Abstract
The 4-chloro- and 2,4-dichlorophenol-degrading strain Rhodococcus opacus 1CP has previously been shown to acquire, during prolonged adaptation, the ability to mineralize 2-chlorophenol. In addition, homogeneous chlorocatechol 1,2-dioxygenase from 2-chlorophenol-grown biomass has shown relatively high activity towards 3-chlorocatechol. Based on sequences of the N terminus and tryptic peptides of this enzyme, degenerate PCR primers were now designed and used for cloning of the respective gene from genomic DNA of strain 1CP. A 9.5-kb fragment containing nine open reading frames was obtained on pROP1. Besides other genes, a gene cluster consisting of four chlorocatechol catabolic genes was identified. As judged by sequence similarity and correspondence of predicted N termini with those of purified enzymes, the open reading frames correspond to genes for a second chlorocatechol 1,2-dioxygenase (ClcA2), a second chloromuconate cycloisomerase (ClcB2), a second dienelactone hydrolase (ClcD2), and a muconolactone isomerase-related enzyme (ClcF). All enzymes of this new cluster are only distantly related to the known chlorocatechol enzymes and appear to represent new evolutionary lines of these activities. UV overlay spectra as well as high-pressure liquid chromatography analyses confirmed that 2-chloro-cis,cis-muconate is transformed by ClcB2 to 5-chloromuconolactone, which during turnover by ClcF gives cis-dienelactone as the sole product. cis-Dienelactone was further hydrolyzed by ClcD2 to maleylacetate. ClcF, despite its sequence similarity to muconolactone isomerases, no longer showed muconolactone-isomerizing activity and thus represents an enzyme dedicated to its new function as a 5-chloromuconolactone dehalogenase. Thus, during 3-chlorocatechol degradation by R. opacus 1CP, dechlorination is catalyzed by a muconolactone isomerase-related enzyme rather than by a specialized chloromuconate cycloisomerase.
Chloroaromatic compounds as a class tend to be relatively persistent in the environment and often are degraded slowly or incompletely. Nevertheless, a large number of bacteria are known to utilize chloroaromatic compounds as sources of carbon and energy. For many of the mono- and dichlorinated aromatic compounds and for some of the more chlorinated ones, it has been shown that under aerobic conditions they may be degraded via chlorocatechols as central ring cleavage intermediates (38, 40).
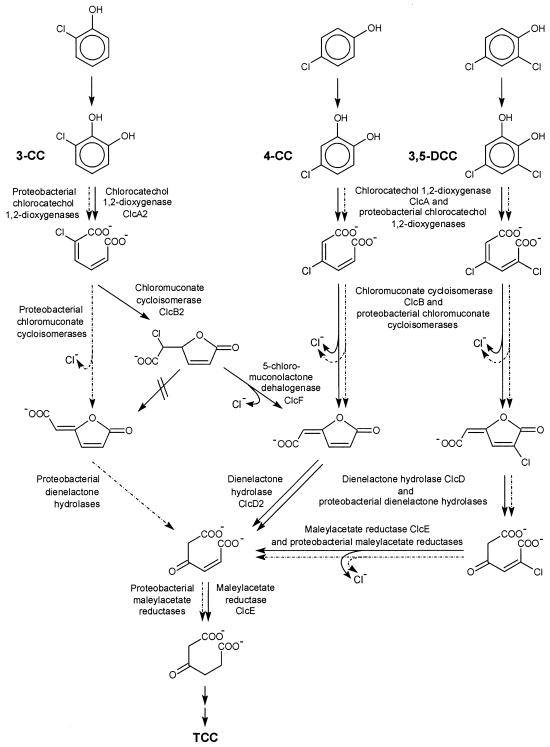
The catabolism of chlorocatechols has mainly been investigated with Proteobacteria. While productive meta cleavage pathways have recently been shown to occur (17, 26), the majority of known strains use a modified ortho cleavage pathway. In this pathway, 3-chlorocatechol is cleaved by chlorocatechol 1,2-dioxygenase (EC 1.13.11.1) to give 2-chloro-cis,cis-muconate (Fig. 1). In proteobacterial chlorocatechol pathways, this intermediate is converted by chloromuconate cycloisomerase (EC 5.5.1.7) to the so-called trans-dienelactone (trans-4-carboxymethylenebut-2-en-4-olide). This reaction comprises a cycloisomerization to 2-chloro- or 5-chloromuconolactone and a dehalogenation of the latter to the trans-dienelactone (42, 49). Since proteobacterial muconate cycloisomerases (EC 5.5.1.1), in contrast, convert 2-chloro-cis,cis-muconate only to a mixture of 2-chloro- and 5-chloromuconolactone, the dehalogenation is enzyme catalyzed, and this ability had to be evolved during divergence of proteobacterial muconate and chloromuconate cycloisomerases (47). The trans-dienelactone resulting from dehalogenation is cleaved by a dienelactone hydrolase (EC 3.1.1.45) to give maleylacetate, which is then reduced by maleylacetate reductase (EC 1.3.1.32) to give 3-oxoadipate, an intermediate of usual pathways for the catabolism of aromatic compounds (Fig. 1).
FIG. 1.
Degradative pathways for 3-chlorocatechol (3-CC), 4-chlorocatechol (4-CC), and 3,5-dichlorocatechol (3,5-DCC), the central intermediates in chlorophenol degradation, as found in R. opacus 1CP (solid lines). Dashed arrows show the known proteobacterial 3-chlorocatechol pathway for comparison. Enzyme names and their designations as gene products are given.
4-Chlorocatechol may be cleaved by chlorocatechol 1,2-dioxygenase to 3-chloro-cis,cis-muconate. From this intermediate, chloromuconate cycloisomerases generate the cis-dienelactone (Fig. 1). In this case, the enzymes had to evolve the ability to avoid formation of the toxic protoanemonin which is generated from 3-chloro-cis,cis-muconate by usual muconate cycloisomerases (1). Obviously, in chloromuconate cycloisomerases the rate of chloride elimination from the enol-enolate reaction intermediate was enhanced relative to the rate of proton addition to it (21). The cis-dienelactone, like the trans isomer, is again hydrolyzed by dienelactone hydrolase to maleylacetate and then reduced to 3-oxoadipate (Fig. 1). 3,5-Dichlorocatechol, generated, for example, from the herbicide 2,4-dichlorophenoxyacetate, is converted basically like 4-chlorocatechol, the difference being that the intermediates carry an additional chlorine substituent (Fig. 1). The latter is eliminated from chloromaleylacetate by maleylacetate reductase, which in a first reduction generates maleylacetate, which is then reduced to 3-oxoadipate (18, 50).
Among the Actinobacteria, chlorocatechol catabolism has been investigated in some detail only for Rhodococcus opacus 1CP. This strain was originally isolated based on its ability to utilize 2,4-dichlorophenol and 4-chlorophenol, which are degraded via 3,5-dichlorocatechol and 4-chlorocatechol, respectively (11). The enzymes which strain 1CP employs for the further catabolism of these chlorocatechols turned out to be unusual in various ways. (i) The enzymes of R. opacus 1CP, especially the chloromuconate cycloisomerase and the dienelactone hydrolase, were found to have a much higher substrate specificity than their proteobacterial counterparts (23, 24, 44). The latter showed high specificity constants for 3-chloro- and 2,4-dichloromuconate or cis-dienelactone and 2-chloro-cis-dienelactone but extremely low turnover of the metabolites expected to be generated from 3-chlorocatechol, that is, 2-chloromuconate and trans-dienelactone. (ii) The chloromuconate cycloisomerase turned out to be unable to dehalogenate 2-chloro-cis,cis-muconate, while 3-chloro- and 2,4-dichloro-cis,cis-muconate were converted to the expected (2-chloro-)cis-dienelactone (44). (iii) Sequencing of the 4-chlorocatechol-3,5-dichlorocatechol catabolic gene cluster revealed a low sequence similarity to the corresponding proteobacterial genes and, together with biochemical evidence, suggested that the chlorocatechol pathway of strain 1CP should have evolved to be functionally convergent to the pathway in proteobacteria (8).
A few years ago, it was found that R. opacus 1CP could be adapted to utilize 2-chlorophenol, 3-chlorophenol, and 3-chlorobenzoate in addition to the known substrates, 4-chloro- and 2,4-dichlorophenol (30; S. Lakner personal communication). Under aerobic conditions, 2-chlorophenol is most likely degraded via a 3-chlorocatechol pathway, and 3-chlorocatechol and 2-chloro-cis,cis-muconate were formed from 2-chlorophenol by washed cells (30). However, as explained above, chloromuconate cycloisomerase and the dienelactone hydrolase from 4-chlorocatechol-3,5-dichlorocatechol catabolism of 1CP were obviously not useful for the known 3-chlorocatechol degradation pathway, and the chlorocatechol 1,2-dioxygenase also converted 3-chlorocatechol with poor activity. Thus, the occurrence of a separate pathway for 3-chlorocatechol conversion had to be investigated. Recently, we reported the purification and characterization of a second chlorocatechol 1,2-dioxygenase, a second chloromuconate cycloisomerase, and a second dienelactone hydrolase from 2-chlorophenol-grown cells of R. opacus 1CP (29). Unexpectedly, the second dienelactone hydrolase also was specific for cis-dienelactone and did not convert the trans isomer. The second chloromuconate cycloisomerase was specific for 2-chloro-cis,cis-muconate, while 3-chloro- and 2,4-dichloro-cis,cis-muconate were not substrates for the enzyme. Initial evidence that the second chloromuconate cycloisomerase of 1CP also converts 2-chloro-cis,cis-muconate only to 5-chloromuconolactone was obtained. A 5-chloromuconolactone-converting enzyme was highly purified and appeared to generate the cis-dienelactone from 5-chloromuconolactone, thus completing the pathway (29).
The work presented here aimed at the identification of the genetic background of the 3-chlorocatechol degradation by strain 1CP. In addition, the relatedness of the genes comprising it to known genes had to be elucidated. Although some aspects of the biochemistry of the involved genes were previously presented (29), the unexpected dehalogenation reaction had to be characterized in a more detailed way, for example, with respect to the possible formation of by-products. Since the 5-chloromuconolactone-converting enzyme turned out to be highly similar to muconolactone isomerases (EC 5.3.3.4), its ability to convert muconolactone had to be determined, thus providing information on the degree of evolutionary adaptation and specialization of this 5-chloromuconolactone dehalogenase.
(Some of the results presented here have previously been reported in a preliminary communication [O. V. Moiseeva, S. R. Kaschabek, L. Golovleva, and M. Schlömann, Biospektrum special issue, poster no. 13, p. 47, 2000].)
MATERIALS AND METHODS
Strains, plasmids, and cultivation conditions.
R. opacus 1CP was previously isolated from an enrichment culture with 2,4-dichlorophenol as the sole carbon source (11). Cultivation of R. opacus 1CP on 2-chlorophenol was performed as described previously (29). For DNA isolation, R. opacus 1CP was grown at 30°C in a mineral medium (pH 7.2) (4) with a doubled phosphate buffer concentration supplied with 20 g of glycine per liter and 2 g of glucose per liter. Pseudomonas putida PRS2000 (32) was grown in the same medium without glycine and glucose but with 5 mM benzoate as a carbon source. Escherichia coli DH5α was obtained from GIBCO BRL. Usually, E. coli DH5α was grown aerobically with constant shaking at 37°C in Luria-Bertani medium (39) containing 100 μg of ampicillin per ml.
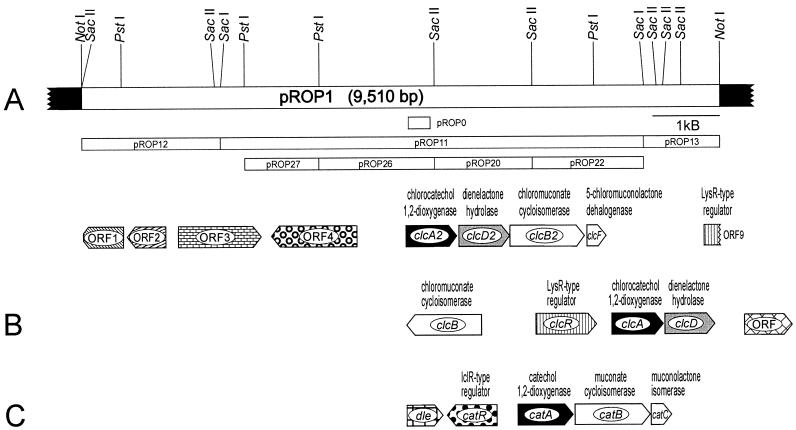
pBluescript II SK(+) is a phagemid derived from pUC19 (Plac lacZ′ Apr; f1[+] origin, ColE1 origin) and was purchased from Stratagene. pROP0 was obtained by cloning the ca. 0.33-kb PCR product of primers fw-om1 and rev-om1 with R. opacus 1CP DNA into the EcoRV site of pBluescript II SK(+). pROP1 was obtained by cloning a ca. 9.5-kb NotI fragment of R. opacus 1CP DNA in pBluescript II SK(+). For DNA sequencing, several subclones were generated from pROP1, the most important of which are shown in Fig. 2. Additional subclones were constructed by using BamHI, EcoRV, EcoRI, HindIII, KpnI, SalI, SmaI, and XhoI restriction fragments.
FIG. 2.
(A) Restriction map of the insert of pROP1 carrying 9,510 bp of R. opacus 1CP DNA (white bar) in the multiple cloning site of pBluescript II SK(+) (black bars). Shown below the map are the most important subclones and the probe pROP0. The locations and orientations of ORFs on pROP1 are indicated. (B and C) For comparison, R. opacus 1CP gene clusters for 4-chloro- and 3,5-dichlorocatechol catabolism (B) and for catechol catabolism (C) are shown. Homologous genes are presented in the same shade.
Preparation of cell extract.
Frozen cells of R. opacus strain 1CP grown on 2-chlorophenol (wet weight, 6 g) were thawed and resuspended in 3 ml of 50 mM Tris-HCl buffer (pH 7.2)-2 mM MnSO4. Disruption was achieved by grinding of the cell suspension with glass beads (diameter, 200 to 300 μm) in a type MM2 mill (Retsch GmbH, Haan, Germany) at 4°C for 15 min. The glass beads were separated from the rest of the mixture by filtration through a glass filter funnel and washed with the same buffer. Cell debris and unbroken cells were removed from this filtrate by centrifugation (100,000 × g, 30 min, 4°C). The clarified supernatant solution was used as crude cell extract for the purification of chloromuconate cycloisomerase ClcB2, dienelactone hydrolase ClcD2, and the 5-chloromuconolactone-converting enzyme ClcF.
Enzyme assays and estimation of protein concentration.
The activities of chlorocatechol 1,2-dioxygenase ClcA2 and chloromuconate cycloisomerase ClcB2 were measured spectrophotometrically at 260 nm with 3-chlorocatechol and 2-chloro-cis,cis-muconate, respectively, by procedures described previously (23, 44). A specific ɛ260 of 17,100 M−1 cm−1 was used for 2-chloro-cis,cis-muconate (5).
The activity of the 5-chloromuconolactone-converting enzyme ClcF in the absence of dienelactone hydrolase ClcD2 was measured spectrophotometrically at 280 nm by the formation of cis-dienelactone, using 0.1 mM 5-chloromuconolactone as the substrate. In crude extract or preparations containing additional dienelactone hydrolase activity, ClcF was only qualitatively detected by the spectral change of the absorption by 5-chloromuconolactone.
Dienelactone hydrolase ClcD2 was assayed at 280 nm according to a modification of the procedure of Schmidt and Knackmuss (42) in the presence of 50 mM Tris-HCl (pH 7.2) and 0.1 mM cis-dienelactone (ɛ260 = 17,000 M−1 cm−1 [41]).
Muconolactone isomerase was assayed at 230 nm according to a modification of the procedure of Ornston (32) in the presence of an excess of partially purified 3-oxoadipate enol-lactone hydrolase as auxiliary enzyme. A specific ɛ230 of 1,430 M−1 cm−1 was used.
3-Oxoadipate enol-lactone hydrolase activity was measured basically by the same procedure as for muconolactone isomerase but with an excess of the latter enzyme.
Protein concentrations were determined by the method of Bradford (2) with bovine serum albumin as the standard.
Protein purifications.
The purification of chlorocatechol 1,2-dioxygenase ClcA2, chloromuconate cycloisomerase ClcB2, the 5-chloromuconolactone-converting enzyme ClcF, and dienelactone hydrolase ClcD2 was performed by a previously described procedure (29).
Muconolactone isomerase and 3-oxoadipate enol-lactone hydrolase, which were necessary as a positive control or auxiliary enzyme, respectively, to detect muconolactone-converting activity of ClcF, were partially purified from an extract of benzoate-grown cells of P. putida PRS2000. The extract was first subjected to anion-exchange chromatography on a Q-Sepharose HP column, during which the two enzymes coeluted at 220 mM NaCl. The most active fractions were then combined, precipitated by ammonium sulfate treatment (final concentration, 3 M), and redissolved in 50 mM Tris-HCl buffer (pH 7.5) containing 1.2 M (NH4)2SO4. Hydrophobic interaction chromatography on a phenyl-Sepharose HP column led to the separation of the two activities. Muconolactone isomerase and 3-oxoadipate enol-lactone hydrolase eluted at 850 and 670 mM (NH4)2SO4, respectively. The fraction with highest isomerase activity was used to determine the relative substrate specificities of muconolactone isomerase against different substrates, and the fraction with highest enol-lactone hydrolase activity was used in muconolactone isomerase assays as an auxiliary enzyme removing the enol-lactone from the equilibrium.
SDS-polyacrylamide gel electrophoresis and Western blotting.
For purity tests of enzyme preparations discontinuous sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was carried out as previously described (7). Gels were stained with Coomassie brilliant blue R-250.
For N-terminal sequencing of the enzymes, ca. 2 μg of the purified preparations were run on an SDS-polyacrylamide gel (12% polyacrylamide for ClcA2 and ClcB2 and 15% for ClcF). Transfer of the proteins onto an Immobilon-P membrane (Millipore) was achieved by following the protocol from Moos (31). Electrotransfer was performed for 120 min at 0.01 A and 4°C in a Bio-Rad Trans-Blot cell. Staining and destaining of the membranes were performed as described previously (9). The visible protein bands were cut out, air dried, and subjected to N-terminal sequencing.
Protein cleavage, isolation of peptides, and sequencing of peptides and N termini.
Trypsin digestion of the homogeneous chlorocatechol 1,2-dioxygenase (dry weight, 49 μg) and subsequent separation of tryptic peptides by reversed-phase high-pressure liquid chromatography (HPLC) were performed basically by the procedure described previously (7). Selected peptide-containing fractions from reversed-phase HPLC were subjected to automated sequencing (Applied Biosystems model 473A).
Analytical methods.
HPLC analysis of metabolites was performed on a Dionex HPLC system with a diode array detector (model UVD 340) and Chromeleon software. As the stationary phase, a Eurospher-100 C18 reversed-phase column (Knauer, Berlin, Germany) with a 4-mm internal diameter and 125-mm length was used. The mobile phase was an aqueous solution of 5% (vol/vol) methanol and 0.1% (wt/vol) H3PO4. The retention times for standards at a flow rate of 1 ml/min were as follows: maleylacetic acid, 1.87 min; 5-chloromuconolactone, 2.92 min; trans-dienelactone, 3.66 min; 2-chloromuconolactone, 4.11 min; cis-dienelactone, 5.20 min; and 2-chloro-cis,cis-muconic acid, 7.47 min. The progress of the chromatography was monitored simultaneously at between 200 and 400 nm.
UV spectroscopic enzyme assays and the formation of intermediates were performed with a Kontron Uvikon 941 Plus UV-visible spectrophotometer or a Varian Cary 50 UV-visible spectrophotometer. Spectra were recorded at between 200 and 350 nm.
Purification and general in vitro manipulations of genomic and plasmid DNAs.
Genomic DNA from R. opacus 1CP was prepared as described previously (7). Plasmid DNA from E. coli DH5α was isolated with a Pharmacia Flexiprep kit. Vector DNA digested with only one enzyme was dephosphorylated prior to ligation. For cloning of PCR products, a T-vector was prepared as described by Marchuk et al. (25). Insert DNA for ligation or digoxigenin labeling was isolated from gels by use of an Easy Pure kit (Biozym). Preparation of competent cells of E. coli DH5α and subsequent transformation were achieved by the method of Inoue et al. (16). Loss of LacZ complementation by insertion of DNA into vectors was detected by using Luria-Bertani plates with 0.13 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and 32 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) per ml.
PCR.
Oligonucleotides were custom synthesized according to the sequences of the determined N terminus of ClcA2 and the sequence of one of its internal peptides. The reaction mixture (50 μl) contained 50 pmol of each primer, 0.5 μg of genomic template DNA, a 25 μM concentration of each deoxynucleoside triphosphate, 1× PCR buffer (MBI Fermentas), 2.0 U of DNA Taq polymerase (MBI Fermentas), and 3 mM MgCl2. The PCR was performed with a touchdown thermocycle program: an initial denaturation (98°C, 2 min) before addition of the polymerase; 9 cycles with decreasing annealing temperature (55 to 47°C, 30 s), polymerization (72°C, 1 min), and denaturation (98°C, 45 s); 20 more cycles with 45°C as the annealing temperature; and an additional 5 min of polymerization during the last cycle.
Cloning strategy and hybridization procedures.
The PCR product was initially ligated into the T-tailed EcoRV site of pBluescript II SK(+), yielding pROP0. The insert of pROP0 was labeled with digoxigenin by using a DIG DNA Labeling and Detection Kit Nonradioactive (Boehringer). The labeling and hybridization procedure was performed as described in the Boehringer manual. The probe was then used to detect the corresponding fragment on a Southern blot of 0.44 μg of NotI-digested R. opacus DNA run on a 1% agarose gel with TAE (4.48 g of Tris base, 1.14 ml of glacial acetic acid, and 2 ml of 0.5 M EDTA [pH 8] per liter). Prior to blotting of the DNA onto a 0.45-μm-pore-size Hybond N+ transfer membrane (Amersham), the gel had been incubated twice in 0.25 M HCl for 15 min and subsequently in 1.5 M NaCl-0.5 M NaOH for denaturation. From a second gel, an area which corresponded in size to the hybridization signal was excised, and the included DNA was eluted and ligated into pBluescript II SK(+). After transformation of the ligation mixture into E. coli DH5α, the labeled insert of pROP0 was used to identify the correct clones by colony hybridization as described previously (7).
DNA sequence analysis.
The nucleotide sequence of the overlapping inserts of pROP1 was determined by sequencing subclones in pBluescript II SK(+) by the dideoxy chain termination method, using a Pharmacia (Uppsala, Sweden) T7 sequencing kit. Subsequent electrophoresis and analysis were performed in an ALF Express II sequencer (Pharmacia). Computer-based sequence analyses were performed mainly with the Lasergene program package (DNASTAR Inc. Madison, Wis.). BlastX was used under default conditions to screen databases, while multiple sequence alignments were calculated by ClustalX 1.8 downloaded from the European Molecular Biology Laboratory home page. Dendrograms were generated using programs (Protdist and Fitch) of the PHYLIP package (version 3.5c).
Chemicals.
cis-Dienelactone, cis,cis-muconate, and 2-chloro-cis,cis-muconate were chemically prepared (19, 20) and kindly provided by W. Reineke (Wuppertal, Germany). (+)-5-Chloromuconolactone was available from previous synthesis (47). Muconolactone was biologically prepared from incubation of cis,cis-muconate with purified muconate cycloisomerase from P. putida PRS2000 (47). 3-Chlorocatechol was obtained by chemical synthesis from 2,3-dimethoxybenzonitrile (27).
Nucleotide sequence accession number.
The GenBank accession number for the sequence reported in this paper is AJ439407.
RESULTS
Cloning of a chlorocatechol 1,2-dioxygenase-encoding region from R. opacus 1CP.
In order to obtain sufficient amounts of protein for the isolation of tryptic fragments, the chlorocatechol 1,2-dioxygenase was purified to homogeneity from an extract of 2-chlorophenol-grown cells of R. opacus 1CP basically by the same procedure described previously (29).
An aliquot of the purified chlorocatechol 1,2-dioxygenase was subjected to trypsin digestion, and the resulting peptide mixture was separated by reversed-phase HPLC. Sequence information was obtained for the N terminus [(S)TDYTGNIVGKMIAAINAVIKDEKVQY-(E)(Y)(K)(A)] and for the isolated peptides 11 [T(I)VPAPY(E)IPK], 21 [GSQSS(I)QGP(Y)F(I)PGAPELSIP(Y)], 22 [GEV(V)DQEGAPLADVLLDM(M)QADAAGE(Y)SFINP], and 24 [ASTG(W)LISVGEQNEWPL-(D)(D)V(F)F(E)E] (parentheses indicate uncertain amino acids, and a hyphen indicates that no amino acid was detected in this step). The underlined segments of the N terminus and of peptide 22 were used for the design of the PCR primers fm-om1 (5′-GGC AAC ATC GTS GGX AAR ATG-3′) and rev-om1 (5′-CGG GGC GCC YCY TGR TC-3′), respectively. With both primers a fragment with the expected size of ca. 0.33 kb was obtained. This fragment was cloned into pBluescript II SK(+), yielding pROP0. DNA sequencing of the cloned insert demonstrated that it contained the respective primers and between them a DNA fragment with sequence similarities to known (chloro)catechol 1,2-dioxygenases. The insert in pROP0 was digoxigenin labeled and then used for the identification of corresponding bands on a Southern blot as well as for the identification of colonies of the desired clones. A clone carrying a 9.5-kb NotI insert in pBluescript II SK(+) was isolated and designated pROP1 (Fig. 2).
Sequence analysis of pROP1.
DNA sequencing of the 9.5-kb insert of pROP1 revealed nine open reading frames (ORFs) (Fig. 2), which were all compared on the basis of their predicted amino acid sequences. The predicted amino acid sequences of the incomplete first ORF (positions 599 to 1) and of the second ORF (positions 1229 to 648) show remarkably high sequence similarity to, respectively, RmpA and RmpB (a 3-hexulose-6-phosphate synthase and a 6-phospho-3-hexuloisomerase from Mycobacterium gastri MB19 [accession no. AB034913] [28]), with which they share 57 and 59% identical positions, respectively. Both genes are part of the ribulose monophosphate pathway of formaldehyde fixation, which exists in a wide range of methylotrophic bacteria that can grow on C1 compounds (37). The protein encoded by the third ORF (positions 1417 to 2658) is similar to several transcription regulators of the LuxR family.
The fourth ORF (positions 4085 to 2802) presumably encodes a glucose-6-phosphate 1-dehydrogenase. The predicted protein shares around 55 to 56% identical positions with corresponding proteins from Mycobacterium tuberculosis H37RV (accession no. O08407) (3) and Streptomyces coelicolor strains (accession no. T35160 and T36009).
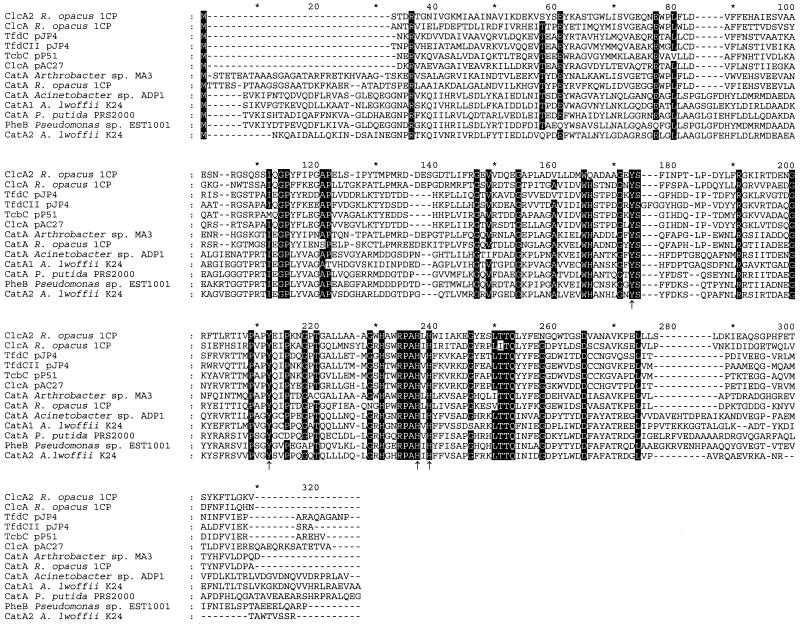
The deduced amino acid sequence of ORF5 (positions 4818 to 5582; predicted subunit mass, 28,105 Da) shows significant sequence similarities to known catechol and chlorocatechol 1,2-dioxygenases (Fig. 3), especially ClcA of R. opacus 1CP (accession no. AF003948) (8), with which it shares 51% identical positions in the alignment shown in Fig. 3. The similarity to other dioxygenases shown was in the range of from 25 to 49%. All four peptides derived from trypsin digestion and the N terminus of the purified chlorocatechol 1,2-dioxygenase were identified in the sequence predicted from this ORF, which was therefore termed clcA2. Despite the relatively low similarity of ClcA2 and the other chlorocatechol 1,2-dioxygenases, it shares with them an N terminus which lacks 24 to 29 amino acids compared to other known catechol 1,2-dioxygenases (Fig. 3).
FIG. 3.
Sequence alignment of chlorocatechol 1,2-dioxygenases (ClcA, TfdC, and TcbC) and catechol 1,2-dioxygenases (CatA and PheB). The alignment was calculated using all sequences in Fig. 8 by using ClustalX1.8. Sequences not shown here were removed afterwards, and resulting column gaps were eliminated. Numbers above the sequences refer to positions in the alignment, not in individual sequences. Amino acids in positions in which at least 11 of the 13 sequences are identical are highlighted. Amino acids involved in iron binding are indicated by arrows (46). Amino acids shaded in gray are exclusively conserved in proteobacterial chloromuconate cycloisomerases able to dechlorinate 2-chloro-cis,cis-muconate. Accession numbers for the published sequences are as follows: ClcA from R. opacus 1CP, AF003948; TfdC from pJP4, M35097; TfdCII from pJP4, U16782; TcbD from pP51, M57629; ClcA from pAC27, M16964; CatA from Arthrobacter sp. strain mA3, AJ000187; CatA from R. opacus 1CP, X99622; CatA from Acinetobacter sp. strain ADP1, AF009224; CatA from A. lwoffii K24, U77658; CatA from P. putida PRS2000, U12557; PheB from Pseudomonas sp. strain EST1001, M57500; and CatA2 from Acinetobacter lwoffii K24, U77659.
The deduced protein of ORF6 (positions 5608 to 6372; predicted subunit mass, 27,400 Da) is similar to several dienelactone hydrolases, especially ClcD from R. opacus 1CP (accession no. AF003948) (8), with which it shares 40% identical positions. It was therefore termed clcD2.
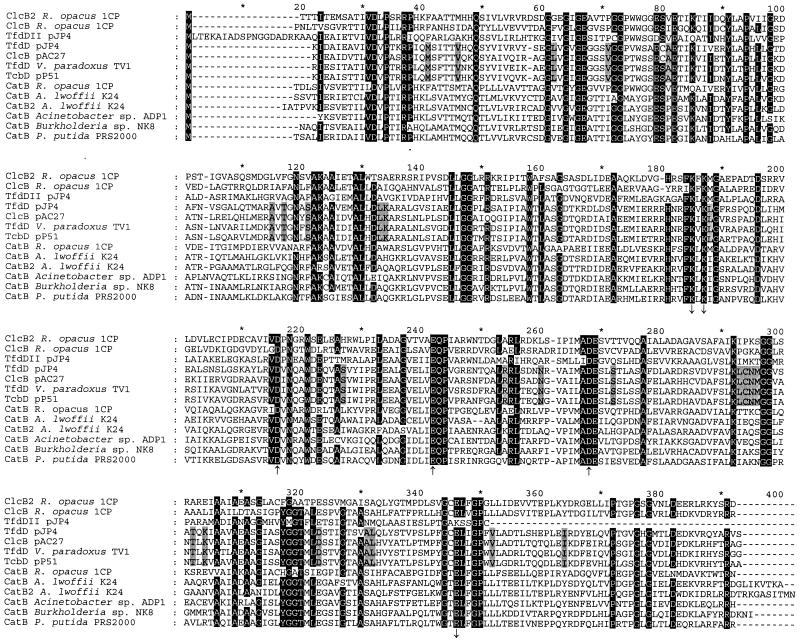
ORF7 (positions 6369 to 7484) encodes a protein (predicted subunit mass, 39,539 Da) that is similar to several chloromuconate and muconate cycloisomerases (Fig. 4). A similarity of 46% identical positions was found with the muconate cycloisomerase CatB from P. putida PRS2000 (accession no. U12557) (15), and a similarity of 45% identical positions was found with the chloromuconate cycloisomerase ClcB of R. opacus strain 1CP (accession no. AF003948) (8). The similarity to other cycloisomerases in Fig. 4 ranged from 36 to 46% identical positions. The predicted N terminus of ORF7 was identical to that of the purified chloromuconate cycloisomerase from 2-chlorophenol-grown cells of strain 1CP, which was determined as to be TXXITEMSATIVDLPS(R)(R) (X indicates an unknown amino acid, and parentheses indicate an uncertain amino acid). Therefore, ORF7 was designated clcB2.
FIG. 4.
Alignment of chloromuconate cycloisomerases (ClcB, TfdD, and TcbD) and muconate cycloisomerases (CatB). The alignment was calculated by using ClustalX1.8 with the outgroups shown in Fig 8. Outgroups and column gaps resulting from outgroup removal are not shown. Numbers above the sequences refer to positions in the alignment, not in individual sequences. Residues involved in manganese coordination (upward-pointing arrows) and residues directly involved in the enzyme mechanism (downward-pointing arrows) are indicated (10, 12, 14). Amino acids in positions in which at least 11 of the 13 sequences are identical are highlighted. Accession numbers for the published sequences are as follows: ClcB from R. opacus 1CP, AF003948; TfdDII from pJP4, U16782; TfdD from pJP4, M35097; ClcB from pAC27, M16964 (corrected as published by Vollmer et al. [48]); TfdD from Variovorax paradoxus TV1, AB028643; TcbD from pP51, M57629; CatB from R. opacus 1CP, X99622; CatB from A. lwoffii K24, U77658; CatB2 from A. lwoffii K24, U77659; CatB from Acinetobacter sp. strain ADP1, AF009224; CatB from Burkholderia sp. strain NK8, AB024746; and CatB from P. putida PRS2000, U12557.
The protein encoded by ORF8 (positions 7520 to 7804; predicted subunit mass, 11,193 Da) shows significant sequence similarities to several muconolactone isomerases (Fig. 5), especially to a methylmuconolactone isomerase of Ralstonia eutropha JMP134 (accession no. X99639) (6) (55% identical positions). The predicted amino acid sequence of the N terminus of this protein was identical to that of the purified 5-chloromuconolactone-converting enzyme from 2-chlorophenol-grown cells of strain 1CP, which was determined to be MLYLVRMTVNLPRNLDP(R)EEEXL(K)(A)(S). The gene for this new chlorocatechol degradative enzyme was named clcF.
FIG. 5.
Sequence alignment of 5-chloromuconolactone dehalogenase (ClcF) with muconolactone isomerases as calculated by using ClustalX1.8 and used for dendrogram calculations (Fig. 8). Numbers above the sequences refer to positions in the alignment, not in individual sequences. Amino acids in positions in which at least 10 of the 11 sequences are identical are highlighted. Accession numbers for the published sequences are as follows: MmlJ (methylmuconolactone isomerase) from R. eutropha JMP134, X99639; CatC from Burkholderia sp. strain NK8, AB024746; CatC from Acinetobacter sp. strain ADP1, AF009224; CatC from A. lwoffii K24, U77658; CatC from R. opacus 1CP, X99622; CatC from Streptomyces setonii, AF277051; CatC from Mycobacterium smegmatis mc2-155, AF144091; CatC2 from A. lwoffii K24, U77659; CatC from P. putida PRS2000, U12557; and CatC from Pseudomonas sp. strain CA10, AB047272.
A ninth, incomplete ORF (positions 9263 to 9510) presumably codes for a regulator belonging to the LysR family. The predicted protein shares 60% identical positions with a presumed transcriptional regulator, PA5029, from Pseudomonas aeruginosa PAO1 (accession no. AE004916) (45).
No ORF encoding a maleylacetate reductase was detected on pROP1.
HPLC and spectral analysis of the conversion of 2-chloro-cis,cis-muconate by ClcB2, ClcF, and ClcD2.
To verify the biochemical function of ClcB2, ClcF, and ClcD2, as well as to identify the occurrence of possible by-products during cycloisomerization or dehalogenation, sequential incubations of 2-chloro-cis,cis-muconate with the enzyme preparations were analyzed by reversed-phase HPLC and UV-visible spectrophotometry.
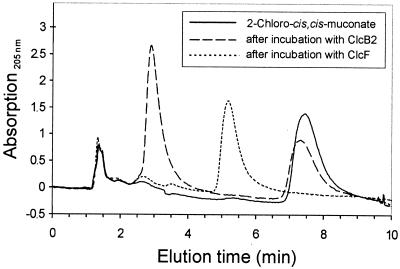
As documented by HPLC (Fig. 6), the addition of homogeneous chloromuconate cycloisomerase ClcB2 to 2-chloro-cis,cis-muconate (retention time = 7.47 min; λmax = 278 nm) resulted in a decrease of this compound. A single new peak appeared (retention time = 2.92 min; λmax = 202 nm), which was identical to that of 5-chloromuconolactone. The reaction was not quantitative, indicating an equilibrium between the two compounds. After subsequent addition of ClcF to the equilibrium mixture, both the 5-chloromuconolactone and the 2-chloro-cis,cis-muconic acid signals disappeared completely. cis-Dienelactone (retention time = 5.20 min; λmax = 276 nm) was identified as the sole product of this dechlorination. trans-Dienelactone was not detected.
FIG. 6.
Overlaid HPLC chromatograms of 2-chloro-cis,cis-muconate before and after sequential incubation with chloromuconate cycloisomerase ClcB2 and with the 5-chloromuconolactone-converting enzyme ClcF. At least 0.1 U of the homogeneous enzymes was added in sequence to a 1-ml assay mixture containing 0.2 μmol of 2-chloro-cis,cis-muconate and 50 μmol of Tris-HCl buffer (pH 7.5). Samples, which were taken from this mixture before enzyme addition, 10 min after incubation with ClcB2 alone, and 10 min after further incubation with ClcF, were quenched by addition of 10% (vol/vol) ortho-phosphoric acid and subjected to reversed-phase HPLC. The retention times for the following compounds at a flow rate of 1 ml/min were as follows: 5-chloromuconolactone, 2.92 min; cis-dienelactone, 5.20 min; and 2-chloro-cis,cis-muconic acid, 7.47 min. The signal at 1.5 min refers to the injection peak.
Further incubation of cis-dienelactone with the partially purified dienelactone hydrolase ClcD2 yielded maleylacetate and was accompanied by a shift of the absorption maximum from 280 nm to 240 to 250 nm (34).
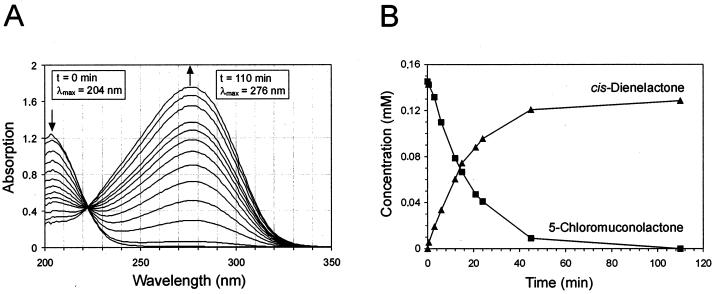
To test if the dehalogenation by ClcF of 5-chloromuconolactone to cis-dienelactone is quantitative or yields additional products, a mixture of 5-chloromuconolactone and the homogeneous enzyme was simultaneously analyzed by UV-visible spectrophotometry and by HPLC. By means of repeated chromatography (Fig. 7B), it was shown that only about 90% of the theoretical yield of cis-dienelactone was formed. The facts that no detectable amount of 5-chloromuconolactone remained and no other by-product was detected after incubation strongly indicated that the reaction was quantitative. Because of the small amount of available 5-chloromuconolactone, there might have been a relatively high error in the preparation of the standard stock solution. An overlay of simultaneously recorded UV spectra (Fig. 7A) from the reaction showed the presence of only one isosbestic point, which indicated that no side reaction took place and no stable intermediates occurred during conversion.
FIG. 7.
(A) Spectral changes during the ClcF-catalyzed conversion of 5-chloromuconolactone to cis-dienelactone. (B) Stoichiometry of the reaction, estimated by reversed-phase HPLC. A 0.2-μmol amount of 5-chloromuconolactone was incubated with 0.01 μg of purified ClcF (7 × 10−3 U as measured with 5-chloromuconolactone) in phosphate buffer (pH 7.2) at room temperature. UV spectra were recorded at 0, 0.5, 3, 6, 9, 12, 15, 18, 21, 24, 45, and 110 min. From an identical second reaction mixture, samples were taken at similar intervals. After quenching by addition of 10% (vol/vol) ortho-phosphoric acid, those samples were subjected to HPLC.
Lack of muconolactone conversion by ClcF.
The 5-chloromuconolactone-converting enzyme ClcF was initially purified based on its activity with 5-chloromuconolactone and was found to have a sequence with high similarity to those of muconolactone isomerases. In order to test the muconolactone-isomerizing activity of the purified enzyme, 2.65 U of ClcF (as measured with 5-chloromuconolactone) was incubated with 0.2 mM muconolactone in the presence of an excess of partially purified 3-oxoadipate enol-lactone hydrolase from P. putida in 50 mM Tris-HCl buffer (pH 7.5). Interestingly, the measured value of 6 × 10−3 U indicated no significant activity, since the detection limit of the assay under the conditions used was determined to be in the same range. In contrast, muconolactone isomerase partially purified from P. putida showed activities of 14 U/mg with 5-chloromuconolactone and 12 U/mg with muconolactone.
DISCUSSION
Based on the purification of a second chlorocatechol 1,2-dioxygenase induced as the only detectable intradiol-cleaving enzyme in R. opacus 1CP during growth with 2-chlorophenol (29) and on sequences of tryptic peptides, it was possible to clone a second chlorocatechol gene cluster of strain 1CP into E. coli. As shown by the correspondence of N termini from purified enzymes and N termini predicted from DNA sequences, the enzymes encoded by this gene cluster are clearly functional in 2-chlorophenol degradation via a 3-chlorocatechol pathway. The substrate specificities as determined by Moiseeva et al. (29) fit very well into this picture. Those authors had shown that the second chlorocatechol 1,2-dioxygenase ClcA2 converts 3-chlorocatechol three times better than 4-chlorocatechol (as judged by kcat/Km ratios) and that the second chloromuconate cycloisomerase converts 2-chloro-cis,cis-muconate but not 3-chloro- and 2,4-dichloro-cis,cis-muconate.
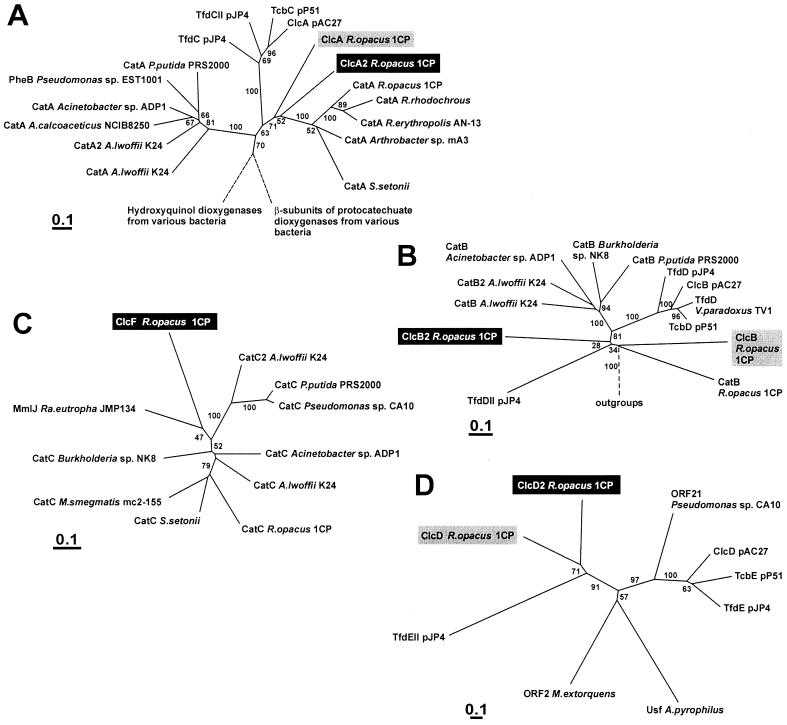
As in the known chlorocatechol gene cluster of R. opacus 1CP for 4-chloro- and 3,5-dichlorocatechol conversion, a maleylacetate reductase gene was not detected in the gene cluster (Fig. 8). However, a separate maleylacetate reductase gene has previously been cloned and characterized from R. opacus 1CP, and evidence that yet another maleylacetate reductase may be induced by this strain has been obtained (43). Thus, the lack of a reductase gene in the new gene cluster neither is surprising nor renders the cluster nonfunctional.
FIG. 8.
Dendrograms showing the relatedness of catechol 1,2-dioxygenases (CatA and PheB) and chlorocatechol 1,2-dioxygenases (ClcA, TfdC, and TcbC) (A), muconate cycloisomerase (CatB) and chloromuconate cycloisomerases (ClcB, TfdD, and TcbD) (B), muconolactone isomerases (CatC and MmIJ) and 5-chloromuconolactone dehalogenase (ClcF) (C), and dienelactone hydrolases (ClcD, TfdE, and TcbE) (D). The dendrograms were calculated using programs (Protdist and Fitch) of the PHYLIP package (version 3.5c) based on sequence alignments calculated by ClustalX1.8. Bootstrap values were derived from Seqboot (PHYLIP) calculations with 100 replicates. For the cycloisomerases, the muconolactone isomerases, and the dienelactone hydrolases, the complete alignments were used for dendrogram calculation (Fig. 4 and 5). For the dioxygenases, only positions 112 to 259 in Fig. 3 were used. Sequences examined in this study are highlighted black; sequences already known for chlorocatechol degradation in R. opacus 1CP are highlighted gray. Broken lines indicate that branches were cut. Accession numbers for catechol 1,2-dioxygenases not shown in Fig. 3 are as follows: CatA from A. calcoaceticus NCIB8250, Z36909; CatA from R. rhodochrous, AF043741; CatA from R. erythropolis AN-13, D83237; and CatA from S. setonii, AF277051. Accession numbers for the dienelactone hydrolases are as follows: ClcD from R. opacus 1CP, AF003948; TfdEII from pJP4, U16782; ORF21 from Pseudomonas sp. strain CA10, AB047548; ClcD from pAC27, M16964; TcbE from pP51, M57629; and TfdE from pJP4, M35097. Accession numbers for sequences used as outgroups are as follows. For panel A, hydroxyquinol dioxygenases from Burkholderia cepacia AC1100 (U19883), Ralstonia pickettii DTP0602 (D86544), and Arthrobacter sp. strain BA-5-17 (AB016258) and β-subunits of protocatechuate 3,4-dioxygenases from Pseudomonas sp. strain HR199 (Y18527), P. putida WCS358 (AJ295623), P. putida NCIMB9869 (U96339), alphaproteobacterium Y3F (AF253466), Agrobacterium tumefaciens A348 (U32867), and P. marginata ATCC10248 (U33634) were used. For panel B, N-acylamino acid racemases from Amycolatopsis orientalis subsp. lurida (AJ292519), Amycolatopsis sp. strain TS-1-60 (D30738), Deinococcus radiodurans R1 (AE001867), and Thermoplasma acidophilum DSM1728 (AL445063); mandelate racemase from P. putida (J05293); mandelate racemase-like protein from Sulfolobus solfataricus (AE006902); starvation-sensing protein RspA from E. coli (D90799); and putative muconate cycloisomerase YkfB from Bacillus subtilis (AJ002571) were used. For panel D, ORF2 from Methylobacterium extorquens AM1 (U72662) and Usf from Aquifex pyrophilus Ko15a (U17575), both with unknown function, were used.
The presence of two different chlorocatechol gene clusters in the same strain has recently been reported also for two proteobacterial degraders of chloroaromatic compounds. Thus, R. eutropha JMP134 possesses two such clusters on its plasmid pJP4 (22, 33), and Delftia acidovorans P4a harbors two clusters on a presumed catabolic transposon on the chromosome (13).
In contrast to all known chlorocatechol gene clusters, the one described here is unique in that it comprises a gene for an enzyme related to muconolactone isomerases which in the degradative pathway acts as a 5-chloromuconolactone dehalogenase, yielding cis-dienelactone. The fact that proteobacterial muconolactone isomerases are able to convert (4R,5R)- and (4R,5S)-5-chloro-3-methyl- and (4R,5S)-5-chloromuconolactone to 3-methyl-trans-dienelactone, 3-methyl-cis-dienelactone, and cis-dienelactone, respectively, has previously been reported by Prucha et al. (35, 36). Conversion of 5-chloromuconolactone to dienelactones by muconolactone isomerase of Acinetobacter sp. strain ADP1 was also reported by Vollmer et al. (47). Prucha et al. (35) even discussed that this reaction could be used to construct a chlorocatechol pathway. However, the enzymes used in those experiments belonged to usual catechol gene clusters, and the reactions found were not necessarily physiologically relevant for the strains. In R. opacus 1CP, 5-chloromuconolactone dehalogenation to cis-dienelactone for the first time was found to be a reaction that was functional in a degradative pathway. The great difference from all other known chlorocatechol pathways is that here an enzyme related to muconolactone isomerases is the dehalogenating enzyme and not a chloromuconate cycloisomerase. Interestingly, activity of ClcF with its original substrate muconolactone was not detectable (below 0.2% of activity with 5-chloromuconolactone). Thus, the original muconolactone isomerase obviously has undergone an evolutionary adaptation and specialization for 5-chloromuconolactone as a substrate. Overall, the data show that the gene cluster described here codes for a new variant of 3-chlorocatechol ortho-cleavage pathways with new enzymes involved in dechlorination.
As found for the clcABD gene cluster for 4-chloro- and 3,5-dichlorocatechol catabolism of R. opacus 1CP (8), the enzymes encoded by the new clcA2B2D2F gene cluster also represent very deep branches in their respective dendrograms (Fig. 8). The enzymes are obviously not closely related to those of the previously known chlorocatechol gene cluster of 1CP, and according to the available data they appear to represent yet another independent evolutionary line leading to productive chlorocatechol catabolism. Thus, based on a divergent evolution of the enzymes, chlorocatechol degradative pathways may have arisen even three times independently, once among the Proteobacteria and twice among the Actinobacteria.
Acknowledgments
We thank Hans-Joachim Knackmuss for valuable support and helpful advice. We are indebted to Rolf Schmid and Volker Nödinger for the amino acid sequencing.
The work was supported by grants 436 RUS 113/59/0 and SCHL 284/2-2 from the Deutsche Forschungsgemeinschaft and by funds from the Deutsche Bundesstiftung Umwelt.
REFERENCES
- 1.Blasco, R., R. M. Wittich, M. Mallavarapu, K. N. Timmis, and D. H. Pieper. 1995. From xenobiotic to antibiotic, formation of protoanemonin from 4-chlorocatechol by enzymes of the 3-oxoadipate pathway. J. Biol. Chem. 270:29229-29235. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, S. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, S. Skelton, S. Squares, R. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 4.Dorn, E., M. Hellwig, W. Reineke, and H.-J. Knackmuss. 1974. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch. Microbiol. 99:61-70. [DOI] [PubMed] [Google Scholar]
- 5.Dorn, E., and H.-J. Knackmuss. 1978. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem. J. 174:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erb, R. W., K. N. Timmis, and D. H. Pieper. 1998. Characterization of a gene cluster from Ralstonia eutropha JMP134 encoding metabolism of 4-methylmuconolactone. Gene 206:53-62. [DOI] [PubMed] [Google Scholar]
- 7.Eulberg, D., L. A. Golovleva, and M. Schlömann. 1997. Characterization of catechol catabolic genes from Rhodococcus erythropolis 1CP. J. Bacteriol. 179:370-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eulberg, D., E. M. Kourbatova, L. A. Golovleva, and M. Schlömann. 1998. Evolutionary relationship between chlorocatechol catabolic enzymes from Rhodococcus opacus 1CP and their counterparts in proteobacteria: sequence divergence and functional convergence. J. Bacteriol. 180:1082-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eulberg, D., S. Lakner, L. A. Golovleva, and M. Schlömann. 1998. Characterization of a protocatechuate catabolic gene cluster from Rhodococcus opacus 1CP: evidence for a merged enzyme with 4-carboxymuconolactone-decarboxylating and 3-oxoadipate enol-lactone-hydrolyzing activity. J. Bacteriol. 180:1072-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerlt, J. A., and P. G. Gassman. 1992. Understanding enzyme-catalyzed proton abstraction from carbon acids: details of stepwise mechanisms for β-elimination reactions. J. Am. Chem. Soc. 114:5928-5934. [Google Scholar]
- 11.Gorlatov, S. N., O. V. Maltseva, V. I. Shevchenko, and L. A. Golovleva. 1989. Degradation of chlorophenols by a culture of Rhodococcus erythropolis. Mikrobiologiya 58:802-806. [Google Scholar]
- 12.Helin, S., P. C. Kahn, B. L. Guha, D. G. Mallows, and A. Goldman. 1995. The refined X-ray structure of muconate lactonizing enzyme from Pseudomonas putida PRS2000 at 1.85 Å resolution. Comput. Appl. Biol. Sci. 5:151-153. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann, D. 2001. Genetische Organisation der produktiven Entgiftung von 2,4-Dichlorphenoxyessigsäure bei einem alkalitoleranten Bakterium. Ph.D. thesis. University of Leipzig, Leipzig, Germany.
- 14.Hoier, H., M. Schlömann, A. Hammer, J. P. Glusker, H. L. Carrell, A. Goldman, J. J. Stezowski, and U. Heinemann. 1994. Crystal structure of chloromuconate cycloisomerase from Alcaligenes eutrophus JMP134 (pJP4) at 3 Å resolution. Acta Cryst. D50:75-84. [DOI] [PubMed]
- 15.Houghton, J. E., T. M. Brown, A. J. Appel, E. J. Hughes, and L. N. Ornston. 1995. Discontinuities in the evolution of Pseudomonas putida cat genes. J. Bacteriol. 177:401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue, H., H. Nojima, and H. Okayama. 1990. One step preparation of competent E. coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. USA 86:2172-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaschabek, S. R., T. Kasberg, D. Müller, A. E. Mars, D. B. Janssen, and W. Reineke. 1998. Degradation of chloroaromatics: purification and characterization of a novel type of chlorocatechol 2,3-dioxygenase of Pseudomonas putida GJ31. J. Bacteriol. 180:296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaschabek, S. R., and W. Reineke. 1992. Maleylacetate reductase of Pseudomonas sp. strain B13: dechlorination of chloromaleylacetates, metabolites in the degradation of chloroaromatic compounds. Arch. Microbiol. 158:412-417. [DOI] [PubMed] [Google Scholar]
- 19.Kaschabek, S. R., and W. Reineke. 1994. Synthesis of bacterial metabolites from haloaromatic degradation. 1. Fe(III)-catalyzed peracetic acid oxidation of halocatechols, a facile entry to cis,cis-2-halo-2,4-hexadienedioic acids and 3-halo-5-oxo-2(5H)-furanylideneacetic acids. J. Org. Chem. 59:4001-4003. [Google Scholar]
- 20.Kaschabek, S. R., and W. Reineke. 1995. Maleylacetate reductase of Pseudomonas sp. strain B13: specificity of substrate conversion and halide elimination. J. Bacteriol. 177:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaulmann, U., S. R. Kaschabek, and M. Schlömann. 2001. Mechanism of chloride elimination from 3-chloro- and 2,4-dichloro-cis,cis-muconate: new insight obtained from analysis of muconate cycloisomerase variant CatB-K169A. J. Bacteriol. 183:4551-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli, C. M., J. H. Leveau, A. J. Zehnder, and J. R. van der Meer. 2000. Characterization of a second tfd gene cluster for chlorophenol and chlorocatechol metabolism on plasmid pJP4 in Ralstonia eutropha JMP134(pJP4). J. Bacteriol. 182:4165-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maltseva, O. V., I. P. Solyanikova, and L. A. Golovleva. 1994. Chlorocatechol 1,2-dioxygenase from Rhodococcus erythropolis 1CP. Kinetic and immunochemical comparison with analogous enzymes from Gram-negative strains. Eur. J. Biochem. 226:1053-1061. [DOI] [PubMed] [Google Scholar]
- 24.Maltseva, O. V., I. P. Solyanikova, L. A. Golovleva, M. Schlömann, and H.-J. Knackmuss. 1994. Dienelactone hydrolase from Rhodococcus erythropolis 1CP: purification and properties. Arch. Microbiol. 162:368-374. [Google Scholar]
- 25.Marchuk, D., M. Drumm, A. Saulino, and F. S. Collins. 1991. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 19:1154.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mars, A. E., T. Kasberg, S. R. Kaschabek, M. H. van Agteren, D. B. Janssen, and W. Reineke. 1997. Microbial degradation of chloroaromatics: use of the meta-cleavage pathway for mineralization of chlorobenzene. J. Bacteriol. 179:4530-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason, H. S. 1947. The allergenic principles of poison ivy. VI. Note on the synthesis of 3-substituted catechols. J. Am. Chem. Soc. 69:2241.. [DOI] [PubMed] [Google Scholar]
- 28.Mitsui, R., Y. Sakai, H. Yasueda, and N. Kato. 2000. A novel operon encoding formaldehyde fixation: the ribulose monophosphate pathway in the gram-positive facultative methylotrophic bacterium Mycobacterium gastri MB19. J. Bacteriol. 182:944-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moiseeva, O. V., O. V. Belova, I. P. Solyanikova, M. Schlömann, and L. A. Golovleva. 2001. Enzymes of a new modified ortho-pathway utilizing 2-chlorophenol in Rhodococcus opacus 1CP. Biokhimiya 66:678-687. [DOI] [PubMed] [Google Scholar]
- 30.Moiseeva, O. V., E. V. Lin'ko, B. P. Baskunov, and L. A. Golovleva. 1999. Degradation of 2-chlorophenol and 3-chlorobenzoate by Rhodococcus opacus 1CP. Mikrobiologiya 68:461-466. [Google Scholar]
- 31.Moos, M. 1993. Isolation of proteins for microsequence analysis, p. 10.19.1-10.19.12. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, supplement 21. John Wiley & Sons, Inc., New York, N.Y.
- 32.Ornston, L. N. 1966. The conversion of catechol and protocatechuate to β-ketoadipate by Pseudomonas putida. III. Enzymes of the catechol pathway. J. Biol. Chem. 241:3795-3799. [PubMed] [Google Scholar]
- 33.Perez-Pantoja, D., L. Guzman, M. Manzano, D. H. Pieper, and B. Gonzalez. 2000. Role of tfdC(I)D(I)E(I)F(I) and tfdD(II)C(II)E(II)F(II) gene modules in catabolism of 3-chlorobenzoate by Ralstonia eutropha JMP134(pJP4). Appl. Environ. Microbiol. 66:1602-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pieper, D. H., K. Pollmann, P. Nikodem, B. Gonzalez, and V. Wray. 2002. Monitoring key reactions in degradation of chloroaromatics by in situ 1H nuclear magnetic resonance: solution structures of metabolites formed from cis-dienelactone. J. Bacteriol. 184:1466-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prucha, M., A. Peterseim, K. N. Timmis, and D. H. Pieper. 1996. Muconolactone isomerase of the 3-oxoadipate pathway catalyzes dechlorination of 5-chloro-substituted muconolactones. Eur. J. Biochem. 237:350-356. [DOI] [PubMed] [Google Scholar]
- 36.Prucha, M., V. Wray, and D. H. Pieper. 1996. Metabolism of 5-chlorosubstituted muconolactones. Eur. J. Biochem. 237:357-366. [DOI] [PubMed] [Google Scholar]
- 37.Quayle, J. R., and T. Ferenci. 1978. Evolutionary aspects of autotrophy. Microbiol. Rev. 42:251-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reineke, W., and H. J. Knackmuss. 1988. Microbial degradation of haloaromatics. Annu. Rev. Microbiol. 42:263-287. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 40.Schlömann, M. 1994. Evolution of chlorocatechol catabolic pathways. Biodegradation 5:301-321. [DOI] [PubMed] [Google Scholar]
- 41.Schlömann, M., E. Schmidt, and H.-J. Knackmuss. 1990. Different types of dienelactone hydrolase in 4-fluorobenzoate-utilizing bacteria. J. Bacteriol. 172:5112-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt, E., and H. J. Knackmuss. 1980. Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem. J. 192:339-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seibert, V., E. M. Kourbatova, L. A. Golovleva, and M. Schlömann. 1998. Characterization of the maleylacetate reductase MacA of Rhodococcus opacus 1CP and evidence for the presence of an isofunctional enzyme. J. Bacteriol. 180:3503-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solyanikova, I. P., O. V. Maltseva, M. D. Vollmer, L. A. Golovleva, and M. Schlömann. 1995. Characterization of muconate and chloromuconate cycloisomerase from Rhodococcus erythropolis 1CP: indications for functionally convergent evolution among bacterial cycloisomerases. J. Bacteriol. 177:2821-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrook-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. M. Lim, K. A. Smith, D. H. Spencer, G. K. S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 46.Vetting, M. W., and D. H. Ohlendorf. 2000. The 1.8 Å crystal structure of catechol 1,2-dioxygenase reveals a novel hydrophobic helical zipper as a subunit linker. Structure Fold. Des. 8:429-440. [DOI] [PubMed] [Google Scholar]
- 47.Vollmer, M. D., P. Fischer, H.-J. Knackmuss, and M. Schlömann. 1994. Inability of muconate cycloisomerases to cause dehalogenation during conversion of 2-chloro-cis,cis-muconate. J. Bacteriol. 176:4366-4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vollmer, M. D., U. Schell, V. Seibert, S. Lakner, and M. Schlömann. 1999. Substrate specificities of the chloromuconate cycloisomerases from Pseudomonas sp. B13, Ralstonia eutropha JMP134 and Pseudomonas sp. P51. Appl. Microbiol. Biotechnol. 51:598-605. [DOI] [PubMed] [Google Scholar]
- 49.Vollmer, M. D., and M. Schlömann. 1995. Conversion of 2-chloro-cis,cis-muconate and its metabolites 2-chloro- and 5-chloromuconolactone by chloromuconate cycloisomerases of pJP4 and pAC27. J. Bacteriol. 177:2938-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vollmer, M. D., K. Stadler-Fritzsche, and M. Schlömann. 1993. Conversion of 2-chloromaleylacetate in Alcaligenes eutrophus JMP134. Arch. Microbiol. 159:182-188. [DOI] [PubMed] [Google Scholar]