Abstract
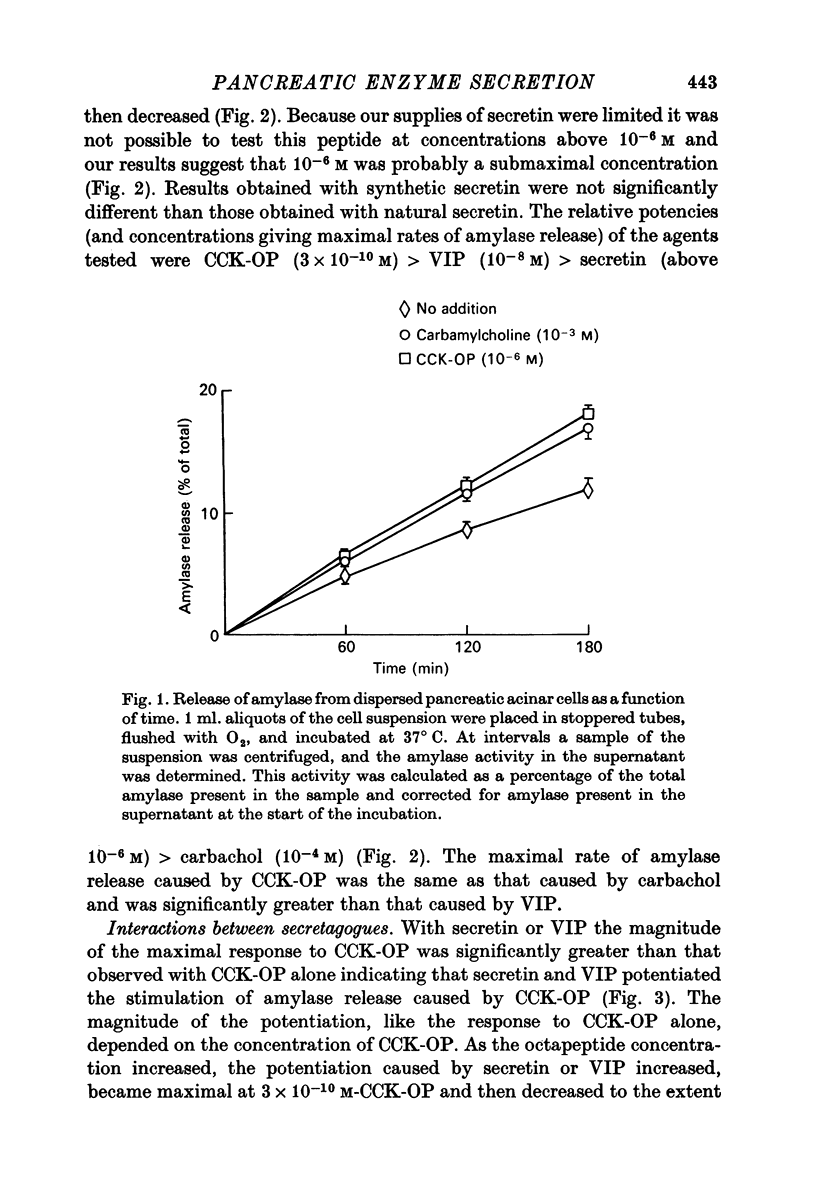
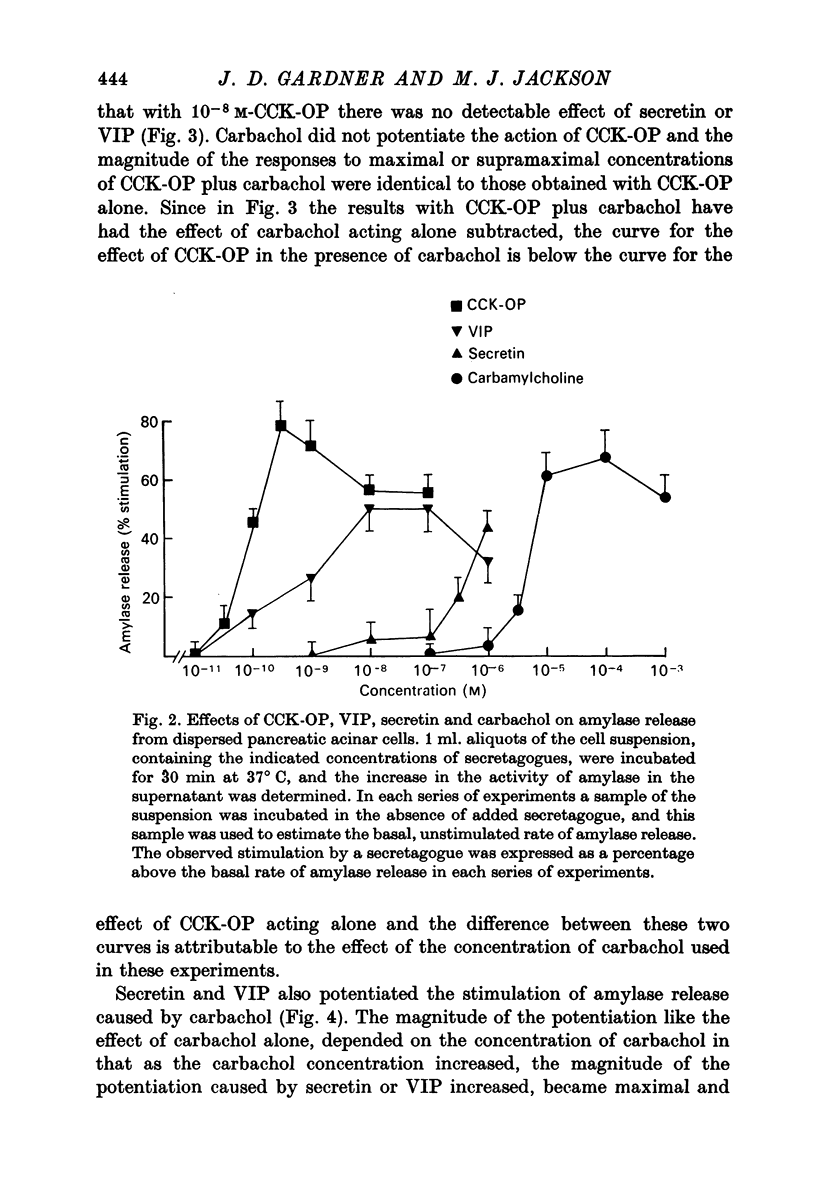
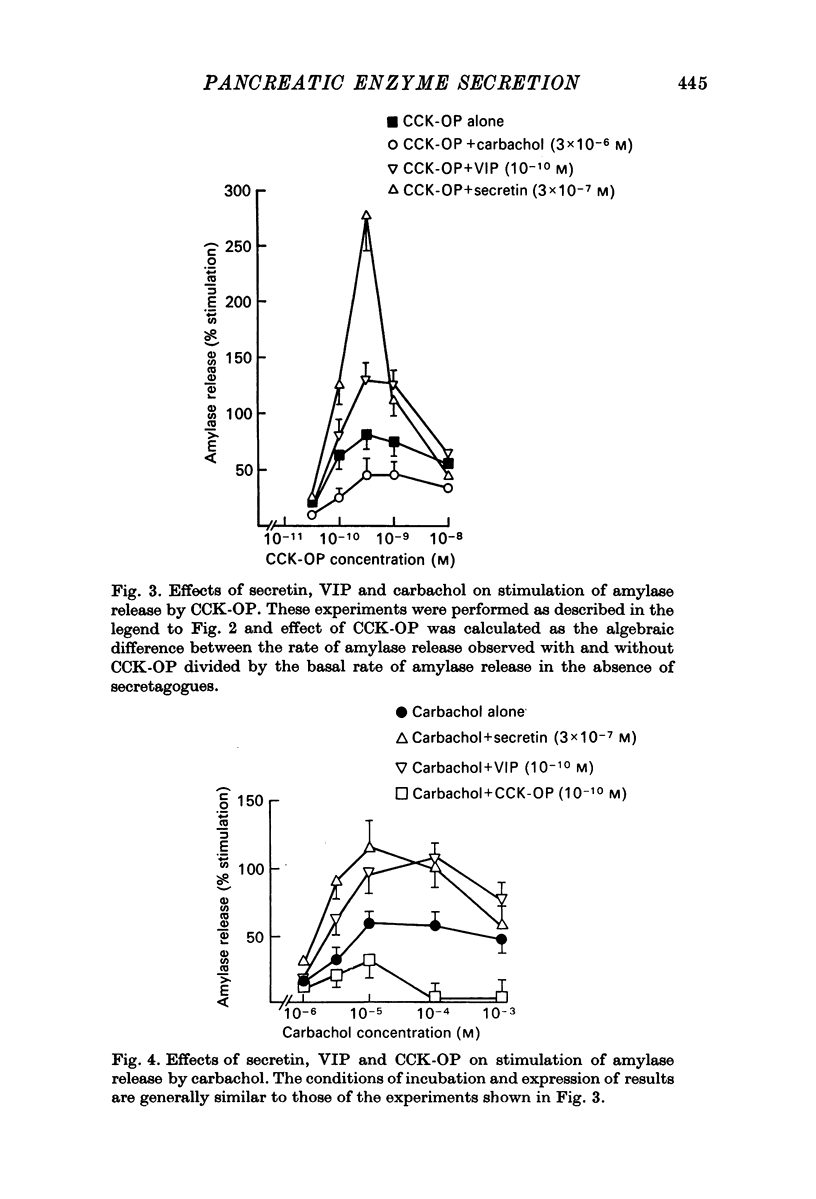
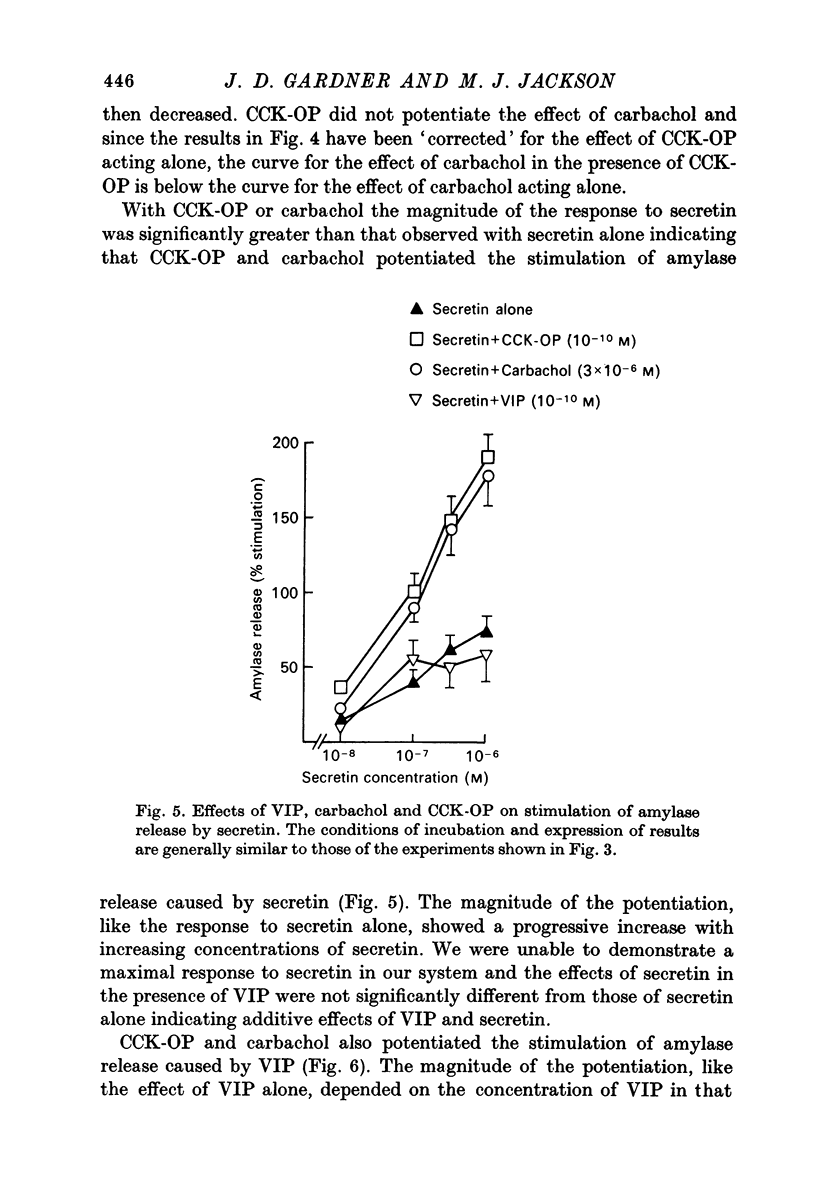
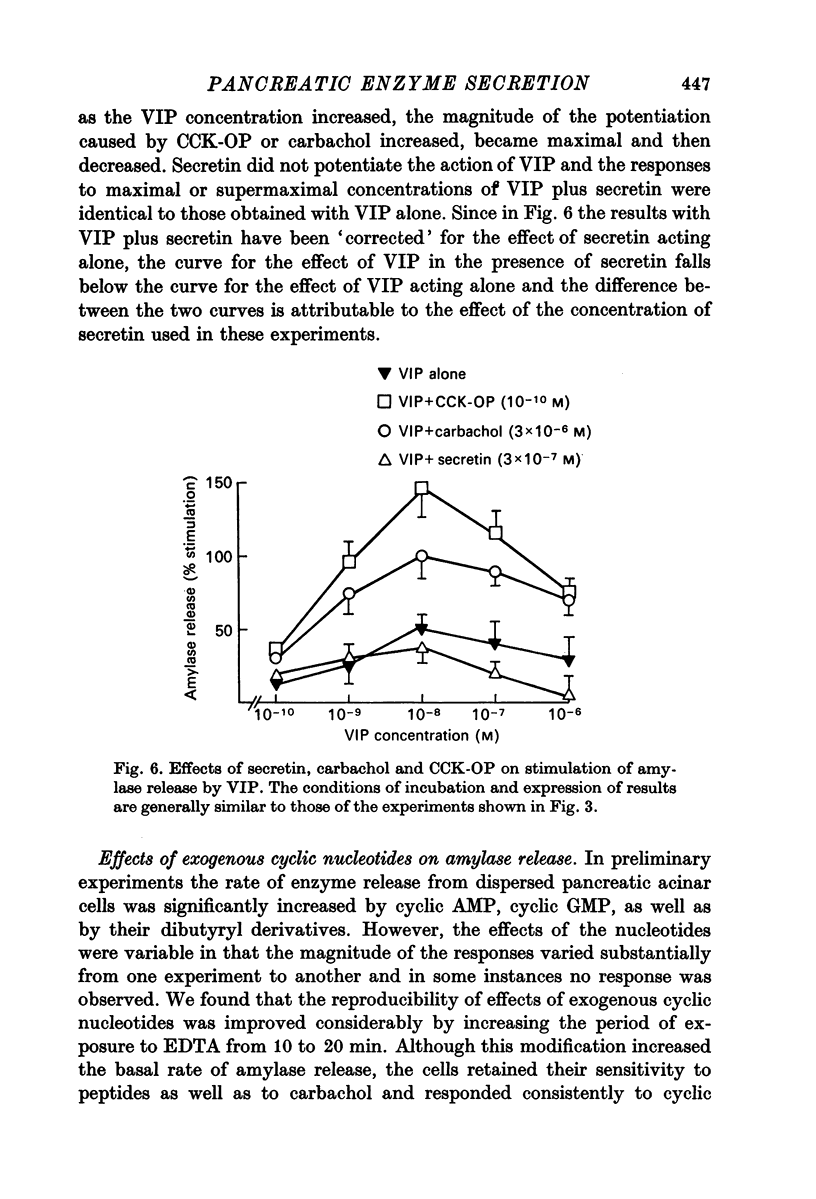
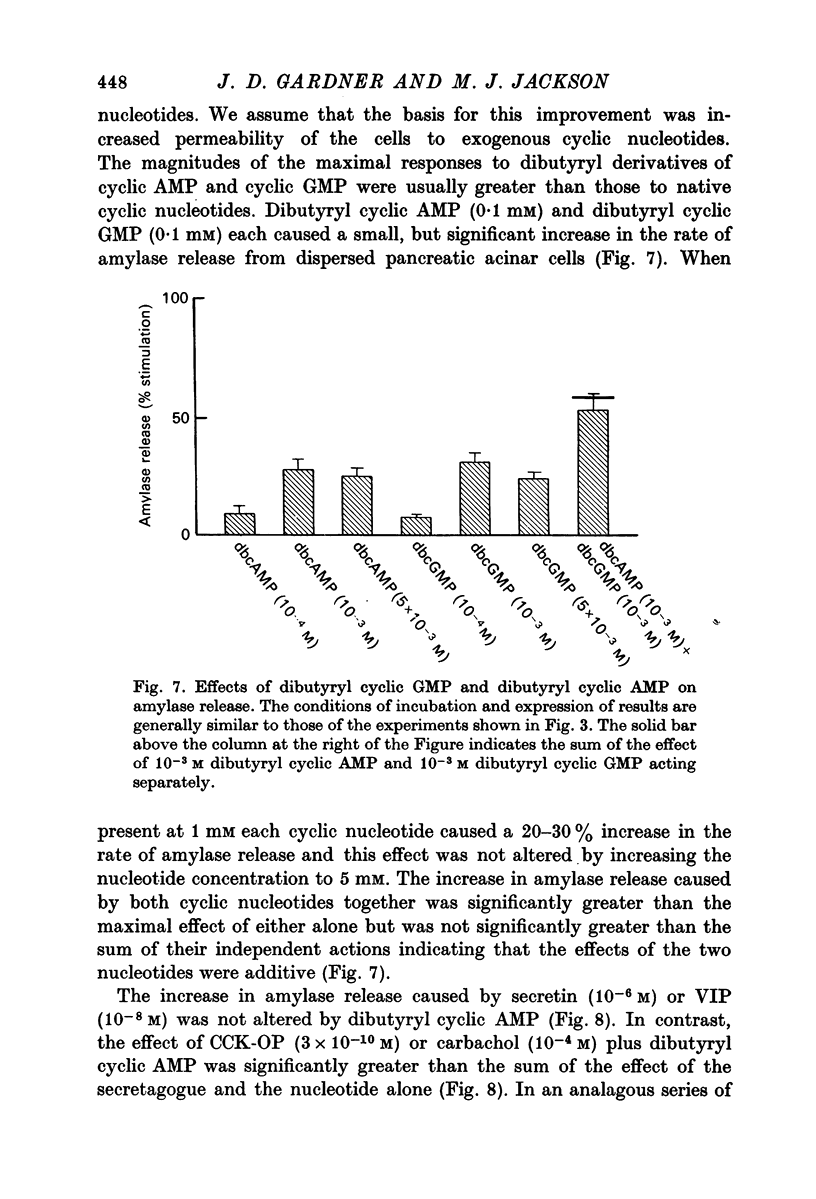
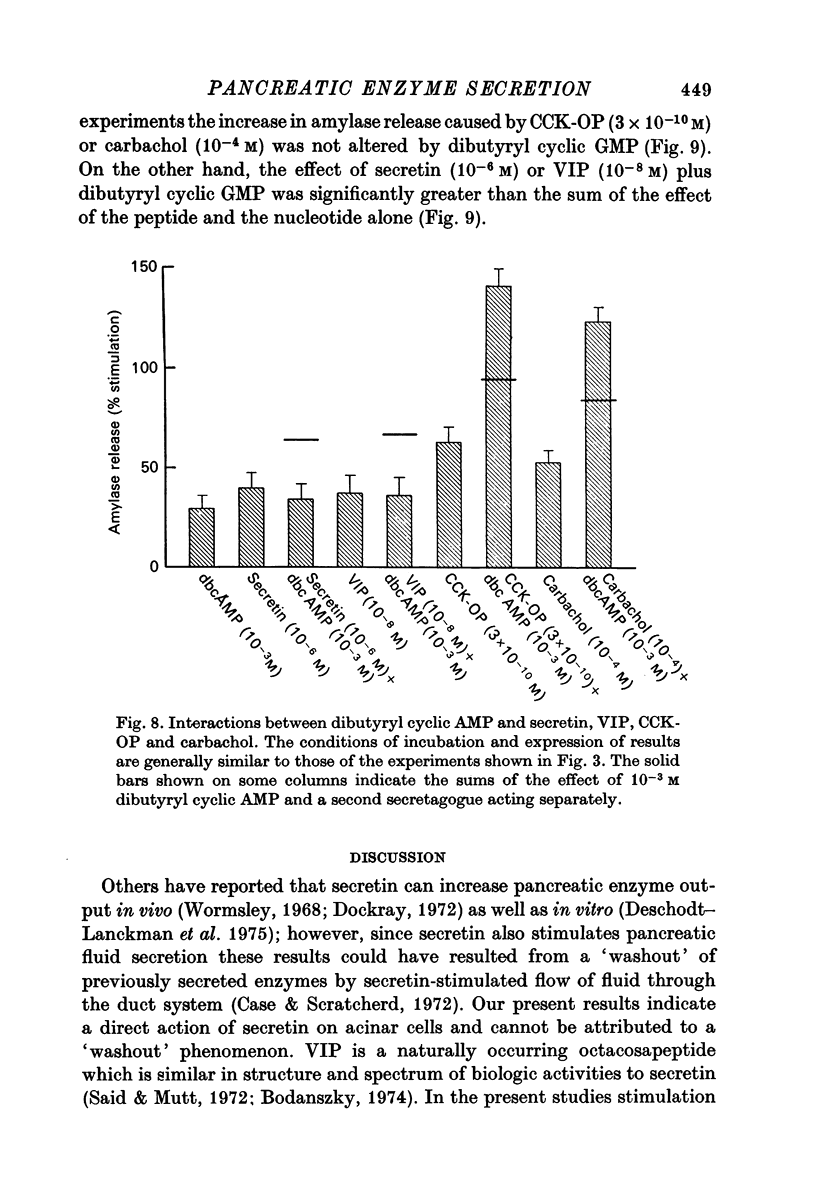
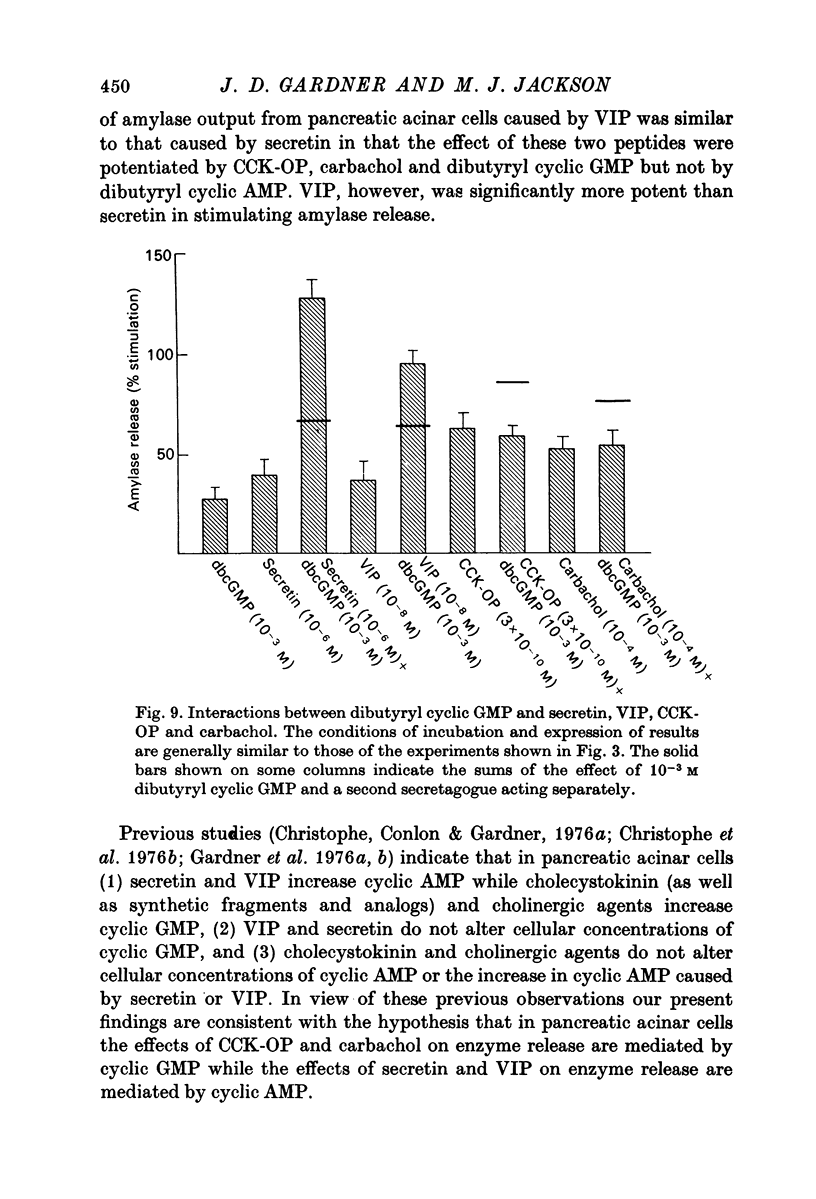
1. A study has been made of factors influencing release of amylase from dispersed pancreatic acinar cells. 2. In the basal, unstimulated, condition cells released 2-3% of the total amylase present in 30 min. 3. The rate of amylase release was stimulated 50-70% by C-terminal octapeptide of cholecystokinin (CCK-OP, maximally effective concentration, 3 X 10(-10) M); carbamylcholine (maximally effective concentration, 10(-5 M); secretin (maximally effective concentration greater than 10(-6) M); vasoactive intestinal peptide (VIP, maximally effective concentration, 10(-8) M); and adenosine 3':5' monophosphate (cyclic AMP) and guanosine 3':5' monophosphate (cyclic GMP) as well as their dibutyryl derivatives (maximally effective concentrations, 10(-3) M). 4. The responses to CCK-OP or carbamylcholine were potentiated by secretin, VIP or dibutyryl cyclic AMP. 5. The responses to secretin or VIP were potentiated by CCK-OP, carbamylcholine, or dibutyryl cyclic GMP. 6. There appear to be two pathways for the regulation of amylase release from pancreatic acinar cells: one pathway can be stimulated by cholecystokinin or cholinergic agonists, and the response to these stimuli is mediated by cyclic GMP; the other pathway can be stimulated by secretin or VIP, and the response to these stimuli is mediated by cyclic AMP.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J., Bhoola K. D., Harvey R. F. Intracellular messenger role of cyclic GMP in exocrine pancreas. Nature. 1976 Jul 29;262(5567):404–406. doi: 10.1038/262404a0. [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Jamieson J. D. Structural and functional characterization of isolated pancreatic exocrine cells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3028–3032. doi: 10.1073/pnas.69.10.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. II. Functional characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1057–1073. doi: 10.1083/jcb.63.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauduin H., Rochus L., Vincent D., Dumont J. E. Role of cyclic 3',5'-amp in the action of physiological secretagogues on the metabolism of rat pancreas in vitro. Biochim Biophys Acta. 1971 Oct;252(1):171–183. doi: 10.1016/0304-4165(71)90106-1. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Harper A. A., Scratcherd T. Potentiation of secretin stimulation of the pancreas. J Physiol. 1967 Jun;190(3):519–530. doi: 10.1113/jphysiol.1967.sp008225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Johnson M., Scratcherd T., Sherratt H. S. Cyclic adenosine 3',5'-monophosphate concentration in the pancreas following stimulation by secretin, cholecystokinin-pancreozymin and acetylcholine. J Physiol. 1972 Jun;223(3):669–684. doi: 10.1113/jphysiol.1972.sp009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T. The actions of dibutyryl cyclic adenosine 3',5'-monophosphate and methyl xanthines on pancreatic exocrine secretion. J Physiol. 1972 Jun;223(3):649–667. doi: 10.1113/jphysiol.1972.sp009867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe J. P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Binding of radioiodinated peptide. J Biol Chem. 1976 Aug 10;251(15):4629–4634. [PubMed] [Google Scholar]
- Christophe J. P., Frandsen E. K., Conlon T. P., Krishna G., Gardner J. D. Action of cholecystokinin, cholinergic agents, and A-23187 on accumulation of guanosine 3':5'-monophosphate in dispersed guinea pig pancreatic acinar cells. J Biol Chem. 1976 Aug 10;251(15):4640–4645. [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Labrie F., Christophe J. In vitro interactions of gastrointestinal hormones on cyclic adenosine 3':5'-monophosphate levels and amylase output in the rat pancreas. Gastroenterology. 1975 Feb;68(2):318–325. [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Lammens M., Christophe J. In vitro action of bombesin and bombesin-like peptides on amylase secretion, calcium efflux, and adenylate cyclase activity in the rat pancreas: a comparison with other secretagogues. J Clin Invest. 1976 Oct;58(4):891–898. doi: 10.1172/JCI108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. The action of scretin, cholecystokinin-pancreozymin and caerulein on pancreatic secretion in the rat. J Physiol. 1972 Sep;225(3):679–692. doi: 10.1113/jphysiol.1972.sp009963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fölsch U. R., Wormsley K. G. Pancreatic enzyme response to secretin and cholecystokinin-pancreozymin in the rat. J Physiol. 1973 Oct;234(1):79–94. doi: 10.1113/jphysiol.1973.sp010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P., Adams T. D. Cyclic AMP in pancreatic acinar cells: effects of gastrointestinal hormones. Gastroenterology. 1976 Jan;70(1):29–35. [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P., Kleveman H. L., Adams T. D., Ondetti M. A. Action of cholecystokinin and cholinergic agents on calcium transport in isolated pancreatic acinar cells. J Clin Invest. 1975 Aug;56(2):366–375. doi: 10.1172/JCI108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig T. H. Regulation of pancreatic acinar function: effects of cyclic AMP, dibutyryl cyclic AMP, and theophylline in vitro. Can J Physiol Pharmacol. 1974 Aug;52(4):780–785. doi: 10.1139/y74-102. [DOI] [PubMed] [Google Scholar]
- Haymovits A., Scheele G. A. Cellular cyclic nucleotides and enzyme secretion in the pancreatic acinar cell. Proc Natl Acad Sci U S A. 1976 Jan;73(1):156–160. doi: 10.1073/pnas.73.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler S., Fast D., Tenenhouse A. Role of Ca 2+ and cyclic AMP in protein secretion from rat exocrine pancreas. Biochim Biophys Acta. 1972 Oct 25;279(3):561–572. doi: 10.1016/0304-4165(72)90178-x. [DOI] [PubMed] [Google Scholar]
- Heisler S., Grondin G. Absence of effects of dibutyryl cyclic guanosine 3',5'-monophosphate on release of alpha-amylase, 45Ca efflux, and protein synthesis in rat pancreas in vitro. Experientia. 1975 Aug 15;31(8):936–938. doi: 10.1007/BF02358862. [DOI] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Kimbert D. V. Cyclic nucleotides and their role in gastrointestinal secretion. Gastroenterology. 1974 Nov;67(5):1023–1064. [PubMed] [Google Scholar]
- Knodell R. G., Toskes P. P., Reber H. A., Brooks F. P. Significance of cyclic AMP in the regulation of exocrine pancreas secretion. Experientia. 1970 May 15;26(5):515–517. doi: 10.1007/BF01898480. [DOI] [PubMed] [Google Scholar]
- Kulka R. G., Sternlicht E. Enzyme secretion in mouse pancreas mediated by adenosine-3'5'-cyclic phosphate and inhibited by adenosine-3'-phosphate. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1123–1128. doi: 10.1073/pnas.61.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. H., Spingola J., Grossman M. I. Endogenous cholecystokinin potentiates exogenous secretin on pancreas of dog. Am J Physiol. 1971 Sep;221(3):742–747. doi: 10.1152/ajplegacy.1971.221.3.742. [DOI] [PubMed] [Google Scholar]
- Ridderstap A. S., Bonting S. L. Cyclic AMP and enzyme secretion by the isolated rabbit pancreas. Pflugers Arch. 1969;313(1):62–70. doi: 10.1007/BF00586329. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Structural requirements for effects of vasoactive intestinal peptide and secretin on cellular adenosine 3':5'-monophosphate. J Biol Chem. 1976 Aug 10;251(15):4635–4639. [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lanckman M., De Neef P., Borgeat P., Christophe J. In vivo effects of pancreozymin, secretin, vasoactive intestinal polypeptide and pilocarpine on the levels of cyclic AMP and cyclic GMP in the rat pancreas. FEBS Lett. 1974 Jul 15;43(2):139–143. doi: 10.1016/0014-5793(74)80986-5. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lanckman M., De Neef P., Christophe J. Effects of somatostatin on pancreatic exocrine function. Interaction with secretin. Biochem Biophys Res Commun. 1975 Nov 3;67(1):315–323. doi: 10.1016/0006-291x(75)90318-6. [DOI] [PubMed] [Google Scholar]
- Said S. I., Mutt V. Isolation from porcine-intestinal wall of a vasoactive octacosapeptide related to secretin and to glucagon. Eur J Biochem. 1972 Jul 13;28(2):199–204. doi: 10.1111/j.1432-1033.1972.tb01903.x. [DOI] [PubMed] [Google Scholar]
- Shelby H. T., Gross L. P., Lichty P., Gardner J. D. Action of cholecystokinin and cholinergic agents on membrane-bound calcium in dispersed pancreatic acinar cells. J Clin Invest. 1976 Dec;58(6):1482–1493. doi: 10.1172/JCI108605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way L. W., Grossman M. I. Pancreatic stimulation by duodenal acid and exogenous hormones in conscious cats. Am J Physiol. 1970 Aug;219(2):449–454. doi: 10.1152/ajplegacy.1970.219.2.449. [DOI] [PubMed] [Google Scholar]
- Wormsley K. G. A comparison of the response to secretin, pancreozymin and a combination of these hormones, in man. Scand J Gastroenterol. 1969;4(5):411–417. [PubMed] [Google Scholar]


