Abstract
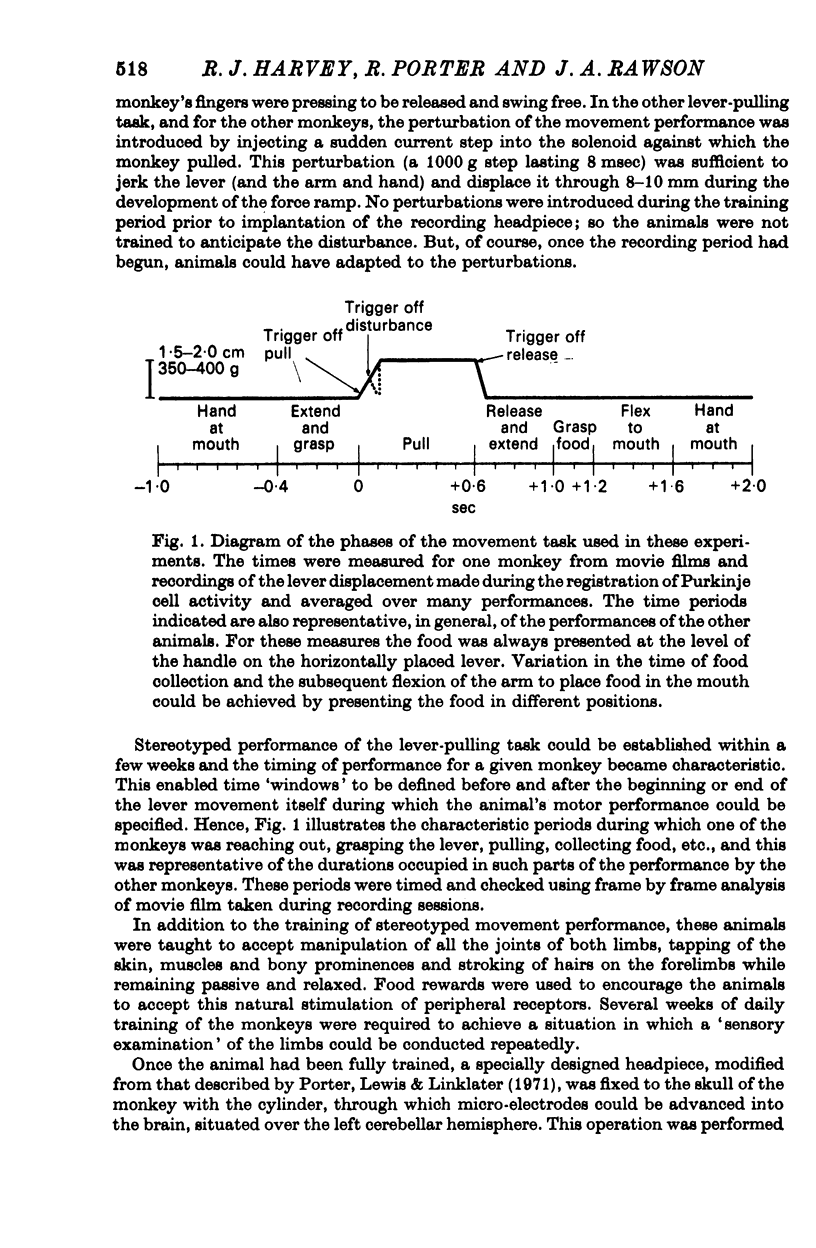
1. Conscious monkeys were trained with food rewards to perform movement tasks with the left hand and to accept manipulation of the joints and muscles and natural non-noxious stimulation of the skin of both forelimbs.
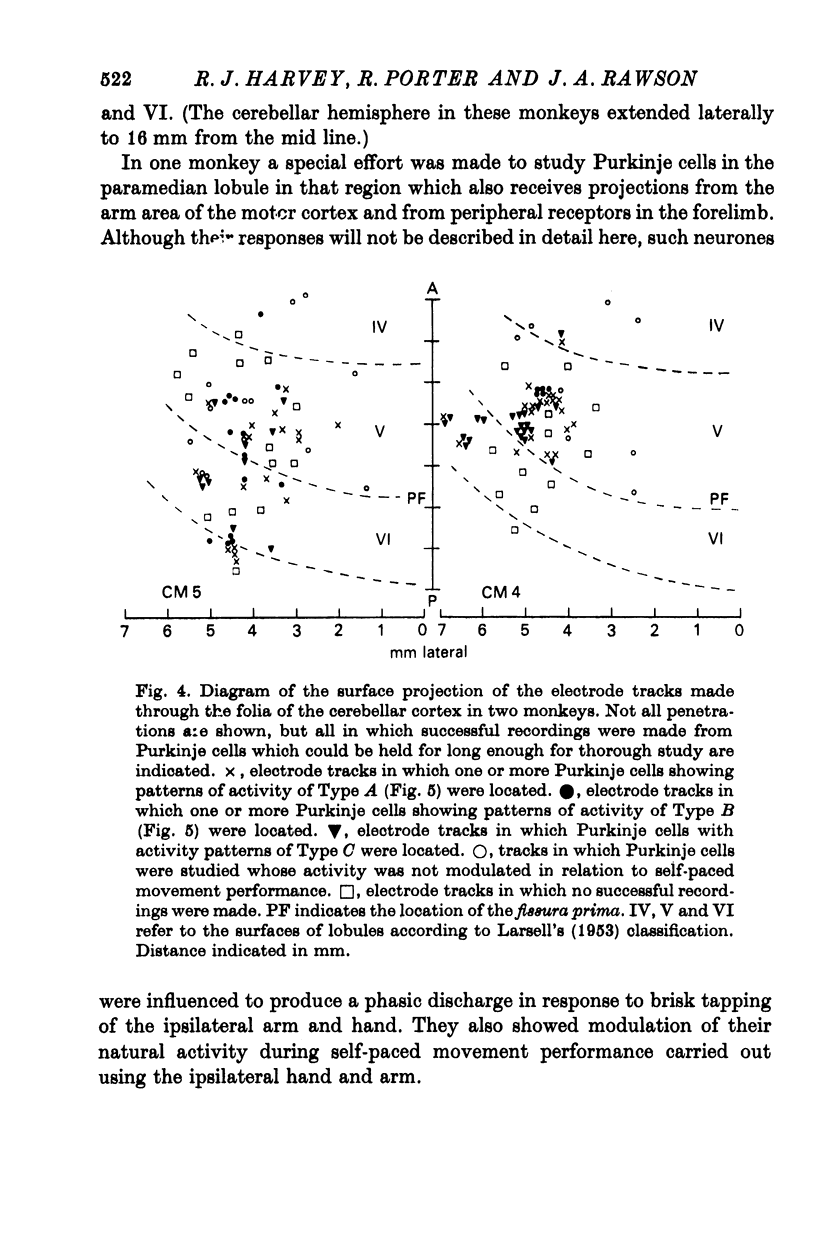
2. Recordings were made from 230 Purkinje cells situated in the paravermal region of lobules V and VI or immediately adjacent folia of the left cerebellum in a region from 2 to 7 mm from the mid line. These neurones were all in a zone which was demonstrated to receive inputs from the ipsilateral hand and which is known to receive projections, via the pontine nuclei from the `arm area' of motor cortex in the right hemisphere.
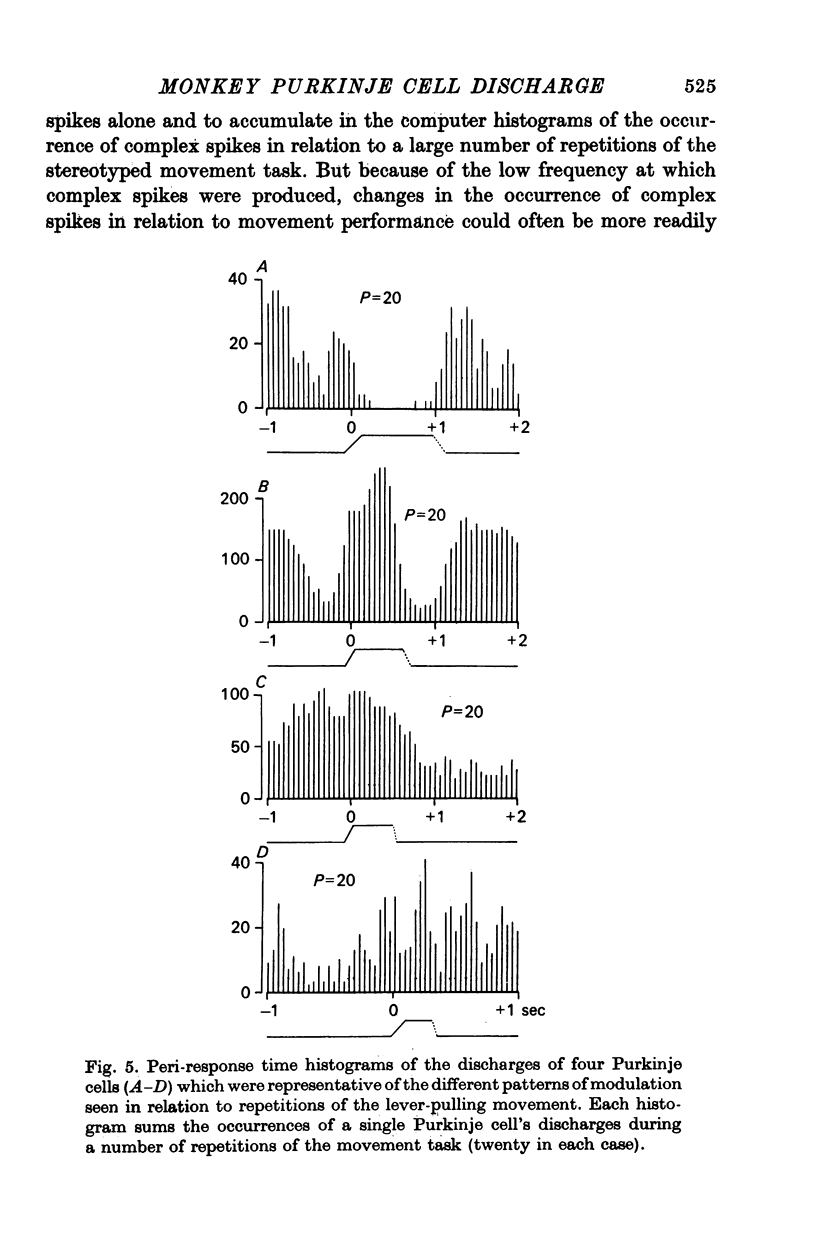
3. Modulation of the natural activity of 182 of these 230 Purkinje cells (79%) occurred in a reproducible manner in temporal association, each with a particular phase of the self-paced movement tasks performed by the animal using the ipsilateral arm and hand. The patterns of modulation of Purkinje cell firing in this limited zone of cerebellar cortex could be classified into one of four groups, and each cell's discharge was associated with a particular aspect of movement such as general arm flexion, shoulder retraction, elbow extension or elbow flexion whenever it occurred.
4. The cells were spontaneously active at rest. Most commonly, marked accelerations of the discharge were related to one direction of the particular aspect of movement and a reduction of activity or even total silence accompanied movement in the opposite direction.
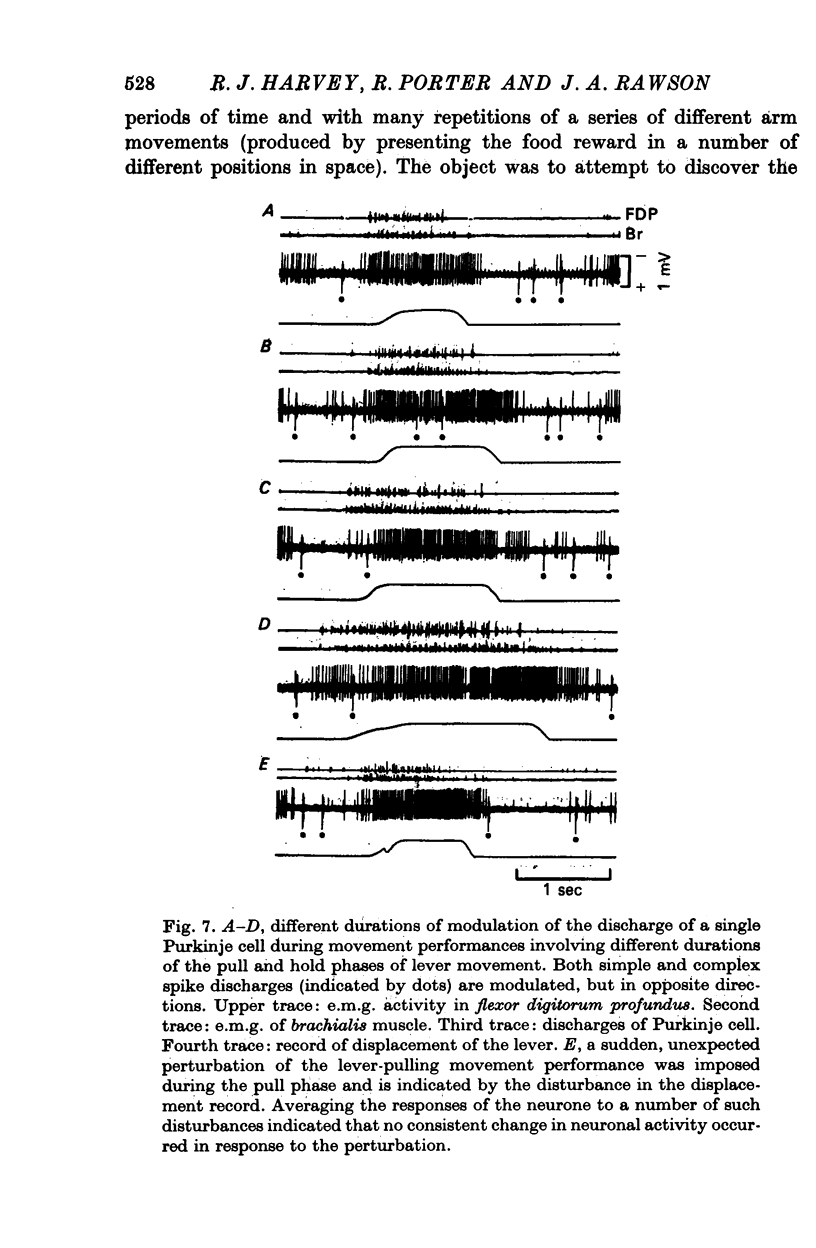
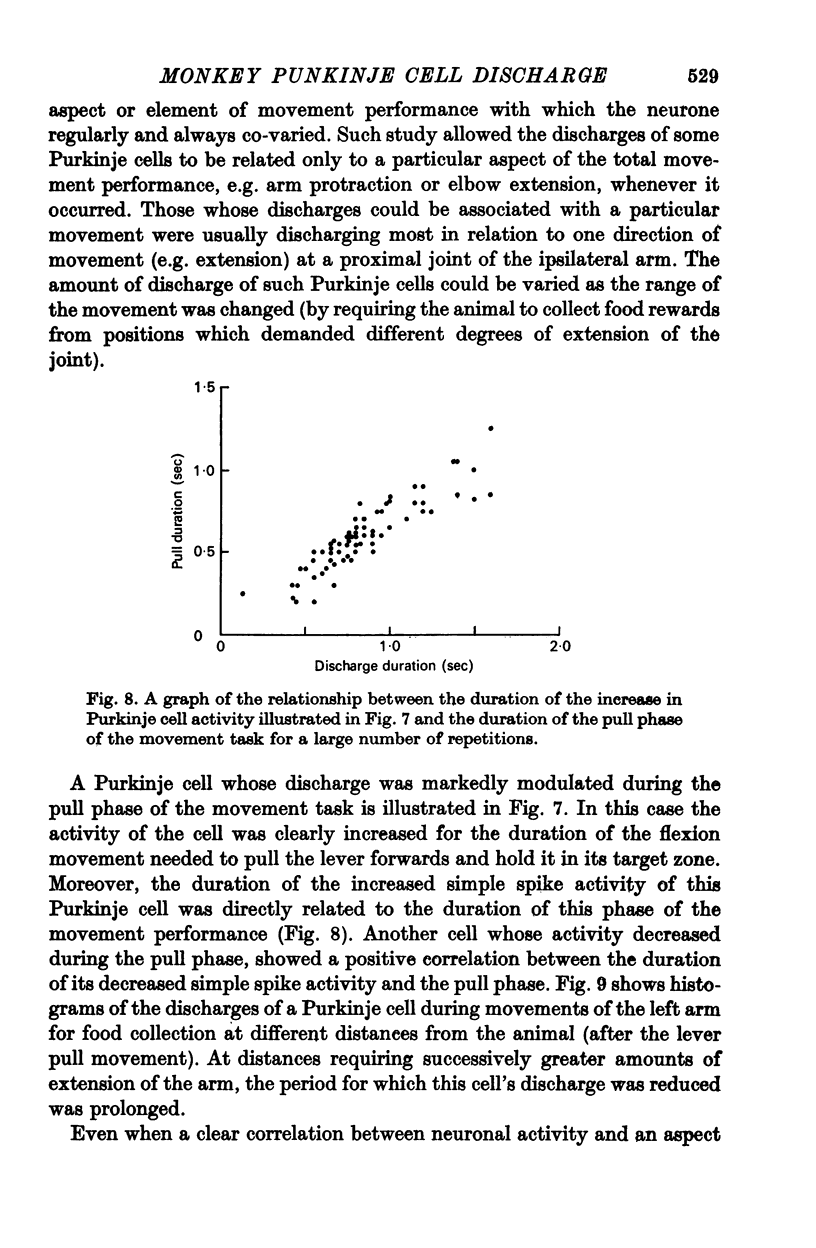
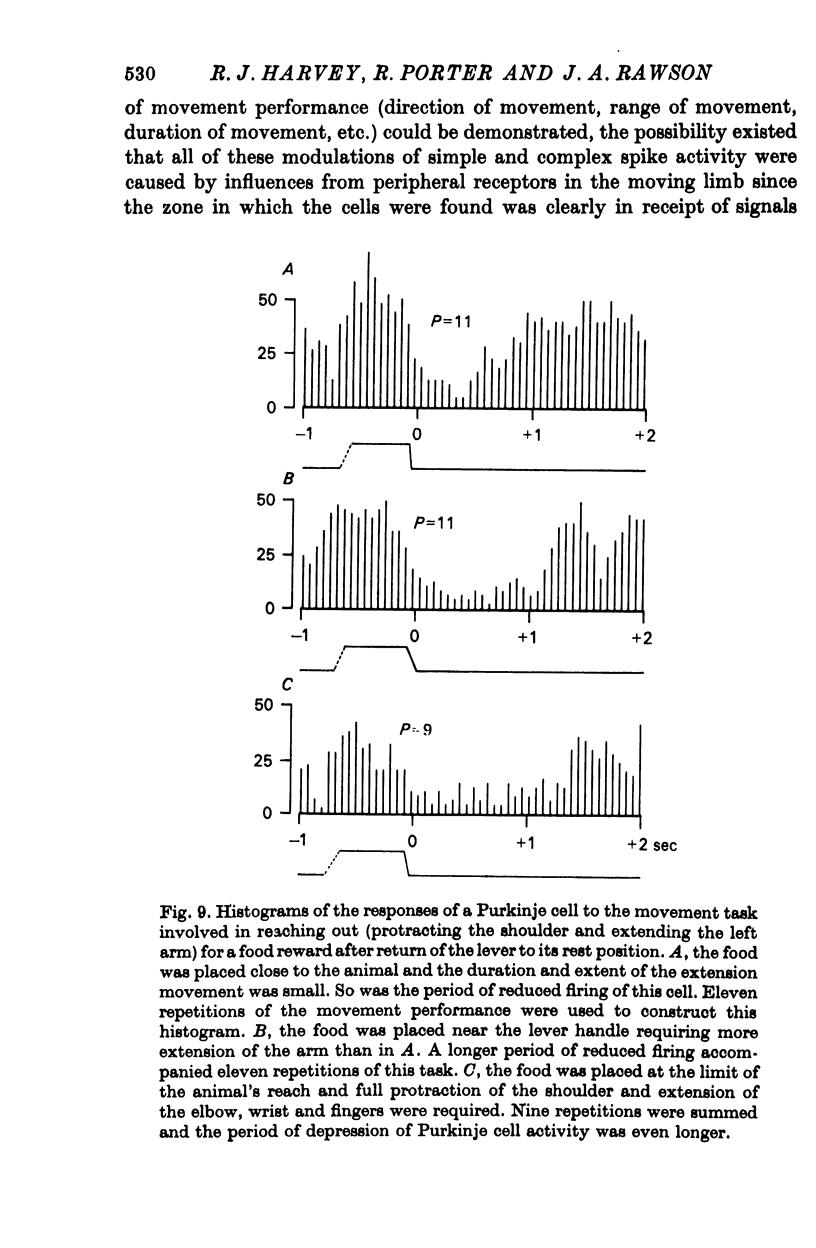
5. Variation of the amount of discharge demonstrated during a movement performance with which this discharge was characteristically associated could be related to the range of the movement or its duration, more activity being characteristic of more prolonged movement performance through larger angles of joint displacement.
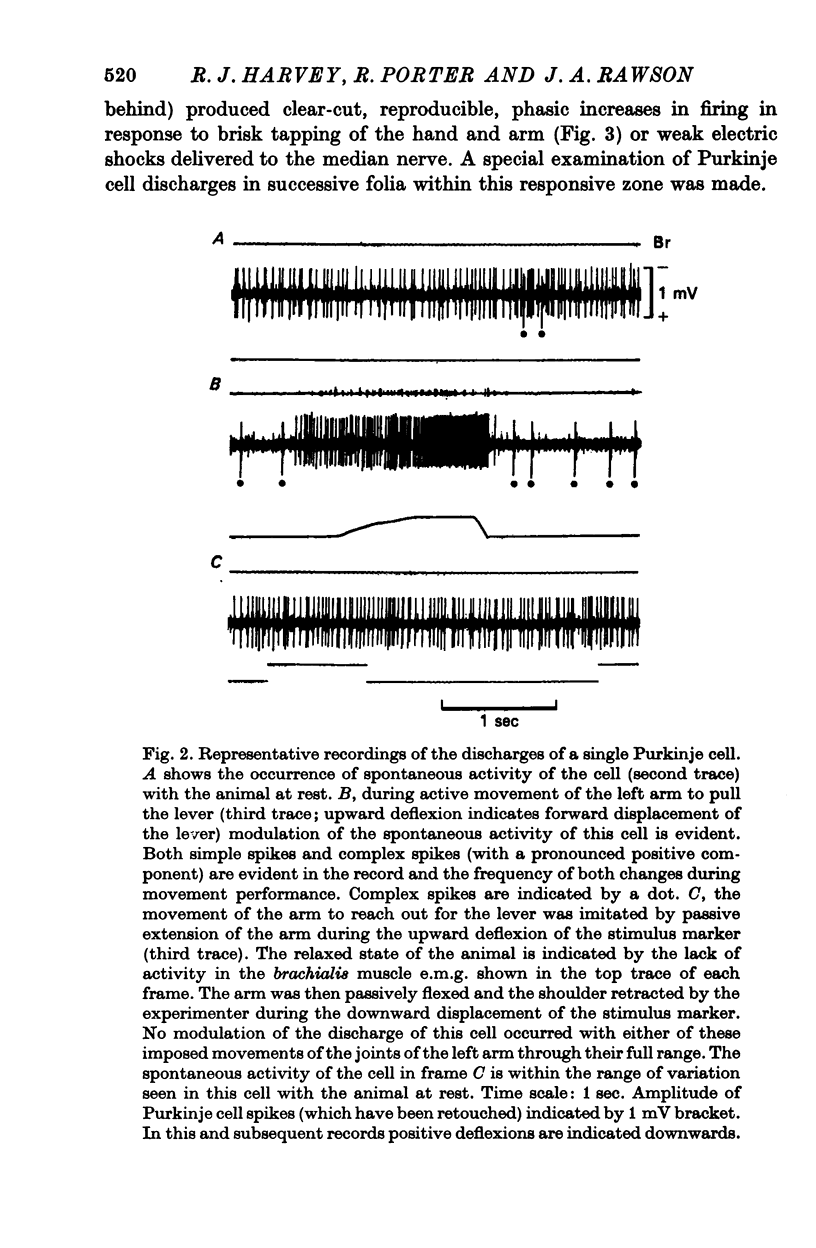
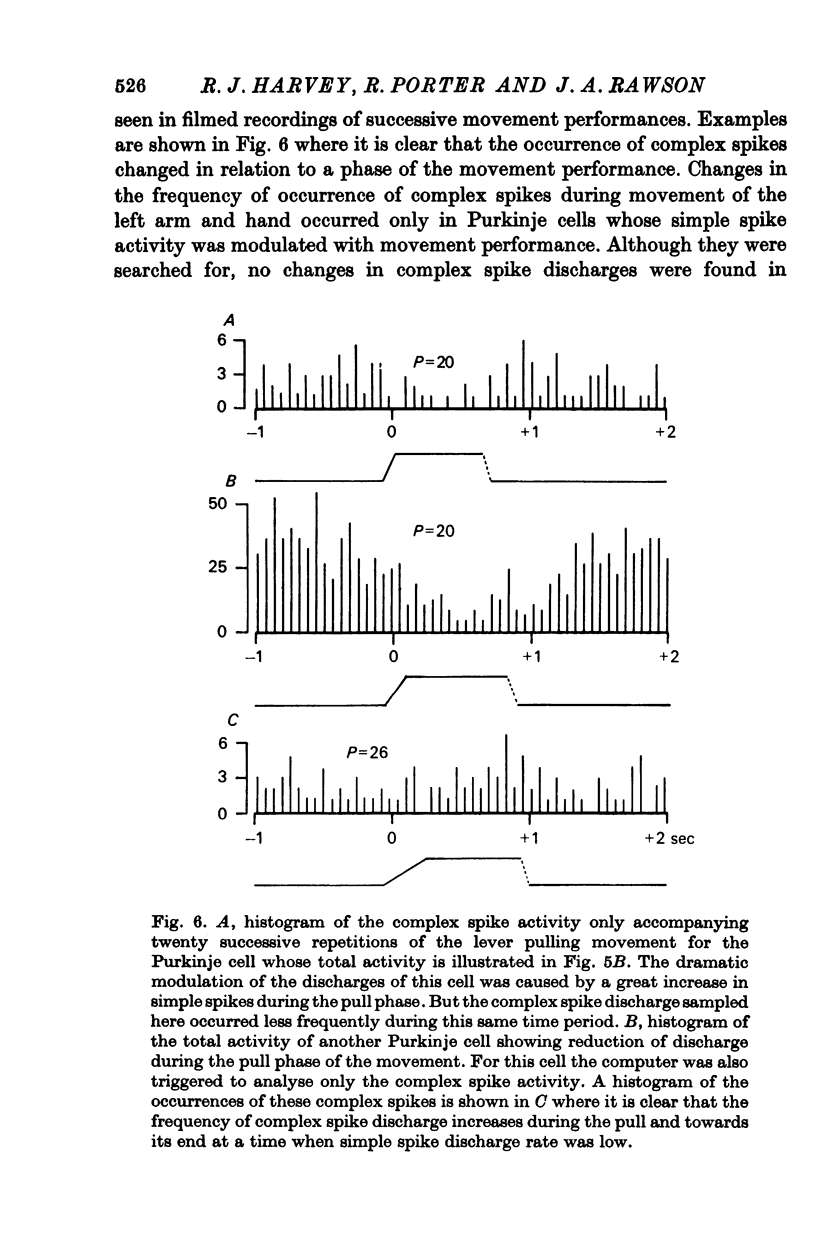
6. Both simple spikes and complex spikes of some cells showed characteristic modulation of their activity during the monkey's self-initiated movements. Cells whose simple spikes did not change in frequency during the movement task, also showed no modification of complex spike discharge.
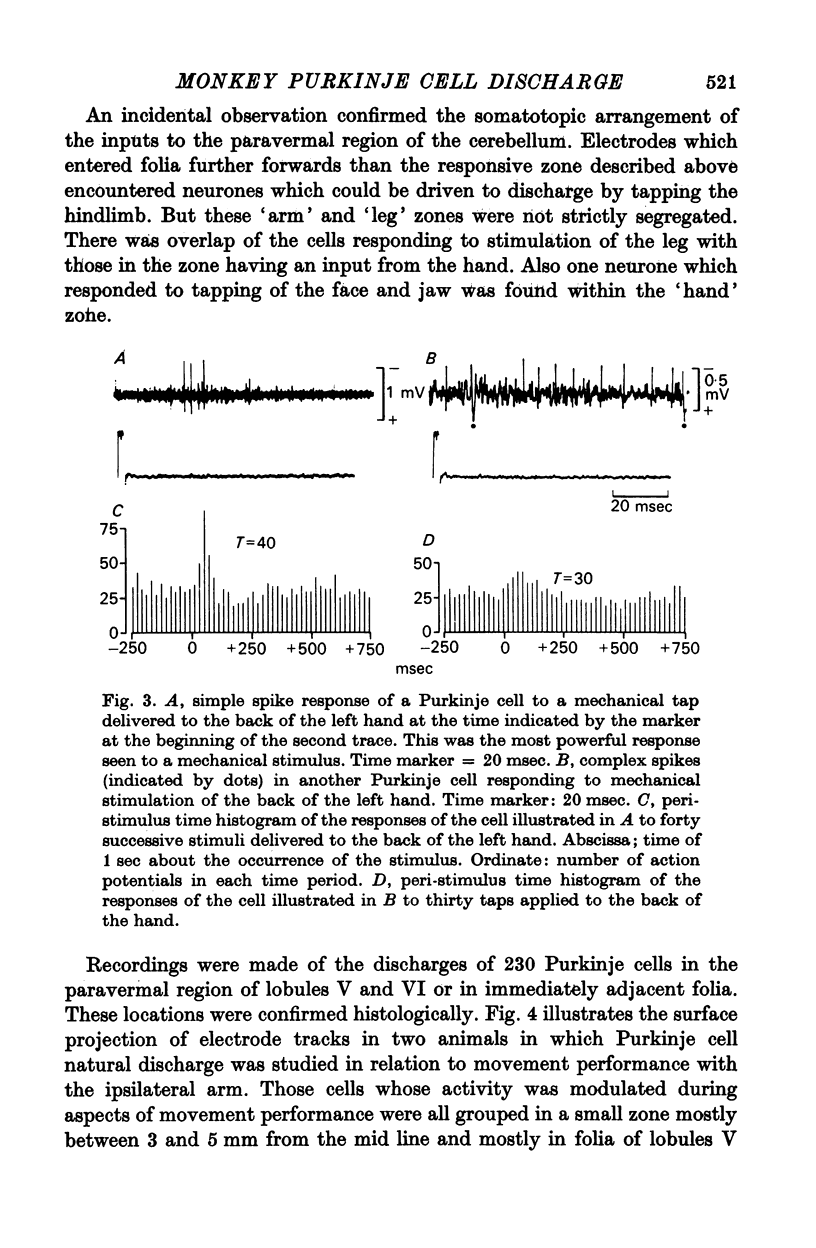
7. Of the 182 neurones whose discharges changed during active movement performance, 105 (roughly 60%) were demonstrated to be in receipt of an input from peripheral receptors in the hand which could be activated by brisk tapping of the skin or brushing of hairs. In contrast, none of the Purkinje cells whose discharges were unchanged during arm movements could be demonstrated to receive such an input.
8. Movement of joints through their full range and prodding of muscles were completely ineffective stimuli for causing changes in Purkinje cell firing in this zone of the cerebellar cortex while the animal was passive and relaxed. Imposed perturbations of movement performance injected unexpectedly during the execution of a movement task were also ineffective in modifying the discharge of these Purkinje cells in relation to the task.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKHART J. M., MORUZZI G., SNIDER R. S. Spike discharges of single units in the cerebellar cortex. J Neurophysiol. 1950 Nov;13(6):465–486. doi: 10.1152/jn.1950.13.6.465. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber H. H. Motor functions of cerebellum and basal ganglia: the cerebellocortical saccadic (ballistic) clock, the cerebellonuclear hold regulator, and the basal ganglia ramp (voluntary speed smooth movement) generator. Kybernetik. 1971 Apr;8(4):157–162. doi: 10.1007/BF00290561. [DOI] [PubMed] [Google Scholar]
- LARSELL O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953 Aug;99(1):135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- Lemon R. N., Hanby J. A., Porter R. Relationship between the activity of precentral neurones during active and passive movements in conscious monkeys. Proc R Soc Lond B Biol Sci. 1976 Oct 29;194(1116):341–373. doi: 10.1098/rspb.1976.0083. [DOI] [PubMed] [Google Scholar]
- Porter R., Lewis M. M., Linklater G. F. A headpiece for recording discharges of neurons in unrestrained monkeys. Electroencephalogr Clin Neurophysiol. 1971 Jan;30(1):91–93. doi: 10.1016/0013-4694(71)90210-0. [DOI] [PubMed] [Google Scholar]
- Porter R., Rack P. M. Timing of the responses in the motor cortex of monkeys to an unexpected disturbance of finger position. Brain Res. 1976 Feb 20;103(2):201–213. doi: 10.1016/0006-8993(76)90794-0. [DOI] [PubMed] [Google Scholar]
- SNIDER R. S., ELDRED E. Cerebrocerebellar relationships in the monkey. J Neurophysiol. 1952 Jan;15(1):27–40. doi: 10.1152/jn.1952.15.1.27. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968 Sep;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol. 1970 Jul;33(4):527–536. doi: 10.1152/jn.1970.33.4.527. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970 Jul;33(4):537–547. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Thach W. T., Jr Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967 Jul;30(4):675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Timing of activity in cerebellar dentate nucleus and cerebral motor cortex during prompt volitional movement. Brain Res. 1975 May 2;88(2):233–241. doi: 10.1016/0006-8993(75)90387-x. [DOI] [PubMed] [Google Scholar]


