Abstract
1. Velocity characteristics of optokinetic nystagmus (OKN) and optokinetic after-nystagmus (OKAN) induced by constant velocity full field rotation were studied in rhesus monkeys. A technique is described for estimating the dominant time constant of slow phase velocity curves and of monotonically changing data. Time constants obtained by this technique were used in formulating a model of the mechanism responsible for producing OKN and OKAN.
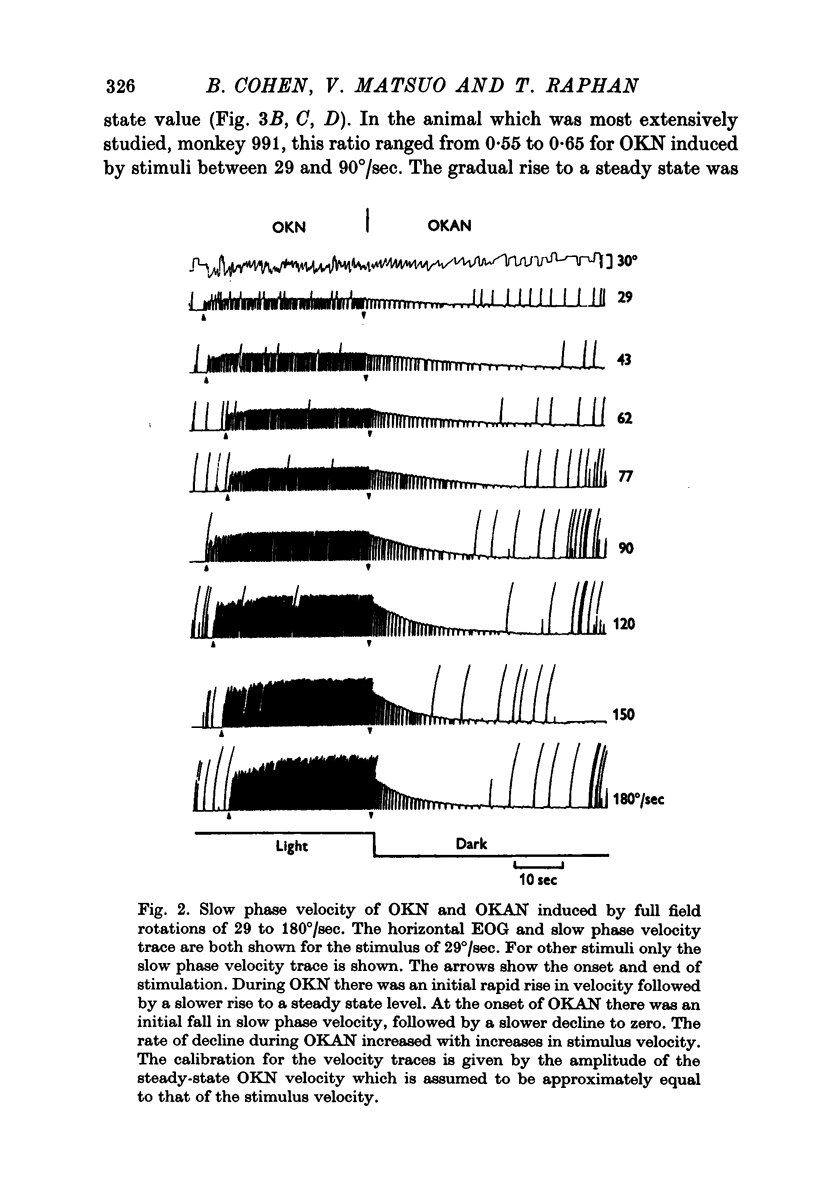
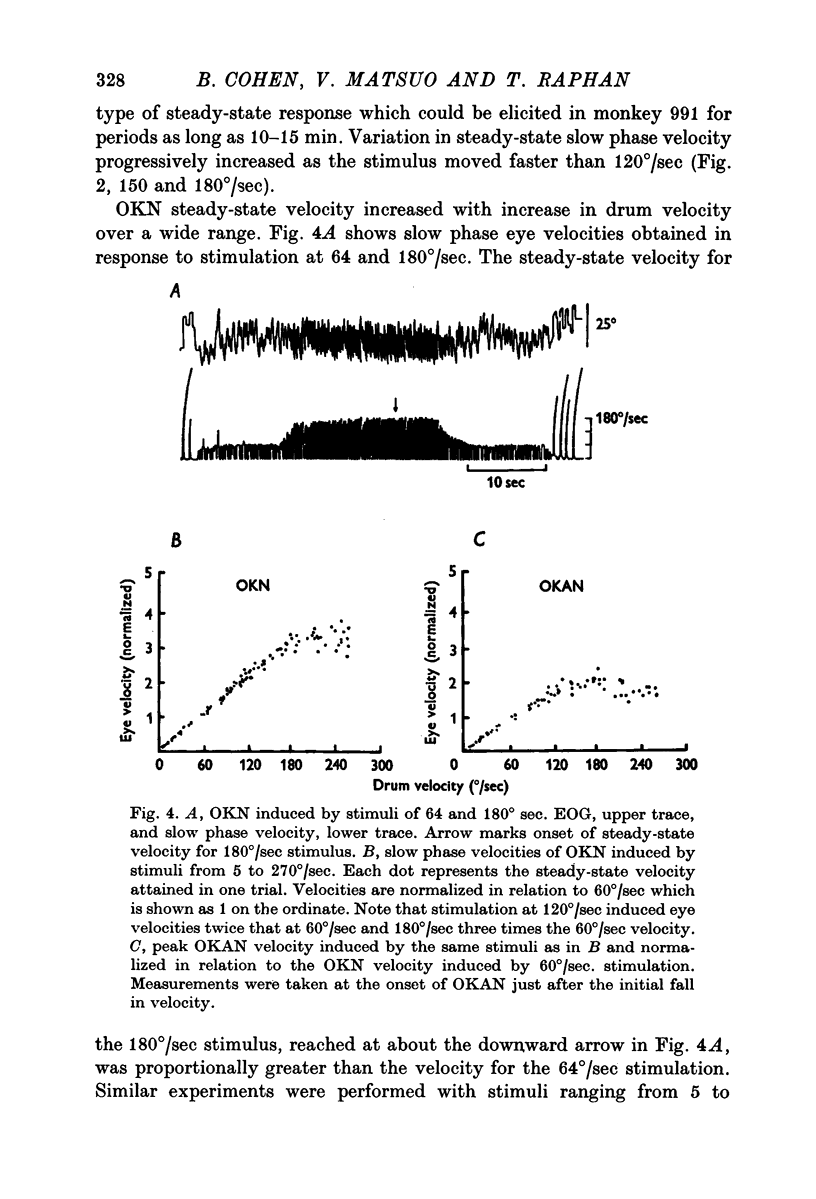
2. Slow phase velocity of optokinetic nystagmus in response to steps in stimulus velocity was shown to be composed of two components, a rapid rise, followed by a slower rise to a steady-state value. Peak values of OKN slow phase velocity increased linearly with increases in stimulus velocity to 180°/sec. Maximum slow phase eye velocities in the monkey are 2-3 times as great as in humans.
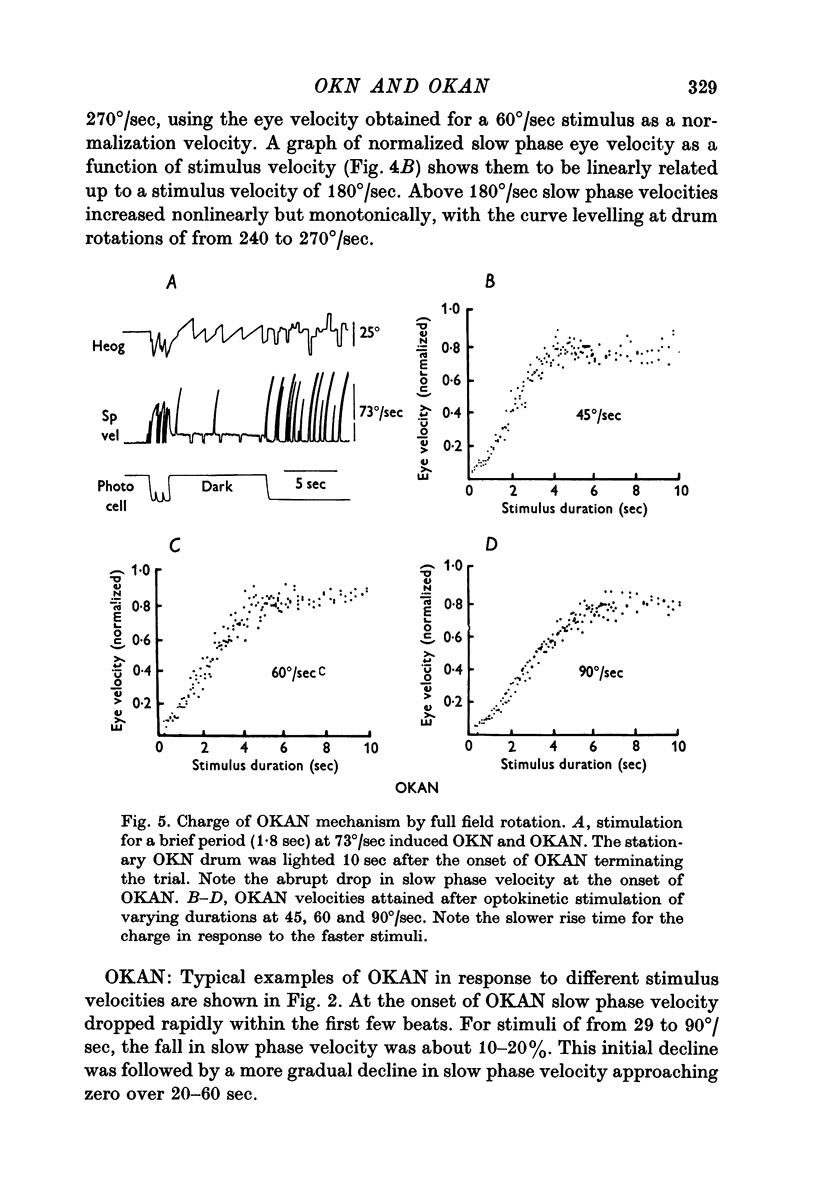
3. At the onset of OKAN, slow phase velocity falls by about 10-20%, followed by a slower decline to zero. Peak OKAN slow phase velocities were linearly related to optokinetic stimulus velocities up to 90-120°/sec. Above 120°/sec OKAN slow phase velocity saturated although OKN slow phase velocity continued to increase.
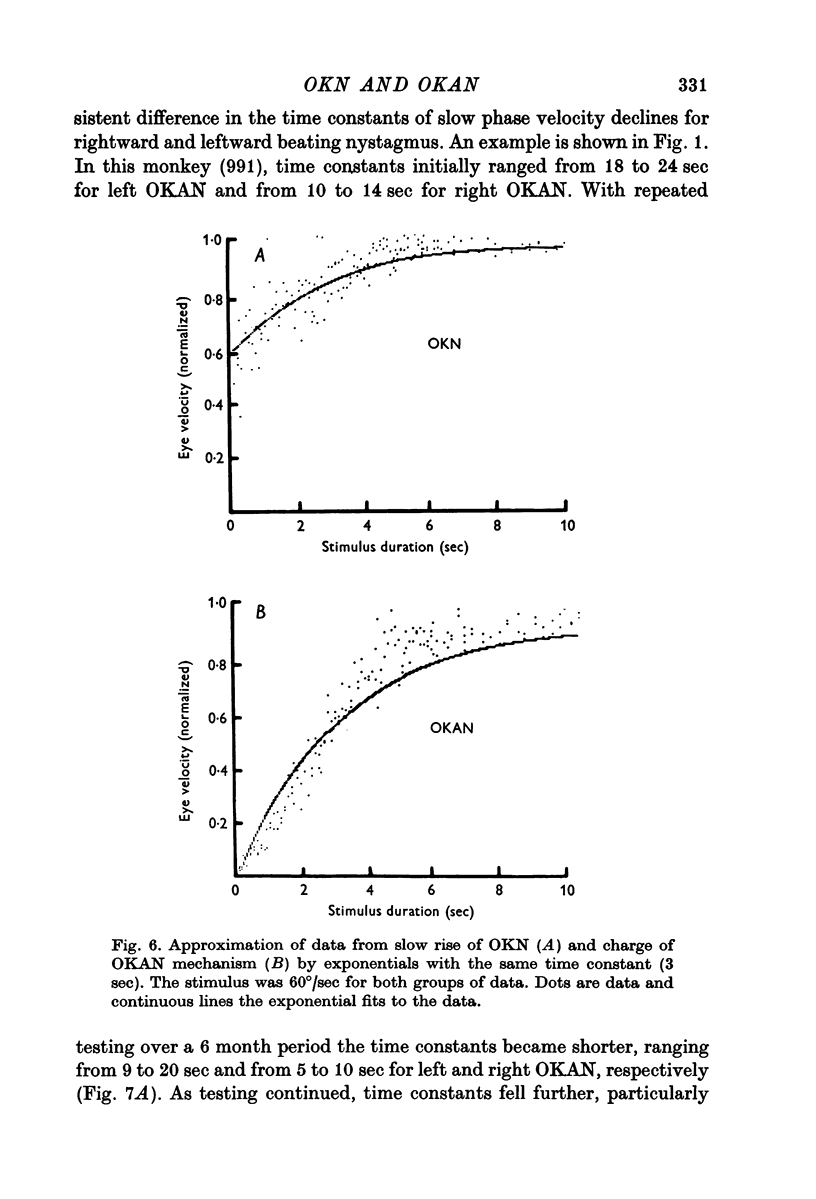
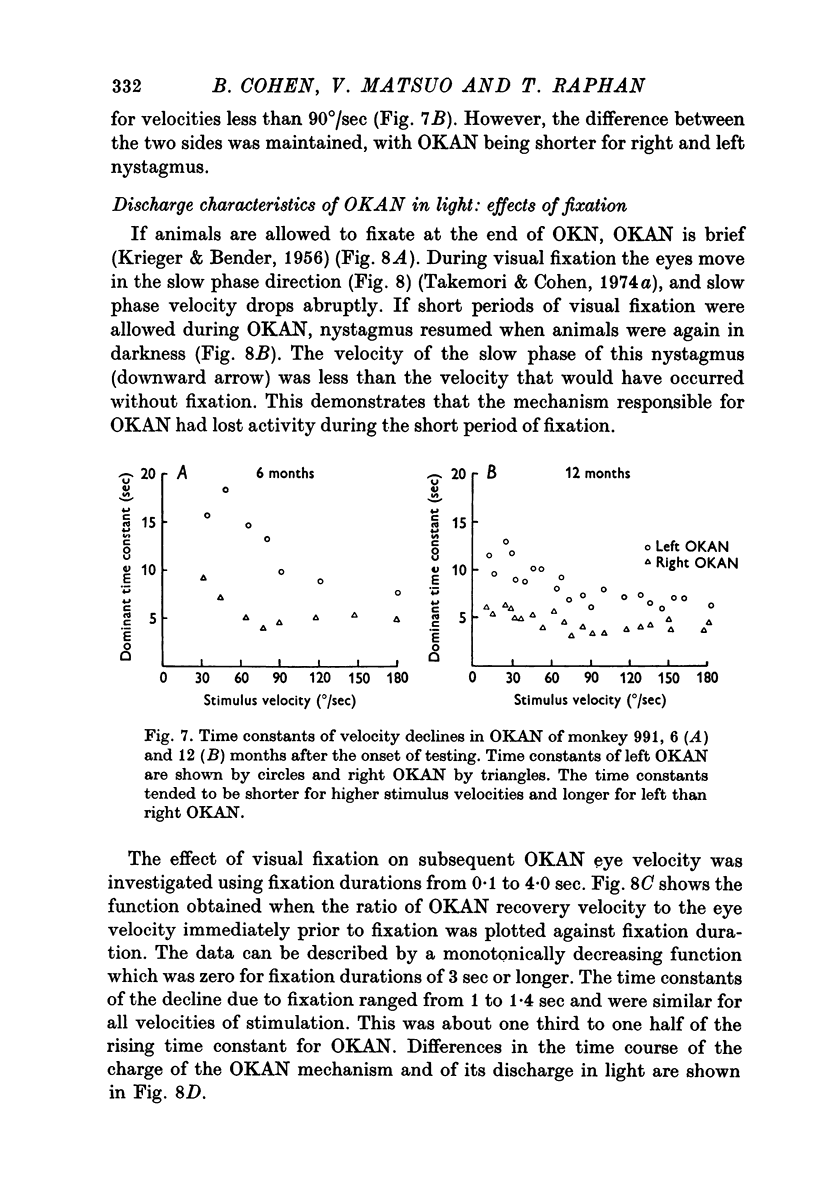
4. The charge and discharge characteristics of OKAN were studied. The OKAN mechanism charged in 5-10 sec and discharged over 20-60 sec in darkness. The time constants of decay in OKAN slow phase velocity decreased as stimulus velocities increased. They also decreased on repeated testing. In several monkeys there was a consistent difference in the rate of decay of OKAN slow phase velocity to the right and left.
5. Extended visual fixation discharged the activity responsible for producing OKAN. Short fixation times caused only a partial discharge of the OKAN mechanism. Following brief periods of fixation, OKAN resumed but with depressed slow phase velocities.
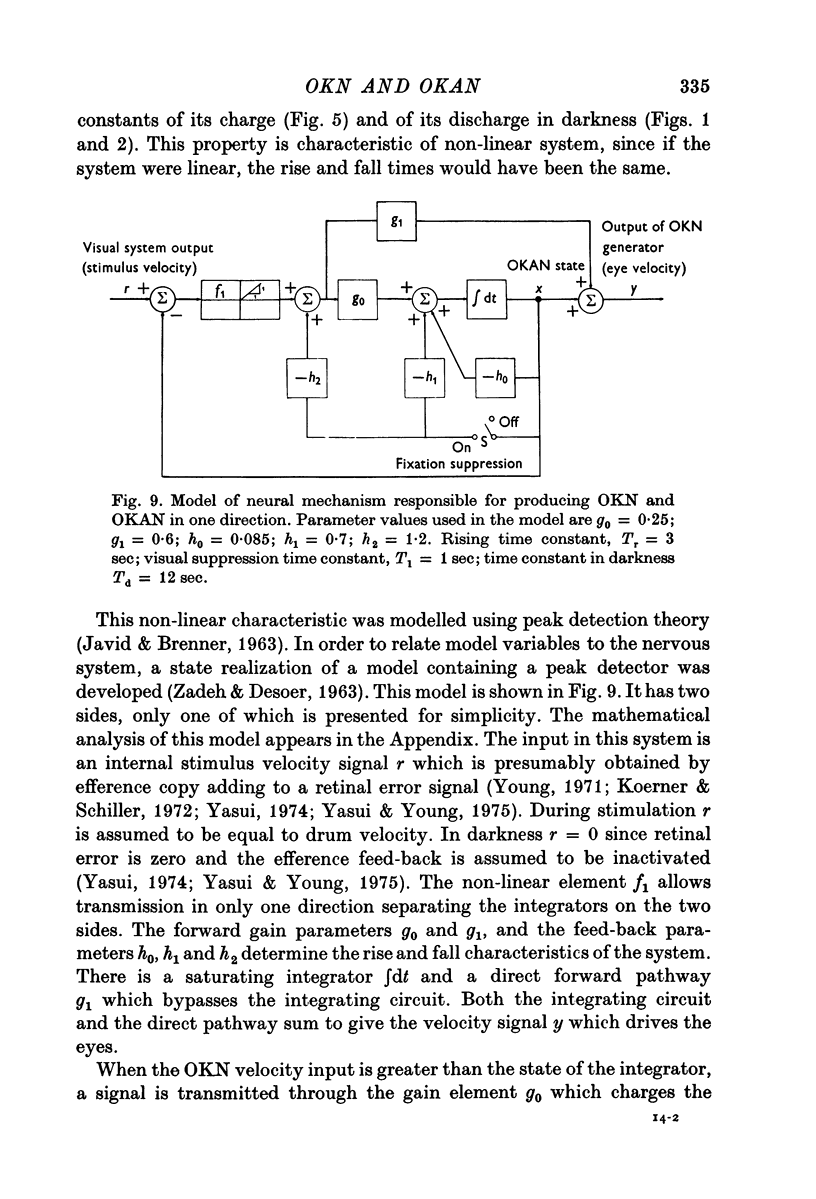
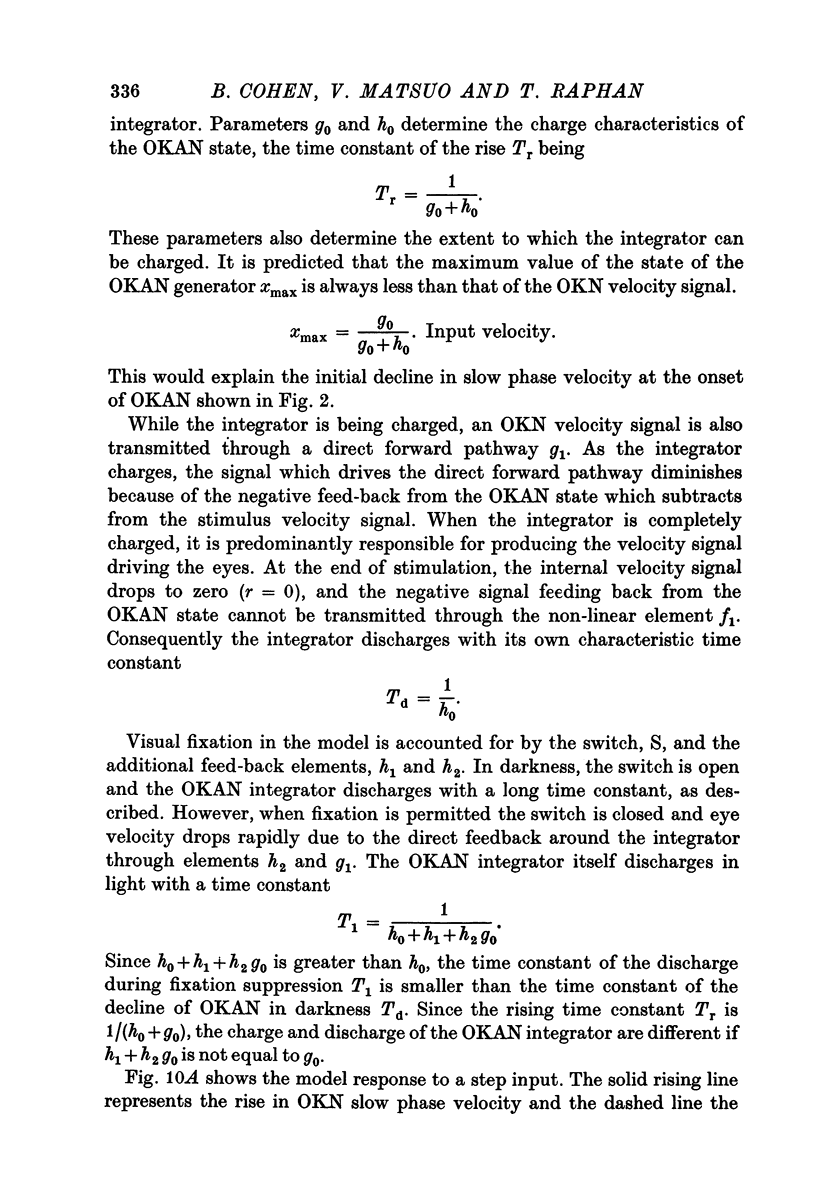
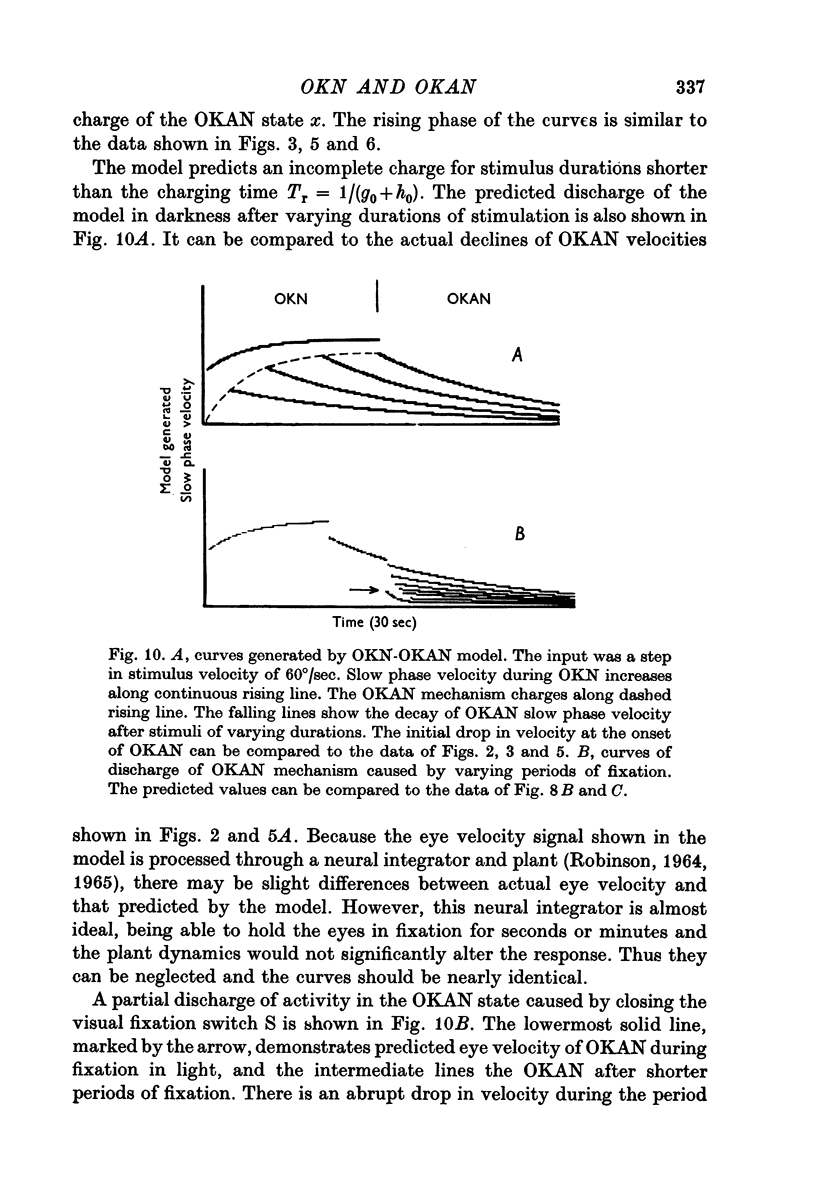
6. A model based on a state realisation of a peak detector was formulated which approximately reproduces the salient characteristics of OKN and OKAN. This model predicts the three dominant characteristics of OKAN: (1) charge over 5-7 sec, (2) slow discharge in darkness, and (3) rapid discharge with visual fixation. With the addition of direct fast forward pathways, it also correctly predicts the rapid and slow rise in OKN. We postulate that OKAN is produced by a central integrator which is also active during OKN. Presumably this integrator acts to maximize velocities during OKN and to smooth and stabilize ocular following during movement of the visual surround.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASCHAN G., BERGSTEDT M., STAHLE J. Nystagmography; recording of nystagmus in clinical neuro-otological examinations. Acta Otolaryngol Suppl. 1956;129:1–103. [PubMed] [Google Scholar]
- Aschoff J. C., Cohen B. Changes in saccadic eye movements produced by cerebellar cortical lesions. Exp Neurol. 1971 Aug;32(2):123–133. doi: 10.1016/0014-4886(71)90056-2. [DOI] [PubMed] [Google Scholar]
- Bond H. W., Ho P. Solid miniature silver-silver chloride electrodes for chronic implantation. Electroencephalogr Clin Neurophysiol. 1970 Feb;28(2):206–208. doi: 10.1016/0013-4694(70)90190-2. [DOI] [PubMed] [Google Scholar]
- Brandt T., Dichgans J., Büchle W. Motion habituation: inverted self-motion perception and optokinetic after-nystagmus. Exp Brain Res. 1974;21(4):337–352. doi: 10.1007/BF00237897. [DOI] [PubMed] [Google Scholar]
- Brandt T., Dichgans J. Circularvektion, optische Pseudocoriolis-Effekte und optokinetischer Nachnystagmus. Eine vergleichende Untersuchung subjektiver und objektiver optokinetischer Nacheffekte. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1972;184(1):42–57. doi: 10.1007/BF00410494. [DOI] [PubMed] [Google Scholar]
- Brandt T., Dichgans J., Koenig E. Differential effects of central verses peripheral vision on egocentric and exocentric motion perception. Exp Brain Res. 1973 Mar 19;16(5):476–491. doi: 10.1007/BF00234474. [DOI] [PubMed] [Google Scholar]
- Büttner U., Waespe W., Henn V. Duration and direction of optokinetic after-nystagmus as a function of stimulus exposure time in the monkey. Arch Psychiatr Nervenkr (1970) 1976 Dec 30;222(4):281–291. doi: 10.1007/BF00343237. [DOI] [PubMed] [Google Scholar]
- Cohen B., Uemura T., Takemori S. Effects of labyrinthectomy on optokinetic nystagmus (OKN) and optokinetic after-nystagmus (OKAN). Int J Equilib Res. 1973 Jun;3(1):88–93. [PubMed] [Google Scholar]
- Collewijn H. An analog model of the rabbit's optokinetic system. Brain Res. 1972 Jan 14;36(1):71–88. doi: 10.1016/0006-8993(72)90767-6. [DOI] [PubMed] [Google Scholar]
- Collewijn H. Latency and gain of the rabbit's optokinetic reactions to small movements. Brain Res. 1972 Jan 14;36(1):59–70. doi: 10.1016/0006-8993(72)90766-4. [DOI] [PubMed] [Google Scholar]
- Gonshor A., Jones G. M. Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol. 1976 Apr;256(2):381–414. doi: 10.1113/jphysiol.1976.sp011330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonshor A., Jones G. M. Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiol. 1976 Apr;256(2):361–379. doi: 10.1113/jphysiol.1976.sp011329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Shiida T., Yagi N., Yamamoto M. Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res. 1974 Jan 4;65(1):170–174. doi: 10.1016/0006-8993(74)90344-8. [DOI] [PubMed] [Google Scholar]
- KRIEGER H. P., BENDER M. B. Optokinetic afternystagmus in the monkey. Electroencephalogr Clin Neurophysiol. 1956 Feb;8(1):97–106. doi: 10.1016/0013-4694(56)90036-0. [DOI] [PubMed] [Google Scholar]
- Koerner F., Schiller P. H. The optokinetic response under open and closed loop conditions in the monkey. Exp Brain Res. 1972;14(3):318–330. doi: 10.1007/BF00816166. [DOI] [PubMed] [Google Scholar]
- Komatsuzaki A., Harris H. E., Alpert J., Cohen B. Horizontal nystagmus of rhesus monkeys. Acta Otolaryngol. 1969 May;67(5):535–551. doi: 10.3109/00016486909125481. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Fuchs A. F. Response of flocculus Purkinje cells to adequate vestibular stimulation in the alert monkey: fixation vs. compensatory eye movements. Brain Res. 1974 Apr 5;69(2):347–353. doi: 10.1016/0006-8993(74)90013-4. [DOI] [PubMed] [Google Scholar]
- Maekawa K., Simpson J. I. Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J Neurophysiol. 1973 Jul;36(4):649–666. doi: 10.1152/jn.1973.36.4.649. [DOI] [PubMed] [Google Scholar]
- Miles F. A., Fuller J. H. Adaptive plasticity in the vestibulo-ocular responses of the rhesus monkey. Brain Res. 1974 Nov 22;80(3):512–516. doi: 10.1016/0006-8993(74)91035-x. [DOI] [PubMed] [Google Scholar]
- ROBINSON D. A. THE MECHANICS OF HUMAN SACCADIC EYE MOVEMENT. J Physiol. 1964 Nov;174:245–264. doi: 10.1113/jphysiol.1964.sp007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. A. The mechanics of human smooth pursuit eye movement. J Physiol. 1965 Oct;180(3):569–591. doi: 10.1113/jphysiol.1965.sp007718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori S., Cohen B. Loss of visual suppression of vestibular nystagmus after flocculus lesions. Brain Res. 1974 Jun 7;72(2):213–224. doi: 10.1016/0006-8993(74)90860-9. [DOI] [PubMed] [Google Scholar]
- Takemori S., Cohen B. Visual suppression of vestibular nystagmus in rhesus monkeys. Brain Res. 1974 Jun 7;72(2):203–212. doi: 10.1016/0006-8993(74)90859-2. [DOI] [PubMed] [Google Scholar]
- Takemori S. The similarities of optokinetic after-nystagmus to the vestibular nystagmus. Ann Otol Rhinol Laryngol. 1974 Mar-Apr;83(2):230–238. doi: 10.1177/000348947408300211. [DOI] [PubMed] [Google Scholar]
- Uemura T., Cohen B. Effects of vestibular nuclei lesions on vestibulo-ocular reflexes and posture in monkeys. Acta Otolaryngol Suppl. 1973;315:1–71. doi: 10.3109/00016487409129565. [DOI] [PubMed] [Google Scholar]
- Yasui S., Young L. R. Perceived visual motion as effective stimulus to pursuit eye movement system. Science. 1975 Nov 28;190(4217):906–908. doi: 10.1126/science.1188373. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Yee R. D., Robinson D. A. Optokinetic responses in labyrinthine-defective human beings. Brain Res. 1976 Aug 27;113(2):423–428. doi: 10.1016/0006-8993(76)90955-0. [DOI] [PubMed] [Google Scholar]


