 18 |
| pCPF7 | Contains UUU-AAU frameshifting window fused to lacZ | 18 |
| pCPF8 | Contains UUU-CAU frameshifting window fused to lacZ | 18 |
| pTHF32 | Contains CCC-AAA frameshifting window fused to lacZ | 58 |
| pTHF33 | Contains CCC-AAG frameshifting window fused to lacZ | 58 |
| pJC27tet CUU-UAU | Contains CUU-UAU frameshifting window fused to lacZ | 58 |
| pJC27tet CUU-UAC | Contains CUU-UAC frameshifting window fused to lacZ | 58 |
| pJC27tet UAU-UAG | Contains UAU-UAG frameshifting window fused to lacZ | 58 |
| pJC27tet UAC-UAG | Contains UAC-UAG frameshifting window fused to lacZ | 58 |
| F′ am117 | F′ Δ-14 lacl am117 proA+B+ | 36 |
| F′ am121 | F′ Δ-14 lacl am121 proA+B+ | 36 |
| pTrc99A | Plasmid vector | Pharmacia |
| pTrc99A-truB | Contains the truB+ gene | 23 |
| pTrc99A-truBD48C | Contains the truBD48C gene | 23 |
Growth conditions and genetic procedures.
Cultures were grown in either Luria-Bertani (LB) medium (2), rich-MOPS (37a), or MOPS-glucose medium (37). For growth of S. flexneri, MOPS-glucose medium was supplemented with 0.005% nicotinic acid (NA). As solid medium, TYS (10 g of Trypticase peptone, 5 g of yeast extract, 5 g of NaCl, and 15 g of agar per liter) or BHI (37 g of brain heart infusion [Difco Laboratories, Detroit, Mich.] and 15 g of agar per liter) was used. When needed, carbenicillin (50 μg/ml), chloramphenicol (15 μg/ml), or tetracycline (15 μg/ml) was added to the growth media. Phage P1 transductions and F′ conjugations in E. coli were performed as described previously (35). S. flexneri strains were constructed by P1-mediated transduction with E. coli strains as donors.
Analysis of modified nucleotides in tRNA by TLC.
Bacteria were grown in 10 ml of LB medium at 37°C, harvested at a cell density of about 4 × 108 cells/ml by centrifugation, washed once with buffer B (25 mM Tris-HCl [pH 7.4], 10 mM Mg[CH3COO]2, 0.1 mM dithiothreitol, 1 mM EDTA, 10% [by volume] ethylene glycol) (40), and resuspended in 0.5 ml of buffer B. Cells were disrupted by sonication three times for 5 s at 20% power on a VCX400 sonicator (Sonics and Materials Inc., Danbury, Conn.). Cell debris was removed by centrifugation with an Eppendorf centrifuge (5415D) for 15 min at 4°C, and the supernatant was transferred to new tube. The obtained supernatant was used as enzyme extract. To assay enzymatic activity of the TruB protein and/or TrmA protein, [α-32P]UTP-labeled transcripts of tRNAVal (about 104 cpm) were incubated with a mixture containing 80 μl of enzyme extract, 50 μM S-adenosylmethionine, 25 mM Tris-HCl, pH 8.0, 2.5 mM MgCl2, and 0.1 mM dithiothreitol in a final volume of 100 μl for 30 min at 37°C. The reaction was stopped by adding equal volumes of Tris-buffered phenol, pH 7.5, and chloroform-isoamyl alcohol (24:1) and vortexing. The tRNA in the aqueous phase was precipitated by adding 20 μg of yeast RNA as a carrier and 2 volumes of ethanol. The precipitate was washed with 70% ethanol, dried, and digested to nucleotides by P1 nuclease (21). The distribution of modified uridine derivatives was determined by two-dimensional thin-layer chromatography (TLC) as described previously (38). The radioactive compounds were detected with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Analysis of modified nucleotides in tRNA by HPLC and determination of growth rates.
High-pressure liquid chromatography (HPLC) analysis was performed as described previously (20, 21). Growth rates at 37°C in rich-MOPS and MOPS-glucose were determined as described previously (4).
Determination of frameshifting levels.
The level of frameshifting was determined by using a system which contains the lacZ gene placed downstream of a short frameshifting window in such a way that the β-galactosidase activity is a direct measurement of the frequency with which the ribosomes shift reading frame (58).
Determination of suppression by tyrT (supF) tRNA.
The efficiency of supF amber (UAG) suppressor tRNA, which is a mutated tRNATyr, was measured by introducing an F′ plasmid with a lacI-lacZ fusion with or without nonsense mutations in the lacI part into a tyrT (supF) mutant E. coli (36). Strains were grown in LB medium, and the β-galactosidase activity was determined as described previously (35).
Determination of sensitivity to amino acid analogs.
Strains were grown at 37°C overnight in LB medium. A sample of each culture (0.1 ml) was mixed with 2 ml of 0.5% agar in 0.9% NaCl, and the mixture was poured onto plates containing medium E plus 0.2% glucose. Paper disks (6 mm in diameter) were placed on the surfaces of the plates, and 50 μg of various amino acid analogs was applied to each disk. The plates were incubated at 37°C for 24 h before being scored. Analogs to which strains responded equally were dl-aspartic-β-hydroxamate, 1,2,4-triazole, azaserine, l-glutamic acid-γ-hydrazide, l-methionine-dl-sulfoximine, 1,2,4-dl-triazole-3-alanine, 3-amino-1,2,4-triazole, β-chloro-l-alanine, 4-aza-dl-leucine, 5,5,5-trifluoro-dl-leucine, S-2-aminoethyl-l-cysteine, l-methionine, dl-methionine-hydroxamate, α-methyl-dl-methionine, l-norleucine, m-fluoro-dl-phenylalanine, p-fluoro-dl-phenylalanine, β-(2-thienyl)-dl-alanine, β-3-thienyl-dl-alanine, l-2-acetidine-carboxylic acid, thioproline, dl-serine-hydroxamate, dl-β-hydroxynorvaline, dl-7-azatryptophane, dl-5-fluoro-tryptophane, 5-methyl-dl-tryptophane, 3-amino-l-tyrosine, m-fluoro-dl-tyrosine, 3-nitro-l-tyrosine, azatyrosine, and fluoracetate.
Measurement of carbon source oxidation.
Cells were grown at 37°C in either LB or MOPS-glucose medium to a density of about 5 × 108 to 1 × 109 cells/ml. Cells were harvested on ice, pelleted, and diluted to about 5 × 107 cells/ml in 0.9% NaCl. Samples (150 μl) were transferred to each well of an ES Microplate purchased from Biolog Inc. (Hayward, Calif.). The Biolog Microplate tests the ability of bacteria to oxidize 95 different compounds. Oxidation is measured as transfer of electrons from NADH to a tetrazolium dye, which results in formation of a purple color. After inoculation, plates were incubated at 37°C for 15 to 18 h, and optical density at 620 nm (OD620) was measured by a Titertrek Multiscan MCC/340 reader.
Virulence phenotype assays.
S. flexneri strains were grown at 37°C. The contact hemolytic assay (52) was used to measure the production of invasins. Expression of the mxiC gene was measured by using a vir-83::MudI1734 (mxiC-lacZ) operon fusion (34).
Immunoblotting.
Bacterial samples were prepared as described previously (16). Bacterial proteins were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis. Separated proteins were blotted onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, Calif.). Antisera specific to VirF (15) and IpaBCD (43) have been described previously. The ECF Western blotting kit (Amersham Life Science Ltd., Little Chalfont, Buckinghamshire, England) was used for detection of primary antibodies. The PVDF membrane was scanned with a Storm 860 optical scanner and quantified with ImageQuant software (Molecular Dynamics).
Statistical analysis.
A t test of the means with two tails was used to evaluate whether different parameters were statistically different for the wild type and the respective mutant (P < 0.05; see Tables 3 to 5 and Fig. 2, 3, 4A, and 5), except when measuring carbon source oxidation (see Table 6), where parameters to determine differences in values were set arbitrarily.
TABLE 3.
Growth rate determination for the truB, trmA, and trmH mutants, lacking the Ψ55, m5U54, and Gm18 tRNA modifications
| Strain | Genotype | Growth rate (% difference from wt)a in indicated medium at:
|
|||||
|---|---|---|---|---|---|---|---|
| 30°C
|
37°C
|
42°C, rich-MOPS | 40°Cb, MOPS-glucose | ||||
| Rich-MOPS | MOPS-glucose | Rich-MOPS | MOPS-glucose | ||||
| MW100 | wt | 0 (1.04 ± 0.01) | 0 (0.62 ± 0.05) | 0 (1.44 ± 0.06) | 0 (0.81 ± 0.03) | 0 (1.35 ± 0.04) | 0 (0.70 ± 0.02) |
| GOB113 | truB | −6 | 0 | −7 | 0 | −17 | 0 |
| GRB1882 | trmA | 0 | 0 | 0 | 0 | 0 | 0 |
| GRB1756 | trmH | −29 | 0 | −33 | 0 | −27 | −11 |
| GRB1737 | trmH truB | −27 | −15 | −9 | −33 | −30 | −46 |
| GRB1814 | trmH trmA | −27 | −16 | −35 | 0 | −33 | −9 |
| GRB1887 | truB trmA | 0 | 0 | −18 | 0 | −7 | 0 |
| GRB1777 | trmH truB trmA | −29 | −19 | −17 | −33 | −37 | −44 |
The results presented are averages from three independent experiments. Specific growth constant values (in parentheses; averages ± standard deviations) are presented only for wild-type (wt) bacteria.
In MOPS-glucose medium, the growth rate of the wild-type strain was reduced more at 40°C than it was in rich-MOPS at 42°C (cf. rich-MOPS at 37 and 42°C with MOPS-glucose at 37 and 40°C); this is why 40°C was chosen instead of 42°C for MOPS-glucose medium.
TABLE 5.
Sensitivity of the truB, trmA, and trmH mutants, lacking the Ψ55, m5U54, and Gm18 modifications, to 3,4-dehydro-dl-prolinea
| Strain | Genotype | Inhibition zone diam (mm) |
|---|---|---|
| MW100 | wt | 30.5 ± 0.7 |
| GOB113 | truB | 33.5 ± 0.7 |
| GRB1882 | trmA | 32.0 ± 1.4 |
| GRB1756 | trmH | 31.5 ± 4.9 |
| GRB1737 | trmH truB | 39.0 ± 1.4 |
| GRB1887 | truB trmA | 34.0 ± 4.2 |
| GRB1814 | trmH trmA | 32.5 ± 3.5 |
| GRB1777 | trmH truB trmA | 37.0 ± 0.0 |
Fifty micrograms of 3,4-dehydro-dl-proline was placed on a 6-mm-diameter paper disk in the center of each agar plate. Diameters of zones without growth of bacteria were scored after 24 h of growth at 37°C. Average values of two experiments ± standard deviations are presented. Cases of significant difference between the wild type (wt) and mutant are in boldface.
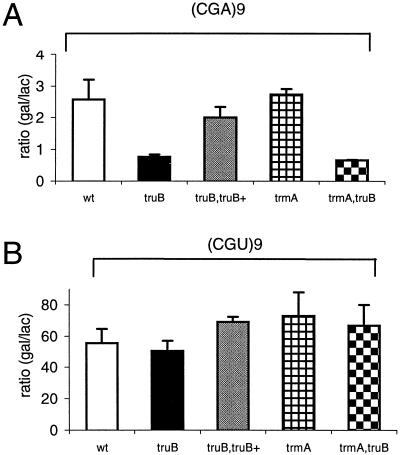
FIG. 2.
Effect of the truB and trmA mutations on translation of the nine consecutive CGA (A) and CGU (B) codons. The results presented are averages from three independent experiments and are expressed as ratios of the β-galactosidase (gal) to β-lactamase (lac) enzyme activities. Variations are standard deviations. wt, wild type.
TABLE 6.
Carbon source utilization in the truB, trmA, and trmH mutants, lacking the Ψ55, m5U54, and Gm18 modificationsa
| Strain | Genotype | OD620 for oxidation in indicated medium of:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
dl-Malic acid
|
d-Malic acid
|
l-Malic acid
|
Monomethyl succinate
|
||||||
| LB | MOPS-Glu | LB | MOPS-Glu | LB | MOPS-Glu | LB | MOPS-Glu | ||
| MW100 | wt | 0.02 | 0.01 | 0.00 | ∗ | 0.02 | 0.02 | 0.01 | ∗ |
| GOB113 | truB | 0.16 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| GRB1882 | trmA | 0.12 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| GRB1756 | trmH | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| GRB1737 | trmH truB | 0.15 | 0.06 | 0.11 | ∗ | 0.09 | ∗ | 0.14 | ∗ |
| GRB1887 | truB trmA | 0.27 | 0.16 | 0.31 | ∗ | 0.15 | ∗ | 0.23 | ∗ |
| GRB1814 | trmH trmA | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 0.10 | ∗ |
| GRB1777 | trmH truB trmA | 0.17 | ∗ | 0.15 | ∗ | 0.11 | 0.12 | 0.17 | ∗ |
The carbon sources shown are those that are oxidized inefficiently by the wild-type (wt) strain (OD620 < 0.03) and that are oxidized more efficiently (OD620 > 0.05) by the mutant in respective experiment. The results are averages of two separate experiments. The actual OD620 values are shown when a difference between the wild-type strain and the respective mutant was observed. ∗, no efficient oxidation in wild type and mutants. Glu, glucose.
RESULTS
Construction of strains containing the trmH::Kmr, trmA5, truB2422::mini-Tn10Cm mutations and their combinations.
To determine the influence of Gm18, m5U54, and Ψ55 on the physiology of E. coli, we transferred one or more of the trmH::Kmr, trmA5, and truB2422::mini-Tn10Cm mutations into different genetic backgrounds by P1 transduction. Since the trmA gene is essential (44), we used the trmA5 allele, which abolishes the synthesis of m5U54 (3). Therefore, to address the function of the methyl group of m5U, the trmA5 allele is suitable. We showed by TLC and HPLC analyses that the levels of Gm18, m5U54, and Ψ55 were not detectable in mutants containing the respective mutations (data not shown).
The growth rate is decreased in an E. coli mutant lacking both Gm18 and Ψ55.
Strains MW100 (wild type), GOB113 (truB2422::mini-Tn10Cm), GRB1882 (trmA5), GRB1756 (trmH::Kmr), GRB1737 (truB2422::mini-Tn10Cm trmH::Kmr), GRB1887 (truB2422::mini-Tn10Cm trmA5), GRB1814 (trmH::Kmr trmA5), and GRB1777 (truB2422::mini-Tn10Cm trmH::Kmr trmA5) were grown under steady-state conditions in rich-MOPS or MOPS-glucose medium at 30, 37, or 42°C (rich-MOPS) or at 40°C (MOPS-glucose). The trmA single mutation did not reduce the growth rate at any of the conditions tested, consistent with earlier results (4), whereas the truB and trmH mutations reduced the growth rate in rich-MOPS (Table 3). In glucose minimal medium, only the trmH mutation reduced the growth rate and then only at 40°C. The trmH truB and the trmH trmA double mutants showed a reduced growth rate in the rich medium that was similar to that of the trmH single mutant but not to that of the trmH truB double mutant at 37°C (Table 3). In MOPS-glucose medium the truB mutation augmented at all temperatures the reduction of growth rate observed for the single trmH mutant (Table 3; 30, 37, and 40°C). In this medium the trmA mutation also augmented the trmH effect, but only at 30°C. The growth rate of the trmH truB trmA triple mutant was in general similar to that of the trmH truB double mutant in both rich and minimal media. We conclude that the parallel lack of Gm18 and Ψ55 in the tRNA reduced the growth rate, consistent with the suggestion that the presence of these modifications stabilizes the tRNA structure. However, lack of m5U54 had no, or only a minor, influence on the growth rates of strains lacking the other two modifications.
Ψ55, but not m5U54, improves the reading of the CGA codon.
To measure the efficiency of translation of arginine CGA and CGU codons, we introduced plasmids containing nine CGA or CGU codons in a row (9) into the wild-type strain, the trmA and truB single mutants, and the trmA truB double mutant. Since Gm18 is not present in tRNAs reading these codons, the plasmids were not introduced into the trmH mutant. The nine CGA or CGU codons are translationally coupled to the lacZ gene such that the ribosomes translate the CGA- or CGU-containing cistrons, terminate, and reinitiate at lacZ. Therefore, β-galactosidase activity is a measure of the rate with which the ribosome translates these nine codons in a row. The more efficiently the codons upstream of the initiation site of the lacZ mRNA are translated, the more ribosomes will initiate lacZ mRNA and thus the more β-galactosidase that is synthesized. The truB mutation reduced the translation of the (CGA)9 codons 3.4-fold, whereas the trmA mutation had no effect on the translation of these codons (Fig. 2A). The effect mediated by the truB mutation was not influenced by the trmA mutation. However, there was no influence on the translation of the (CGU)9 codons by any of the mutations (Fig. 2B). Strain GRB1655 [DUP(truB+)(truB)] contains both the truB+ allele and the truB mutant allele in a chromosomal duplication (6). Accordingly, this strain contains Ψ55 in its tRNA, as shown by TLC analysis (data not shown). The level of β-galactosidase in this strain was the same as that observed in wild-type cells (Fig. 2A, truB+ truB). We conclude that presence of Ψ55 in tRNA (or the TruB protein) improves the translation of CGA codons but not that of CGU codons, whereas m5U54 does not influence the reading of these codons.
Ψ55 does not affect expression of the thr operon.
The expression of the threonine (thrABC) operon of E. coli is regulated by an attenuator located upstream of the first gene, thrA (51). The rate with which the ribosomes traverse through four Ile and eight Thr control codons in the leader mRNA regulates the expression of the thrABC operon (32). The truB mutation was introduced into a strain containing a thrA-lacZ transcription fusion in the thrA gene. Thus, the activity of β-galactosidase reflects the level of transcription of the thr operon (51). The fact that the levels of expression of β-galactosidase in strains GRB1212 (wild type) and GRB1536 (truB) grown in either rich-MOPS or MOPS-glucose minimal medium were the same suggests that Ψ55 does not influence translation of the Ile and/or Thr codons (data not shown).
A-site selection of Gm18-deficient 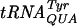 is decreased.
is decreased.
The rate of selection of aminoacyl-tRNA in the A site of the ribosome during translation influences frameshifting by the P-site tRNA (11). To study the importance of Ψ55, m5U54, and Gm18 on A-site selection, we used several assay systems containing a short frameshifting window: the nonprogrammed +1 frameshifting in the argI gene at a UUU-U/CAU site (18), the tRNAPro-induced +1 frameshifting system at CCC-NNN sites (24), and the prfB gene-based +1 frameshifting at CUU-NNN sites (11). In these assay systems, the first three letters denote a codon in the P site and next three letters denote a codon in the A site. The lacZ gene is placed downstream of the frameshifting window such that the β-galactosidase activity is a direct measure of the frequency with which the ribosome shifts frame within this window.
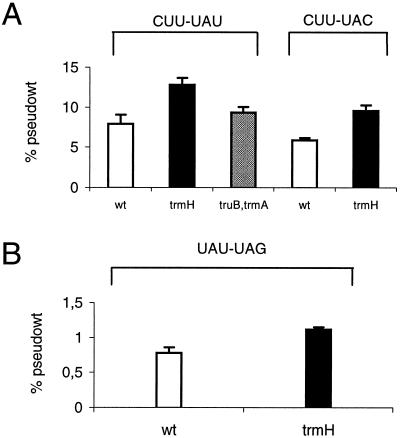
Plasmids pCPF4 (UUU-UAU), pCFP7 (UUU-AAU), pCFP8 (UUU-CAU), pTHF32 (CCC-AAA), and pTHF33 (CCC-AAG) were introduced into strains GBEC384 (wild type) and GRB1490 (truB). There was no difference in the level of frameshifting between the wild type and the truB mutant at any of these sites tested (data not shown). The 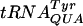 , which reads codon UAU/C, contains the Ψ55, m5U54, and Gm18 modifications. We introduced plasmids pJC27tet CUU-UAU and CUU-UAC into strains lacking one, two, or all three of the modifications. Frameshifting was increased by 60% at the CUU-UAU and -UAC sites in the single trmH mutant. In the truB trmA double mutant frameshifting was increased by 20% at the CUU-UAU site (Fig. 3A), whereas no difference at the CUU-UAC site was observed (data not shown). No difference in the level of frameshifting between the wild-type strain and the other mutants tested (trmA, truB, trmA trmH, truB trmH, and trmA truB trmH mutants) was observed at either of these two sites (data not shown).
, which reads codon UAU/C, contains the Ψ55, m5U54, and Gm18 modifications. We introduced plasmids pJC27tet CUU-UAU and CUU-UAC into strains lacking one, two, or all three of the modifications. Frameshifting was increased by 60% at the CUU-UAU and -UAC sites in the single trmH mutant. In the truB trmA double mutant frameshifting was increased by 20% at the CUU-UAU site (Fig. 3A), whereas no difference at the CUU-UAC site was observed (data not shown). No difference in the level of frameshifting between the wild-type strain and the other mutants tested (trmA, truB, trmA trmH, truB trmH, and trmA truB trmH mutants) was observed at either of these two sites (data not shown).
FIG. 3.
(A) 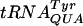 selection in the wild-type (wt) and trmH and truB trmA mutants, lacking Gm18 (trmH) or Ψ55 and m5U54 (truB trmA) tRNA modifications. The results are expressed as the activities of the β-galactosidase produced from the test plasmids pJC27tet CUU-UAU and -UAC compared to that from pseudo-wild-type plasmid pJC27tet. (B) Influence on P-site slippage by Gm18-deficient
selection in the wild-type (wt) and trmH and truB trmA mutants, lacking Gm18 (trmH) or Ψ55 and m5U54 (truB trmA) tRNA modifications. The results are expressed as the activities of the β-galactosidase produced from the test plasmids pJC27tet CUU-UAU and -UAC compared to that from pseudo-wild-type plasmid pJC27tet. (B) Influence on P-site slippage by Gm18-deficient 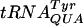 . The results are expressed as the activities of the β-galactosidase produced from the test plasmid pJC27tet CUU-UAU compared to that from pseudo-wild-type plasmid pJC27tet. The results (A and B) are averages from three independent experiments, and variations are standard deviations.
. The results are expressed as the activities of the β-galactosidase produced from the test plasmid pJC27tet CUU-UAU compared to that from pseudo-wild-type plasmid pJC27tet. The results (A and B) are averages from three independent experiments, and variations are standard deviations.
P-site slippage of Gm18-deficient 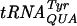 is increased.
is increased.
Recently, we have shown that undermodification of tRNA leads to an increased slippage in the P site of the ribosome, resulting in an elevated level of frameshifting (58). To measure the P-site effect, a stop codon was placed just downstream of the P-site codon (8). Since the release factor acts at the A site (55) and since the lacZ gene is placed in the +1 frame downstream of this site, β-galactosidase activity is a measure of a P-site event. We introduced plasmids pJC27tet UAU-UAG and UAC-UAG into strains lacking either Gm18, m5U54, or Ψ55, any two of them, or all three. A lack of Gm18 increased the level of frameshifting by 40% at the UAU-UAG site (Fig. 3B), whereas no difference in frameshifting level compared to the wild-type strain was observed for any of the other mutant strains (trmA, truB, truB trmA, trmA trmH, truB trmH, trmA truB trmH mutants; data not shown).
The Gm18-deficient tyrT (supF) tRNA reads inefficiently stop codon UAG.
The amber (UAG) supF suppressor 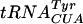 , which is a mutated
, which is a mutated 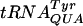 , contains Ψ55, m5U54, and Gm18 modifications. We measured the suppressor activity of this tRNA in various mutants lacking one to three of these modifications. For this purpose, we introduced two different F′ plasmids harboring an in-frame lacI′-′lacZ fusion with or without amber nonsense codons in the lacI part (36) into strains lacking one to three of these modifications. The suppression efficiency was measured at three different temperatures, since we suspected that the tRNA modifications tested could have different effects at different temperatures. Lack of Gm18 resulted in a reduced efficiency of the supF
, contains Ψ55, m5U54, and Gm18 modifications. We measured the suppressor activity of this tRNA in various mutants lacking one to three of these modifications. For this purpose, we introduced two different F′ plasmids harboring an in-frame lacI′-′lacZ fusion with or without amber nonsense codons in the lacI part (36) into strains lacking one to three of these modifications. The suppression efficiency was measured at three different temperatures, since we suspected that the tRNA modifications tested could have different effects at different temperatures. Lack of Gm18 resulted in a reduced efficiency of the supF 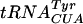 in reading the lacI am117 codon at 37°C and the lacI am121 codon at 30 and 37°C but not at 42°C (Table 4). Also, decreased suppression was observed in the mutant lacking both Gm18 and m5U54. These results suggest that the major contributor to the reduced efficiency was the lack of Gm18. Increased suppression was observed when Gm18 and Ψ55 (lacI am121 at 42°C) or Ψ55 and m5U54 (lacI am121 at 37°C) were lacking. Thus, of these three modifications, only the Gm18 modification alone influenced the efficiency of the supF
in reading the lacI am117 codon at 37°C and the lacI am121 codon at 30 and 37°C but not at 42°C (Table 4). Also, decreased suppression was observed in the mutant lacking both Gm18 and m5U54. These results suggest that the major contributor to the reduced efficiency was the lack of Gm18. Increased suppression was observed when Gm18 and Ψ55 (lacI am121 at 42°C) or Ψ55 and m5U54 (lacI am121 at 37°C) were lacking. Thus, of these three modifications, only the Gm18 modification alone influenced the efficiency of the supF 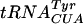 , whereas a lack of two modifications both increased and decreased efficiency. Surprisingly, lack of all three modifications did not influence the efficiency of suppression at either lacI am117 or lacI am121 codons at any temperature. Although the reason for the lack of effect in the triple mutant is not fully understood, the fact that we observed both an increase and a decrease in the efficiency of suppression caused by modification deficiency suggests that at certain conditions the effect of one modification can counteract the effect of another modification.
, whereas a lack of two modifications both increased and decreased efficiency. Surprisingly, lack of all three modifications did not influence the efficiency of suppression at either lacI am117 or lacI am121 codons at any temperature. Although the reason for the lack of effect in the triple mutant is not fully understood, the fact that we observed both an increase and a decrease in the efficiency of suppression caused by modification deficiency suggests that at certain conditions the effect of one modification can counteract the effect of another modification.
TABLE 4.
Suppression ability of tyrT (supF) tRNA in the truB, trmA, and trmH mutants, lacking the Ψ55, m5U54, and Gm18 tRNA modifications
| Strain | Genotype | Relative activity (%)a with mutation in indicated lacI codon at:
|
|||||
|---|---|---|---|---|---|---|---|
| 30°C
|
37°C
|
42°C
|
|||||
| am117 | am121 | am117 | am121 | am117 | am121 | ||
| UB585 | wt | 14.0 ± 0.6 | 45.0 ± 3.5 | 11.7 ± 1.5 | 62.4 ± 2.5 | 2.7 ± 0.5 | 19.4 ± 1.1 |
| GRB1734 | truB | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| GRB1743 | trmA | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| GRB1893 | trmH | ∗ | 35.1 ± 0.6 | 8.2 ± 1.4 | 50.1 ± 2.7 | ∗ | ∗ |
| GRB1754 | trmH truB | ∗ | ∗ | ∗ | ∗ | ∗ | 34.0 ± 0.7 |
| GRB1894 | truB trmA | ∗ | ∗ | ∗ | 84.3 ± 7.5 | ∗ | ∗ |
| GRB1819 | trmH trmA | 11.8 ± 0.6 | ∗ | 8.2 ± 0.2 | 51.2 ± 5.3 | ∗ | ∗ |
| GRB1783 | trmH truB trmA | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
The results are averages from three independent experiments ± standard deviations. The results are expressed as the activity of β-galactosidase expressed from the F′lacZ amber mutants relative to that of β-galactosidase expressed from the F′lacZ+ plasmid. ∗, no difference in the efficiency of suppression between the wild type (wt) strain and the respective mutants.
Lack of both Ψ55 and Gm18 causes an increased sensitivity to an amino acid analog, whereas lack of Ψ55 is the major cause for increased oxidation of some carbon sources.
It is known that undermodified tRNA influences the regulation of the synthesis of several amino acids (5, 22, 56, 61). We tested 31 different amino acid analogs for the growth response of the trmH trmA5 truB triple mutant (strain GRB1777). One of these analogs, 3,4-dehydro-dl-proline, to which the triple mutant was sensitive, was then tested on strains having one or two mutations. The inhibition zones for the triple mutant and the trmH truB double mutant were increased by 25% compared to that of the wild-type strain (Table 5), suggesting that the lack of both Gm18 and Φ55 in the tRNA influences the metabolism of the 3,4-dehydro-dl-proline in the cell.
Levels of oxidation of various carbon compounds by the wild-type and mutants lacking Ψ55, m5U54, and/or Gm18 were compared. When cells were grown in the rich LB medium, all mutants, except the trmH and the trmA trmA mutants, oxidized dl-malic acid more efficiently than the wild-type cells (Table 6). The triple mutant and the truB trmH and truB trmA double mutants oxidized the three variants of malic acids and monomethylsuccinate more efficiently than the wild type. In general mutants pregrown in MOPS-glucose medium oxidized these compounds similarly to the wild-type cell. Apparently, the lack of Ψ55 is the major cause of the observed increased oxidation ability when cells were pregrown in LB medium.
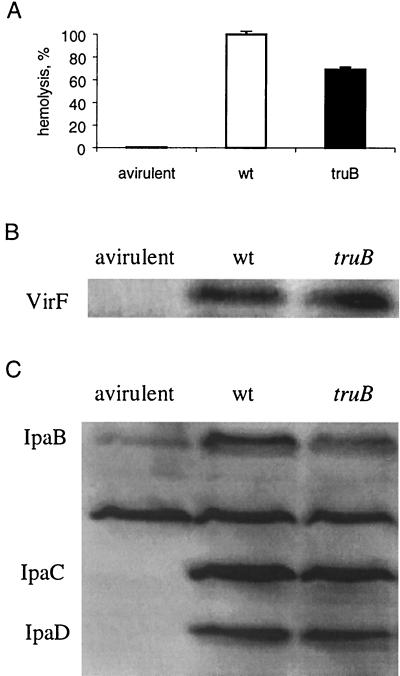
A truB mutation reduces the expression of some virulence-associated genes of S. flexneri.
The virulence of Shigella depends on the activity of the VirF protein, which belongs to the AraC family of transcription factors. The VirF protein activates directly the synthesis of two other transcription factors, VirG and VirB. In turn, VirB activates the ipa operon, which encodes invasines IpaB, -C, and -D, and the mxi operon. The hemolytic activity of the truB mutant was reduced about 30% compared with that of S. flexneri wild-type strain 2457T, although the level of VirF was the same (Fig. 4A and B). Of the three proteins, IpaB, -C, and -D, encoded in the ipa operon, only the level of IpaB was reduced (Fig. 4C). Since the vector influences hemolytic activity in the wild-type cells, we were unable to do a proper complementation experiment. To monitor a possible translational effect upstream of the regulation of the mxi operon, we used an mxiC-lacZ transcriptional fusion. Indeed, the truB mutation reduced the expression of the mxi operon by 25% in MOPS-glucose medium (Fig. 5), but not when cells were grown in rich-MOPS (data not shown). The decreased expression of the mxi operon in the truB mutant was restored by a plasmid containing the coding sequence for wild-type TruB but not by a plasmid containing the coding sequence for the TruBD48C mutant protein, which is able to bind to the tRNA but not to modify it (47). These results indicate that it was the lack of Ψ55 in tRNA rather than the absence of the TruB protein which was responsible for the reduced transcription of the mxi operon. In summary, our results suggest that Ψ55 in tRNA improves the translation of the IpaB protein and a protein(s) required for efficient expression of the mxi operon.
FIG. 4.
Effect of the truB mutation on the expression of virulence-associated genes of S. flexneri. (A) Hemolytic activity of strains 2457T (wild type [wt]) and GBOB8 (truB) grown in MOPS-glucose-NA medium at 37°C. The contact hemolytic activity of the 2457T strain was arbitrarily defined as 100%. Each bar shows the mean and standard deviation of four measurements. Avirulent strain YSH6200 was used as a negative control. (B) Western blot of the VirF protein. An identical amount of total protein from each strain grown in MOPS-glucose-NA medium at 37°C was electrophoresed in sodium dodecyl sulfate-12% polyacrylamide gel and transferred to a PVDF membrane before being immunostained with antibodies specific to VirF. The quantification of the VirF-specific bands was performed by fluorescence scanning analysis (Storm), and values for different strains were compared. Lanes: left, YSH6200 (avirulent); middle, 2457T (wild type); right, GBOB8 (truB). (C) Western blot of the Ipa proteins. The procedure was the same as that for panel B except that antibodies specific to Ipa proteins were used. Lanes: left, YSH6200 (avirulent); middle, 2457T (wild type); right, GBOB8 (truB). The value for the avirulent strain, YSH6200, was subtracted when calculating the amounts of IpaB in the wild-type and truB strains.
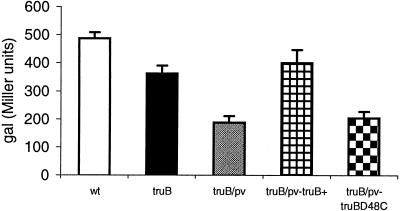
FIG. 5.
Expression from the mxiC-lacZ transcriptional fusion in S. flexneri strains BS184 (wild type [wt]), GBOB4 (truB), and GBOB4 (truB) with plasmid Trc99A (pv) derivatives expressing the TruB+ or TruBD48C protein. The results presented are averages from three independent experiments, and variations are standard deviations. Results are expressed as β-galactosidase (gal) activity.
The orp gene in P. aeruginosa is homologous to the truB gene of E. coli.
An orp::Tn5Tc mutant version of opportunistic human pathogen P. aeruginosa is impaired in growth on BHI plates at 43°C and has reduced amounts of virulence factor phospholipase C compared to wild-type strain PAO1 (50). The predicted orp gene product possesses sequence similarity to the truB gene product of E. coli. Therefore, we tested whether the enzyme extracts from P. aeruginosa strains PAO1 (orp+) and Tn5T1 (orp::Tn5Tc) have a Ψ55-modifying activity. The extract from the orp+ strain was able to catalyze the synthesis of Ψ55 in vitro, whereas the extract from the orp::Tn5Tc mutant was not (Fig. 6). These results show that the orp gene is most likely the homologue of the truB gene of E. coli, and therefore the orp::Tn5Tc mutant should lack Ψ55 in its tRNA. Therefore, we suggest that the orp gene in P. aeruginosa be renamed truB.
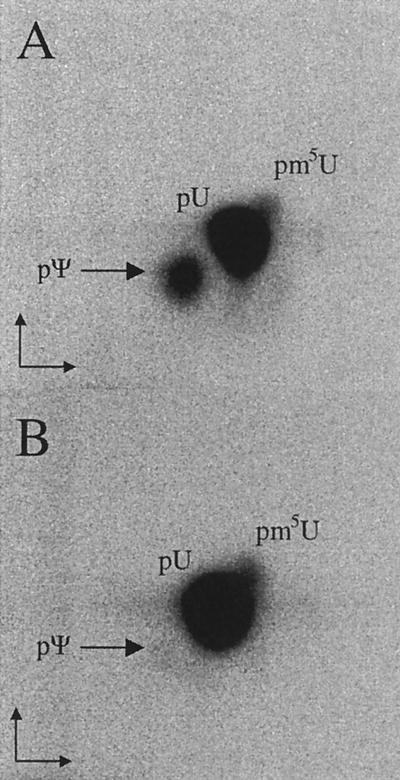
FIG. 6.
Formation of Ψ55 in P. aeruginosa wild-type (wt) strain PAO1 and an orp mutant. In vitro-synthesized tRNAVal, labeled by incorporation of [α-32P]UTP, was incubated with the S16 enzyme extract prepared from strain PAO1 (A) and the orp mutant (B). After incubation, labeled tRNA was completely digested by nuclease P1 and two-dimensional TLC was performed. Arrows, positions of pΨ55.
DISCUSSION
In this work we show that a parallel lack of Gm18, m5U54, and Ψ55 in tRNA of E. coli affects growth rate, translation of certain codons, sensitivity to amino acid analogs, and oxidation of some carbon compounds. Since nucleosides Gm18, m5U54, and Ψ55 are located close to each other in the three-dimensional structure of tRNA and thereby stabilize it (12, 30, 46, 59), lack of these modifications could destabilize the tRNA. If so, one might see an effect on the physiology of E. coli. Indeed, a strain lacking all three of these modifications showed reduced growth rate in both rich-MOPS and MOPS-glucose minimal medium at all temperatures (Table 3). The major contributor to the growth rate reduction in rich-MOPS was the lack of Gm18. This was surprising since it was previously demonstrated that lack of Gm18, as in the trmH mutant, has no effect on the growth rate in the rich LB medium (45). We also did not find any growth rate difference between our wild-type strain MW100 and its trmH derivative when growth in LB medium was monitored (data not shown). Thus, the observed growth rate reduction caused by lack of Gm18 is medium dependent and not the result of differences in strain backgrounds among the different experiments. No growth rate difference between wild-type strain MW100 and its trmH derivative was observed for MOPS-glucose minimal medium, except at 40°C, confirming that the observed growth rate difference is medium dependent. Previously, it was reported that truB+ and truB mutants grow with equal rates in LB and M-9 glucose minimal media (23), whereas we observed a minor growth rate reduction in rich-MOPS caused by the truB mutation (Table 3). Possibly, differences between the media used or differences in strain backgrounds might explain the different results. In some cases, when the lack of one of the Gm18, m5U54, and Ψ55 modifications did not have any effect on the growth rate, a lack of two of the modifications resulted in a reduced growth rate, suggesting a cooperative effect by these modifications. These results suggest that small conformational changes in the tRNA may lead to changed interaction with rRNA (62). Absence of Gm18, m5U54, and Ψ55 had no or only small effects on A-site-mediated frameshifting at codons UAU and UAC (Tyr), AUA (Asn), CAU (His), and Lys (AAA and AAG); peptidyl-tRNA slippage at codons UAU and UAC; and reading of the UAG stop codon by the tyrT tRNA. The observed effects can be explained by the effect of tRNA modification(s) on the stability of tRNA. We find such an explanation to our results unlikely, since single mutations in Saccharomyces cerevisiae resulting in Gm18, m5U54, or Ψ55 deficiency have no major effect on the stability of 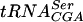 (25). An alternative explanation may be that the lack of modification(s) cause a small conformational change of the tRNA, which in turn could affect its interaction with rRNA and could result in decreased efficiency of translation (62). This can also explain both the increased and decreased reading of the UAG stop codon by the tyrT tRNA lacking Gm18, m5U54, and/or Ψ55 (Table 4). Since the observed effects were small, we placed several copies of the same codon in a row to enhance the effect on translation by lack of these modifications. Indeed, the absence of Ψ55, but not of m5U54, caused decreased efficiency in the translation of nine consecutive arginine CGA codons but not of nine consecutive arginine CGU codons (Fig. 2). Both CGA and CGU are read by the same
(25). An alternative explanation may be that the lack of modification(s) cause a small conformational change of the tRNA, which in turn could affect its interaction with rRNA and could result in decreased efficiency of translation (62). This can also explain both the increased and decreased reading of the UAG stop codon by the tyrT tRNA lacking Gm18, m5U54, and/or Ψ55 (Table 4). Since the observed effects were small, we placed several copies of the same codon in a row to enhance the effect on translation by lack of these modifications. Indeed, the absence of Ψ55, but not of m5U54, caused decreased efficiency in the translation of nine consecutive arginine CGA codons but not of nine consecutive arginine CGU codons (Fig. 2). Both CGA and CGU are read by the same 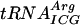 , but CGA is decoded inefficiently due to the poor I34:A(III) [I34, I in position 34 (wobble position, first position of the anticodon) of the tRNA; A(III), third nucleoside of the codon] base pairing, whereas CGU uses the more efficient I34:U(III) base pairing (9). Apparently, the presence of the Ψ55 in
, but CGA is decoded inefficiently due to the poor I34:A(III) [I34, I in position 34 (wobble position, first position of the anticodon) of the tRNA; A(III), third nucleoside of the codon] base pairing, whereas CGU uses the more efficient I34:U(III) base pairing (9). Apparently, the presence of the Ψ55 in 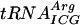 is more important for the poorly decoded CGA than for the more efficiently decoded CGU. It is unlikely that the observed decrease of the β-galactosidase activity is due to frameshifting instead of a decreased efficiency of translation of the nine-codon cistron. Previously, it was demonstrated that the CGA codon is not prone to frameshift or to premature termination by RF2 (9, 10). In addition, we have not observed any effect of Ψ55 on both +1 (see above) and −1 (57) frameshifting. Moreover, frameshifting in either +1 or −1 directions would create two mismatches in the new frame, including a purine-purine clash in the first position of the codon (CGA to GAC [+1] and CGA to ACG [−1]).
is more important for the poorly decoded CGA than for the more efficiently decoded CGU. It is unlikely that the observed decrease of the β-galactosidase activity is due to frameshifting instead of a decreased efficiency of translation of the nine-codon cistron. Previously, it was demonstrated that the CGA codon is not prone to frameshift or to premature termination by RF2 (9, 10). In addition, we have not observed any effect of Ψ55 on both +1 (see above) and −1 (57) frameshifting. Moreover, frameshifting in either +1 or −1 directions would create two mismatches in the new frame, including a purine-purine clash in the first position of the codon (CGA to GAC [+1] and CGA to ACG [−1]).
The requirement for Ψ55 for the efficient translation of the poorly decoded CGA codon and for plcH mRNA, encoding phospholipase C in P. aeruginosa (50), suggests that Ψ55 may also improve translation of the virF mRNA in S. flexneri. Surprisingly, whereas the expression of the ipaB and mxiC genes was reduced in the truB mutant, (Fig. 4C and 5), the amount of the VirF protein was the same as that in the wild type (Fig. 4B), which is in contrast to the effect caused by lack of Q34 or ms2i6A37 (14, 16). Thus, whereas Q34 and ms2i6A37 exert their effects on virulence by reducing the translation of the virF mRNA, the lack of Ψ55 did not affect the synthesis of the main regulator VirF but affected the expression of VirF-regulated virulence genes virB and ipaB and/or a protein(s) required for efficient transcription of the mxi operon. Lack of Q34 in the tgt mutant of S. flexneri caused a delayed response in an animal infection model (Serény test) (42). Since the effect of Ψ55 was similar to that of Q34 in a contact hemolytic assay, the truB mutant may also influence infection of the host by S. flexneri.
It has been shown that various modified nucleosides have different impacts on the activities of various tRNA species; e.g., the lack of m1G37 in 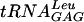 did not influence A-site selection but the lack of the same modification in
did not influence A-site selection but the lack of the same modification in 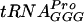 severely reduced the same reaction (31). Similar observations were also noted for Ψ in the anticodon stem (31). Here we show that Ψ55 improved the activity of
severely reduced the same reaction (31). Similar observations were also noted for Ψ in the anticodon stem (31). Here we show that Ψ55 improved the activity of 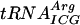 (Fig. 2) but not that of
(Fig. 2) but not that of 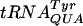 (Fig. 3, Table 4). Clearly, to clarify the function of some of the modified nucleosides, one will have to monitor the activity of specific tRNA species. Nonetheless, the fact that a reduced growth rate was observed for the triple mutant as well as for some of the double and single mutants demonstrates an important function of these modified nucleosides in the ability of the bacterium to grow and compete efficiently in the environment.
(Fig. 3, Table 4). Clearly, to clarify the function of some of the modified nucleosides, one will have to monitor the activity of specific tRNA species. Nonetheless, the fact that a reduced growth rate was observed for the triple mutant as well as for some of the double and single mutants demonstrates an important function of these modified nucleosides in the ability of the bacterium to grow and compete efficiently in the environment.
Acknowledgments
We thank G. Bylund, N. Gutgsell, L. Isaksson, M. Vasil, and M. Wikström for providing strains and plasmids, R. Ghebrezgiabher, M. Rosengren, and D. Ågren for assistance in some of the experiments, and A. Byström, M. Johansson, B. E. Uhlin, and M. Wikström for critical reading of the manuscript.
This work was supported by grants from the Swedish Cancer Society (Project 680) and the Swedish Research Council (Project B-BU 2930).
REFERENCES
- 1.Auffinger, P., and E. Westhof. 1997. RNA hydration: three nanoseconds of multiple molecular dynamics simulations of the solvated tRNAAsp anticodon hairpin. J. Mol. Biol. 269:326-341. [DOI] [PubMed] [Google Scholar]
- 2.Bertani, G. 1951. Studies on lysogenesis. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Björk, G. R., and F. C. Neidhardt. 1973. Evidence for the utilization of host tRNA(m5U)methylase to modify tRNA coded by phage T4. Virology 52:507-519. [DOI] [PubMed] [Google Scholar]
- 4.Björk, G. R., and F. C. Neidhardt. 1975. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J. Bacteriol. 124:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck, M., and E. Griffiths. 1982. Iron mediated methylthiolation of tRNA as a regulator of operon expression in Escherichia coli. Nucleic Acids Res. 10:2609-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bylund, G. O., J. M. Lövgren, and P. M. Wikström. 2001. Characterization of mutations in the metY-nusA-infB operon that suppress the slow growth of a ΔrimM mutant. J. Bacteriol. 183:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bylund, G. O., L. C. Wipemo, L. A. C. Lundberg, and P. M. Wikström. 1998. RimM and RbfA are essential for efficient processing of 16S rRNA in Escherichia coli. J. Bacteriol. 180:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran, J. F. 1993. Analysis of effects of tRNA:message stability on frameshift frequency at the Escherichia coli RF2 programmed frameshift site. Nucleic Acids Res. 21:1837-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran, J. F. 1995. Decoding with the A-I wobble pair is inefficient. Nucleic Acids Res. 23:683-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran, J. F., and B. L. Gross. 1994. Evidence that GHN phase bias does not constitute a framing code. J. Mol. Biol. 235:389-395. [DOI] [PubMed] [Google Scholar]
- 11.Curran, J. F., and M. Yarus. 1989. Rates of aminoacyl-tRNA selection at 29 sense codons in vivo. J. Mol. Biol. 209:65-77. [DOI] [PubMed] [Google Scholar]
- 12.Davanloo, P., M. Sprinzl, K. Watanabe, M. Albani, and H. Kersten. 1979. Role of ribothymidine in the thermal stability of transfer RNA as monitored by proton magnetic resonance. Nucleic Acids Res. 6:1571-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis, D. R. 1995. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res. 23:5020-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durand, J. M., G. R. Björk, A. Kuwae, M. Yoshikawa, and C. Sasakawa. 1997. The modified nucleoside 2-methylthio-N6-isopentenyladenosine in tRNA of Shigella flexneri is required for expression of virulence genes. J. Bacteriol. 179:5777-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durand, J. M., B. Dagberg, B. E. Uhlin, and G. R. Björk. 2000. Transfer RNA modification, temperature and DNA superhelicity have a common target in the regulatory network of the virulence of Shigella flexneri: the expression of the virF gene. Mol. Microbiol. 35:924-935. [DOI] [PubMed] [Google Scholar]
- 16.Durand, J. M., N. Okada, T. Tobe, M. Watarai, I. Fukuda, T. Suzuki, N. Nakata, K. Komatsu, M. Yoshikawa, and C. Sasakawa. 1994. vacC, a virulence-associated chromosomal locus of Shigella flexneri, is homologous to tgt, a gene encoding tRNA-guanine transglycosylase (Tgt) of Escherichia coli K-12. J. Bacteriol. 176:4627-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Formal, S. B., P. Gemski, L. S. Baron, and E. H. LaBrec. 1971. A chromosomal locus which controls the ability of Shigella flexneri to evoke keratoconjunctivitis. Infect. Immun. 3:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu, C., and J. Parker. 1994. A ribosomal frameshifting error during translation of the argI mRNA of Escherichia coli. Mol. Gen. Genet. 243:434-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gefter, M. L. 1969. The in vitro synthesis of 2′-O-methylguanosine and 2-methylthio-N6-(γ,γ,dimethylallyl)adenosine in transfer RNA of Escherichia coli. Biochem. Biophys. Res. Commun. 36:435-441. [DOI] [PubMed] [Google Scholar]
- 20.Gehrke, C. W., and K. C. Kuo. 1990. Ribonucleoside analysis by reversed-phase high performance liquid chromatography, p. A3-A71. In C. W. Gehrke and K. C. T. Kuo (ed.), Chromatography and modification of nucleosides. Part A. Analytical methods for major and modified nucleosides. Elsevier, Amsterdam, The Netherlands.
- 21.Gehrke, C. W., K. C. Kuo, R. A. McCune, K. O. Gerhardt, and P. F. Agris. 1982. Quantitative enzymatic hydrolysis of tRNAs: reversed-phase high-performance liquid chromatography of tRNA nucleosides. J. Chromatogr. 230:297-308. [PubMed] [Google Scholar]
- 22.Gowrishankar, J., and J. Pittard. 1982. Regulation of phenylalanine biosynthesis in Escherichia coli K-12: control of transcription of the pheA operon. J. Bacteriol. 150:1130-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutgsell, N., N. Englund, L. Niu, Y. Kaya, B. G. Lane, and J. Ofengand. 2000. Deletion of the Escherichia coli pseudouridine synthase gene truB blocks formation of pseudouridine 55 in tRNA in vivo, does not affect exponential growth, but confers a strong selective disadvantage in competition with wild-type cells. RNA 6:1870-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagervall, T. G., T. M. Tuohy, J. F. Atkins, and G. R. Björk. 1993. Deficiency of 1-methylguanosine in tRNA from Salmonella typhimurium induces frameshifting by quadruplet translocation. J. Mol. Biol. 232:756-765. [DOI] [PubMed] [Google Scholar]
- 25.Johansson, M. J., and A. S. Byström. 2002. Dual function of the tRNA(m5U54)methyltransferase in tRNA maturation. RNA 8:324-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kammen, H. O., C. C. Marvel, L. Hardy, and E. E. Penhoet. 1988. Purification, structure, and properties of Escherichia coli tRNA pseudouridine synthase I. J. Biol. Chem. 263:2255-2263. [PubMed] [Google Scholar]
- 27.Kawai, G., H. Ue, M. Yasuda, K. Sakamoto, T. Hashizume, J. A. McCloskey, T. Miyazawa, and S. Yokoyama. 1991. Relation between functions and conformational characteristics of modified nucleosides found in tRNAs. Nucleic Acids Symp. Ser. 49-50. [PubMed]
- 28.Kawai, G., Y. Yamamoto, T. Kamimura, T. Masegi, M. Sekine, T. Hata, T. Iimori, T. Watanabe, T. Miyazawa, and S. Yokoyama. 1992. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2′-hydroxyl group. Biochemistry 31:1040-1046. [DOI] [PubMed] [Google Scholar]
- 29.Kim, S. H., F. L. Suddath, G. J. Quigley, A. McPherson, J. L. Sussman, A. H. Wang, N. C. Seeman, and A. Rich. 1974. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science 185:435-440. [DOI] [PubMed] [Google Scholar]
- 30.Ladner, J. E., A. Jack, J. D. Robertus, R. S. Brown, D. Rhodes, B. F. Clark, and A. Klug. 1975. Structure of yeast phenylalanine transfer RNA at 2.5 Å resolution. Proc. Natl. Acad. Sci. USA 72:4414-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, J., B. Esberg, J. F. Curran, and G. R. Björk. 1997. Three modified nucleosides present in the anticodon stem and loop influence the in vivo aa-tRNA selection in a tRNA-dependent manner. J. Mol. Biol. 271:209-221. [DOI] [PubMed] [Google Scholar]
- 32.Lynn, S. P., W. S. Burton, T. J. Donohue, R. M. Gould, R. I. Gumport, and J. F. Gardner. 1987. Specificity of the attenuation response of the threonine operon of Escherichia coli is determined by the threonine and isoleucine codons in the leader transcript. J. Mol. Biol. 194:59-69. [DOI] [PubMed] [Google Scholar]
- 33.Massenet, S., A. Mougin, and C. Branlant. 1998. Posttranscriptional modifications in the U small nuclear RNAs, p. 201-227. In H. Grosjean and R. Benne (ed.), Modification and editing of RNA. ASM Press, Washington, D.C.
- 34.Maurelli, A. T., and P. J. Sansonetti. 1988. Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc. Natl. Acad. Sci. USA 85:2820-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Miller, J. H., and A. M. Albertini. 1983. Effects of surrounding sequence on the suppression of nonsense codons. J. Mol. Biol. 164:59-71. [DOI] [PubMed] [Google Scholar]
- 37.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Neidhardt, F. C., P. L. Bloch, S. Pedersen, and S. Reeh. 1977. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli J. Baceriol. 129:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura, S. 1979. Structures of modified nucleosides found in tRNA, p. 547-549. In P. R. Schimmel, D. Söll, and J. N. Abelson (ed.), Transfer RNA: structure, properties, and recognition. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 39.Nurse, K., J. Wrzesinski, A. Bakin, B. G. Lane, and J. Ofengand. 1995. Purification, cloning, and properties of the tRNA Ψ55 synthase from Escherichia coli. RNA 1:102-112. [PMC free article] [PubMed] [Google Scholar]
- 40.Ny, T., H. R. Lindström, T. G. Hagervall, and G. R. Björk. 1988. Purification of transfer RNA (m5U54)-methyltransferase from Escherichia coli. Association with RNA. Eur. J. Biochem. 177:467-475. [DOI] [PubMed] [Google Scholar]
- 41.Ofengand, J., and M. J. Fournier. 1998. The pseudouridine residues of rRNA: number, location, biosynthesis, and function, p. 229-253. In H. Grosjean and R. Benne R. (ed.), Modification and editing of RNA. ASM Press, Washington, D.C.
- 42.Okada, N., C. Sasakawa, T. Tobe, M. Yamada, S. Nagai, K. A. Talukder, K. Komatsu, S. Kanegasaki, and M. Yoshikawa. 1991. Virulence-associated chromosomal loci of Shigella flexneri identified by random Tn5 insertion mutagenesis. Mol. Microbiol. 5:187-195. [DOI] [PubMed] [Google Scholar]
- 43.Pal, T., S. B. Formal, and T. L. Hale. 1989. Characterization of virulence marker antigen of Shigella spp. and enteroinvasive Escherichia coli. J. Clin. Microbiol. 27:561-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Persson, B. C., C. Gustafsson, D. E. Berg, and G. R. Björk. 1992. The gene for a tRNA modifying enzyme, m5U54-methyltransferase, is essential for viability in Escherichia coli. Proc. Natl. Acad. Sci. USA 89:3995-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persson, B. C., G. Jäger, and C. Gustafsson. 1997. The spoU gene of Escherichia coli, the fourth gene of the spoT operon, is essential for tRNA (Gm18) 2′-O-methyltransferase activity. Nucleic Acids Res. 25:4093-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quigley, G. J., A. H. Wang, N. C. Seeman, F. L. Suddath, A. Rich, J. L. Sussman, and S. H. Kim. 1975. Hydrogen bonding in yeast phenylalanine transfer RNA. Proc. Natl. Acad. Sci. USA 72:4866-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramamurthy, V., S. L. Swann, J. L. Paulson, C. J. Spedaliere, and E. G. Mueller. 1999. Critical aspartic acid residues in pseudouridine synthases. J. Biol. Chem. 274:22225-22230. [DOI] [PubMed] [Google Scholar]
- 48.Raychaudhuri, S., L. Niu, J. Conrad, B. G. Lane, and J. Ofengand. 1999. Functional effect of deletion and mutation of the Escherichia coli ribosomal RNA and tRNA pseudouridine synthase RluA. J. Biol. Chem. 274:18880-18886. [DOI] [PubMed] [Google Scholar]
- 49.Rozenski, J., P. F. Crain, and J. A. McCloskey. 1999. The RNA Modification Database: 1999 update. Nucleic Acids Res. 27:196-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sage, A. E., A. I. Vasil, and M. L. Vasil. 1997. Molecular characterization of mutants affected in the osmoprotectant-dependent induction of phospholipase C in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 23:43-56. [DOI] [PubMed] [Google Scholar]
- 51.Saint-Girons, I. 1978. New regulatory mutations affecting the expression of the threonine operon in Escherichia coli K-12. Mol. Gen. Genet. 162:95-100. [DOI] [PubMed] [Google Scholar]
- 52.Sansonetti, P. J., A. Ryter, P. Clerc, A. T. Maurelli, and J. Mounier. 1986. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect. Immun. 51:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasakawa, C., K. Kamata, T. Sakai, S. Makino, M. Yamada, N. Okada, and M. Yoshikawa. 1988. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J. Bacteriol. 170:2480-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sprinzl, M., C. Horn, M. Brown, A. Ioudovitch, and S. Steinberg. 1998. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 26:148-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tate, W. P., E. S. Poole, and S. A. Mannering. 1996. Hidden infidelities of the translational stop signal. Prog. Nucleic Acid Res. Mol. Biol. 52:293-335. [DOI] [PubMed] [Google Scholar]
- 56.Turnbough, C. L. J., R. J. Neill, R. Landsberg, and B. N. Ames. 1979. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J. Biol. Chem. 254:5111-5119. [PubMed] [Google Scholar]
- 57.Urbonavičius, J. 2002. Influence of tRNA modification on translation, metabolism, and virulence in eubacteria. Ph.D. thesis. Umeå University, Umeå, Sweden.
- 58.Urbonavičius, J., Q. Qian, J. M. Durand, T. G. Hagervall, and G. R. Björk. 2001. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 20:4863-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, S., and E. T. Kool. 1995. Origins of the large differences in stability of DNA and RNA helices: C5-methyl and 2′-hydroxyl effects. Biochemistry 34:4125-4132. [DOI] [PubMed] [Google Scholar]
- 60.Wrzesinski, J., K. Nurse, A. Bakin, B. G. Lane, and J. Ofengand. 1995. A dual-specificity pseudouridine synthase: an Escherichia coli synthase purified and cloned on the basis of its specificity for Ψ746 in 23S RNA is also specific for Ψ32 in tRNAPhe. RNA 1:437-448. [PMC free article] [PubMed] [Google Scholar]
- 61.Yanofsky, C., and L. Soll. 1977. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J. Mol. Biol. 113:663-677. [DOI] [PubMed] [Google Scholar]
- 62.Yusupov, M. M., G. Z. Yusupova, A. Baucom, K. Lieberman, T. N. Earnest, J. H. Cate, and H. F. Noller. 2001. Crystal structure of the ribosome at 5.5 Å resolution. Science 292:883-896. [DOI] [PubMed] [Google Scholar]