Abstract
1. Synaptic transfer between photoreceptors and impulse-generating cells was studied in isolated eyecups from turtles. Single red-sensitive cones or rods were stimulated by current passed through an intracellular electrode, and impulses generated by the resulting synaptic action were recorded with an external micro-electrode. This technique permits study of retinal transmission without the operation of the visual transduction mechanism. Antidromic stimulation of the optic nerve indicated that most of the impulse-generating cells were ganglion cells.
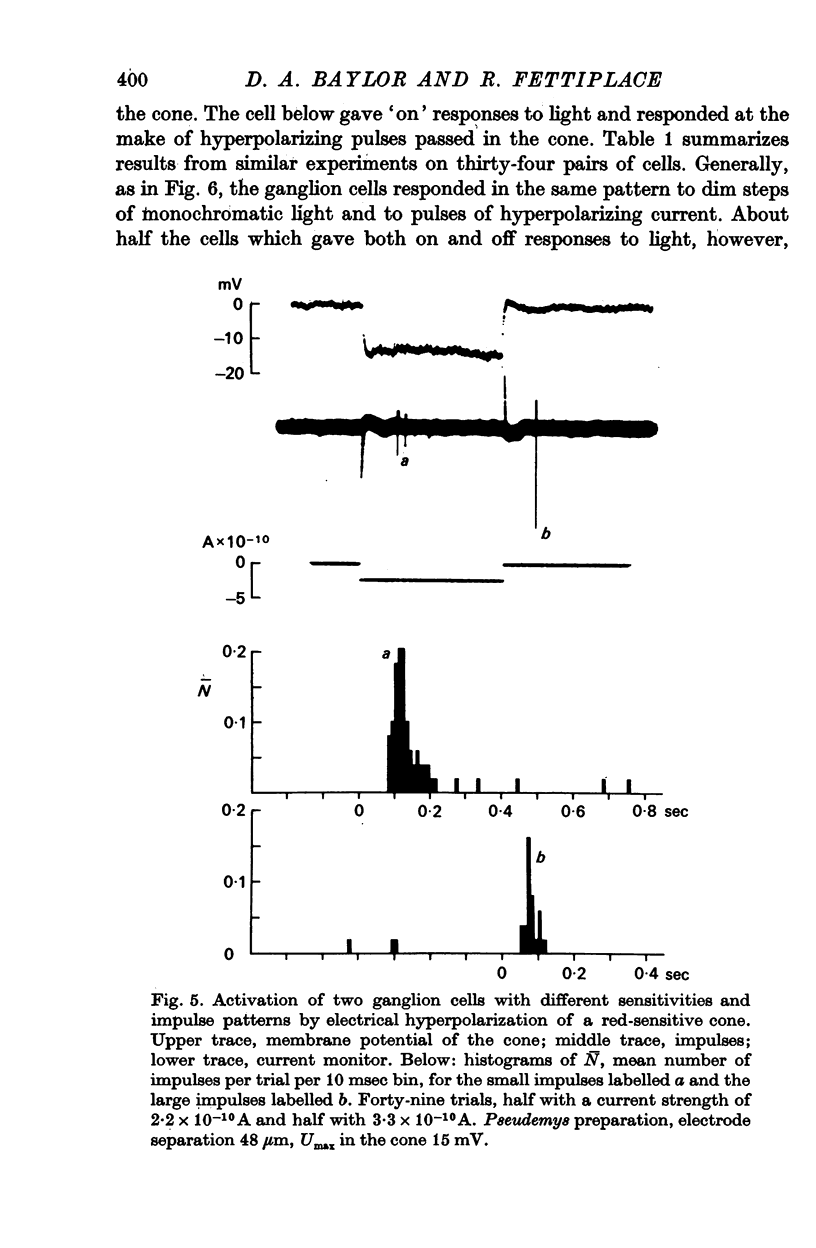
2. Individual ganglion cells responded transiently to changes in the membrane potential of a receptor and could be classified into three groups on the basis of the direction of the effective change in potential. Off centre ganglion cells responded selectively to depolarizations of a receptor, while on centre ganglion cells responded selectively to hyperpolarizations. On-off ganglion cells responded to both depolarizations and hyperpolarizations of a receptor.
3. Ganglion cells gave the same pattern of response to electrical hyperpolarization of a receptor and to light in the centre of their receptive fields. Subthreshold depolarizing currents passed in a receptor antagonized the ganglion cell's response to light, and subthreshold hyperpolarizing currents reinforced the response. These observations are consistent with the view that the hyperpolarization generated by visual transduction is responsible for regulating the release of transmitter at the first retinal synapse.
4. When a receptor was stimulated with weak current pulses of fixed intensity the number and latency of the ganglion cell impulses fluctuated randomly in successive trials. The relation between the fraction of trials yielding a response and the stimulus intensity was broad. These results indicate that the link between retinal input and output is noisy.
5. In the most sensitive pairs of cells, a response of one or more impulses could be obtained in half the trials with a current of about 2 × 10-11 A, which changed the potential of the receptor by 1-2 mV. A current of similar magnitude would be developed by about 130 photoisomerizations in a red-sensitive cone or 50 photoisomerizations in a rod.
6. Dim background light producing a steady hyperpolarization of a few millivolts in the rods raised the threshold for electrically-evoked transmission from a rod to a ganglion cell. In experiments on red-sensitive cones, background light raised the threshold in the off pathway, in which depolarization was the effective stimulus, and lowered the threshold in the on pathway, in which hyperpolarization was the effective stimulus. These changes in sensitivity were not accompanied by obvious changes in the input resistance of the stimulated receptor. Regulation of retinal sensitivity in background light thus involves changes in synaptic transfer as well as changes in the sensitivity of the visual transduction mechanism.
Full text
PDF
































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R., Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res. 1971;Suppl 3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Light path and photon capture in turtle photoreceptors. J Physiol. 1975 Jun;248(2):433–464. doi: 10.1113/jphysiol.1975.sp010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Transmission of signals from photoreceptors to ganglion cells in the eye of the turtle. Cold Spring Harb Symp Quant Biol. 1976;40:529–536. doi: 10.1101/sqb.1976.040.01.049. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Functional characteristics of lateral interactions between rods in the retina of the snapping turtle. J Physiol. 1976 Jul;259(2):251–282. doi: 10.1113/jphysiol.1976.sp011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hecht S., Shlaer S., Pirenne M. H. ENERGY, QUANTA, AND VISION. J Gen Physiol. 1942 Jul 20;25(6):819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Electrophysiological study of single neurons in the inner nuclear layer of the carp retina. Vision Res. 1969 Jan;9(1):37–55. doi: 10.1016/0042-6989(69)90030-3. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Spatial properties of horizontal cell responses in the turtle retina. J Physiol. 1976 Dec;263(2):239–255. doi: 10.1113/jphysiol.1976.sp011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L. Centrifugal actions on amacrine and ganglion cells in the retina of the turtle. J Physiol. 1976 Feb;255(1):137–155. doi: 10.1113/jphysiol.1976.sp011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. I. Intracellular analysis of chloride-sensitive electrogenic properties of receptors, horizontal cells, bipolar cells, and amacrine cells. J Gen Physiol. 1976 Jun;67(6):639–659. doi: 10.1085/jgp.67.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Simon E. J. Properties of centre-hyperpolarizing, red-sensitive bipolar cells in the turtle retina. J Physiol. 1975 Jun;248(2):317–334. doi: 10.1113/jphysiol.1975.sp010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Electrical properties of the rod syncytium in the retina of the turtle. J Physiol. 1976 May;257(2):379–406. doi: 10.1113/jphysiol.1976.sp011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Organization of on-off cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):1–14. doi: 10.1113/jphysiol.1973.sp010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of bipolar cells in the retina of the turtle. J Physiol. 1974 Jan;236(1):211–224. doi: 10.1113/jphysiol.1974.sp010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J., Lamb T. D., Hodgkin A. L. Spontaneous voltage fluctuations in retinal cones and bipolar cells. Nature. 1975 Aug 21;256(5519):661–662. doi: 10.1038/256661a0. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]


