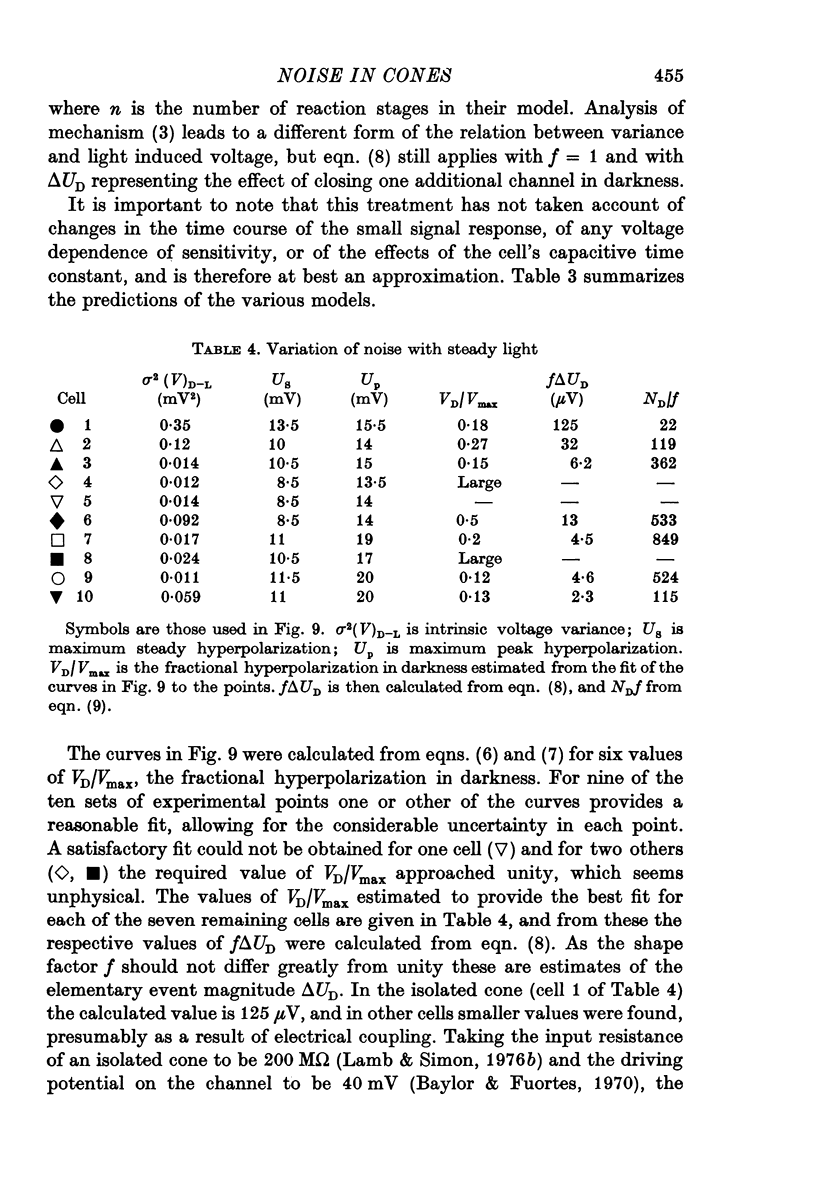
Abstract
1. Properties of the light-sensitive voltage noise in cones in the retina of the turtle, Pseudemys scripta elegans, have been studied by intracellular recording.
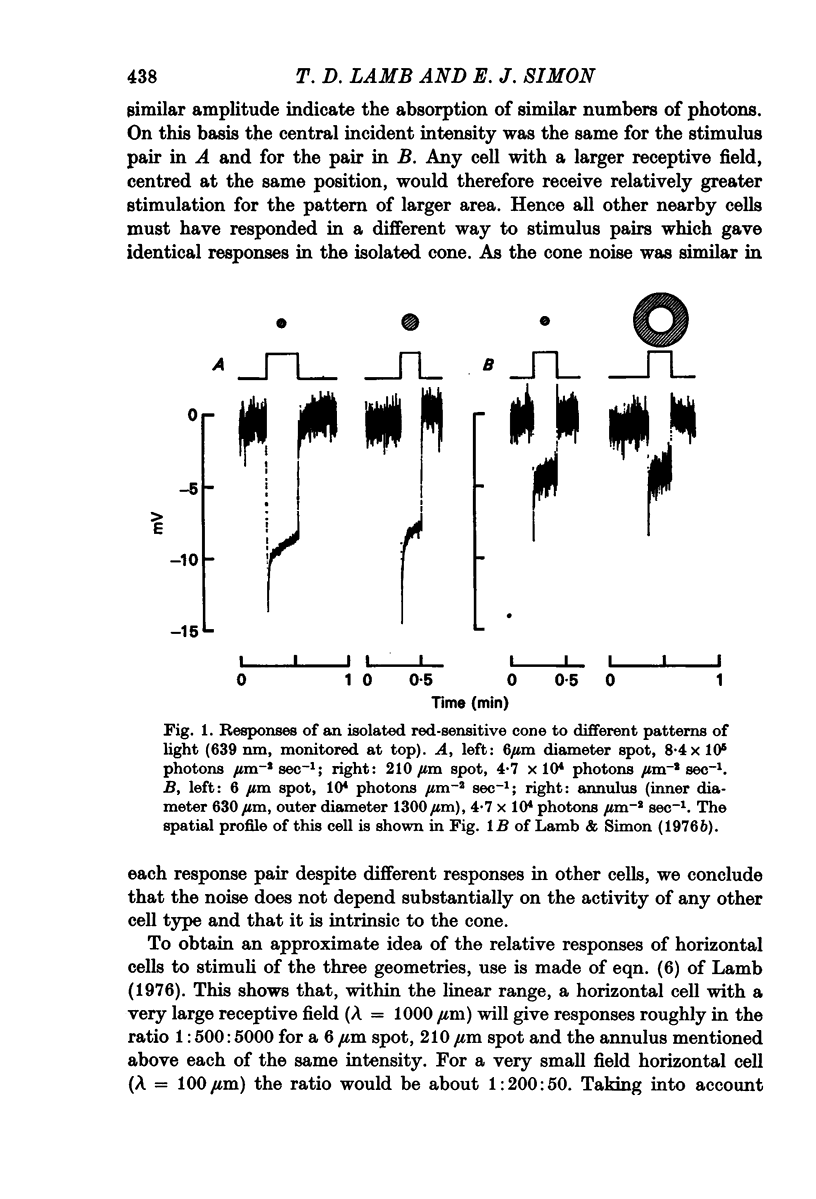
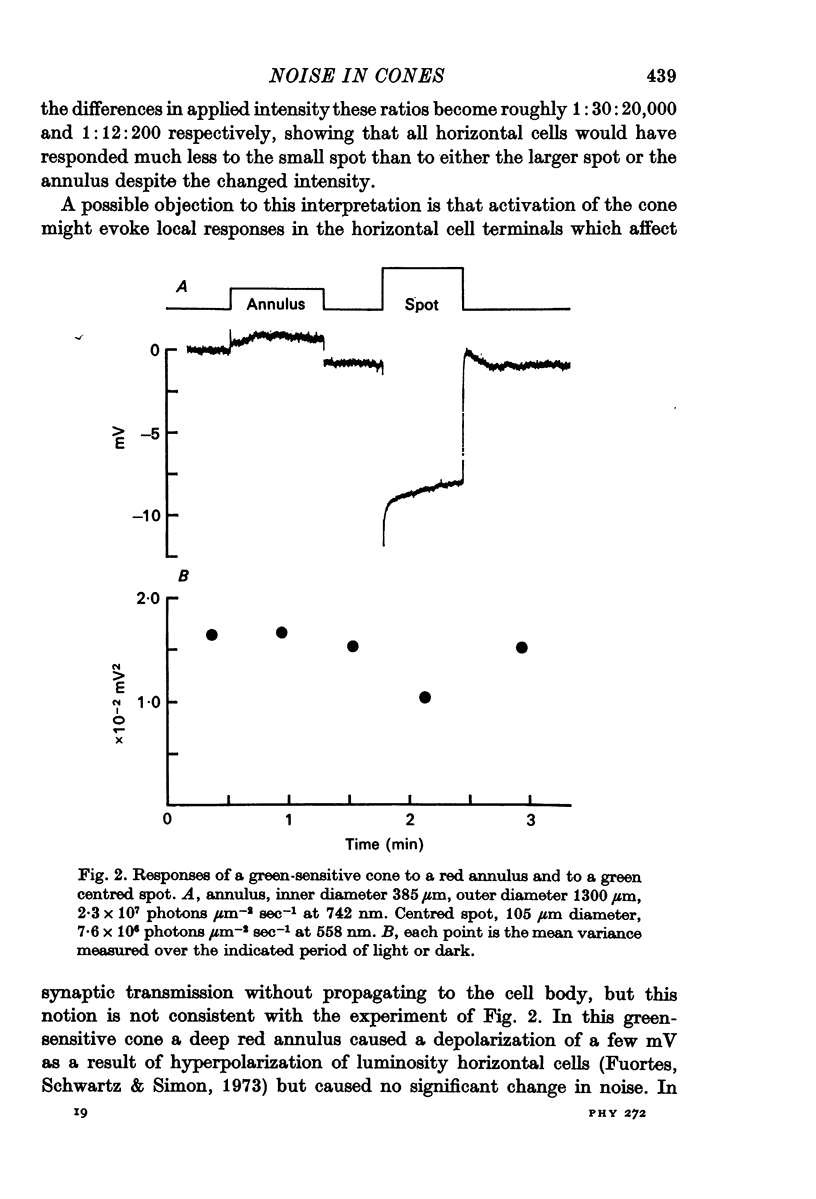
2. Suppression of the noise by light was a function of the hyperpolarizing response of a cone but not of the size or pattern of illumination.
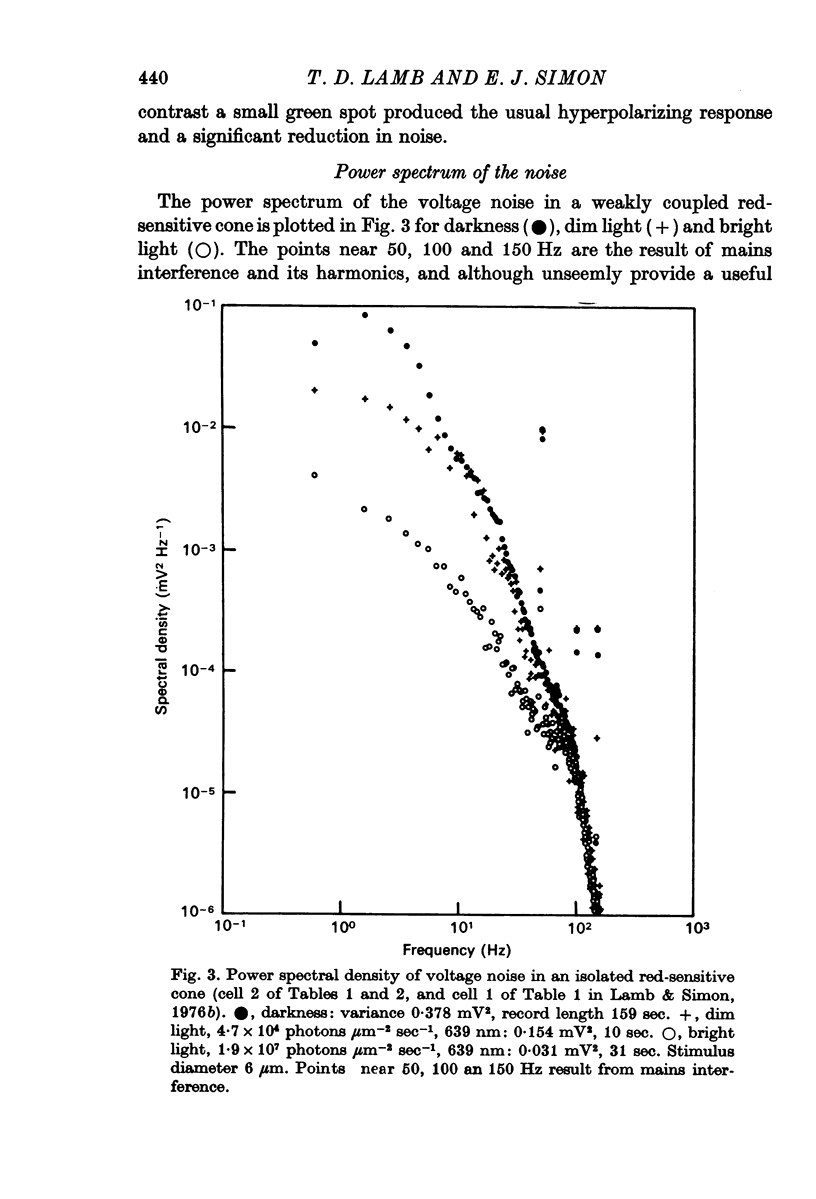
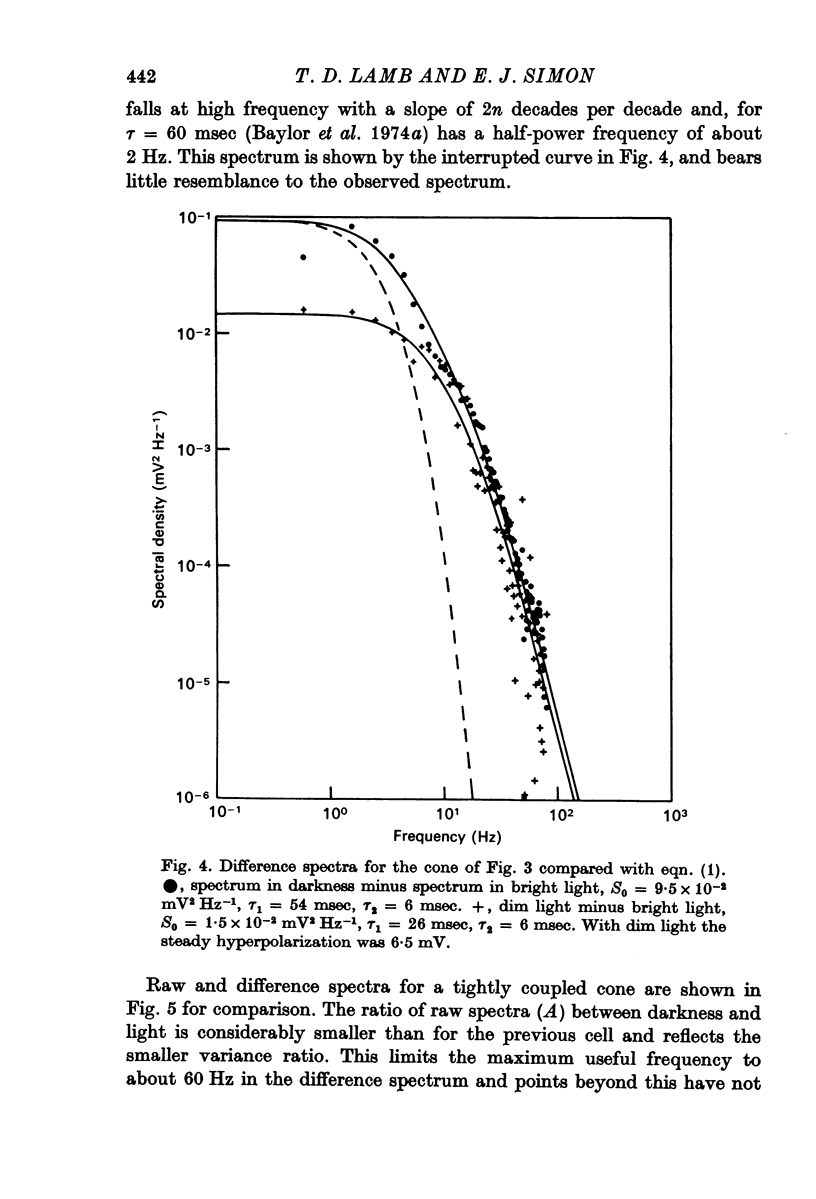
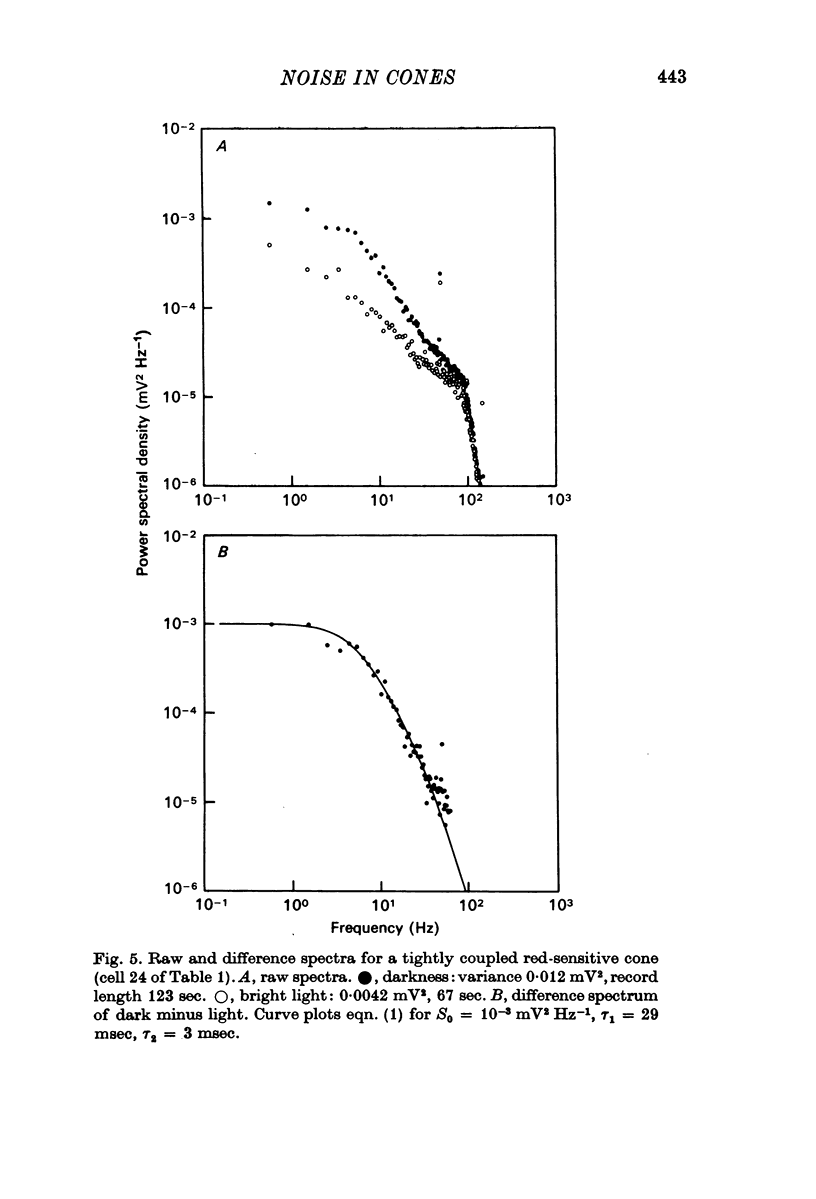
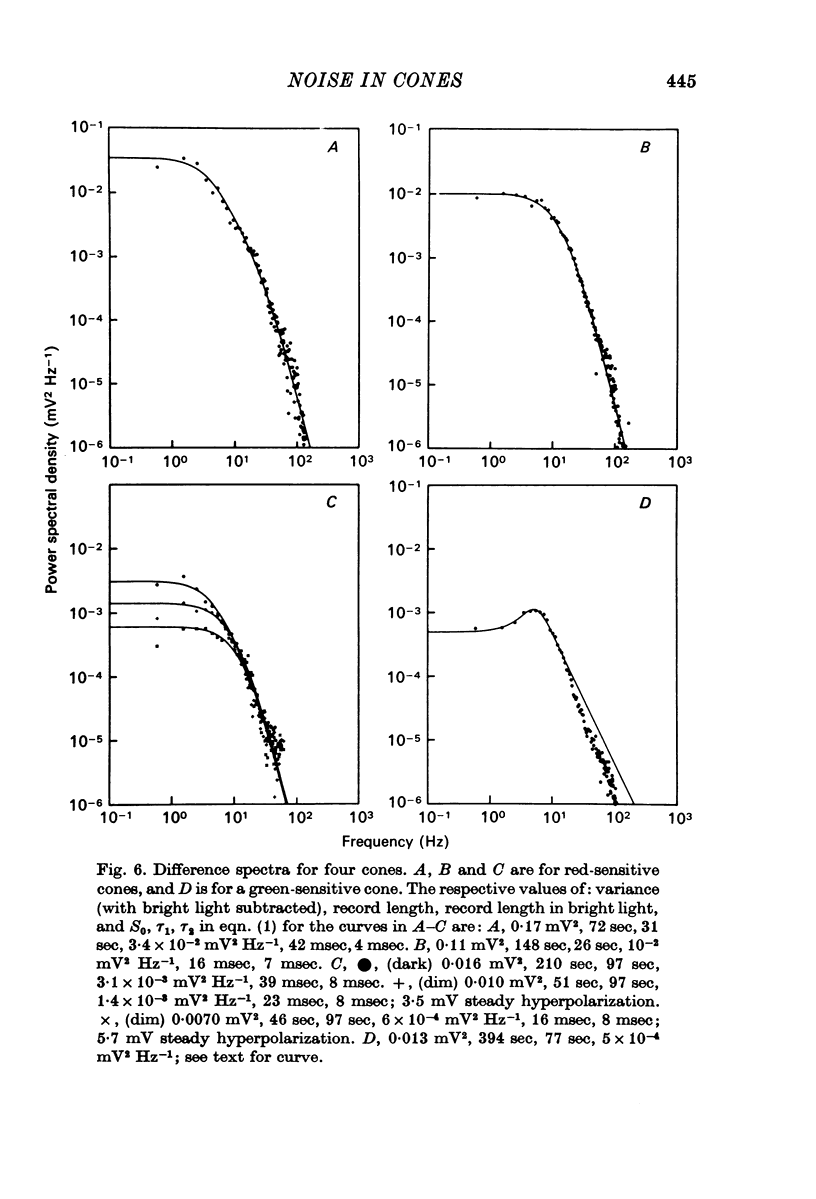
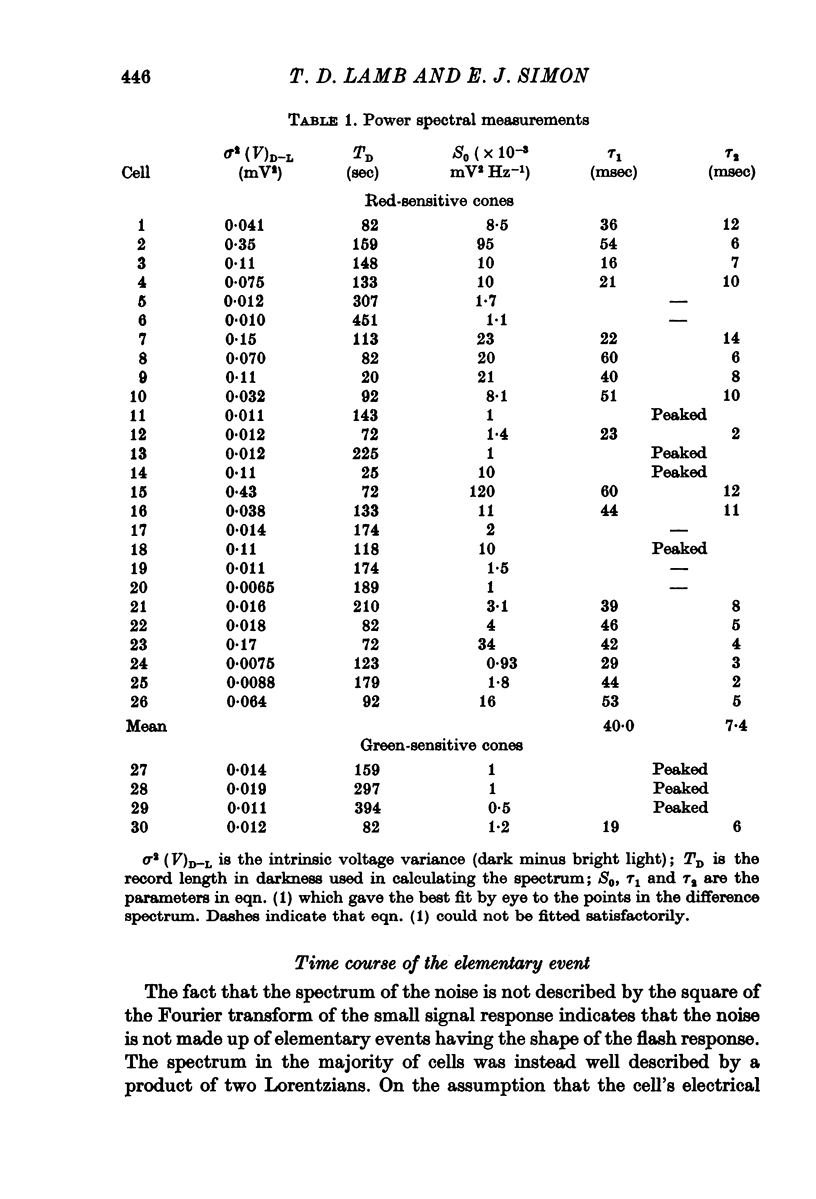
3. Power density spectra of the noise were fitted in many cones by the product of two Lorentzians with characteristic time constants τ1 and τ2 averaging 40 and 7 msec respectively. The spectra of some cells were peaked and could be fitted by a resonance curve.
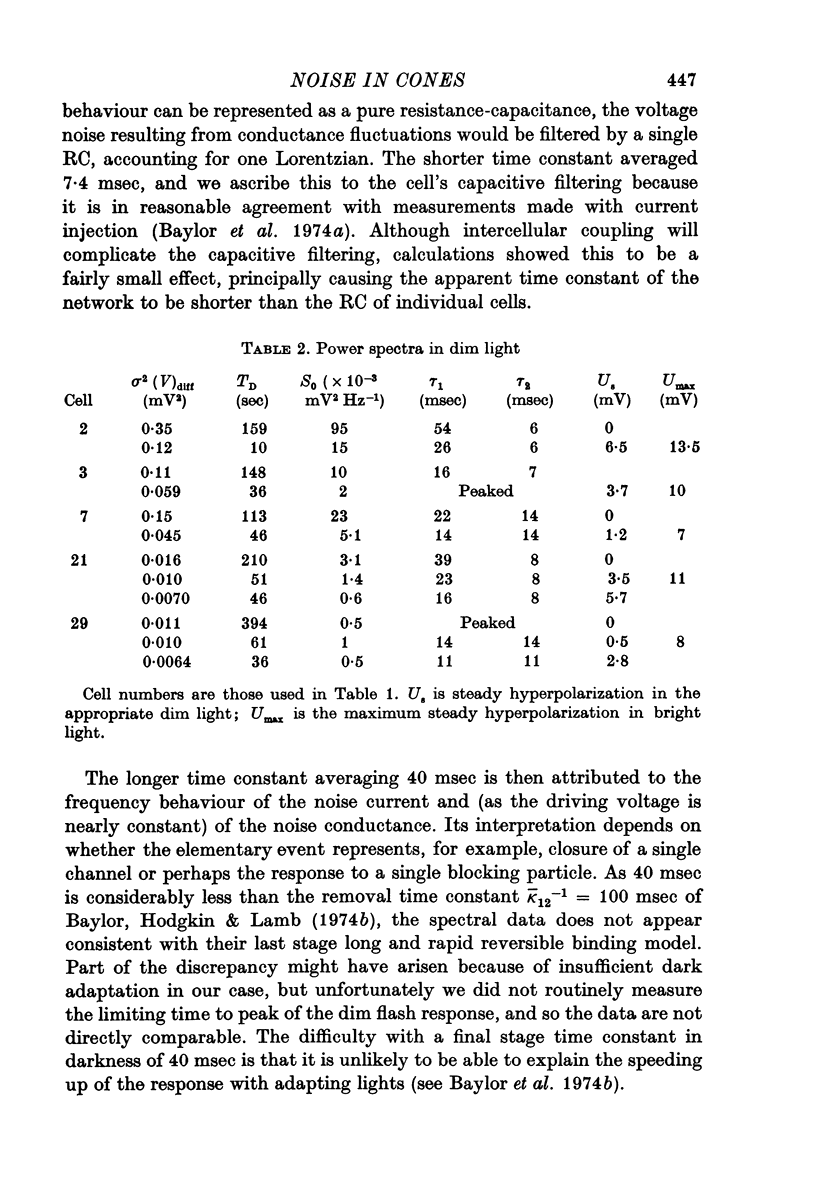
4. Spectra in dim light exhibited decreased low frequency power. They could often be fitted by a product of two Lorentzians using the same value of τ2 as used in darkness but decreasing τ1 and the zero frequency asymptote. An e-fold reduction in τ1 occurred with lights which hyperpolarized by 4-7 mV.
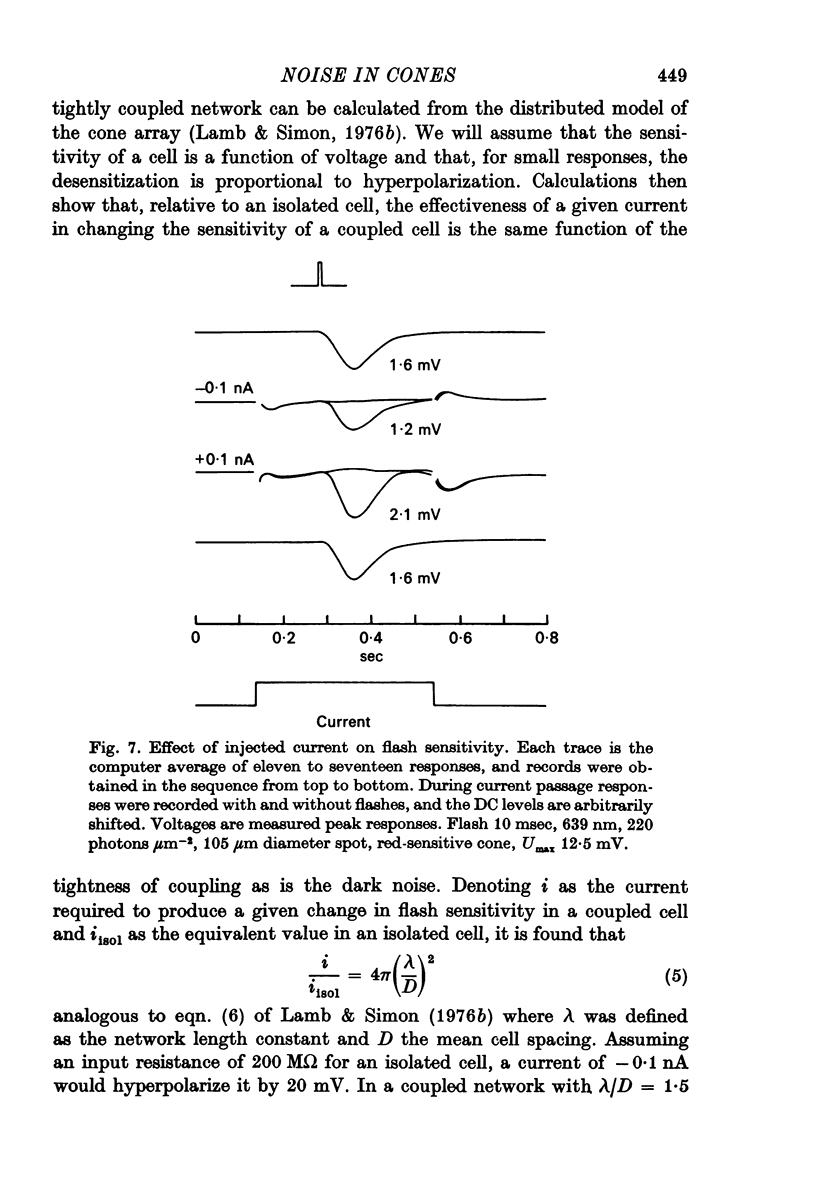
5. Injection of hyperpolarizing currents of about 0·1-0·2 nA into weakly coupled cones reduced the noise, and also reduced the sensitivity to dim flashes.
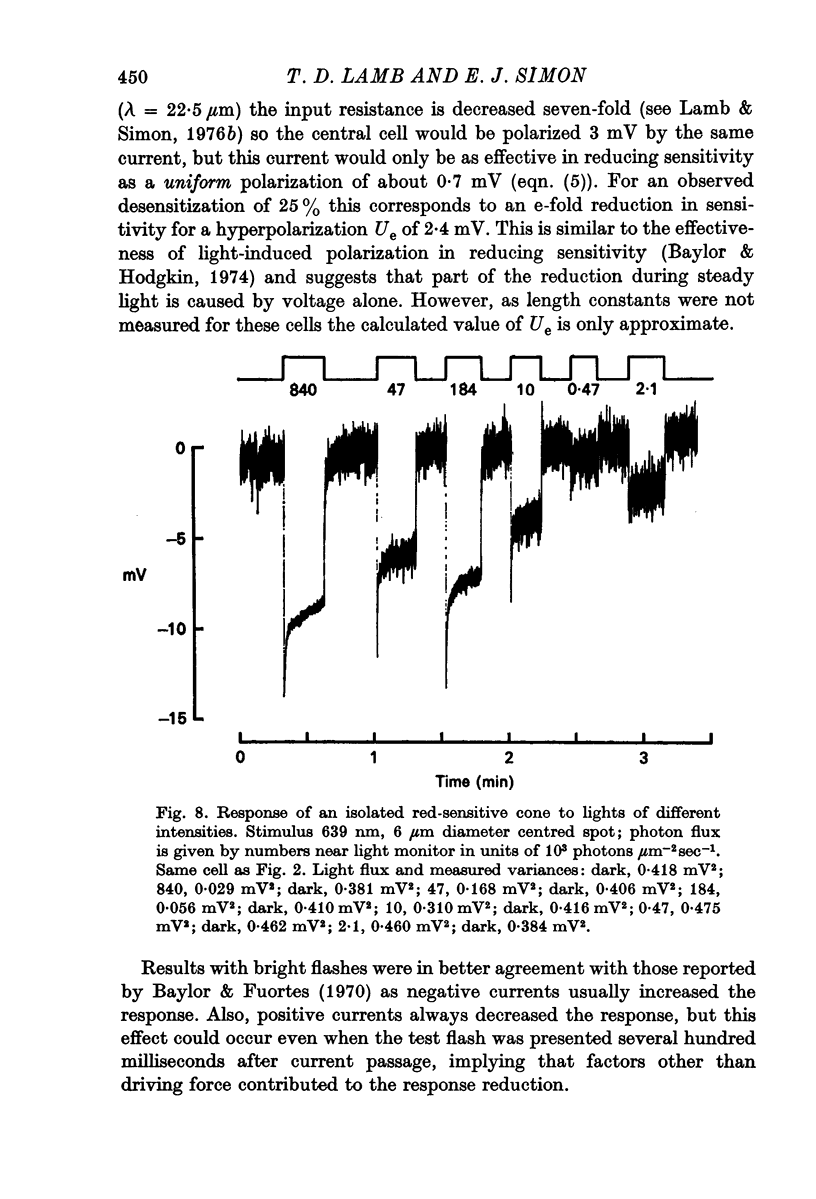
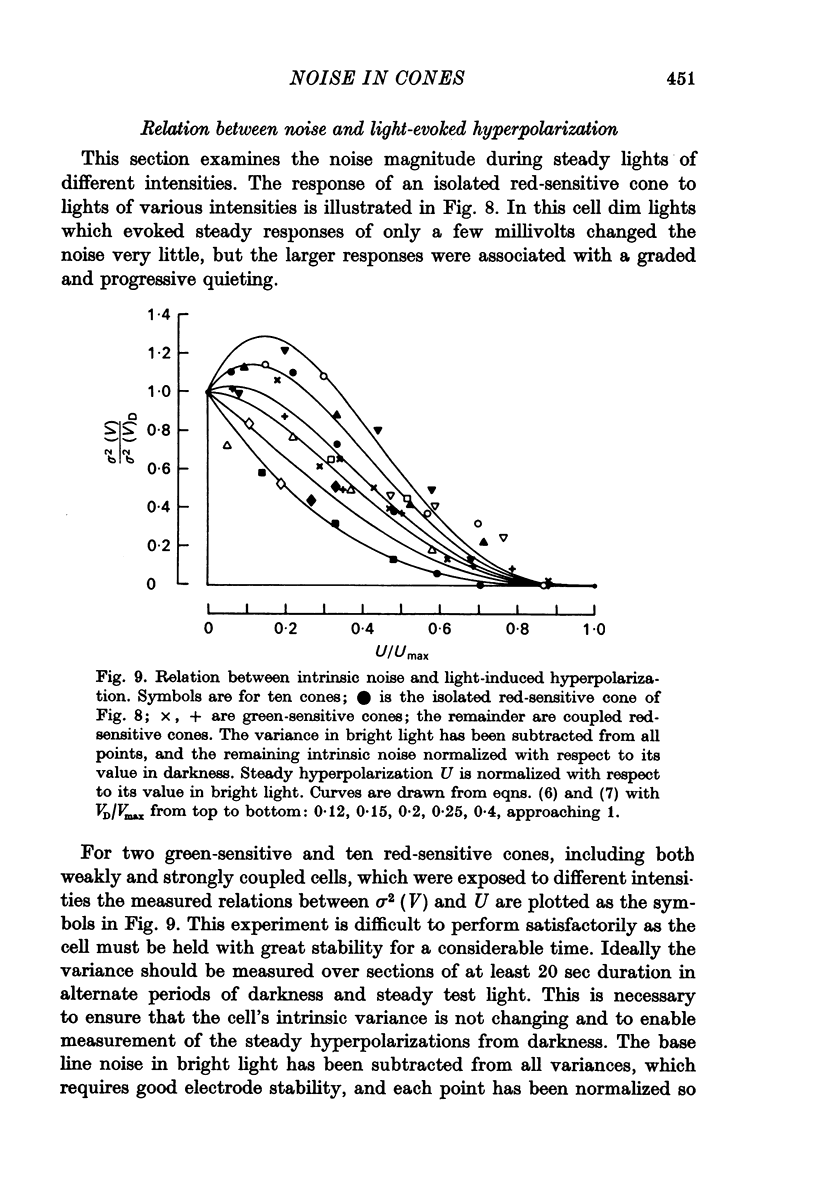
6. The variance-voltage relation during steady illumination of different intensities differed from cone to cone. Dim lights increased the noise in some cells and decreased it in others, but moderately bright lights which gave steady responses of more than about one third maximal reduced the noise in all cells.
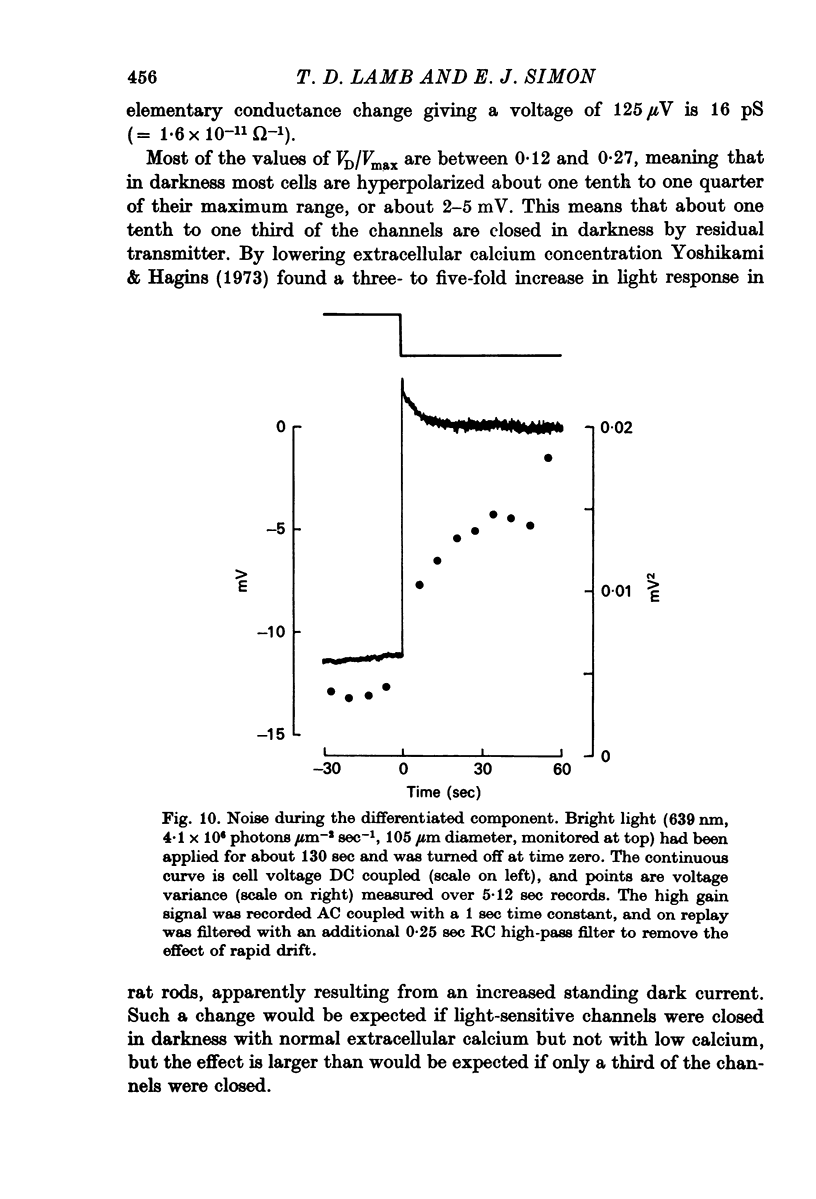
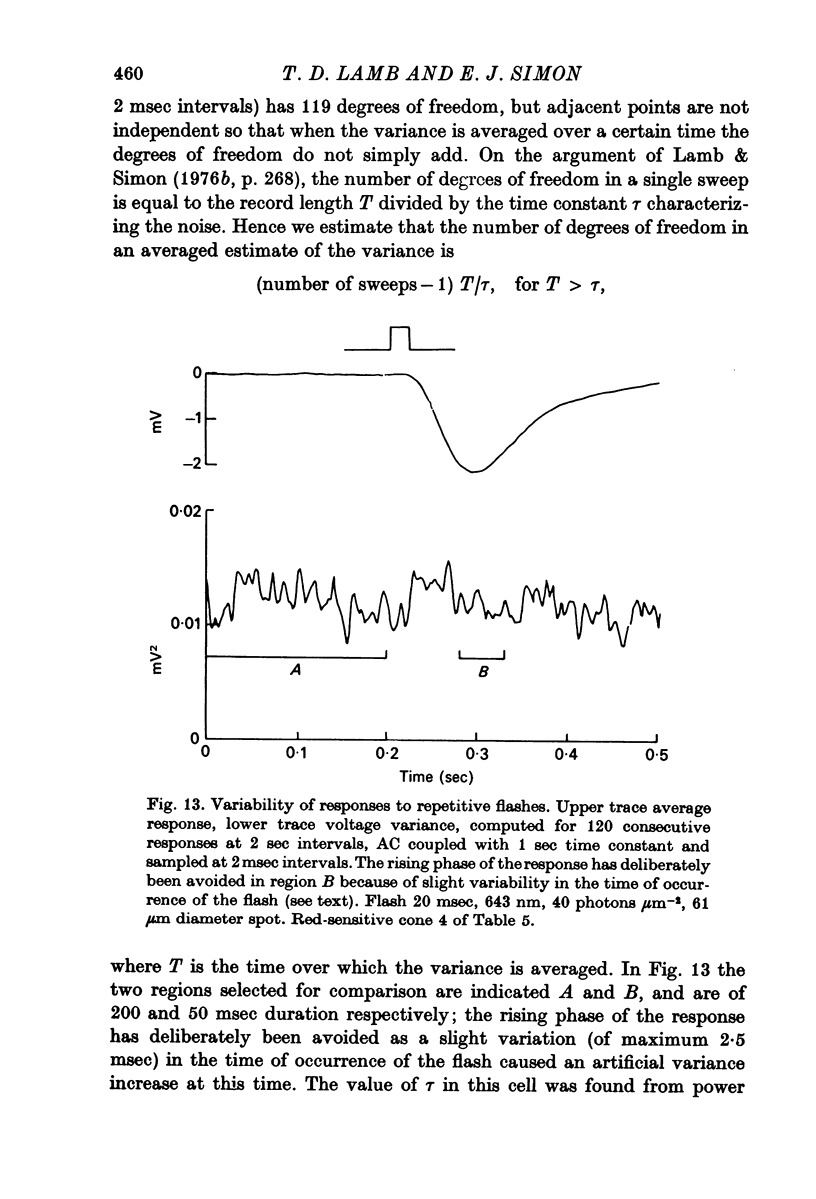
7. When the cell was transiently depolarized during the differentiated component following steady illumination, the noise was less than it was after prolonged darkness.
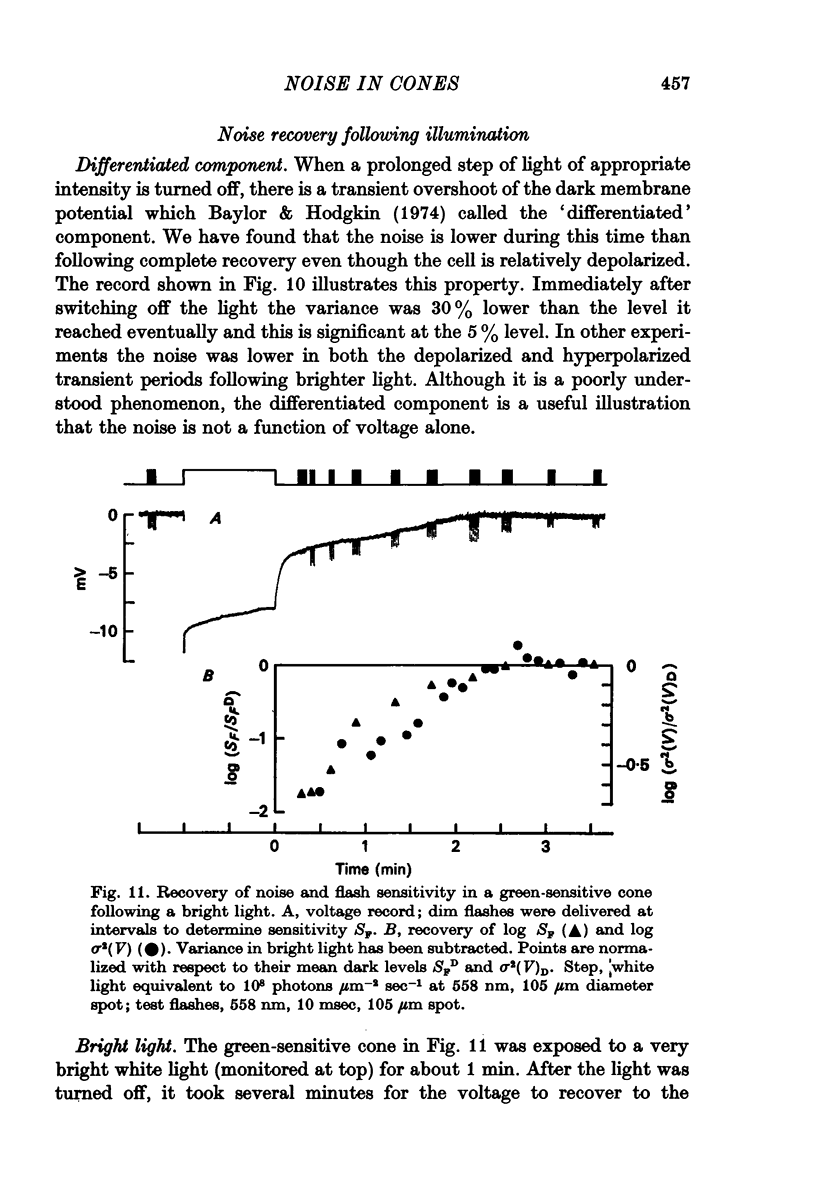
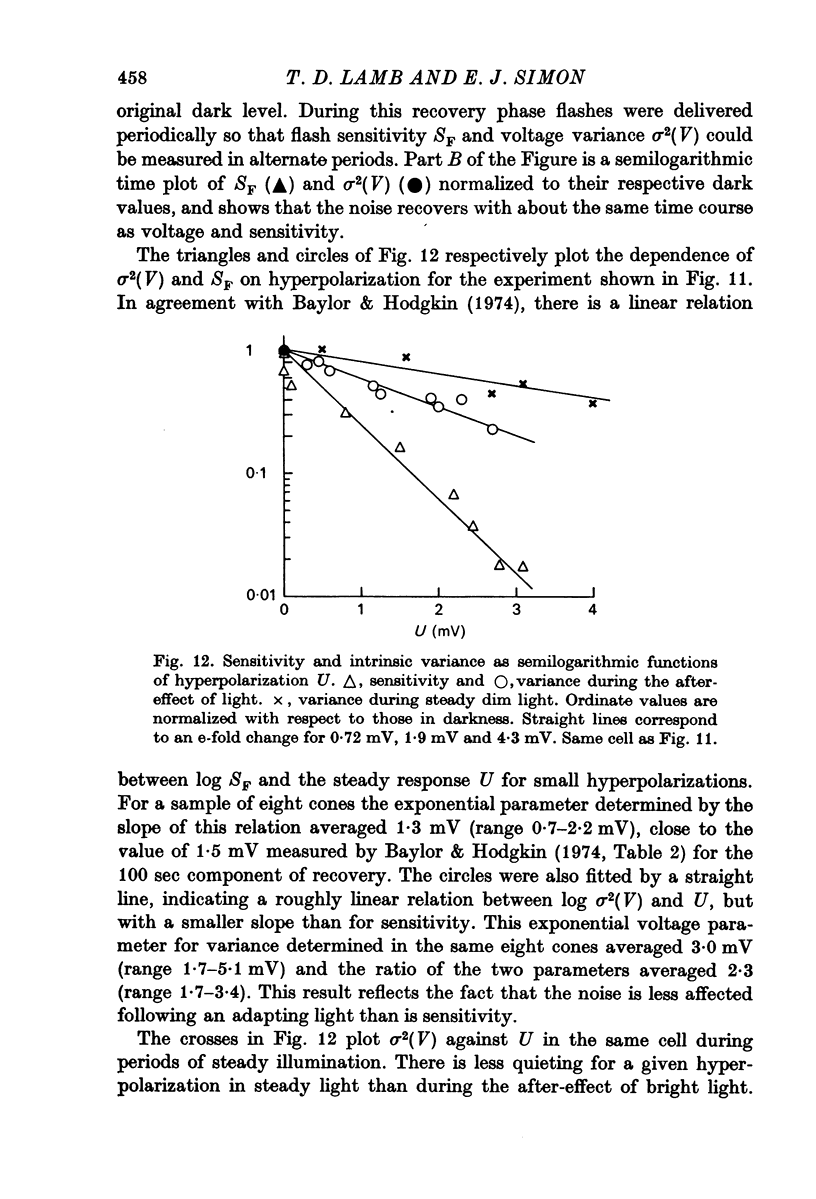
8. In the after-effect of bright light, the time course of recovery of noise was the same as that of flash sensitivity and voltage. The noise was reduced e-fold for hyperpolarizations averaging 3 mV while for sensitivity this reduction occurred for 1·3 mV. For a given hyperpolarization the noise was lower during the after-effect than during steady dim illumination.
9. When a series of dim flashes was delivered to a cone, no significant increase in variance over the dark noise was detected during the photo-response. This implies that each photoisomerization evokes no more than about 1·5 μV at the peak of the response in a coupled cone, corresponding to about 50 μV in an isolated cone.
10. The elementary shot events underlying the noise are about 100 μV in amplitude in an isolated cone, have a characteristic time constant of 16-60 msec and reflect unit conductance fluctuations of about 16 pS (S, Siemen ≡ Ω-1).
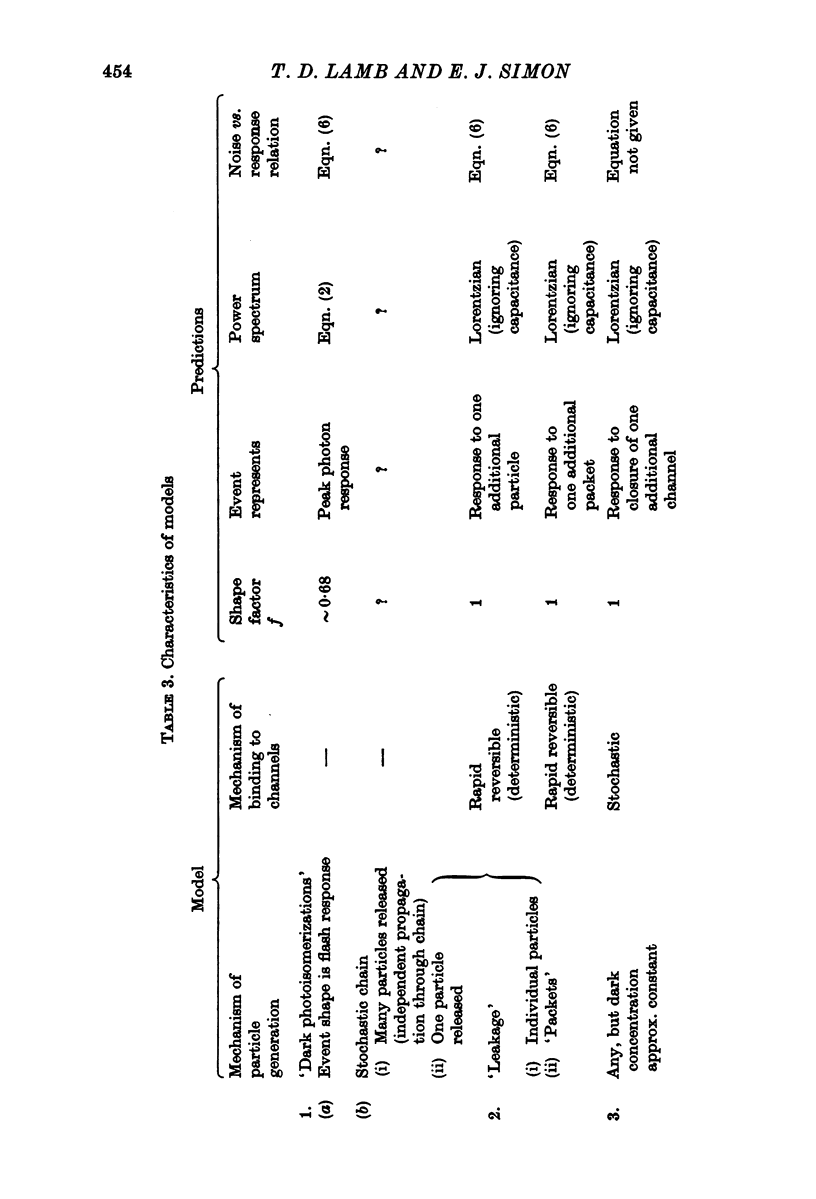
11. It is concluded that the noise source is internal to the cones. We postulate that the noise arises from opening and closing of the light-sensitive ionic channels in the outer segment, and that in darkness there is a residual concentration of the blocking substance which on average closes up to about one third of the channels. It seems likely that the unit event involves a considerable number of blocking molecules and ionic channels.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Levinson S. R. Tetrodotoxin binding to normal depolarized frog muscle and the conductance of a single sodium channel. J Physiol. 1975 May;247(2):483–509. doi: 10.1113/jphysiol.1975.sp010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Kinetics of synaptic transfer from receptors to ganglion cells in turtle retina. J Physiol. 1977 Oct;271(2):425–448. doi: 10.1113/jphysiol.1977.sp012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. Reconstruction of the electrical responses of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Wanke E. Channel noise in nerve membranes and lipid bilayers. Q Rev Biophys. 1975 Nov;8(4):451–506. doi: 10.1017/s0033583500001967. [DOI] [PubMed] [Google Scholar]
- Dipolo R., Requena J., Brinley F. J., Jr, Mullins L. J., Scarpa A., Tiffert T. Ionized calcium concentrations in squid axons. J Gen Physiol. 1976 Apr;67(4):433–467. doi: 10.1085/jgp.67.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. D. On the theory of ion transport across the nerve membrane. IV. Noise from the open-close kinetics of K + channels. Biophys J. 1972 Aug;12(8):948–959. doi: 10.1016/S0006-3495(72)86136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. Power spectral measurements of noise in the turtle tetina [proceedings]. J Physiol. 1976 Dec;263(1):103P–105P. [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol. 1976 Dec;263(2):257–286. doi: 10.1113/jphysiol.1976.sp011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Spatial properties of horizontal cell responses in the turtle retina. J Physiol. 1976 Dec;263(2):239–255. doi: 10.1113/jphysiol.1976.sp011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Voltage noise observed in rods of the turtle retina. J Physiol. 1977 Nov;272(2):217–246. doi: 10.1113/jphysiol.1977.sp012042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J., Lamb T. D., Hodgkin A. L. Spontaneous voltage fluctuations in retinal cones and bipolar cells. Nature. 1975 Aug 21;256(5519):661–662. doi: 10.1038/256661a0. [DOI] [PubMed] [Google Scholar]
- Simon E. J. Two types of luminosity horizontal cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):199–211. doi: 10.1113/jphysiol.1973.sp010183. [DOI] [PMC free article] [PubMed] [Google Scholar]


