Abstract
1. Pulse synchronous bursts of multi-unit sympathetic activity was recorded from median or peroneal muscle nerve fascicles in fourteen subjects resting in the recumbent position. The neural activity was quantitated in terms of burst incidence, i.e. the number of bursts in the mean voltage neurogram/100 heart beats, during successive rest periods of 2-4 min.
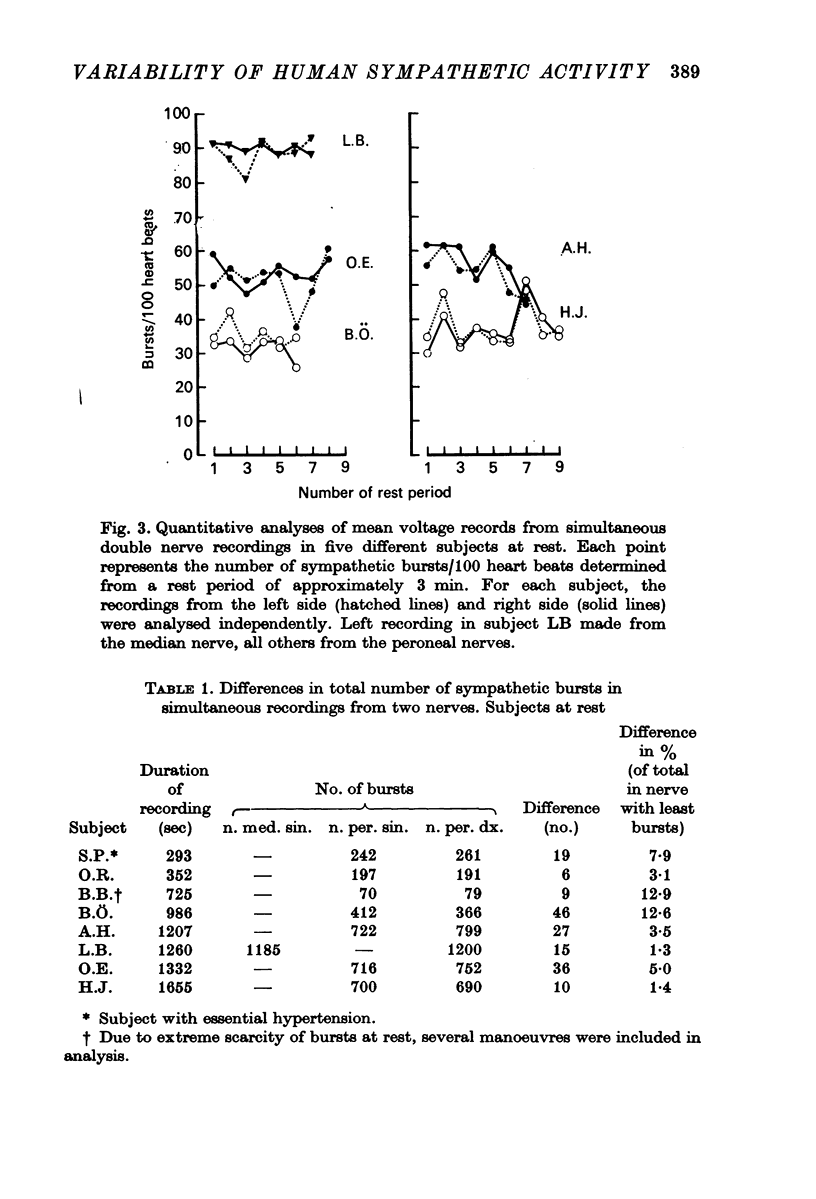
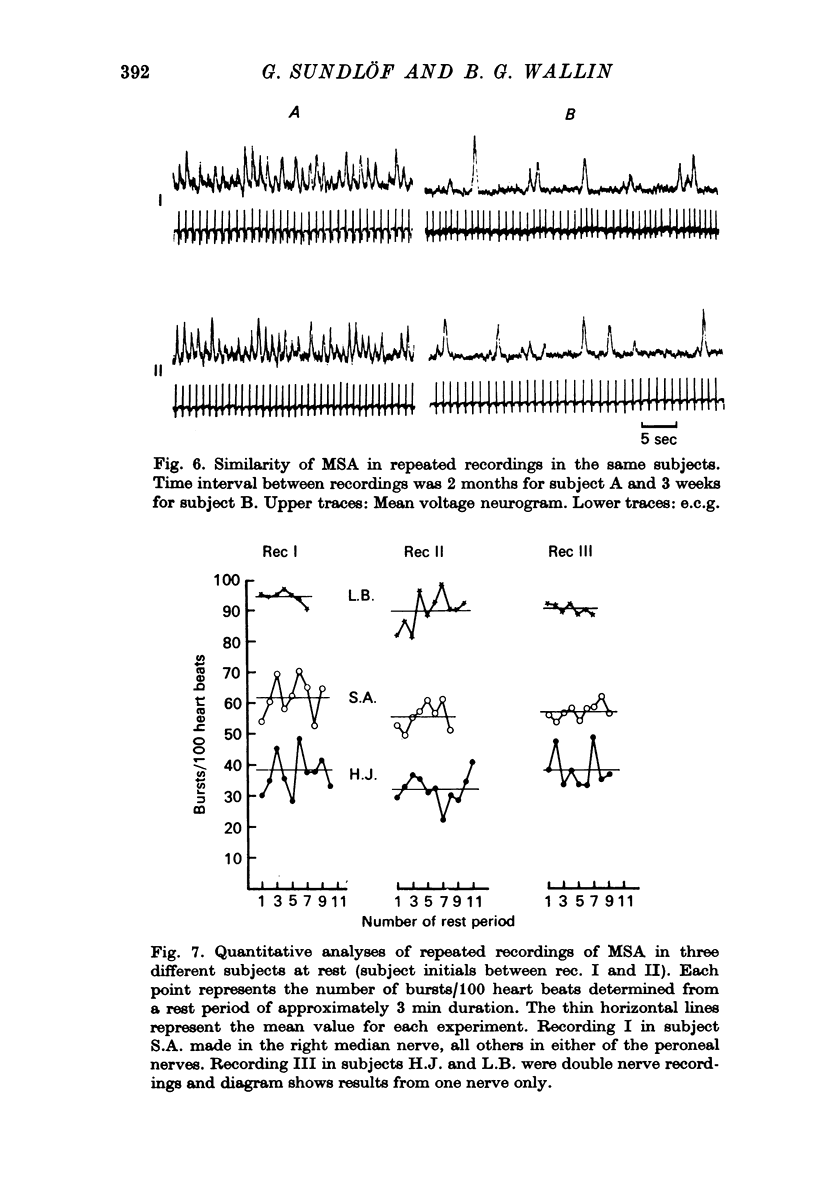
2. For each individual the burst incidence was fairly constant between different rest periods but the mean burst incidence varied widely between individuals, the range being from less than 10 to more than 90 bursts/100 heart beats.
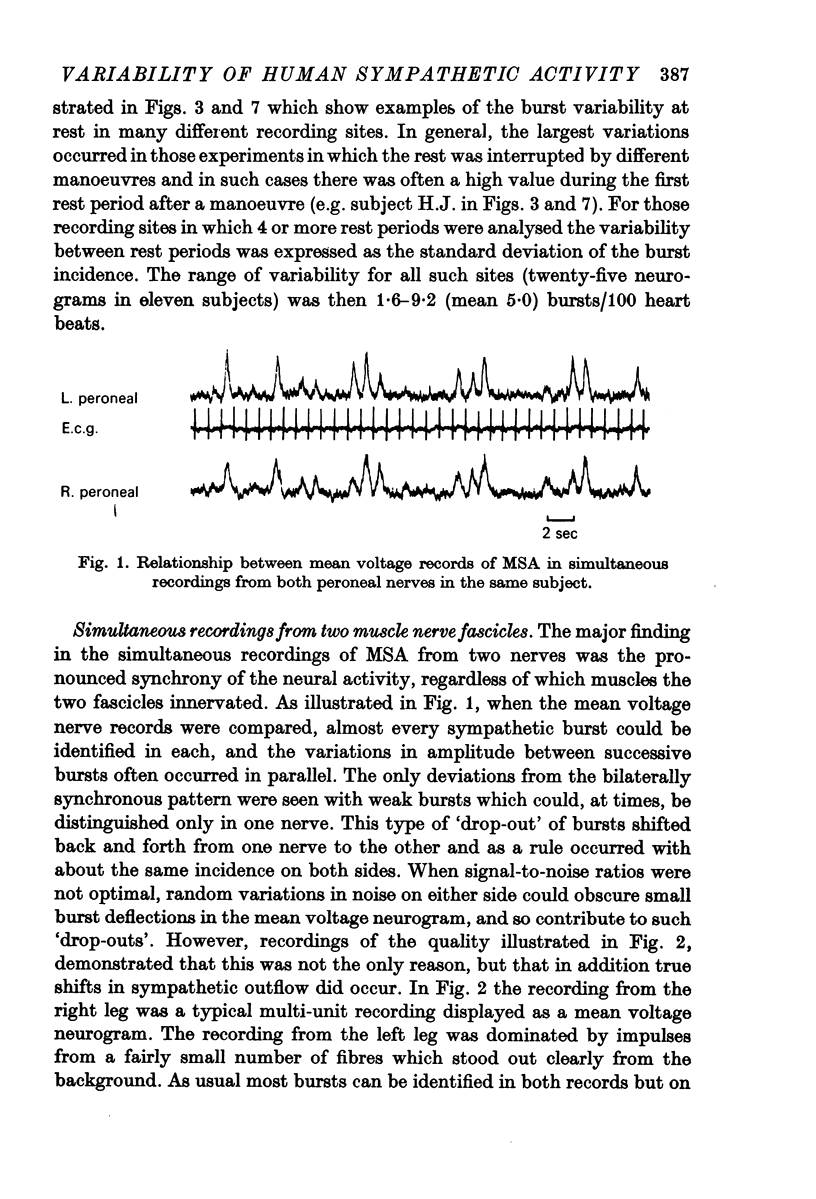
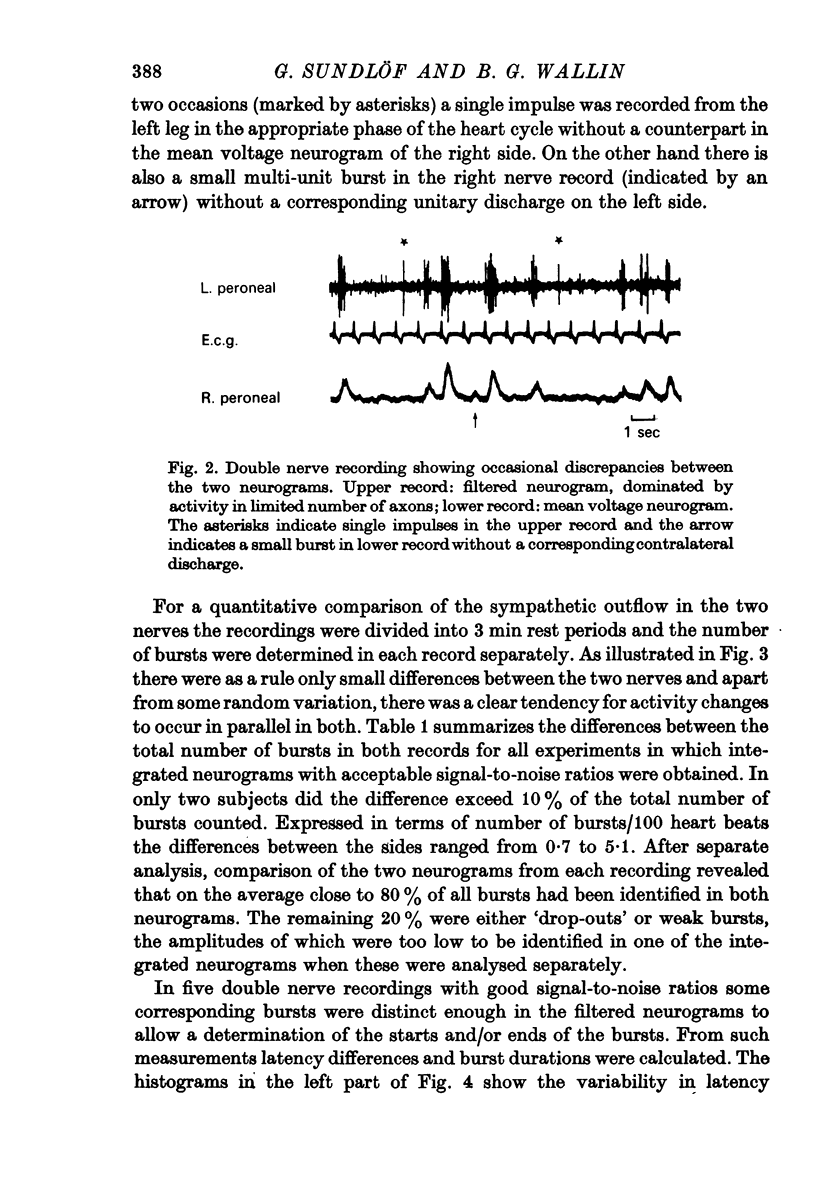
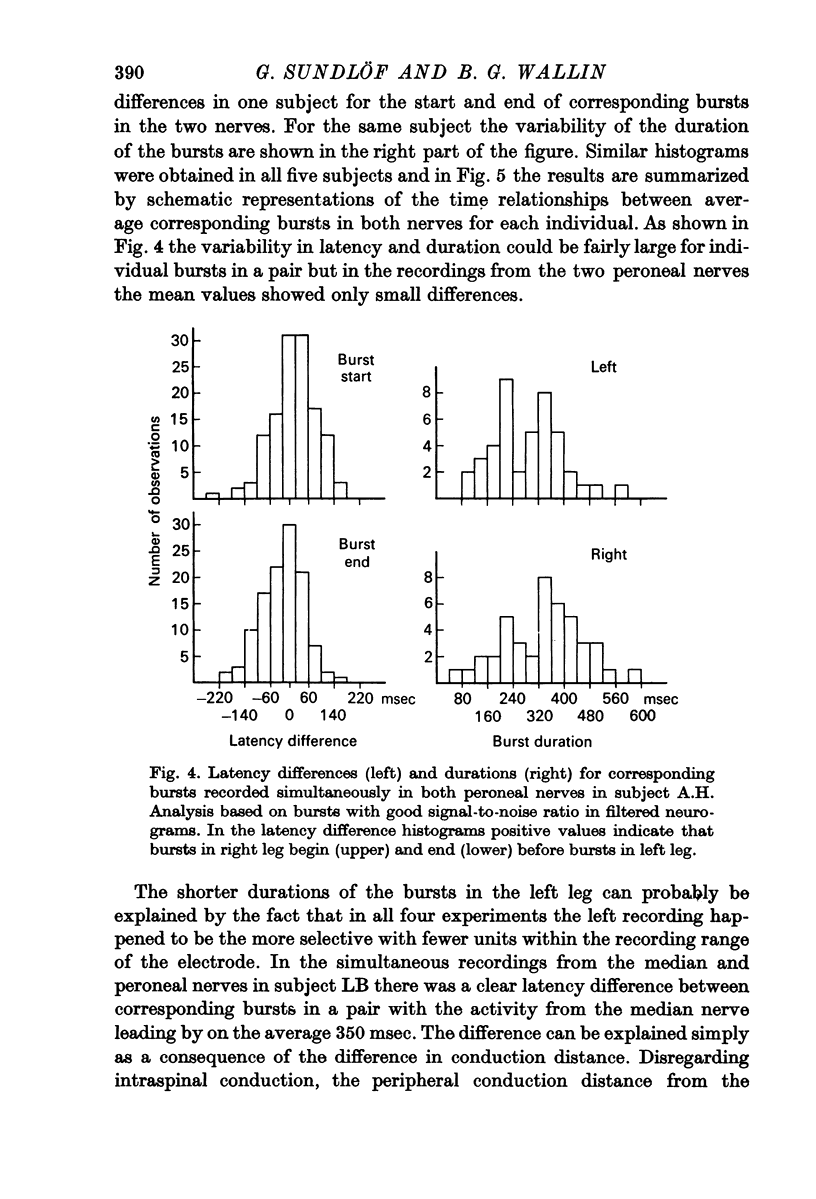
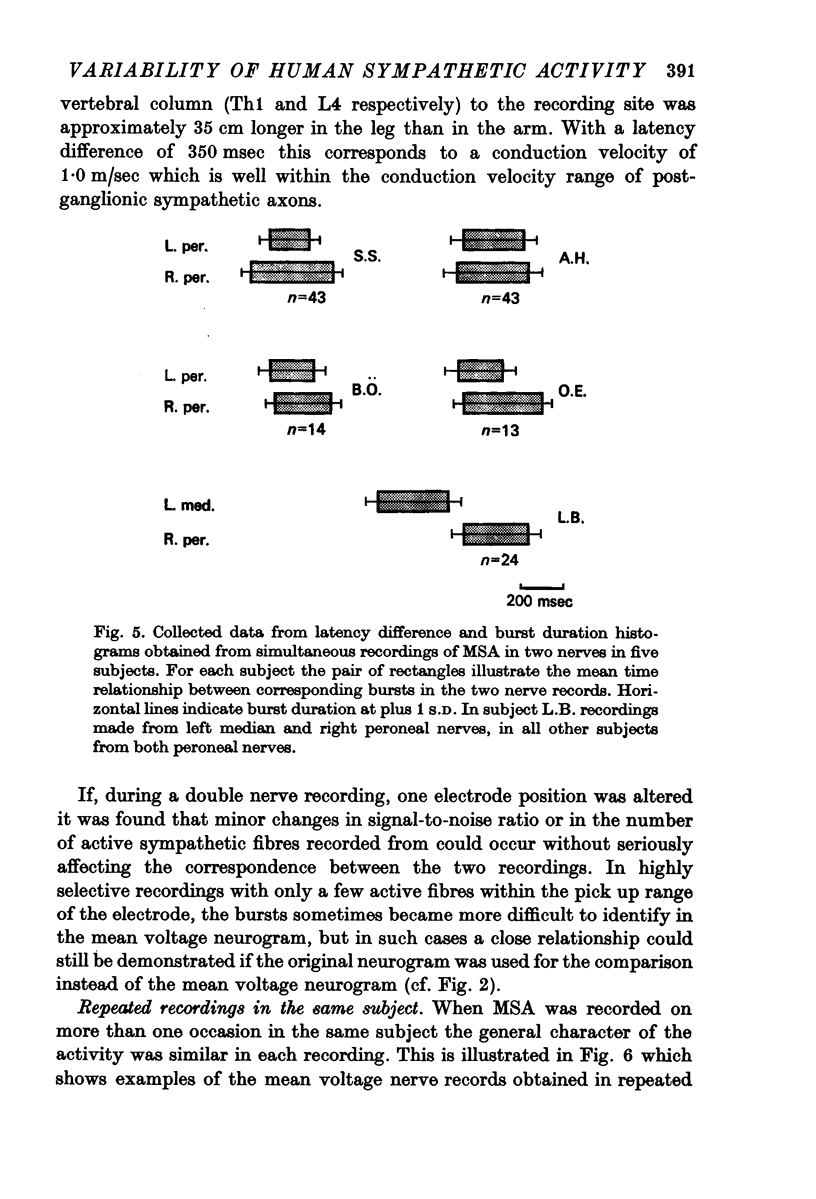
3. Simultaneous double nerve recordings were made on one subject from median and peroneal nerves and on eight subjects from the two peroneal nerves. There was always close similarity between the two records in such experiments regardless of which muscles the nerve fascicles innervated. When analysed separately the difference in burst incidence between the two sides ranged from 0·7 to 5·1 bursts/100 heart beats. The findings suggest that sympathetic neurones destined to skeletal muscles are subjected to a homogenous central drive and that contributions to the activity from ganglionic or segmental sources are of lesser importance.
4. On seven subjects repeated recordings at rest were made with intervals of 3 weeks—21 months between recordings. In each subject mean burst incidences were similar in all recordings (range of differences 0·5-11·2 bursts/100 heart beats) suggesting an individually constant level of sympathetic activity in muscle nerves.
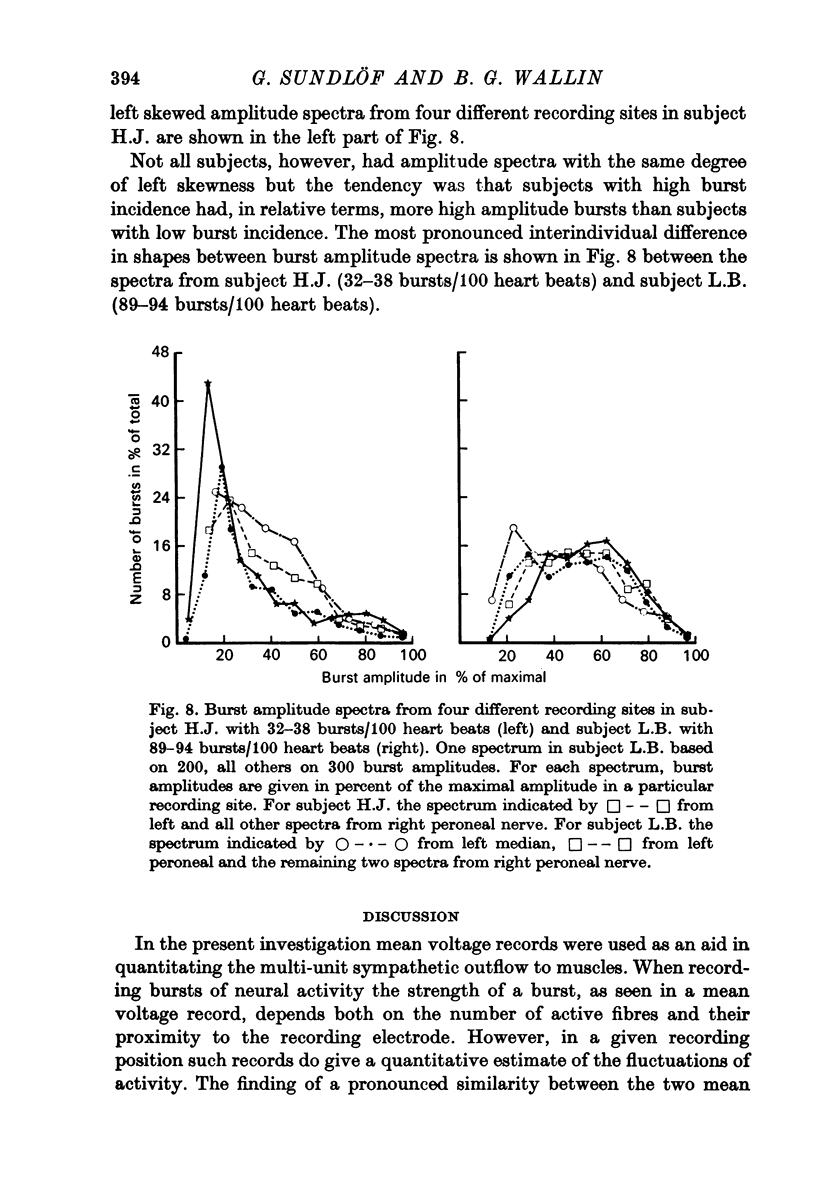
5. For each individual the variability of burst amplitudes in the mean voltage neurogram was described by burst amplitude spectra. Most subjects had a relatively larger proportion of small than high amplitude bursts, but there was a tendency for more even amplitude distributions in subjects with high burst incidence. The finding may be an indication of interindividual differences in the average number of impulses/burst.
6. It is concluded that the multi-unit recording technique can be used for comparisons of the level of muscle nerve `sympathetic tone' between different subjects.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWSE N. L. Effect of bed rest on resting calf blood flow of healthy adult males. Br Med J. 1962 Jun 23;1(5294):1721–1723. doi: 10.1136/bmj.1.5294.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolme P., Fuxe K. Adrenergic and cholinergic nerve terminals in skeletal muscle vessels. Acta Physiol Scand. 1970 Jan;78(1):52–59. doi: 10.1111/j.1748-1716.1970.tb04638.x. [DOI] [PubMed] [Google Scholar]
- Delius W., Hagbarth K. E., Hongell A., Wallin B. G. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand. 1972 Jan;84(1):65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Delius W., Hagbarth K. E., Hongell A., Wallin B. G. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand. 1972 Jan;84(1):82–94. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- GOLENHOFEN K., HILDERBRANDT G. Uber spontan-rhythmische Schwankungen der Muskeldurchblutung des Menschen. Z Kreislaufforsch. 1957 Apr;46(7-8):257–270. [PubMed] [Google Scholar]
- Hagbarth K. E., Hallin R. G., Hongell A., Torebjörk H. E., Wallin B. G. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972 Feb;84(2):164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hallin R. G., Torebjörk H. E. Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiol Scand. 1974 Nov;92(3):303–317. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- Honma S. Functional differentiation in sB and sC neurons of toad sympathetic ganglia. Jpn J Physiol. 1970 Jun 15;20(3):281–295. doi: 10.2170/jjphysiol.20.281. [DOI] [PubMed] [Google Scholar]
- Marwitt R., Pilar G., Weakly J. N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971 Jan 22;25(2):317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Studies on sympathetic B and C neurons and patterns of pregnaglionic innervation. J Cell Physiol. 1965 Aug;66(1):19–32. doi: 10.1002/jcp.1030660103. [DOI] [PubMed] [Google Scholar]
- Wallin B. G., Delius W., Hagbarth K. E. Comparison of sympathetic nerve activity in normotensive and hypertensive subjects. Circ Res. 1973 Jul;33(1):9–21. doi: 10.1161/01.res.33.1.9. [DOI] [PubMed] [Google Scholar]
- Wallin B. G., Delius W., Sundlöf G. Human muscle nerve sympathetic activity in cardiac arrhythmias. Scand J Clin Lab Invest. 1974 Dec;34(4):293–300. doi: 10.3109/00365517409049883. [DOI] [PubMed] [Google Scholar]


