Abstract
The symbiotic, nitrogen-fixing bacterium Sinorhizobium meliloti favors succinate and related dicarboxylic acids as carbon sources. As a preferred carbon source, succinate can exert catabolite repression upon genes needed for the utilization of many secondary carbon sources, including the α-galactosides raffinose and stachyose. We isolated lacR mutants in a genetic screen designed to find S. meliloti mutants that had abnormal succinate-mediated catabolite repression of the melA-agp genes, which are required for the utilization of raffinose and other α-galactosides. The loss of catabolite repression in lacR mutants was seen in cells grown in minimal medium containing succinate and raffinose and grown in succinate and lactose. For succinate and lactose, the loss of catabolite repression could be attributed to the constitutive expression of β-galactoside utilization genes in lacR mutants. However, the inactivation of lacR did not cause the constitutive expression of α-galactoside utilization genes but caused the aberrant expression of these genes only when succinate was present. To explain the loss of diauxie in succinate and raffinose, we propose a model in which lacR mutants overproduce β-galactoside transporters, thereby overwhelming the inducer exclusion mechanisms of succinate-mediated catabolite repression. Thus, some raffinose could be transported by the overproduced β-galactoside transporters and cause the induction of α-galactoside utilization genes in the presence of both succinate and raffinose. This model is supported by the restoration of diauxie in a lacF lacR double mutant (lacF encodes a β-galactoside transport protein) grown in medium containing succinate and raffinose. Biochemical support for the idea that succinate-mediated repression operates by preventing inducer accumulation also comes from uptake assays, which showed that cells grown in raffinose and exposed to succinate have a decreased rate of raffinose transport compared to control cells not exposed to succinate.
Bacteria belonging to the genera Sinorhizobium, Rhizobium, and Bradyrhizobium are members of the α-proteobacteria, a fascinating group of organisms, many of which are intracellular symbionts or pathogens. Sinorhizobium meliloti can grow in soil as free-living organisms but can also live as nitrogen-fixing symbionts inside root nodules of alfalfa and a few other plants belonging to the family Leguminosae (3, 9, 15, 19, 22, 34).
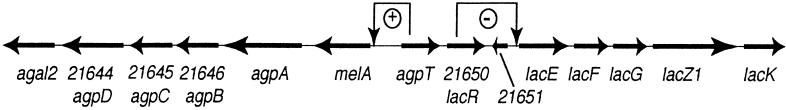
Free-living S. meliloti, like many heterotrophic bacteria, utilizes a wide variety of compounds as sources of carbon for growth. S. meliloti can utilize α-galactosides in laboratory medium and also when growing in the rhizospheres of host and nonhost plants (4). The utilization of α-galactosides requires genes which are part of an operon located on pSymB, a 1.7-Mb plasmid, in S. meliloti (Fig. 1) (7, 12). The agpA gene encodes a 77-kDa periplasmic protein that is required for α-galactoside transport and that is similar to periplasmic binding protein components of the oligopeptide family of permeases (Fig. 1) (12). Immediately downstream of the agpA gene is agpB, which encodes a protein similar to transmembrane proteins of the oligopeptide family of permeases. Downstream of agpB are genes encoding a second transmembrane permease and an ATP binding protein. Thus, this operon appears to encode a complete ATP binding cassette-type transport system for α-galactosides. The agpA gene is preceded by the melA gene, which encodes a 55-kDa α-galactosidase (12). The melA and agp genes are cotranscribed and are referred to in this study as the melA-agp operon. Induction of the melA-agp operon by α-galactosides requires the action of an AraC-type transcriptional activator, AgpT, which is encoded upstream of melA (5).
FIG. 1.
Map of the region of pSymB involved with the transport and utilization of α-galactosides and β-galactosides. ORFs are indicated along with the official ORF names (top row) and names indicating the functions of the proteins encoded by the ORFs (bottom row). Regulatory roles of AgpT and LacR are indicated above the map.
S. meliloti favors succinate and related dicarboxylic acids as carbon and energy sources. Succinate, fumarate, and malate are brought into the cell via membrane-bound permease DctA (28, 38). The expression of dctA is inducible by dicarboxylic acids and structurally related compounds and requires the action of a two-component signal transduction system composed of DctB, the sensor kinase, and DctD, the response regulator (25, 27, 29, 38). As a favored carbon source, succinate often exerts catabolite repression upon genes needed for the utilization of secondary carbon sources. Secondary carbon sources include compounds such as glucose, fructose, galactose, lactose, myo-inositol, and several pentoses and polyols (16, 17, 24, 37). This preference for succinate can manifest itself as diauxie when S. meliloti is grown on succinate plus a secondary carbon source (16, 37).
Succinate also exerts catabolite repression on the consumption of α-galactosides in S. meliloti. The expression of the melA-agp operon is repressed by succinate (12), and S. meliloti shows diauxie when grown on a combination of succinate and the α-galactoside raffinose or stachyose. While the phenomenon of succinate-mediated catabolite repression in S. meliloti is well documented, most of the molecular details of its operation remain to be elucidated. This is also true for organisms of other genera, such as Pseudomonas, which utilize succinate and other tricarboxylic acid cycle intermediates in preference to other carbon sources (8, 14, 23, 35).
It is known that succinate transport is important for the establishment of catabolite repression in S. meliloti. S. meliloti dctA mutants are unable to repress the expression of the lac operon in the presence of succinate plus lactose, indicating that succinate transport, or at least the interaction of succinate with the DctA permease, is required for the establishment of catabolite repression (17).
In order to find genes needed to establish succinate-mediated catabolite repression, we screened for mutants unable to repress the melA-agp operon when grown on a combination of succinate and raffinose. lacR mutants, which constitutively overexpress β-galactoside utilization genes, were isolated in this screen. We show that the effects of lacR mutations on the succinate-mediated catabolite repression of the melA-agp operon are due to the overexpression of β-galactoside transport genes, which likely overwhelms inducer exclusion or inducer expulsion mechanisms (26, 30, 36). The genetic and biochemical experiments presented in this study support this idea and support the conclusion that succinate-mediated catabolite repression acts, in part, through the regulation of intracellular inducer accumulation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
Bacterial strains, plasmids, and their relevant characteristics are listed in Table 1. Cells were grown in tryptone-yeast (TY), Luria-Bertani, or M9 minimal medium with various carbon sources (32). Antibiotics were used at the following concentrations: ampicillin, 50 μg/ml; chloramphenicol, 5 μg/ml for S. meliloti and 100 μg/ml for Escherichia coli; gentamicin, 30 μg/ml; neomycin, 200 μg/ml; spectinomycin, 100 μg/ml; and streptomycin, 500 μg/ml.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| S. meliloti | ||
| Rm1021 | Wild type (Smr) | 21 |
| Rm8002 | pho derivative of Rm1021 | 18 |
| DG73 | Rm1021 agpA::TnphoA-Spr; the Nmr gene of agpA::TnphoA was swapped with the Spr region of Tn5-233 | Gage lab |
| RB21 | Rm1021 lacR::Tn5lacZ (Smr Gmr) | This study |
| RB33 | Rm1021 lacR::Tn5-233 (Smr Gmr Spr) | This study |
| RB45 | Rm1021 lacF::pRB69 | This study |
| RB46 | Rm1021 lacF::pRB69 lacR::Tn5-233 | This study |
| RB47 | Rm1021 lacF::pRB69 agpA::TnphoA | This study |
| SG2001 | Rm1021 agpA::TnphoA | Gage lab |
| E. coli | ||
| XL1Blue | Used for cloning (Tcr) | Stratagene |
| MT616 | Helper strain for conjugal transfer of plasmids (Cmr) | 13 |
| MM294a | Used to deliver pRK607 | 13 |
| Plasmids | ||
| pRB27 | pmelA::gfp fusion in pMB393 (Spr Cmr) | 4 |
| pRB69 | Suicide vector pMB438 containing a 594-bp internal piece of lacF (Apr Nmr/Kmr) | This study |
| pMB393 | Broad-host-range plasmid | 2 |
| pMB438 | Suicide vector (Apr Nmr/Kmr) | 2 |
| pB22 | Plasmid used to deliver Tn5lacZ (Gmr) | 33 |
| pRK607 | Plasmid used to deliver Tn5-233 (Nmr/Kmr Gmr/Kmr Spr/Smr) | 10 |
| pJGJ54 | lacR-complementing plasmid | 17 |
| pJGJ86 | lacR-complementing plasmid | 17 |
| pGEM-T Easy | PCR product cloning vector | Promega |
Construction of S. meliloti strains.
Strain RB21 (agpA::TnphoA lacR::Tn5lacZ) was isolated from the following genetic screen. S. meliloti strain SG2001 (agpA::TnphoA) was mutagenized by triparental mating with E. coli strain S17-1 λpir/pB22 (Tn5lac) and E. coli helper strain MT616. The three strains were streaked together on a TY plate without antibiotics and incubated for 24 h at 30°C. Swaths of bacteria were picked from the plate, suspended in 100 μl of M9 salts, and spread on M9 plates containing 0.2% succinate, 0.02% raffinose, 80 μg of 5-bromo-4-chloro-3-indolyl phosphate (X-Phos)/ml, neomycin, and gentamicin. Deep blue colonies were retained for further study; one of these was strain RB21.
Strain RB33 (lacR::Tn5-233) was also isolated from a genetic screen. S. meliloti wild-type strain Rm1021 was mutagenized by mating with MM294a/pRK607; pRK607 is a self-transmissible plasmid harboring Tn5-233. The strains were streaked together on a TY plate without antibiotics and incubated for 24 h at 30°C. Swaths of bacteria were picked from the plate, suspended in 100 μl of M9 salts, and spread on M9 plates containing 0.2% succinate, 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal)/ml, streptomycin, and gentamicin. The lacR mutants were expected to be blue on this medium because they constitutively expressed the endogenous S. meliloti lacZ gene. One of the resultant deep blue colonies was strain RB33. Southern hybridization confirmed that the Tn5-233 transposon was in the lacR gene of strain RB33 (data not shown).
Strain RB45 is a lacF mutant constructed by inserting suicide plasmid pRB69 into the lacF gene of S. meliloti wild-type strain Rm1021. Suicide plasmid pRB69 was constructed as follows. A 576-bp internal piece of the lacF gene was generated by PCR with primers 5′GCGTGGTCGCTCTGGATGT and 5′TCGTCGAAATGACTGTCGTGAA. The resulting amplification product was inserted into T/A cloning vector pGEM-T Easy. This piece was then removed by EcoRI digestion, and the 594-bp fragment was cloned into broad-host-range suicide vector pMB438, resulting in plasmid pRB69. pRB69 was mobilized from strain XL1Blue/pRB69 into strain Rm1021 by triple mating with E. coli helper strain MT616 and selected on Luria-Bertani medium plus streptomycin and neomycin.
Strain RB46 (lacF lacR) was constructed by transducing the lacF mutation from strain RB45 into strain RB33 (lacR::Tn5-233) with phage N3 (20). Strain RB47 (lacF agpA) was constructed by transducing the lacF mutation from strain RB45 into strain DG73 (agpA::TnphoA-Spr) with phage N3.
Diauxic growth curves.
S. meliloti strains were grown overnight in M9 minimal medium plus antibiotics and with succinate as the sole carbon source. A quantity (1 ml) of cell culture was pelleted, washed thee times with M9 salts to remove residual succinate, and resuspended in 100 μl of M9 salts. Ten microliters of this suspension was used to inoculate 10 ml of M9 minimal medium with 0.05% succinate or with 0.05% succinate plus 0.1% raffinose, maltose, or lactose. Cultures were incubated in 125-ml flasks at 30°C with shaking. Cell density was determined by measuring the absorbance of 100 μl of culture at 415 nm with a Bio-Rad 550 plate reader. Optical densities determined with the plate reader should be multiplied by three to approximate the optical density determined with a spectrophotometer with a standard 1-cm path length.
Quantitation of pmelA::gfp expression.
Bacterial strains were grown overnight in M9 minimal medium with succinate as the sole carbon source. Cells (25 ml) were pelleted, washed three times in M9 salts, and resuspended in 1 ml of M9 salts. Twenty microliters of this cell suspension was used to inoculate 200 ml of M9 minimal medium with 0.1% succinate plus 0.1% raffinose. Growth was monitored by measuring the absorbance of 100 μl of culture at 415 nm with a Bio-Rad 550 plate reader. Periodically, a quantity of culture (1 to 10 ml, depending on the culture density) was pelleted, resuspended in 15% glycerol, and stored at −80°C until all samples had been taken. After all samples were collected, the fluorescence of the pellets was measured by using a CytoFluor 4000 fluorimeter (PerSeptive Biosystems, Foster City, Calif.) with excitation at 485 nm and emission at 508 nm. Relative fluorescence was determined by dividing sample fluorescence by sample optical density at 415 nm. Optical density at 415 nm was determined by using a Bio-Rad 550 plate reader.
Catabolite repression of the pmelA::gfp fusion was measured by growing test strains in tubes (18 by 150 mm) containing 2.5 ml of M9 minimal medium with 0.4% succinate plus 0.4% raffinose. Under these conditions, some succinate remains when cells enter stationary phase and can exert catabolite repression on the pmelA::gfp fusion. After 72 h, 100 μl of culture was removed, and its relative fluorescence was determined as described above.
Raffinose uptake assays.
Bacterial strains were grown overnight in M9 minimal medium with 0.4% succinate or 0.4% raffinose as the sole carbon source. Cells grown in succinate were used to inoculate 25 ml of M9 minimal medium containing 0.4% succinate plus 0.4% raffinose, and cells grown overnight in raffinose were used to inoculate 25 ml of M9 minimal medium containing 0.4% raffinose. Cultures were grown to mid-exponential phase at 30°C with shaking. The raffinose culture was split, and succinate was added to one of the portions to a final concentration of 0.4%. All cultures were incubated for an additional hour. One to 2 ml of culture containing raffinose plus succinate was washed once with M9 salts plus 0.4% succinate, resuspended in 100 μl of M9 salts plus 0.4% succinate, and incubated at room temperature for 5 min. Nine hundred microliters of M9 salts plus 0.4% succinate and 10 μl of 10 mM [3H]raffinose (0.1 mCi/ml) (American Radiolabeled Chemicals, Inc.) were added to the cell suspension. Raffinose-grown cells were prepared in the same way, except that they were washed and suspended in M9 salts without succinate. Samples (100 μl) were removed every 4 min and filtered through 45-μm-pore-size nitrocellulose filters. The filters were immediately rinsed three times with 5 ml of M9 salts containing 10 mM raffinose, and counts were determined with a scintillation counter. Counts were normalized by dividing counts per minute by the optical density of the cell suspension.
RESULTS
Genetic screen for mutants unable to establish succinate-mediated catabolite repression of the melA-agp operon.
To conduct a screen for catabolite repression mutants, we made use of the fact that an agpA::TnphoA reporter is highly expressed when S. meliloti is grown on the α-galactoside raffinose but is repressed when the organism is grown on a combination of succinate and raffinose. S. meliloti strain SG2001 (agpA::TnphoA) was mutagenized with Tn5lac and plated on M9 plates containing selective antibiotics plus succinate, raffinose, and X-Phos. Most colonies were pale blue, indicating that succinate-mediated repression had down-regulated the agpA::TnphoA reporter in spite of the presence of raffinose. However, 12 colonies out of the 20,000 screened were deep blue and were candidates for mutants that had altered succinate-mediated catabolite repression. Transductional mapping and Southern analysis of the Tn5lac mutations showed that two were linked to agpA (data not shown). Sequencing showed that the insertions were in open reading frame (ORF) Y21650, which encodes a lacI-type repressor just downstream of agpT (Fig. 1) (data not shown).
The orientation of Tn5lac in the two ORF Y21650 mutants was such that the lacZ reporter should not have been transcribed. However, we believed that it was still possible for the heterologous lacZ gene, carried on Tn5lac in these strains, to confound the interpretation of experiments investigating galactoside utilization. For this reason, we reisolated an insertional mutation in ORF Y21650 by using Tn5-233. The resulting strain, RB33, was used in the following experiments.
ORF Y21650 encodes the lac repressor LacR.
Jelesko and Leigh reported that LacZ activity in S. meliloti is controlled by a repressor protein, LacR, and that mutations in lacR result in the constitutive expression of β-galactosidase activity (17). The genome sequence of S. meliloti (1, 6, 11) indicates that ORF Y21650 encodes a LacI-type repressor and is about 1,000 bp upstream of the first gene in an operon containing five genes similar to the genes involved in β-galactoside transport and catabolism (http://sequence.toulouse.inra.fr/meliloti.html) (Fig. 1). We believed that it was likely that the ORF Y21650 gene was the same as the lacR gene characterized genetically, but not sequenced, by Jelesko and Leigh (17). The fact that the Tn5-233 mutation in ORF Y21650 caused the constitutive expression of β-galactosidase activity (data not shown) lent weight to this idea. To confirm that ORF Y21650 was the same as lacR, we partially sequenced plasmids pJGJ54 and pJGJ86, which complemented lacR mutations (17), and found that they carried the ORF Y21650 gene. Because the phenotype of an ORF Y21650 mutant is the same as the phenotype of a lacR mutant and because ORF Y21650 is carried on plasmids which can complement lacR, it is likely that ORF Y21650 is the same as the lacR locus described first by Jelesko and Leigh (17).
Mutations in lacR cause aberrations in succinate-mediated catabolite repression of α-galactoside and β-galactoside utilization.
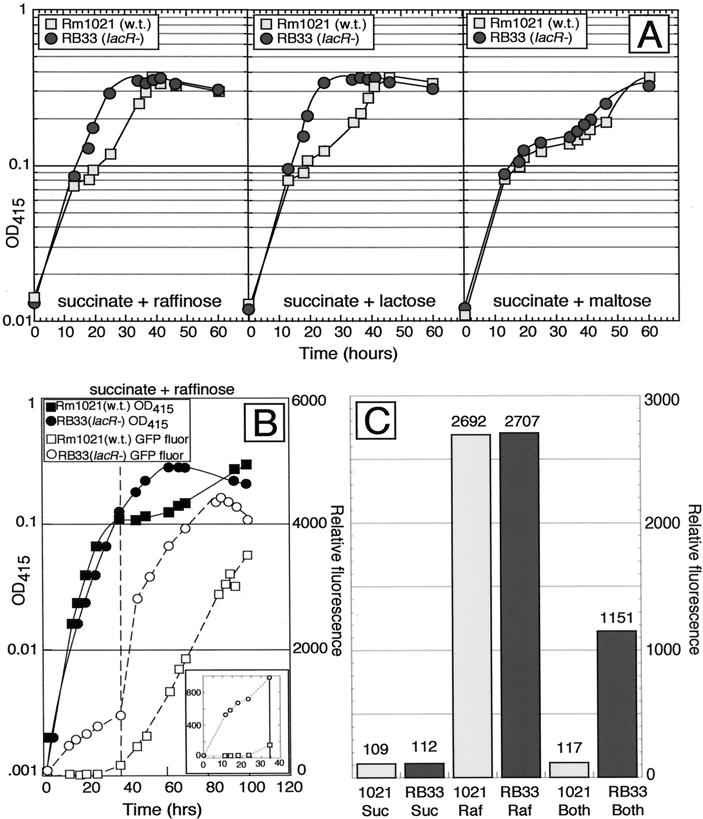
S. meliloti wild-type strain Rm1021 showed diauxie when grown in M9 minimal medium containing a combination of succinate and either raffinose, lactose, or maltose (Fig. 2A). lacR mutant RB33 failed to show diauxie when grown on succinate plus raffinose or on succinate plus lactose. Diauxie on succinate plus maltose was normal, indicating that the abnormal diauxie seen in this strain was not a general phenomenon affecting the utilization of all secondary carbon sources.
FIG. 2.
The lacR::Tn5-233 mutation abolishes succinate-mediated diauxic utilization of α- and β-galactosides. (A) Strains Rm1021 (wild type [w.t.]) and RB33 (lacR::Tn5-233) were grown in medium containing 0.05% succinate and 0.1% the indicated secondary carbon source. Growth was monitored by measuring the optical density at 415 nm (OD415). (B). Strain RB33 (lacR::Tn5-233)/pRB27 (pmelA::gfp) does not exhibit diauxie or repression of the melA::gfp reporter when grown in medium containing succinate plus raffinose. Strains Rm1021/pRB27 (pmelA::gfp) and RB33 (lacR::Tn5-233)/pRB27 (pmelA::gfp) were grown in M9 minimal medium containing 0.1% succinate plus 0.1% raffinose. The OD415 and relative green fluorescent protein (GFP) fluorescence were determined. The vertical broken line marks the time at which growth on succinate stopped in the wild-type strain. The inset shows the relative fluorescence of the strains during the first phase of diauxic growth. (C) The lacR mutation does not cause constitutive expression of the melA-agp promoter. Strains Rm1021/pRB27 (pmelA::gfp) and RB33 (lacR::Tn5-233)/pRB27 (pmelA::gfp) were grown in M9 minimal medium containing 0.4% succinate (Suc), 0.4% raffinose (Raf), or 0.4% succinate plus 0.4% raffinose (Both). After 72 h, cells were harvested, and the relative GFP fluorescence was determined. Numbers above the bars indicate the relative fluorescence value for each culture.
Plasmid pRB27 (pmelA::gfp) contains a transcriptional fusion of the melA promoter to gfp. This fusion was overexpressed in the lacR mutant, relative to the wild type, when succinate and raffinose were present together (Fig. 2B and C). In the experiments depicted in Fig. 2B, the fusion was induced in wild-type cells during the diauxic lag only after succinate had been consumed; however, the fusion was expressed throughout the growth curve for cells of strain RB33 (lacR::Tn5-233), including early times, when succinate was still present.
The lacR::Tn5-233 mutation caused the constitutive expression of β-galactoside utilization genes. If it also caused the constitutive expression of α-galactoside utilization genes, then that would explain its ability to alleviate succinate-mediated catabolite repression in medium containing succinate plus raffinose. The data presented in Fig. 2C show relative fluorescence from the pmelA::gfp fusion when strain Rm1021/pRB27 and strain RB33 (lacR::Tn5-233)/pRB27 were grown for 72 h in M9 minimal medium containing either 0.4% succinate, 0.4% raffinose, or 0.4% succinate plus 0.4% raffinose. In the wild-type strain, the pmelA::gfp fusion was expressed when cells were grown in raffinose but not when cells were grown in succinate or in succinate plus raffinose. In strain RB33 (lacR), the fusion was not expressed when cells were grown in succinate, but it was expressed when cells were grown in raffinose or in succinate plus raffinose. In addition, the pmel::gfp fusion was not expressed in either strain when the medium contained glycerol as the sole carbon source (data not shown). Thus, the lacR::Tn5-233 mutation does not cause merely constitutive expression of the melA-agp genes but causes aberrant expression of these genes when succinate and raffinose are present together.
Abnormal succinate-mediated catabolite repression of the melA-agp operon in a lacR mutant requires a functional β-galactoside transport system.
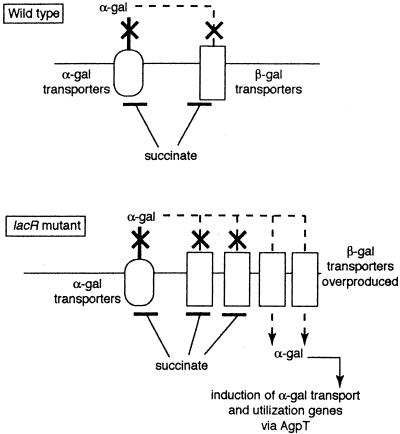
If succinate-mediated catabolite repression acts, at least in part, by preventing the intracellular accumulation of raffinose, then the effect of lacR mutations on the catabolite repression of the melA-agp operon could be explained as follows (Fig. 3). In lacR mutants, β-galactoside transporters are overproduced (17). These may allow some α-galactoside molecules into the cell, where they can interact with AgpT and cause the induction of the melA-agp operon. The overproduction of the β-galactoside transporters may overwhelm the ability of the succinate-mediated catabolite repression system to inhibit the accumulation of α-galactoside inducers. If α-galactoside transport through overproduced β-galactoside transporters is key for the eradication of succinate-mediated control of the melA-agp operon in lacR mutants, then inactivating a β-galactoside transport gene in the lacR::Tn5-233 mutant should alleviate the abnormal catabolite repression of the melA-agp operon caused by the lacR mutation.
FIG. 3.
Model to explain the effects of lacR mutations on succinate-mediated repression of α-galactoside utilization. The model depicts the α-galactoside (α-gal) and β-galactoside (β-gal) transport systems when wild-type (top) and lacR mutant (bottom) cells are growing on succinate plus an α-galactoside. The model postulates that succinate-mediated catabolite repression of the melA-agp operon is due, in part, to succinate causing inducer exclusion of α-galactosides. In the lacR mutant, the overproduction of β-galactoside transporters would allow some α-galactosides into the cell, thus short-circuiting succinate-mediated repression. The details of the model are described in the text.
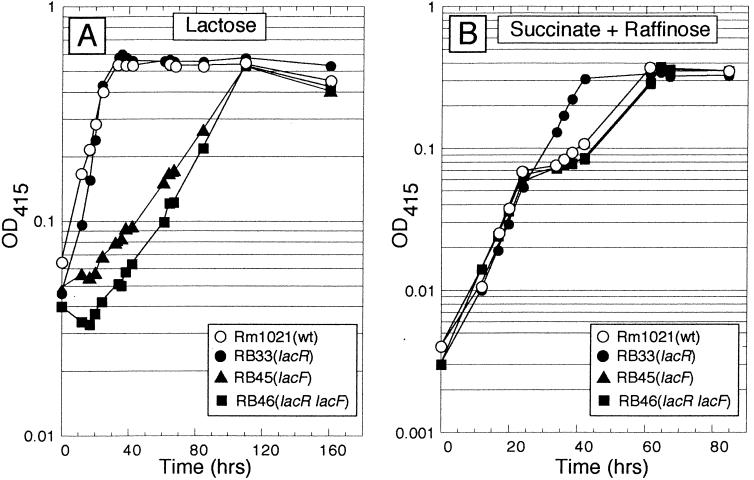
The lacF gene, which is postulated to encode a permease portion of a lactose ATP binding cassette transport system, was mutated by insertion of a suicide plasmid. Strains carrying this mutation grew on lactose with a doubling time of 30 h, compared to 4 h for the wild-type parental strain (Fig. 4A). The lacF mutation was combined with a lacR mutation to produce the double-mutant strain RB46 (lacR lacF), which also grew slowly on lactose. Unlike strain RB33 (lacR), strain RB46 (lacR lacF) showed diauxie when grown in M9 minimal medium containing a combination of succinate and raffinose (Fig. 4B). Thus, the double mutant, with defects in lacR and β-galactoside transport, appears phenotypically normal with respect to succinate-mediated repression of α-galactoside utilization genes. This finding indicated that the transport of some raffinose through β-galactoside transporters is required for the abnormal catabolite repression phenotype seen when a lacR strain is grown on succinate plus raffinose. Even though enough raffinose appears to cross into the cell via the Lac transport system to trigger the induction of the melA-agp genes, the amount must be rather small, because agpA (12) or agpB mutants cannot grow on α-galactosides (data not shown). Even when the Lac transport system is fully induced, not much raffinose crosses into the cell via this system, because lacR agpA double mutants are also unable to grow on α-galactosides (data not shown).
FIG. 4.
The lacR lacF double mutant shows wild-type growth in succinate-plus-raffinose medium. (A) lacF mutants grow very slowly on lactose. The indicated strains were grown in M9 minimal medium containing lactose as the sole source of carbon. OD415, optical density at 415 nm. (B) Succinate-mediated diauxie of α-galactoside utilization is restored in a lacR lacF double mutant. The indicated strains were grown in M9 minimal medium containing 0.05% succinate plus 0.1% raffinose. The lacR mutant failed to undergo diauxic growth, whereas the lacR lacF double mutant exhibited diauxic growth which was indistinguishable from that of wild-type (wt) strain Rm1021.
α-Galactoside accumulation is inhibited by succinate.
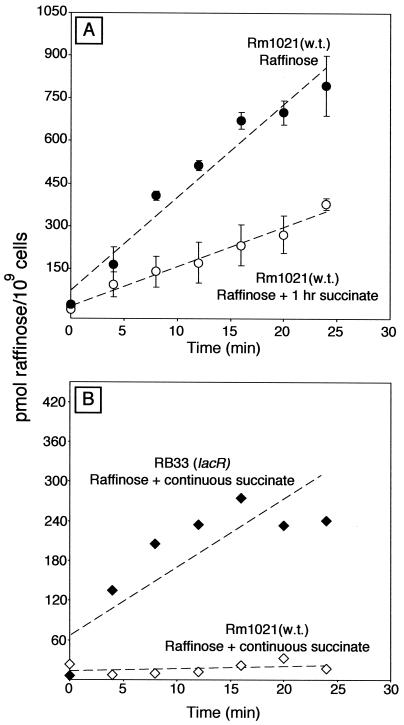
The experiments described above suggested that succinate repression of the melA-agp genes operates by preventing inducer accumulation. We tested this idea directly by conducting raffinose uptake assays with strain Rm1021 grown in M9 minimal medium containing 0.4% raffinose, 0.4% raffinose with 0.4% succinate present continuously, or 0.4% raffinose with 0.4% succinate added 1 h before the uptake assay was started. The results showed that cells grown in raffinose with succinate present continuously did not transport raffinose (Fig. 5B). Cells grown in raffinose and exposed to succinate for 1 h before the uptake assay were restricted in their ability to transport raffinose compared to raffinose-grown cells not exposed to succinate. Such cultures showed a 45 to 80% reduction in the accumulation of raffinose compared to control cultures not exposed to succinate (Fig. 5A).
FIG. 5.
Succinate inhibits the accumulation of raffinose. (A) Strain Rm1021 (wild type [w.t.]) was grown in M9 minimal medium plus 0.4% raffinose. A portion of the culture was exposed to 0.4% succinate for 1 h, and then the uptake of [3H]raffinose was measured in both the raffinose and the raffinose-plus-succinate cultures. Error bars indicate one standard error. (B) Strains Rm1021 and RB33 (lacR::Tn5-233) were grown in M9 minimal medium containing 0.4% raffinose plus 0.4% succinate. Succinate was present continuously as cells were grown in preparation for uptake measurements.
Unlike wild-type strain Rm1021 (Fig. 5A), lacR mutant strain RB33 was able to transport raffinose when grown in M9 minimal medium containing raffinose with succinate present continuously (Fig. 5B).
The biochemical data presented above do not indicate whether succinate decreased raffinose uptake by preventing raffinose from entering the cell or by accelerating its efflux from the cell. That is, succinate may decrease raffinose uptake through inducer exclusion, inducer expulsion, or a combination of the two.
DISCUSSION
Succinate is able to repress the utilization of many different carbon sources, including α-galactosides and β-galactosides in S. meliloti. In order to better understand this phenomenon, we conducted a screen to identify genes involved in the succinate-mediated repression of α-galactoside utilization. This screen revealed that lacR mutants no longer repressed the melA-agp operon when succinate was present along with raffinose. The mutations also eradicated the diauxie normally seen when cells are grown in M9 salts containing succinate plus raffinose. The elimination of succinate-mediated repression of the melA-agp operon in lacR mutant strains was not caused by a general derepression of the melA-agp operon, because these mutants did not exhibit constitutive expression of the melA-agp operon when grown on single carbon sources such as glycerol or succinate.
Experiments were done to determine whether the altered succinate-mediated catabolite repression seen in lacR mutants was general or restricted to the melA-agp operon. These experiments showed that the lacR mutation also eradicated succinate-mediated catabolite repression in a medium containing succinate plus lactose but did not do so in a medium containing succinate plus maltose. The lacR mutant strains constitutively expressed LacZ activity in all media tested. Thus, with succinate plus lactose, the altered succinate-mediated repression seen in the lacR mutants was likely due to the fact that the genes needed for lactose transport and utilization are always overexpressed.
While it is easy to understand how the lacR mutations gave rise to a loss of succinate-mediated repression with respect to lactose, it is not so easy to explain the effects of these mutations on the succinate-mediated repression of α-galactoside utilization. This is because the melA-agp operon is aberrantly expressed only when an α-galactoside is present along with succinate. Our data best support the idea that succinate normally prevents raffinose from accumulating in the cell when both are present together. The overproduction of β-galactoside transporters in the lacR mutants (17) may overwhelm the ability of the succinate-mediated catabolite repression system to inhibit some Lac transporter-dependent accumulation of α-galactoside inducers, even though the succinate-mediated repression system itself is fully operational. The fact that lacR lacF double mutants are normal with respect to the succinate-mediated repression of the melA-agp genes indicates that the overexpression of the Lac transport system is critical for the aberrant succinate-mediated repression of the melA-agp genes observed in the lacR mutants.
Raffinose uptake assays supported the hypothesis that succinate prevents raffinose from accumulating when cells are grown in the presence of both succinate and raffinose. Wild-type cells growing in raffinose and exposed to succinate for 1 h exhibited a 45 to 85% decrease in their rate of raffinose accumulation. Cells grown in raffinose and succinate have a doubling time of about 6 h; thus, the decrease in raffinose uptake seen following the addition of succinate for 1 h cannot be accounted for by a halt of α-galactoside transporter synthesis followed by dilution of the remaining transporters by cell growth. If synthesis of the transporters were shut off completely for 1 h, then one would expect a one-sixth (16%) decline in the rate of raffinose accumulation, because the number of active transporters per culture mass would decline 16%.
Wild-type cells grown in raffinose with succinate continually present did not accumulate raffinose to any significant extent (Fig. 5). The difference in α-galactoside transport between cells grown in raffinose with succinate present continuously and those grown in raffinose and exposed to succinate for 1 h is similar to the effect of preinduction on catabolite repression of the lac system by glucose in E. coli (7a). The more complete shutdown of raffinose transport seen in the cells exposed to raffinose and succinate continuously may have resulted from one or more of the following: (i) active down-regulation of melA-agp expression by transcriptional factors, (ii) down-regulation of melA-agp expression caused by lower internal concentrations of inducer resulting from nearly complete inhibition of α-galactoside transporter activity, or (iii) succinate-dependent degradation of proteins required for α-galactoside transport.
In bacterial species which exhibit glucose-mediated catabolite repression, glucose status is linked to gene repression and/or inducer exclusion by the Hpr or GlcIIA proteins. These two proteins, which are directly involved in glucose transport, also mediate inducer exclusion, cyclic AMP synthesis, and repression of catabolic genes (31, 36). How the information concerning succinate status is transmitted to the regulated transport systems in rhizobia is not yet known. Jelesko and Leigh showed that the dicarboxylic acid transporter DctA was required for the succinate-mediated catabolite repression of the Lac system (17). It is possible that DctA bound to succinate can meditate inducer exclusion directly or that DctA is needed only to bring succinate into the cell, where the presence of succinate then can be signaled by a variety of means: intracellular concentration of tricarboxylic acid cycle intermediates, flux of reducing equivalents through the electron transport chain, or proteins that bind dicarboxylic acids and cause inducer exclusion or expulsion directly.
We have shown that succinate establishes catabolite repression of the melA-agp genes, at least in part, by preventing intracellular inducer accumulation. Currently, we are working on identifying other mutants that are altered in this process. We hope to find mutants that are altered in the systems that convey information about the succinate status of the cell and transmit that information to secondary carbon source transporters. Such mutants should provide information on how succinate-mediated catabolite repression operates in rhizobia and may eventually shed light on how this process works in other species which use dicarboxylic acids as preferred carbon sources.
Acknowledgments
We thank John Leigh for providing plasmids pJGJ54 and pJGJ86, the S. meliloti sequencing consortium for making preliminary data available, Charlie Giardina for use of a fluorescence microplate reader, and Robert Bender for pointing out the work of M. Cohn and K. Horibata.
This work was supported by Department of Energy contract DE-FG02-01ER15175, by a University of Connecticut Research Foundation grant to D.J.G., and by a Heinz Herrmann Graduate Fellowship in Cell Biology from the University of Connecticut Graduate School to R.M.B.
REFERENCES
- 1.Barnett, M. J., R. F. Fisher, T. Jones, C. Komp, A. P. Abola, F. Barloy-Hubler, L. Bowser, D. Capela, F. Galibert, J. Gouzy, M. Gurjal, A. Hong, L. Huizar, R. W. Hyman, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, C. Palm, M. C. Peck, R. Surzycki, D. H. Wells, K. C. Yeh, R. W. Davis, N. A. Federspiel, and S. R. Long. 2001. Nucleotide sequence and predicted functions of the entire Sinorhizobium meliloti pSymA megaplasmid. Proc. Natl. Acad. Sci. USA 98:9883-9888. [DOI] [PMC free article] [PubMed]
- 2.Barnett, M. J., V. Oke, and S. R. Long. 2000. New genetic tools for use in the Rhizobiaceae and other bacteria. BioTechniques 29:240-245. [DOI] [PubMed] [Google Scholar]
- 3.Brewin, N. J. 1991. Development of the legume root nodule. Annu. Rev. Cell Biol. 7:191-226. [DOI] [PubMed] [Google Scholar]
- 4.Bringhurst, R. M., Z. G. Cardon, and D. J. Gage. 2001. Galactosides in the rhizosphere: utilization by Sinorhizobium meliloti and development of a biosensor. Proc. Natl. Acad. Sci. USA 98:4540-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bringhurst, R. M., and D. J. Gage. 2000. An AraC-like transcriptional activator is required for induction of genes needed for α-galactoside utilization in Sinorhizobium meliloti. FEMS Microbiol. Lett. 188:23-27. [DOI] [PubMed] [Google Scholar]
- 6.Capela, D., F. Barloy-Hubler, J. Gouzy, G. Bothe, F. Ampe, J. Batut, P. Boistard, A. Becker, M. Boutry, E. Cadieu, S. Dreano, S. Gloux, T. Godrie, A. Goffeau, D. Kahn, E. Kiss, V. Lelaure, D. Masuy, T. Pohl, D. Portetelle, A. Puhler, B. Purnelle, U. Ramsperger, C. Renard, P. Thebault, M. Vandenbol, S. Weidner, and F. Galibert. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc. Natl. Acad. Sci. USA 98:9877-9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles, T. C., and T. M. Finan. 1991. Analysis of a 1600-kilobase Rhizobium meliloti megaplasmid using defined deletions generated in vivo. Genetics 127:5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Cohn, M., and K. Horibata. 1959. Inhibition by glucose of the induced synthesis of the β-galactoside-enzyme system of Escherichia coli. Analysis of maintenance. J. Bacteriol. 78:601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collier, D. N., P. W. Hager, and P. V. Phibbs, Jr. 1996. Catabolite repression control in the Pseudomonads. Res. Microbiol. 147:551-561. [DOI] [PubMed] [Google Scholar]
- 9.Denarie, J., F. Debelle, and J. C. Prome. 1996. Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65:503-535. [DOI] [PubMed] [Google Scholar]
- 10.De Vos, G. F., G. C. Walker, and E. R. Signer. 1986. Genetic manipulations in Rhizobium meliloti utilizing two new transposon Tn5 derivatives. Mol. Gen. Genet. 204:485-491. [DOI] [PubMed] [Google Scholar]
- 11.Finan, T. M., S. Weidner, K. Wong, J. Buhrmester, P. Chain, F. J. Vorholter, I. Hernandez-Lucas, A. Becker, A. Cowie, J. Gouzy, B. Golding, and A. Puhler. 2001. The complete sequence of the 1,683-kb pSymB megaplasmid from the N2-fixing endosymbiont Sinorhizobium meliloti. Proc. Natl. Acad. Sci. USA 98:9889-9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gage, D. J., and S. R. Long. 1998. α-Galactoside uptake in Rhizobium meliloti: isolation and characterization of agpA, a gene encoding a periplasmic binding protein required for melibiose and raffinose utilization. J. Bacteriol. 180:5739-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glazebrook, J., and G. C. Walker. 1991. Genetic techniques in Rhizobium meliloti. Methods Enzymol. 204:398-418. [DOI] [PubMed] [Google Scholar]
- 14.Hester, K. L., J. Lehman, F. Najar, L. Song, B. A. Roe, C. H. MacGregor, P. W. Hager, P. V. Phibbs, Jr., and J. R. Sokatch. 2000. Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa. J. Bacteriol. 182:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch, A. M. 1992. Developmental biology of legume nodulation. New Phytol. 122:211-237. [DOI] [PubMed] [Google Scholar]
- 16.Hornez, J.-P., M. Timinouni, C. Defives, and J.-C. Derieux. 1994. Unaffected nodulation and nitrogen fixation in carbohydrate pleiotropic mutants of Rhizobium meliloti. Curr. Microbiol. 28:225-229. [Google Scholar]
- 17.Jelesko, J. G., and J. A. Leigh. 1994. Genetic characterization of a Rhizobium meliloti lactose utilization locus. Mol. Microbiol. 11:165-173. [DOI] [PubMed] [Google Scholar]
- 18.Long, S., S. McCune, and G. C. Walker. 1988. Symbiotic loci of Rhizobium meliloti identified by random TnphoA mutagenesis. J. Bacteriol. 170:4257-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long, S. R. 1996. Rhizobium symbiosis: nod factors in perspective. Plant Cell 8:1885-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin, M. O., and S. R. Long. 1984. Generalized transduction in Rhizobium meliloti. J. Bacteriol. 159:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mylona, P., K. Pawlowski, and T. Bisseling. 1995. Symbiotic nitrogen fixation. Plant Cell 7:869-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Gara, F., K. Birkenhead, B. Boesten, and A. M. Fitzmaurice. 1989. Carbon metabolism and catabolite repression in Rhizobium spp. FEMS Microbiol. Rev. 63:93-102. [DOI] [PubMed] [Google Scholar]
- 24.Poole, P. S., A. Blyth, C. J. Reid, and K. Walters. 1994. myo-Inositol catabolism and catabolite repression in Rhizobium leguminosarum bv. viciae. Microbiology 140:2787-2795. [Google Scholar]
- 25.Reid, C. J., and P. S. Poole. 1998. Roles of DctA and DctB in signal transduction by the dicarboxylic acid transport system of Rhizobium leguminosarum. J. Bacteriol. 180:2660-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reizer, J., and M. H. Saier, Jr. 1983. Involvement of lactose enzyme II of the phosphotransferase system in rapid expulsion of free galactosides from Streptococcus pyogenes. J. Bacteriol. 156:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronson, C. W. 1988. Genetic regulation of C-4 dicarboxylate transport in rhizobia, p. 547-551. In H. J. Bothe, F. J. de Bruijn, and W. E. Newton (ed.), Nitrogen fixation: hundred years after. Gustav Fischer Verlag, Stuttgart, Germany.
- 28.Ronson, C. W., and P. M. Astwood. 1985. Genes involved in the carbon metabolism of bacteroids, p. 201-207. In H. J. Evan, P. J. Bottomley, and W. E. Newton (ed.), Nitrogen fixation research progress. Martinus Nijhoff Publishers, Dordrecht, The Netherlands.
- 29.Ronson, C. W., B. T. Nixon, L. M. Albright, and F. M. Ausubel. 1987. Rhizobium meliloti ntrA (rpoN) gene is required for diverse metabolic functions. J. Bacteriol. 169:2424-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saier, M. H., and S. Roseman. 1972. Inducer exclusion and repression of enzyme synthesis in mutants of Salmonella typhimurium defective in enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system. J. Biol. Chem. 247:972-975. [PubMed] [Google Scholar]
- 31.Saier, M. H., Jr. 1998. Multiple mechanisms controlling carbon metabolism in bacteria. Biotechnol. Bioeng. 58:170-174. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Simon, R., J. Quandt, and W. Klipp. 1989. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in gram negative bacteria. Gene 80:161-169. [DOI] [PubMed] [Google Scholar]
- 34.Spaink, H. P. 1995. The molecular basis of infection and nodulation by rhizobia: the ins and outs of sympathogenesis. Annu. Rev. Phytopathol. 33:345-368. [DOI] [PubMed] [Google Scholar]
- 35.Staijen, I. E., R. Marcionelli, and B. Witholt. 1999. The PalkBFGHJKL promoter is under carbon catabolite repression control in Pseudomonas oleovorans but not in Escherichia coli alk+ recombinants. J. Bacteriol. 181:1610-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stulke, J., and W. Hillen. 1999. Carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 37.Ucker, D. S. 1978. Catabolite repression-like phenomenon in Rhizobium meliloti. J. Bacteriol. 136:1197-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarosh, O. K., T. C. Charles, and T. M. Finan. 1989. Analysis of C4-dicarboxylate transport genes in Rhizobium meliloti. Mol. Microbiol. 3:813-823. [DOI] [PubMed] [Google Scholar]