Abstract
1. Six subjects were trained using a one-leg bicycle exercise for 2 months. The untrained leg served as control. After the training period, muscle oxidative capacity, determined as succinate dehydrogenase activity, was 27% higher in the trained (as opposed to the control) leg (P < 0·05).
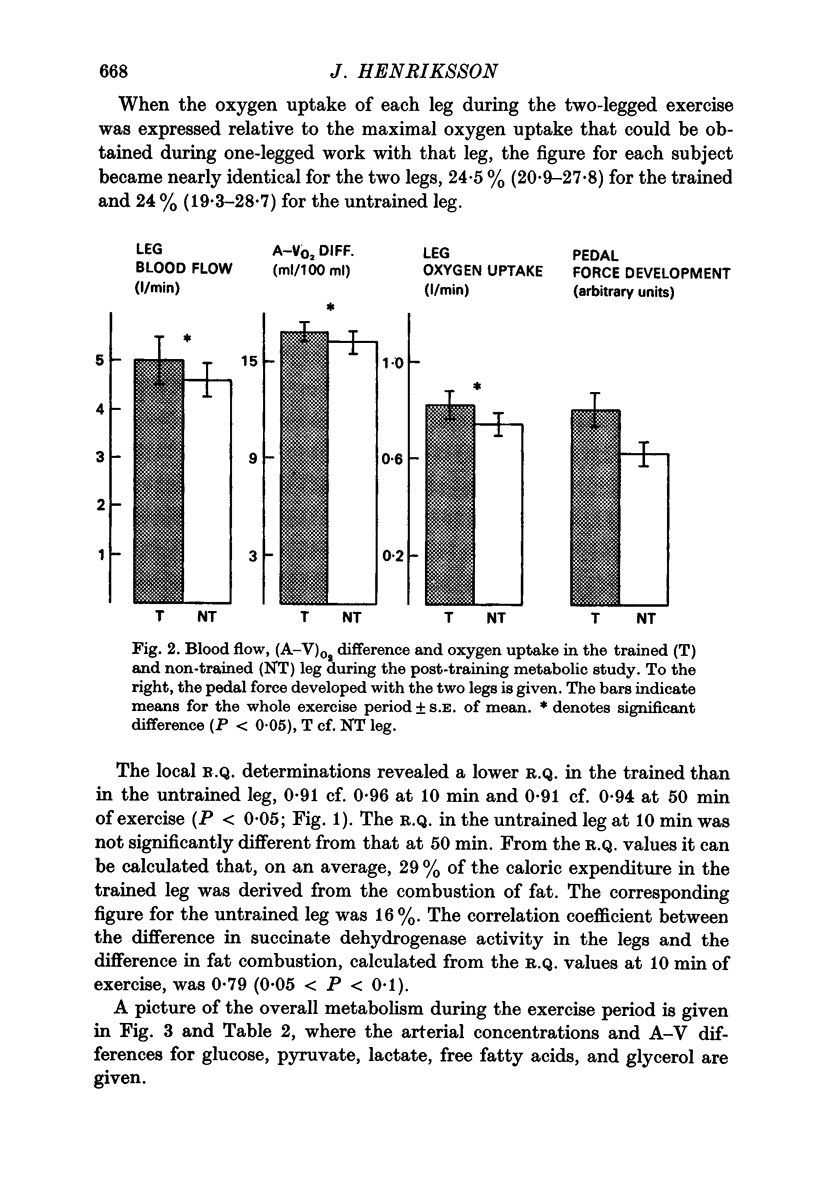
2. When the subjects in this situation performed a 1 h two-legged submaximal bicycle exercise bout (150-225 W), determinations of VO2 of the single leg (leg blood flow × (A—V)O2 difference) revealed that they appeared to choose to work harder with their trained than with their untrained leg, so as to make the relative loads for the two legs the same.
3. Determinations of O2 and CO2 on femoral arterial and venous blood demonstrated that the R.Q. was lower in the trained as compared to the untrained leg, 0·91 cf. 0·96 (10 min) and 0·91 cf. 0·94 (50 min) (P < 0·05).
4. That metabolism of fat was more pronounced in the trained leg was further supported by the finding of a significant net uptake of free fatty acids in this leg only. Moreover, a lower release of lactate from the trained leg was demonstrated.
5. It is suggested that the shift towards a more pronounced metabolism of fat in the trained leg is a function of an increased muscle oxidative capacity.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg G., Hagenfeldt L., Wahren J. Substrate utilization by the inactive leg during one-leg or arm exercise. J Appl Physiol. 1975 Nov;39(5):718–723. doi: 10.1152/jappl.1975.39.5.718. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A., KURFESS N. J. A microspectrophotometric method for the determination of succinic dehydrogenase. J Biol Chem. 1950 Sep;186(1):129–139. [PubMed] [Google Scholar]
- Davies C. T., Sargeant A. J. Effects of training on the physiological responses to one- and two-leg work. J Appl Physiol. 1975 Mar;38(3):377–375. doi: 10.1152/jappl.1975.38.3.377. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Piehl K., Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974 Aug;241(1):45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Henriksson J., Reitman J. S. Time course of changes in human skeletal muscle succinate dehydrogenase and cytochrome oxidase activities and maximal oxygen uptake with physical activity and inactivity. Acta Physiol Scand. 1977 Jan;99(1):91–97. doi: 10.1111/j.1748-1716.1977.tb10356.x. [DOI] [PubMed] [Google Scholar]
- Hoes M. J., Binkhorst R. A., Smeekes-Kuyl A. E., Vissers A. C. Measurement of forces exerted on pedal and crank during work on a bicycle ergometer atdifferent loads. Int Z Angew Physiol. 1968;26(1):33–42. doi: 10.1007/BF00696088. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L., Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971 Nov;41(5):459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- Laurell S., Tibbling G. Colorimetric micro-determination of free fatty acids in plasma. Clin Chim Acta. 1967 Apr;16(1):57–62. doi: 10.1016/0009-8981(67)90269-0. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- SELDINGER S. I. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953 May;39(5):368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- Saltin B., Nazar K., Costill D. L., Stein E., Jansson E., Essén B., Gollnick D. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976 Mar;96(3):289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]


