Abstract
1. The relative isometric tension—pCa relationship has been determined for isolated bundles of barnacle myofibrils under a variety of ionic conditions using [Ca2+]-buffered solutions which also contained an ATP regenerating system (creatine phosphate and creatine kinase).
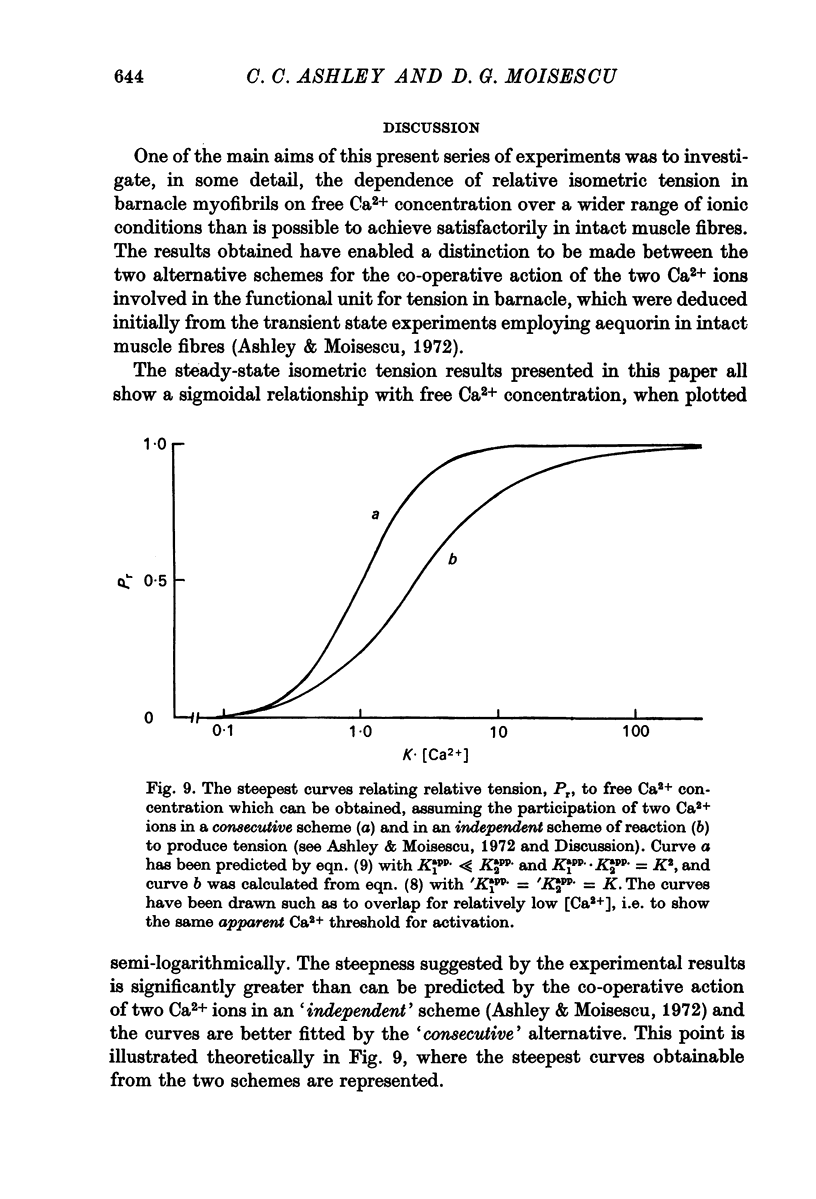
2. The results are in better agreement with the `consecutive' scheme of reaction rather than with the `independent' alternative (Ashley & Moisescu, 1972) for the co-operative action of two Ca2+ ions in the process of tension activation in crustacean skeletal muscle.
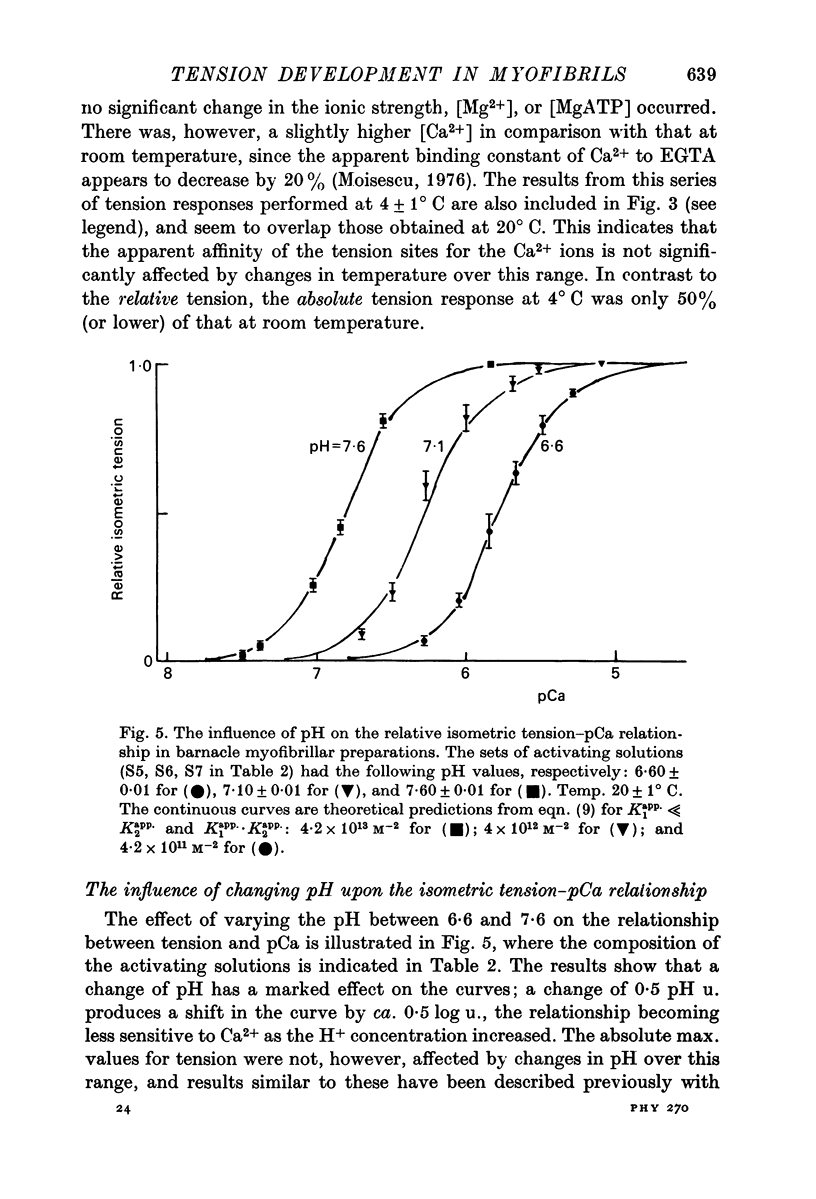
3. Variations in the pH of the activating solutions did have a marked effect on the relative tension—Ca curve, although no effect was observed on the absolute maximum value for isometric tension. A shift in pH by 0·5 u. in the range 6·6-7·6 shifted the Ca2+—activation curve by 0·5 log u. towards lower free Ca2+ concentrations.
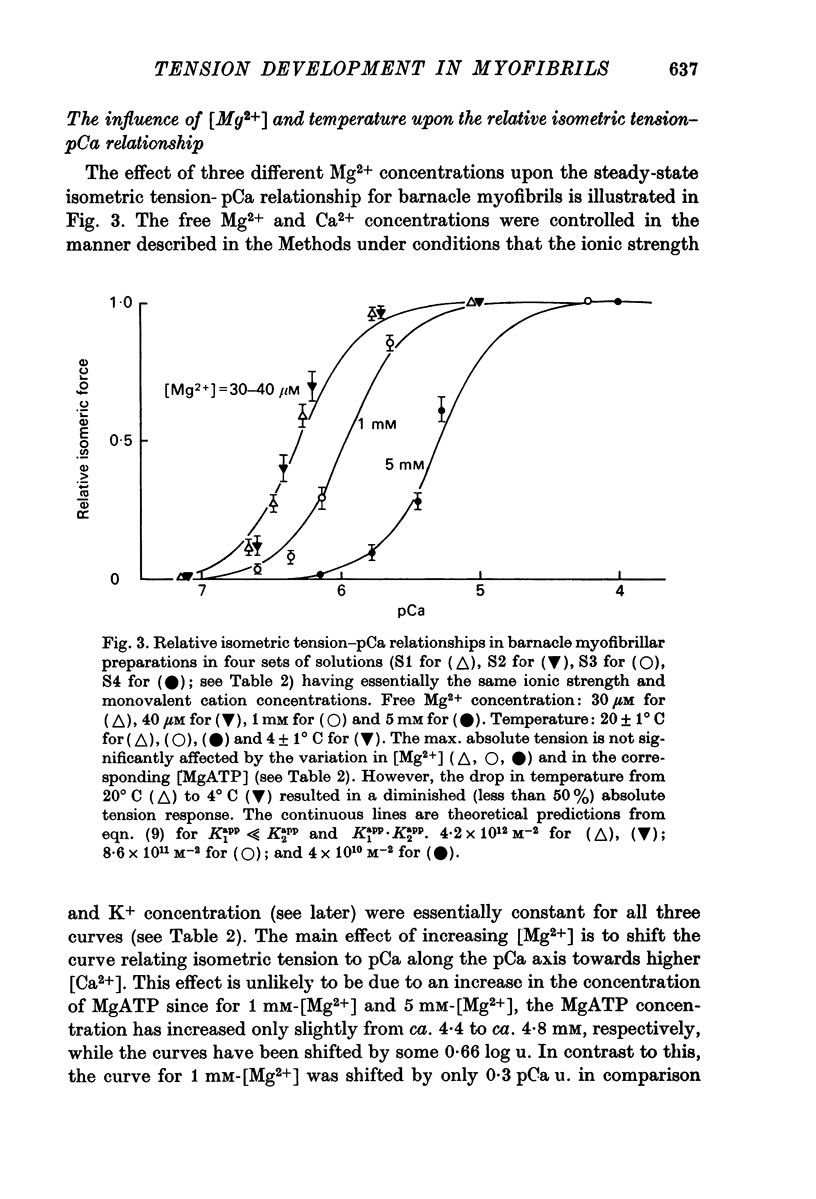
4. Changes in the free Mg2+ concentration of the activating solutions in the millimolar range produced a pronounced shift of the relative tension—pCa curve along the pCa axis. Increasing [Mg2+] from 1 to 5 mM shifted the curve by about 0·7 log u. to higher free Ca2+ concentrations, without significantly modifying its steepness.
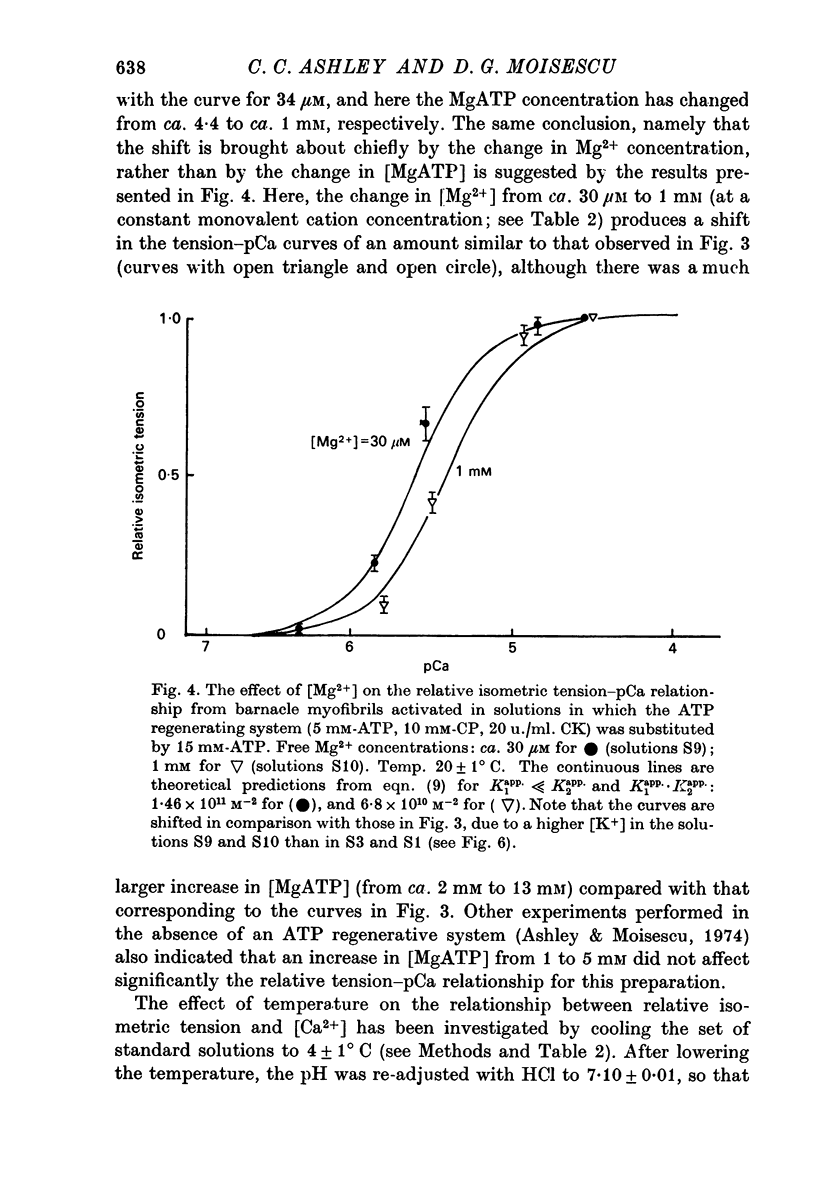
5. Changes in the MgATP concentration of the activating solutions in the range of 1-13 mM had no significant effect on the relative tension—pCa relationship.
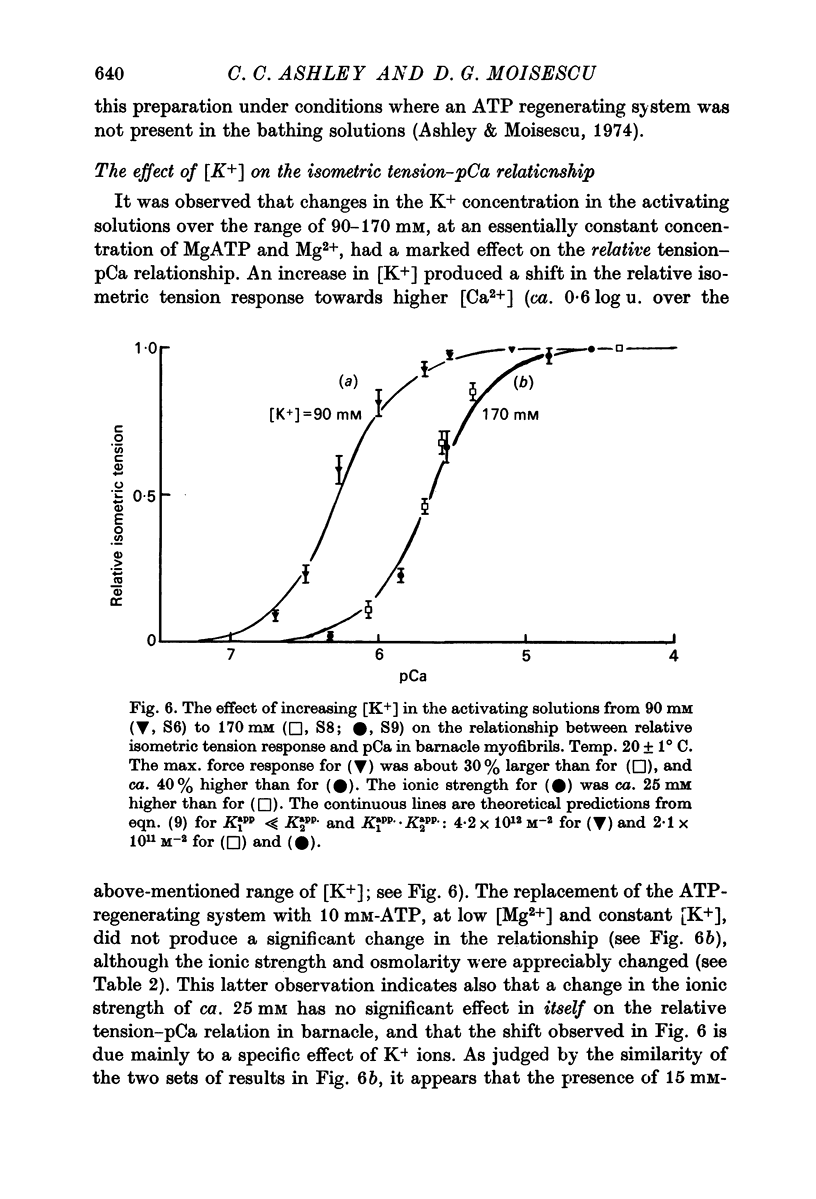
6. Varying the K+ concentration in the activating solutions was also observed to have a marked effect upon the tension—pCa relationship in barnacle. An increase in the K+ concentration from 90 to 170 mM shifted the curve by some 0·6 log u. towards higher free Ca2+ concentrations.
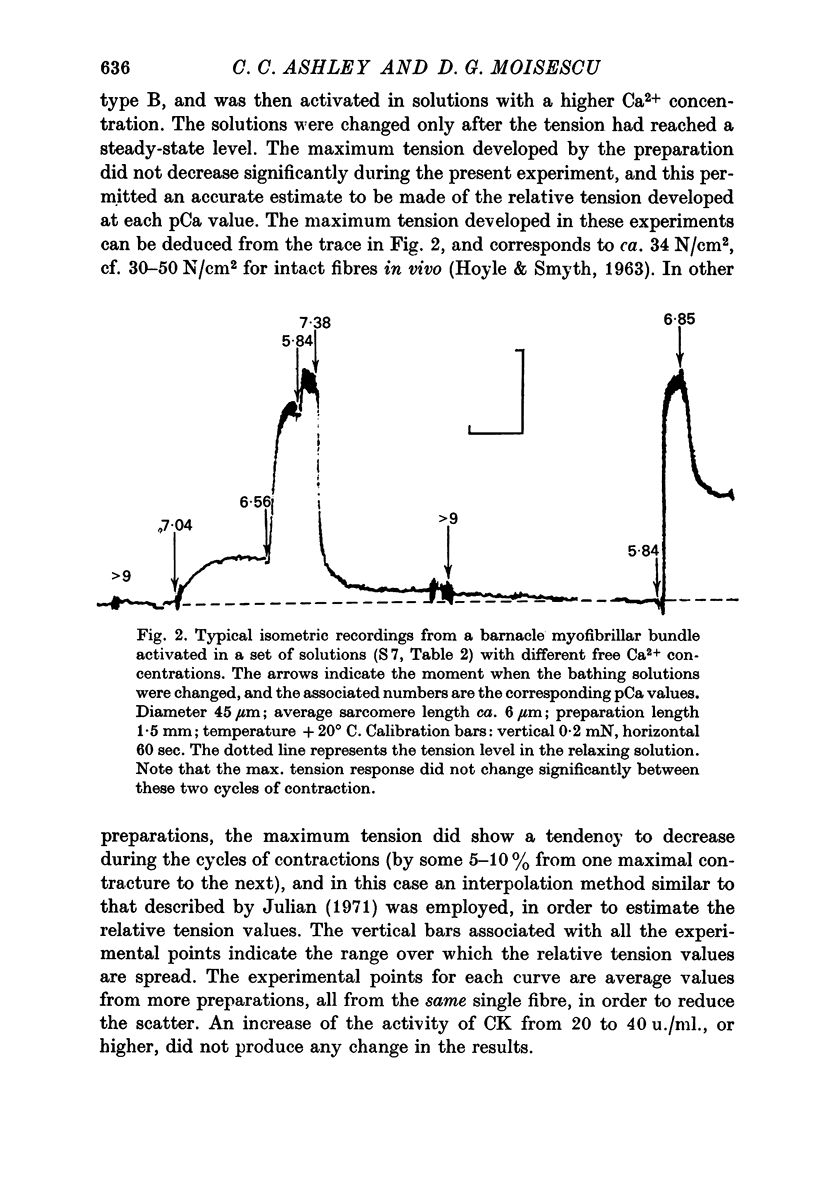
7. Cooling the standard activating solutions from room temperature to +4° C made no apparent difference to the relative tension—pCa relationship, but decreased significantly the absolute tension responses.
8. The results presented show that tonicity by itself has a marked effect upon the absolute steady-state tension levels in isolated bundles of myofibrils.
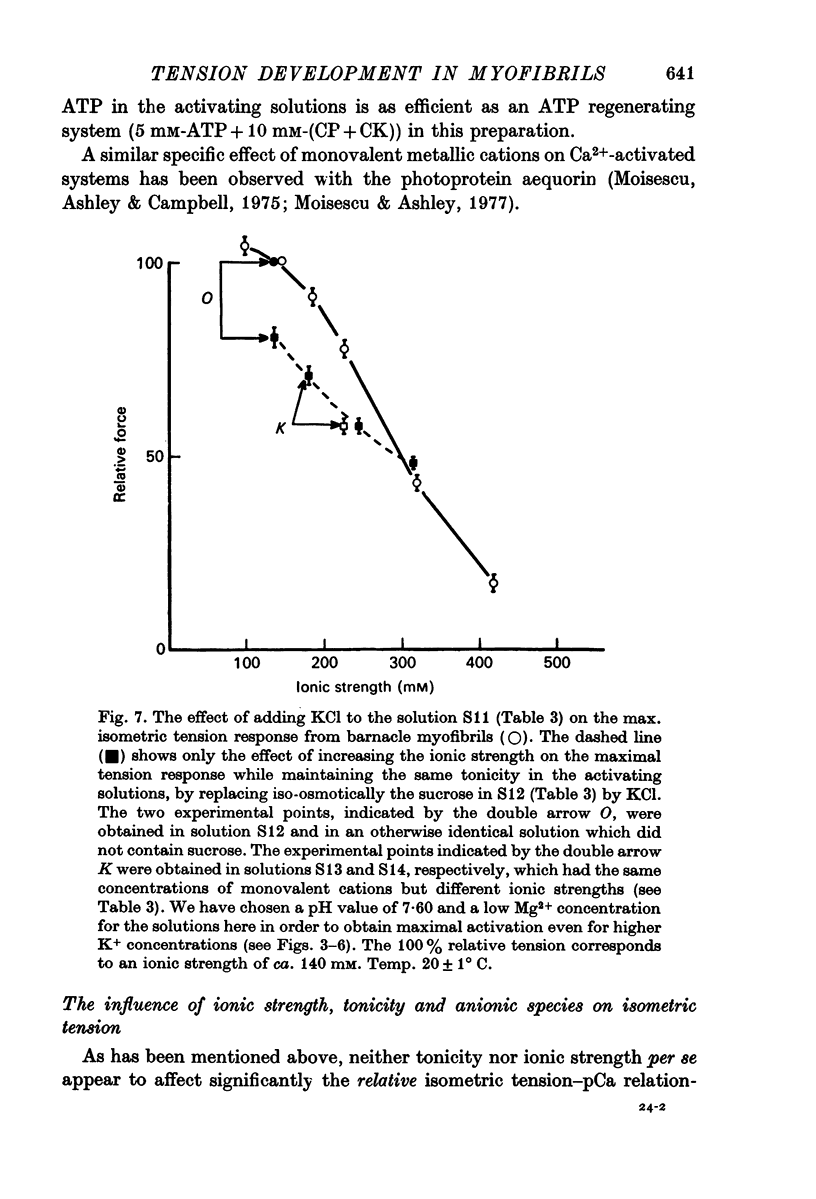
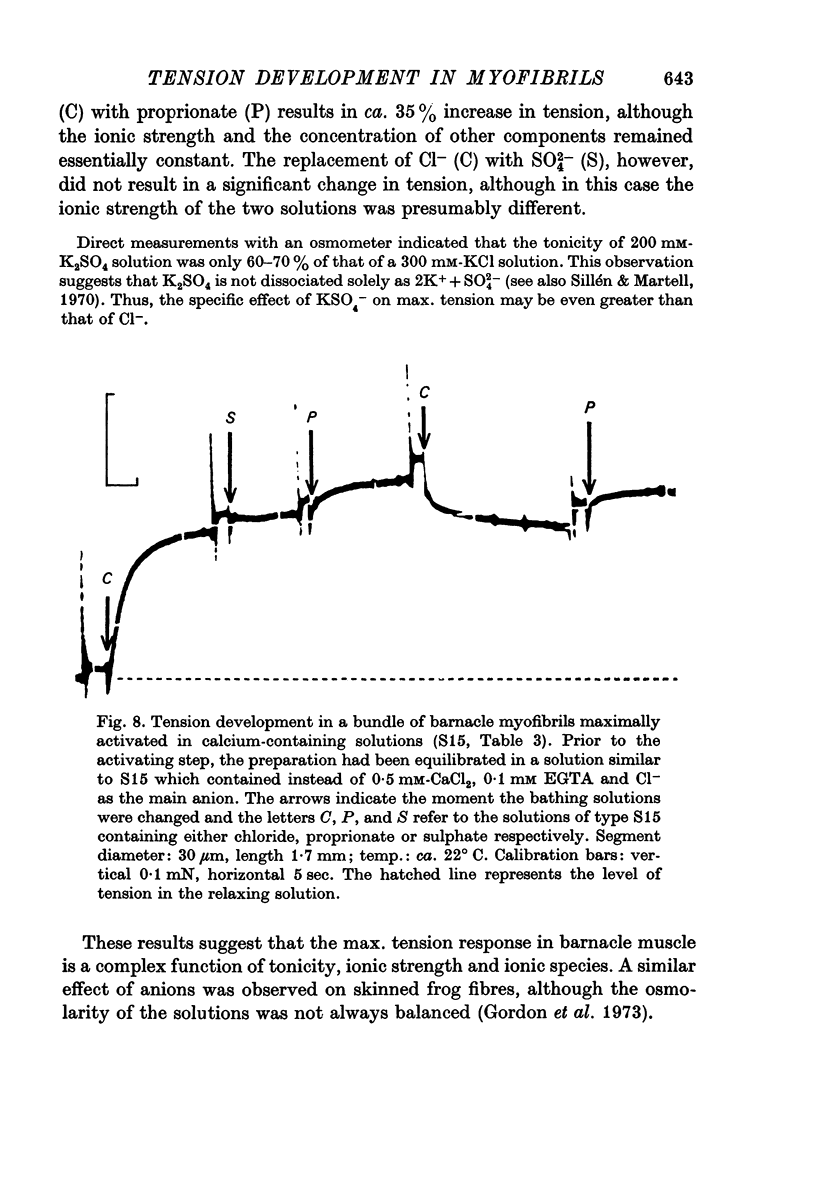
9. Maximum isometric tension in this preparation was not simply related to ionic strength, or to the monovalent cation concentration, but it depended, as well, upon the anionic composition of the activating solution. In addition, a change in ionic strength of 25 mM over the range of 245-270 mM did not appear to modify the relative tension—pCa relationship.
10. The effect of the physiologically occurring cations H+, K+, Mg2+ upon the relative isometric tension—pCa relationship can be accounted for on the basis of a model of competitive inhibition between these cations and Ca2+ for the functional unit for tension. This inhibitory effect appears to involve at least one H+, one Mg2+ and two K+ per each Ca2+ ion participating in the activation process of the functional unit for tension.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- April E. W., Brandt P. W., Elliott G. F. The myofilament lattice: studies on isolated fibers. II. The effects of osmotic strength, ionic concentration, and pH upon the unit-cell volume. J Cell Biol. 1972 Apr;53(1):53–65. doi: 10.1083/jcb.53.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April E. W., Brandt P. W. The myofilament lattice: studies on isolated fibers. 3. The effect of myofilament spacing upon tension. J Gen Physiol. 1973 Apr;61(4):490–508. doi: 10.1085/jgp.61.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April E., Brandt P. W., Reuben J. P., Grundfest H. Muscle contraction: the effect of ionic strength. Nature. 1968 Oct 12;220(5163):182–184. doi: 10.1038/220182a0. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. Model for the action of calcium in muscle. Nat New Biol. 1972 Jun 14;237(76):208–211. doi: 10.1038/newbio237208a0. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. Proceedings: The influence of Mg2+ concentration and of pH upon the relationship between steady-state isometric tension and Ca2+ concentration in isolated bundles of barnacle myofibrils. J Physiol. 1974 Jun;239(2):112P–114P. [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. Tension changes in isolated bundles of frog and barnacle myofibrils in response to sudden changes in the external free calcium concentration. J Physiol. 1973 Aug;233(1):8P–9P. [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Caputo C. Caffeine- and potassium-induced contractures of frog striated muscle fibers in hypertonic solutions. J Gen Physiol. 1966 Sep;50(1):129–139. doi: 10.1085/jgp.50.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. K., Kerrick W. G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975 Oct;66(4):427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Moos C. Actin activation of heavy meromyosin adenosine triphosphatase. Dependence on adenosine triphosphate and actin concentrations. J Biol Chem. 1970 May 10;245(9):2451–2456. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol. 1975 Aug;249(3):497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol. 1972 May;223(1):1–19. doi: 10.1113/jphysiol.1972.sp009830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E., Donaldson S. K., Harris C. E. Tension in skinned frog muscle fibers in solutions of varying ionic strength and neutral salt composition. J Gen Physiol. 1973 Nov;62(5):550–574. doi: 10.1085/jgp.62.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G., SMYTH T., Jr NEUROMUSCULAR PHYSIOLOGY OF GIANT MUSCLE FIBERS OF A BARNACLE, BALANUS NUBILUS DARWIN. Comp Biochem Physiol. 1963 Dec;10:291–314. doi: 10.1016/0010-406x(63)90229-9. [DOI] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The intracellular calcium contents of some invertebrate nerves. J Physiol. 1956 Nov 28;134(2):399–407. doi: 10.1113/jphysiol.1956.sp005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Donaldson S. K. The effects of Mg 2+ on submaximum Ca 2+ -activated tension in skinned fibers of frog skeletal muscle. Biochim Biophys Acta. 1972 Jul 12;275(1):117–122. doi: 10.1016/0005-2728(72)90030-8. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Miller D. J., Moisescu D. G. The effects of very low external calcium and sodium concentrations on cardiac contractile strength and calcium-sodium antagonism. J Physiol. 1976 Jul;259(2):283–308. doi: 10.1113/jphysiol.1976.sp011466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu D. G., Ashley C. C., Campbell A. K. Comparative aspects of the calcium-sensitive photoproteins aequorin and obelin. Biochim Biophys Acta. 1975 Jul 8;396(1):133–140. doi: 10.1016/0005-2728(75)90196-6. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G., Ashley C. C. The effect of physiologically occurring cations upon aequorin light emission. Determination of the binding constants. Biochim Biophys Acta. 1977 May 11;460(2):189–205. doi: 10.1016/0005-2728(77)90206-7. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- PERRY S. V. Relation between chemical and contractile function and structure of the skeletal muscle cell. Physiol Rev. 1956 Jan;36(1):1–76. doi: 10.1152/physrev.1956.36.1.1. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Calcium binding, quantum yield, and emitting molecule in aequorin bioluminescence. Nature. 1970 Sep 26;227(5265):1356–1357. doi: 10.1038/2271356a0. [DOI] [PubMed] [Google Scholar]
- WEBER A., HERZ R. Requirement for calcium in the synaeresis of myofibrils. Biochem Biophys Res Commun. 1961 Dec 20;6:364–368. doi: 10.1016/0006-291x(61)90146-2. [DOI] [PubMed] [Google Scholar]


